Nitrogen-Doped Porous Core-Sheath Graphene Fiber-Shaped Supercapacitors
Abