Bio-Vitrimers for Sustainable Circular Bio-Economy
Abstract
1. Introduction
2. Sustainable Circular Bioeconomy
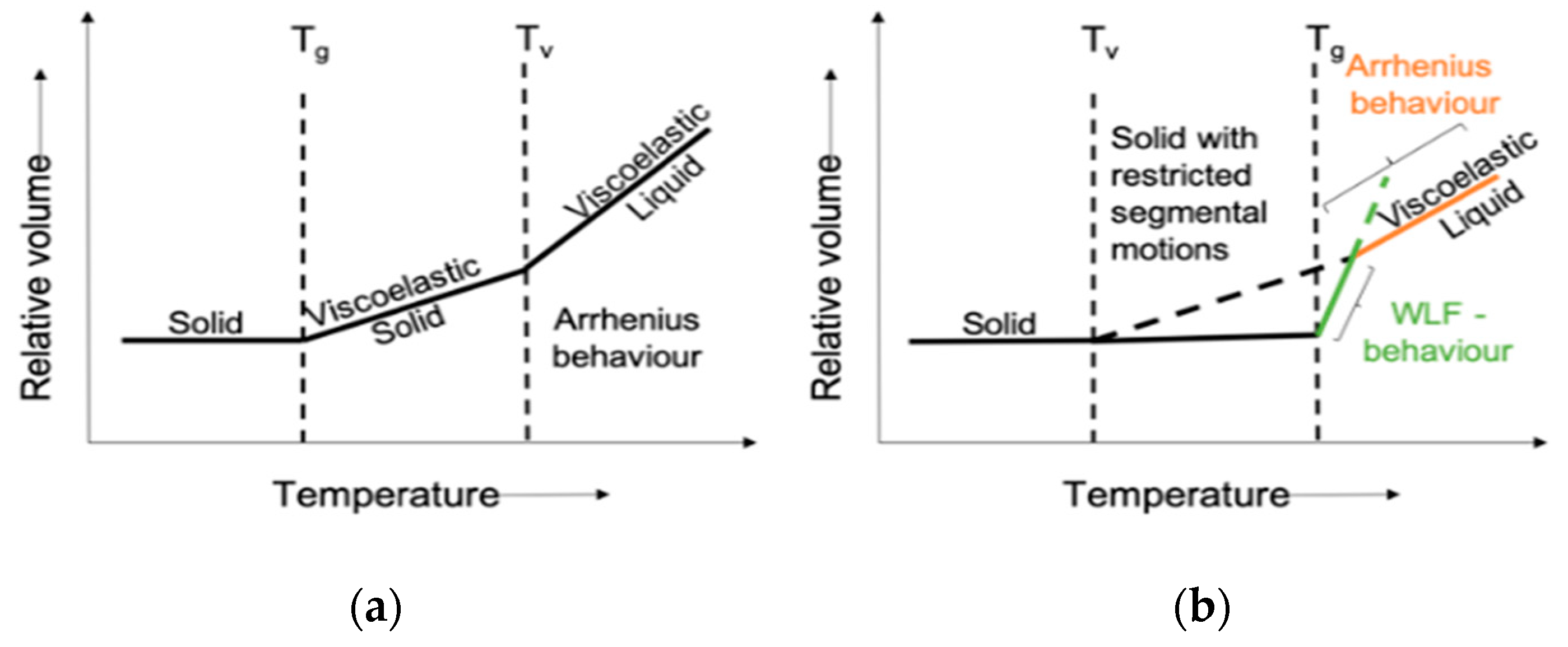
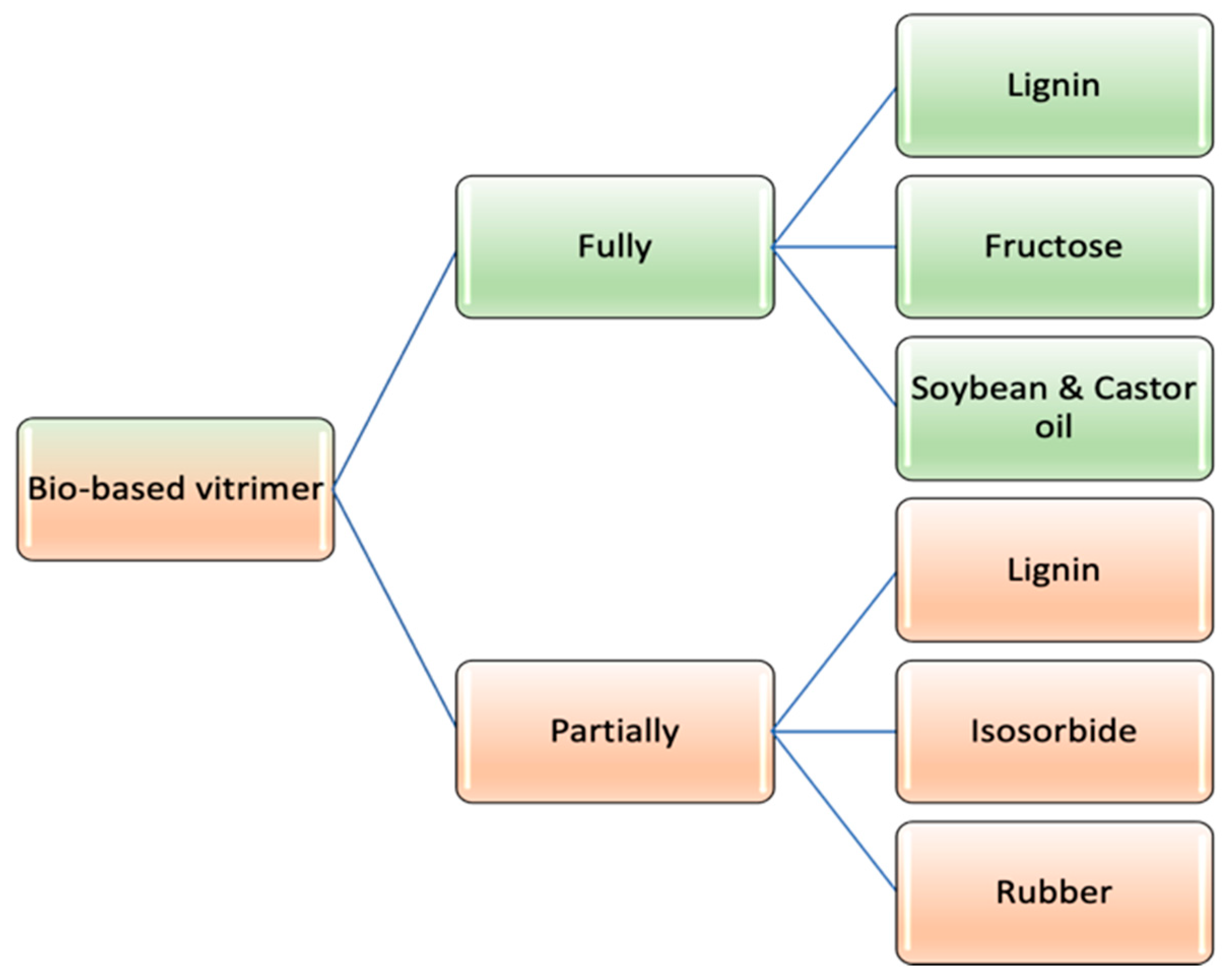
3. Classification of Bio-Vitrimers
3.1. Fully Bio-Based Vitrimer
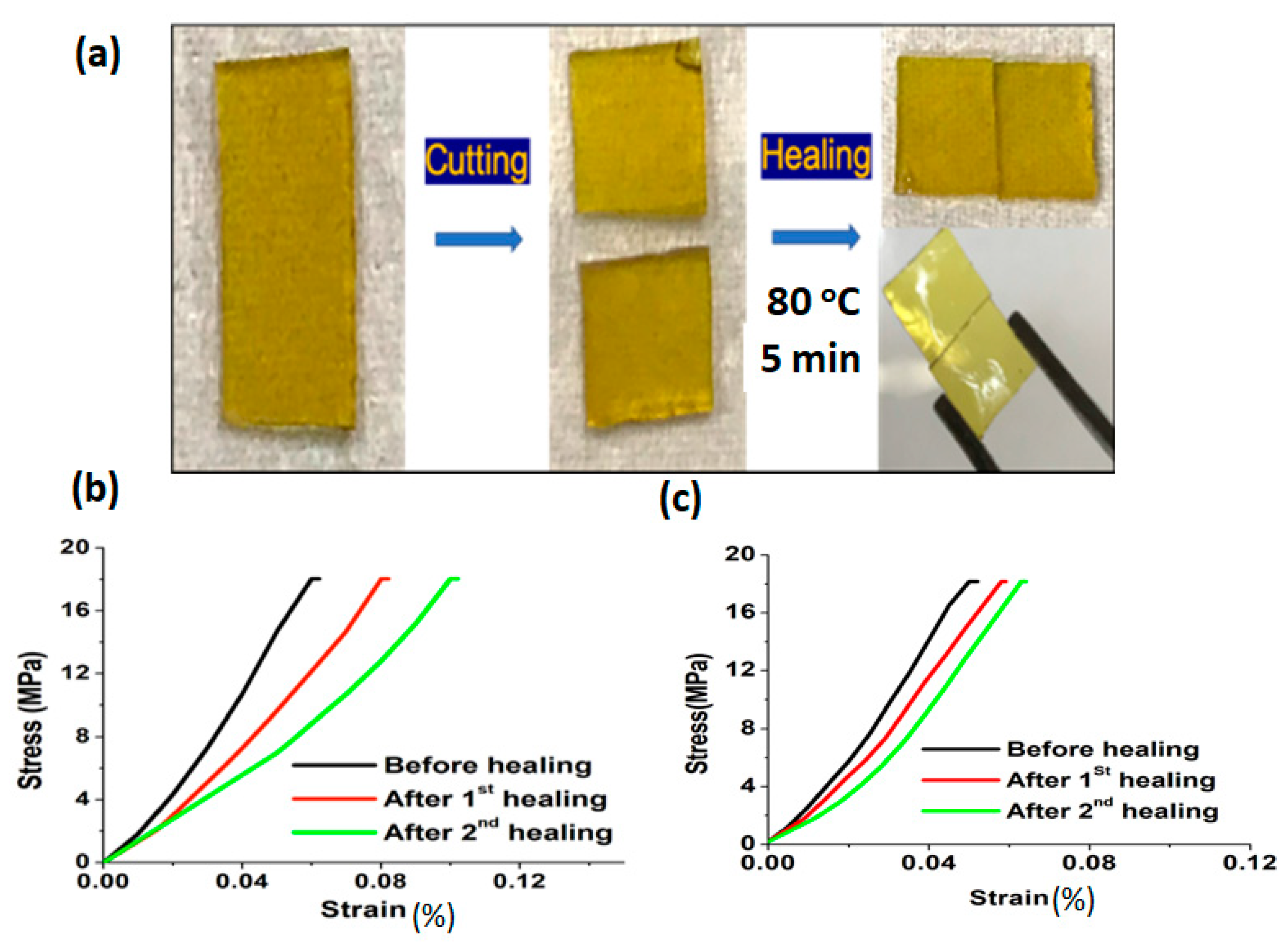
3.1.1. Lignin Derivatives
3.1.2. Fructose Derivatives
3.1.3. Soybean and Castor Oil
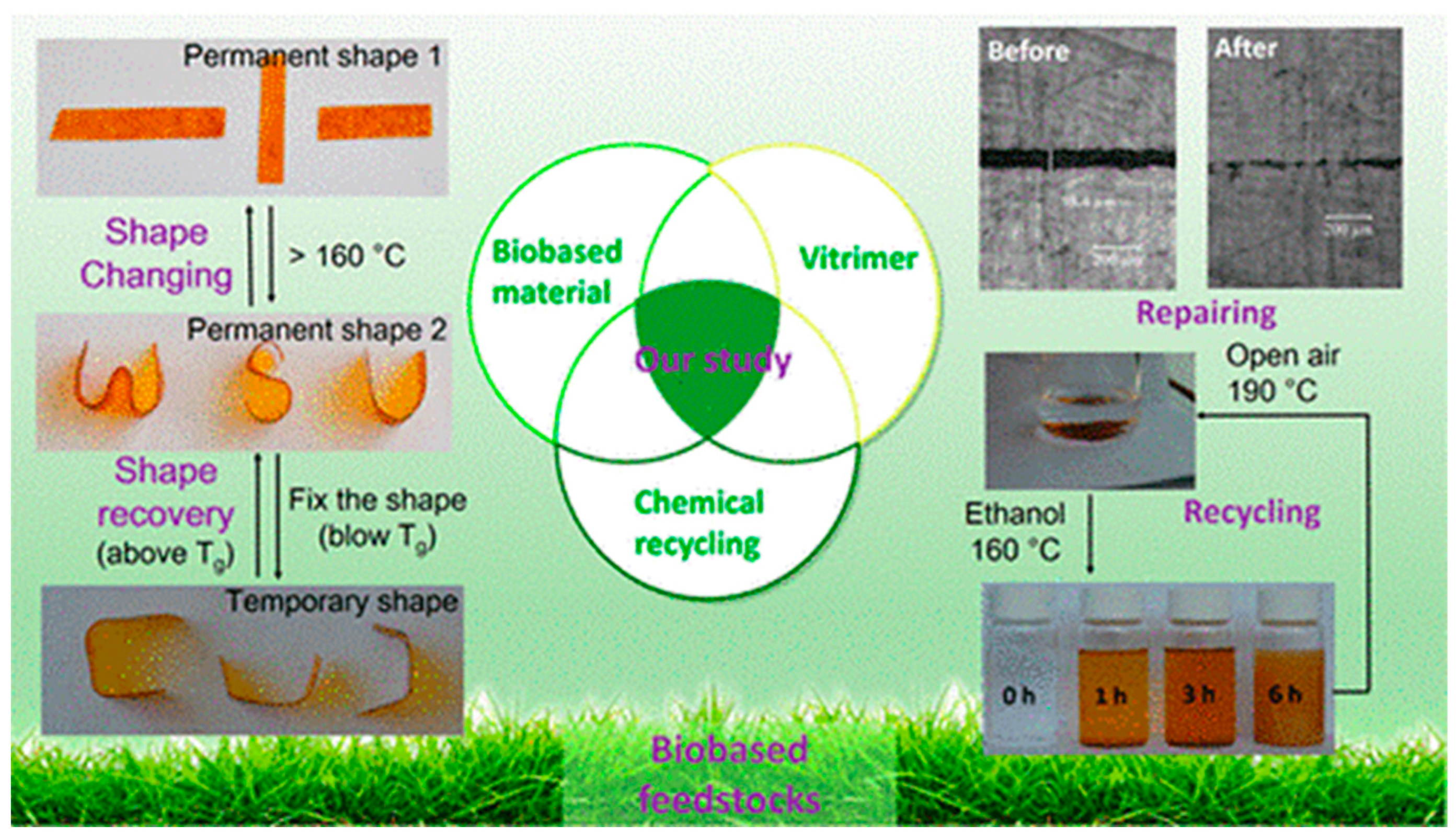
3.2. Partially Bio-Based Vitrimers
3.2.1. Lignin Based Derivatives
3.2.2. Isosorbide Derivative
4. Bio-Based Vitrimer Composites
5. Research Gap and Limitations
6. Outlook and Prospective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Shiota, A.; Ober, C.K. Rigid rod and liquid crystalline thermosets. Prog. Polym. Sci. 1997, 22, 975–1000. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Deléglise, M.; Lacrampe, M.-F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Rutz, B.H.; Berg, J.C. A review of the feasibility of lightening structural polymeric composites with voids without compromising mechanical properties. Adv. Colloid Interface Sci. 2010, 160, 56–75. [Google Scholar] [CrossRef]
- Kaiser, T. Highly crosslinked polymers. Prog. Polym. Sci. 1989, 14, 373–450. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, A.; Rana, S.; Guadagno, L. An Overview on Carbon Fiber-Reinforced Epoxy Composites: Effect of Graphene Oxide Incorporation on Composites Performance. Polymers 2022, 14, 1548. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Zhang, C.; Show, P.-L.; Ho, S.-H. Progress and perspective on algal plastics—A critical review. Bioresour. Technol. 2019, 289, 121700. [Google Scholar] [CrossRef] [PubMed]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.X. Future Directions for Sustainable Polymers. Trends Chem. 2019, 1, 148–151. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.H.; Michael, P.; Rana, S. Reversible self-healing carbon-based nanocomposites for structural applications. Polymers 2019, 11, 903. [Google Scholar] [CrossRef]
- Son, D.H.; Kim, G.Y.; Jeong, J.E.; Lee, S.H.; Park, Y.I.; Kong, H.; Cheong, I.W.; Kim, J.C. Influence of Material Properties on the Damage-Reporting and Self-Healing Performance of a Mechanically Active Dynamic Network Polymer in Coating Applications. Molecules 2021, 26, 2468. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Yin, T.; Rong, M.Z.; Zhang, M.Q. Self healing in polymers and polymer composites. Concepts, realization and outlook: A review. Express Polym. Lett. 2008, 2, 238–250. [Google Scholar] [CrossRef]
- Sordo, F.; Michaud, V. Processing and damage recovery of intrinsic self-healing glass fiber reinforced composites. Smart Mater. Struct. 2016, 25, 84012. [Google Scholar] [CrossRef]
- Guimard, N.K.; Oehlenschlaeger, K.K.; Zhou, J.; Hilf, S.; Schmidt, F.G.; Barner-Kowollik, C. Current trends in the field of self-healing materials. Macromol. Chem. Phys. 2012, 213, 131–143. [Google Scholar] [CrossRef]
- Raimondo, M.; Calabrese, E.; Binder, W.H.; Michael, P.; Rana, S.; Guadagno, L. Tunneling Atomic Force Microscopy Analysis of Supramolecular Self-Responsive Nanocomposites. Polymers 2021, 13, 1401. [Google Scholar] [CrossRef]
- Herbst, F.; Döhler, D.; Michael, P.; Binder, W.H. Self-healing polymers via supramolecular forces. Macromol. Rapid Commun. 2013, 34, 203–2020. [Google Scholar] [CrossRef]
- Chen, S.; Mahmood, N.; Beiner, M.; Binder, W.H. Self-Healing Materials from V- and H-Shaped Supramolecular Architectures. Angew. Chemie Int. Ed. 2015, 54, 10188–10192. [Google Scholar] [CrossRef]
- Herbst, F.; Schröter, K.; Gunkel, I.; Gröger, S.; Thurn-Albrecht, T.; Balbach, J.; Binder, W.H. Aggregation and Chain Dynamics in Supramolecular Polymers by Dynamic Rheology: Cluster Formation and Self-Aggregation. Macromolecules 2010, 43, 10006–10016. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Sottos, N.R.; White, S.R. Nanocapsules for self-healing materials. Compos. Sci. Technol. 2007, 68, 978–986. [Google Scholar] [CrossRef]
- Rana, S.; Döhler, D.; Nia, A.S.; Nasir, M.; Beiner, M.; Binder, W.H. ‘Click’-Triggered Self-Healing Graphene Nanocomposites. Macromol. Rapid Commun. 2016, 37, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef]
- Chen, S.; Binder, W.H. Dynamic Ordering and Phase Segregation in Hydrogen-Bonded Polymers. Acc. Chem. Res. 2016, 49, 1409–1420. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.H.; Michael, P.; Rana, S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Compos. Part B Eng. 2019, 157, 1–13. [Google Scholar] [CrossRef]
- Tamate, R.; Hashimoto, K.; Horii, T.; Hirasawa, M.; Li, X.; Shibayama, M.; Watanabe, M. Self-Healing Micellar Ion Gels Based on Multiple Hydrogen Bonding. Adv. Mater. 2018, 30, 1802792. [Google Scholar] [CrossRef]
- Tian, K.; Bae, J.; Suo, Z.; Vlassak, J.J. Adhesion between Hydrophobic Elastomer and Hydrogel through Hydrophilic Modification and Interfacial Segregation. ACS Appl. Mater. Interfaces 2018, 10, 43252–43261. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Yatsimirsky, A.K. Selectivity in supramolecular host–guest complexes. Chem. Soc. Rev. 2008, 37, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, N.B.; Nando, G.B.; Singha, N.K. Self-healing polymeric gel via RAFT polymerization and Diels–Alder click chemistry. Polymer 2015, 69, 349–356. [Google Scholar] [CrossRef]
- Kötteritzsch, J.; Geitner, R.; Ahner, J.; Abend, M.; Zechel, S.; Vitz, J.; Hoeppener, S.; Dietzek, B.; Schmitt, M.; Popp, J.; et al. Remendable polymers via reversible Diels–Alder cycloaddition of anthracene-containing copolymers with fullerenes. J. Appl. Polym. Sci. 2018, 135, 45916. [Google Scholar] [CrossRef]
- Khan, N.I.; Halder, S.; Gunjan, S.B.; Prasad, T. A review on Diels-Alder based self-healing polymer composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 12007. [Google Scholar] [CrossRef]
- Tang, M.; Li, Z.; Wang, K.; Jiang, Y.; Tian, M.; Qin, Y.; Gong, Y.; Li, Z.; Wu, L. Ultrafast self-healing and self-adhesive polysiloxane towards reconfigurable on-skin electronics. J. Mater. Chem. A 2022, 10, 1750–1759. [Google Scholar] [CrossRef]
- Tang, M.; Zheng, P.; Wang, K.; Qin, Y.; Jiang, Y.; Cheng, Y.; Li, Z.; Wu, L. Autonomous self-healing, self-adhesive, highly conductive composites based on a silver-filled polyborosiloxane/polydimethylsiloxane double-network elastomer. J. Mater. Chem. A 2019, 7, 27278–27288. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Broi, K.; Hecht, S. Lowering the Healing Temperature of Photoswitchable Dynamic Covalent Polymer Networks. Macromol. Rapid Commun. 2018, 39, 1700376. [Google Scholar] [CrossRef]
- Jurowska, A.; Jurowski, K. Vitrimers—The miracle polymer materials combining the properties of glass and plastic? Chemik 2015, 69, 389–394. [Google Scholar]
- Denissen, W.; Winne, J.M.; Prez, F.E.D. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef]
- Hansen, C.J.; White, S.R.; Sottos, N.R.; Lewis, J.A. Accelerated self-healing via ternary interpenetrating microvascular networks. Adv. Funct. Mater. 2011, 21, 4320–4326. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Q.; Lei, X.; Chen, Y.; Zhang, B.; Zhang, Q. A conjugation polyimine vitrimer: Fabrication and performance. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2531–2538. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chemie Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef]
- Krishnan, B.P.; Saalwaechter, K.; Adjedje, V.K.B.; Binder, W.H. Design, Synthesis and Characterization of Vitrimers with Low Topology Freezing Transition Temperature. Polymers 2022, 14, 2456. [Google Scholar] [CrossRef]
- Alabiso, W.; Schlögl, S. The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers: The effect of transesterification reactions on the network structure. Polymers 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Ediger, M.D.; Angell, C.A.; Nagel, S.R. Supercooled Liquids and Glasses. J. Phys. Chem. 1996, 100, 13200–13212. [Google Scholar] [CrossRef]
- Dyre, J.C. Colloquium: The glass transition and elastic models of glass-forming liquids. Rev. Mod. Phys. 2006, 78, 953–972. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Zhang, X.; Gao, L.; Wei, Y.; Ji, Y. Detecting topology freezing transition temperature of vitrimers by AIE luminogens. Nat. Commun. 2019, 10, 3165. [Google Scholar] [CrossRef]
- Dhers, S.; Vantomme, G.; Avérous, L. A fully bio-based polyimine vitrimer derived from fructose. Green Chem. 2019, 21, 1596–1601. [Google Scholar] [CrossRef]
- Li, A.; Fan, J.; Li, G. Recyclable thermoset shape memory polymers with high stress and energy output: Via facile UV-curing. J. Mater. Chem. A 2018, 6, 11479–11487. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Sanka, R.V.S.P.; Binder, W.H.; Parthasarthy, V.; Rana, S.; Karak, N. Vitrimers: Associative dynamic covalent adaptive networks in thermoset polymers. Chem. Eng. J. 2020, 385, 123820. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Sanka, R.S.P.; Binder, W.H.; Park, C.; Jung, J.; Parthasarthy, V.; Rana, S.; Yun, G.J. Catalyst free self-healable vitrimer/graphene oxide nanocomposites. Compos. Part B Eng. 2020, 184, 107647. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A. Biobased Thermosets. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; Elsevier: Boston, MA, USA, 2014; pp. 577–622. [Google Scholar]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Bobade, S.K.; Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Bio-Based Thermosetting Resins for Future Generation: A Review. Polym. Plast. Technol. Eng. 2016, 55, 1863–1896. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Niknam, R.; Ghanbarzadeh, B.; Ayaseh, A.; Hamishehkar, H. Plantago major seed gum based biodegradable films: Effects of various plant oils on microstructure and physicochemical properties of emulsified films. Polym. Test. 2019, 77, 105868. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Lin, X.; Wu, L.; Huang, S.; Qin, Y.; Qiu, X.; Lou, H. Effect of lignin-based amphiphilic polymers on the cellulase adsorption and enzymatic hydrolysis kinetics of cellulose. Carbohydr. Polym. 2019, 207, 52–58. [Google Scholar] [CrossRef]
- Hamada, T.; Yamashita, S.; Omichi, M.; Yoshimura, K.; Ueki, Y.; Seko, N.; Kakuchi, R. Multicomponent-Reaction-Ready Biomass-Sourced Organic Hybrids Fabricated via the Surface Immobilization of Polymers with Lignin-Based Compounds. ACS Sustain. Chem. Eng. 2019, 7, 7795–7803. [Google Scholar] [CrossRef]
- Miura, Y. Design and synthesis of well-defined glycopolymers for the control of biological functionalities. Polym. J. 2012, 44, 679–689. [Google Scholar] [CrossRef]
- Hult, D.; García-Gallego, S.; Ingverud, T.; Andrén, O.C.J.; Malkoch, M. Degradable high Tg sugar-derived polycarbonates from isosorbide and dihydroxyacetone. Polym. Chem. 2018, 9, 2238–2246. [Google Scholar] [CrossRef]
- Saxon, D.J.; Nasiri, M.; Mandal, M.; Maduskar, S.; Dauenhauer, P.J.; Cramer, C.J.; LaPointe, A.M.; Reineke, T.M. Architectural Control of Isosorbide-Based Polyethers via Ring-Opening Polymerization. J. Am. Chem. Soc. 2019, 141, 5107–5111. [Google Scholar] [CrossRef]
- Rudin, A.; Choi, P. Biopolymers. In The Elements of Polymer Science & Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 521–535. [Google Scholar]
- Ciesielski, A. An introduction to rubber technology. Choice Rev. Online 2000, 37, 37–5118. [Google Scholar]
- Mondragón, M.; Arroyo, K.; Romero-García, J. Biocomposites of thermoplastic starch with surfactant. Carbohydr. Polym. 2008, 74, 201–208. [Google Scholar] [CrossRef]
- Carvalho, A.J.F. Starch: Major sources, properties and applications as thermoplastic materials. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 321–342. [Google Scholar]
- Garrison, T.; Murawski, A.; Quirino, R. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Helanto, K.; Matikainen, L.; Rojas, O.J.; Talj, R. Bio-based polymers for sustainable packaging and biobarriers: A critical review. BioResources 2019, 14, 4902–4951. [Google Scholar]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Wautelet, T. Exploring the role of independent retailers in the circular economy: A case study approach. Eur. Univ. Econ. Manag. 2018, 10, 177. [Google Scholar]
- Velenturf, A.P.M.; Purnell, P. Principles for a sustainable circular economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- De Miguel Ramos, C.; Laurenti, R. Towards the Agenda 2030: A Quantitative Analysis of Synergies and Trade-Offs between the SDGs of Spain during 2000–2019. Sustainability 2020, 12, 10506. [Google Scholar] [CrossRef]
- Alarcón, F.; Cortés-Pellicer, P.; Pérez-Perales, D.; Sanchis, R. Sustainability vs. Circular economy from a disposition decision perspective: A proposal of a methodology and an applied example in SMEs. Sustainability 2020, 12, 10109. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; Ripoll-Bosch, R.; Van Zanten, H.H.; Metze, T.A.; Termeer, C.J.; van Ittersum, M.K.; de Boer, I.J. Principles, drivers and opportunities of a circular bioeconomy. Nat. Food 2021, 2, 561–566. [Google Scholar] [CrossRef]
- Kim, S.; Rahman, M.A.; Arifuzzaman, M.; Gilmer, D.B.; Li, B.; Wilt, J.K.; Lara-Curzio, E.; Saito, T. Closed-loop additive manufacturing of upcycled commodity plastic through dynamic cross-linking. Sci. Adv. 2022, 8, eabn6006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, T.; Hao, C.; Wang, L.; Han, J.; Liu, H.; Zhang, J. Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives. Green Chem. 2018, 20, 2995–3000. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Bose, D.; Singh, M.; Sanka, R.V.; Gurunadh, V.V.; Singhal, S.; Parthasarthy, V.; Guadagno, L.; Vijayan, P.P.; Thomas, S.; et al. Sugarcane Bagasse-Derived Activated Carbon- (AC-) Epoxy Vitrimer Biocomposite: Thermomechanical and Self-Healing Performance. Int. J. Polym. Sci. 2021, 2021, 5561755. [Google Scholar] [CrossRef]
- Hao, C.; Liu, T.; Zhang, S.; Brown, L.; Li, R.; Xin, J.; Zhong, T.; Jiang, L.; Zhang, J. A High-Lignin-Content, Removable, and Glycol-Assisted Repairable Coating Based on Dynamic Covalent Bonds. ChemSusChem 2019, 12, 1049–1058. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Singh, M.; Parthasarthy, V.; Park, C.; Sahoo, N.G.; Yun, G.J.; Rana, S. Disulfide exchange assisted self-healing epoxy/PDMS/graphene oxide nanocomposites. Nanoscale Adv. 2020, 2, 2726–2730. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, B.; Zhou, L.; Wang, L.; Majeed, K.; Zhang, B.; Zhou, F.; Zhang, Q. Preparation of environmentally friendly bio-based vitrimers from vanillin derivatives by introducing two types of dynamic covalent C N and S–S bonds. Polymer 2020, 197, 122483. [Google Scholar] [CrossRef]
- Taynton, P.; Zhu, C.; Loob, S.; Shoemaker, R.; Pritchard, J.; Jin, Y.; Zhang, W. Re-healable polyimine thermosets: Polymer composition and moisture sensitivity. Polym. Chem. 2016, 7, 7052–7056. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Liu, W.; Li, P.; Liu, J.; Liu, C.; Zhang, J.; Zhao, N.; Xu, J. Recyclable polybutadiene elastomer based on dynamic imine bond. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2011–2018. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, C.; Zhou, S.; Liu, B. Enabling shape memory and healable effects in a conjugated polymer by incorporating siloxane via dynamic imine bond. Chem. Commun. 2018, 54, 10092–10095. [Google Scholar] [CrossRef]
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.; Qi, H.J.; Zhang, W. Heat- or water-driven malleability in a highly recyclable covalent network polymer. Adv. Mater. 2014, 26, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic Covalent Polymer Networks Based on Degenerative Imine Bond Exchange: Tuning the Malleability and Self-Healing Properties by Solvent. Macromolecules 2016, 49, 6277–6284. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Yuan, W.; Wang, B.; Zhu, J. Robust, Fire-Safe, Monomer-Recovery, Highly Malleable Thermosets from Renewable Bioresources. Macromolecules 2018, 51, 8001–8012. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, B.; Hu, J.; Zuo, H.; Li, J.; Sun, H.; Ning, N.; Tian, M.; Zhang, L. Multifunctional Vitrimer-Like Polydimethylsiloxane (PDMS): Recyclable, Self-Healable, and Water-Driven Malleable Covalent Networks Based on Dynamic Imine Bond. Ind. Eng. Chem. Res. 2019, 58, 1212–1221. [Google Scholar] [CrossRef]
- Quienne, B.; Kasmi, N.; Dieden, R.; Caillol, S.; Habibi, Y. Isocyanate-Free Fully Biobased Star Polyester-Urethanes: Synthesis and Thermal Properties. Biomacromolecules 2020, 21, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Liu, Y.Y.; He, J.; Li, Y.D.; Zhao, X.L.; Zeng, J.B. Biobased, reprocessable and weldable epoxy vitrimers from epoxidized soybean oil. Ind. Crops Prod. 2020, 153, 112576. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Geng, H.; Wang, Y.; Yu, Q.; Gu, S.; Zhou, Y.; Xu, W.; Zhang, X.; Ye, D. Vanillin-Based Polyschiff Vitrimers: Reprocessability and Chemical Recyclability. ACS Sustain. Chem. Eng. 2018, 6, 15463–15470. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Lorwanishpaisarn, N.; Pongsa, U.; Ando, S. Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes. Polymers 2018, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Feng, Z.; Liang, Z.; Lv, Y.; Xiang, F.; Xiong, C.; Duan, C.; Dai, L.; Ni, Y. Vitrimer-Cellulose Paper Composites: A New Class of Strong, Smart, Green, and Sustainable Materials. ACS Appl. Mater. Interfaces 2019, 11, 36090–36099. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, F.; Zhong, J.; Shen, L.; Lin, Y. Renewable castor oil and DL-limonene derived fully bio-based vinylogous urethane vitrimers. Eur. Polym. J. 2020, 135, 109865. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, J.; Zuo, H.; Ning, N.; Zhang, L.; Yu, B.; Tian, M. Photothermal-Induced Self-Healable and Reconfigurable Shape Memory Bio-Based Elastomer with Recyclable Ability. ACS Appl. Mater. Interfaces 2019, 11, 1469–1479. [Google Scholar] [CrossRef]


| Bio-Based Derivatives | Material | Recycling/ Reprocessing | Self-Healing | Shape Memory | Ref. |
|---|---|---|---|---|---|
| Lignin based | Epoxy derived from eugenol with succinic anhydride | 190 °C for 1 h, Low efficiency a,# | 190 °C for 1 h, 90% b | 80 °C for less than minute, 100% c | [95] |
| Dialdehyde monomer derived from vanillin with conventional diamine | 150 °C for 10 min, 71.2% a,* | 150 °C for 1 h, 74.5% b | - | [96] | |
| Dialdehyde derived from vanillin and amine monomers | 60 °C for 20 min, Maximum efficiency a,# | Addition of ethylene diamine | - | [84] | |
| Epoxy derived from sebacic acid and ozone crafted lignin | - | 190 °C for 5 min, 70% b | 80 °C for less than minute, 87–97% c | [80] | |
| Fructose | Furan dialdehyde and fatty acid-based diamine/ triamine | 120 °C for 10 min, Nearly 100% a,* | - | - | [50] |
| Soybean & Castor oil | Fumaropim-aric acid (FPA) derived from Rosin and epoxidized soybean oil (ESO) | 120 °C for 2 h, 88% a,* | 180 °C for 60 min, Nearly 100% | 80 °C for 30 min, 89% | [93] |
| 4,4′-dithiodiphenylamine (APD) cured Epoxidized soybean oil (ESO) | 180 °C for 10 min under 20 MPa, 80% a,~ | - | - | [94] | |
| Vinylogus urethane vitrimer derived from aminate DL-limonene (AL) | 160 °C, 6 MPa for 30 min | - | 70 °C for 1 min, 100% | [99] | |
| Isosorbide | Isosorbide derived epoxy and aromatic diamines | 100 °C for 1 h, 82.6% a,* | 100 °C for 1 h, 100% b | 80 °C for 1 min 100% | [72] |
| Natural rubber | Dodecanedioic acids (DAs) and aniline trimer (ACAT) derived epoxidized natural rubber | 200 °C for 20 min, 88% a,# | NIR and 200 °C for 30 min, 80% b | NIR and 80 °C for less than minute, 95% c | [100] |
| Composite | Cellulose paper from 1,3-Propanediol (PD) and bis (6-membered cyclic carbonate) (BCC) and | HCl at 90 °C for 36 h | 160 °C for 10 s, 75% b,*,^ and 170 °C for 2 h, 4 MPa, 80% b,*,$ | 150 °C for less than minute c | [98] |
| Carbon nano tubes (MWNT) with epoxy/cashew nutshell liquid | - | - | NIR and 60 °C for less than minute, 100% c | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, S.; Solanki, M.; Sahoo, N.G.; Krishnakumar, B. Bio-Vitrimers for Sustainable Circular Bio-Economy. Polymers 2022, 14, 4338. https://doi.org/10.3390/polym14204338
Rana S, Solanki M, Sahoo NG, Krishnakumar B. Bio-Vitrimers for Sustainable Circular Bio-Economy. Polymers. 2022; 14(20):4338. https://doi.org/10.3390/polym14204338
Chicago/Turabian StyleRana, Sravendra, Manisha Solanki, Nanda Gopal Sahoo, and Balaji Krishnakumar. 2022. "Bio-Vitrimers for Sustainable Circular Bio-Economy" Polymers 14, no. 20: 4338. https://doi.org/10.3390/polym14204338
APA StyleRana, S., Solanki, M., Sahoo, N. G., & Krishnakumar, B. (2022). Bio-Vitrimers for Sustainable Circular Bio-Economy. Polymers, 14(20), 4338. https://doi.org/10.3390/polym14204338








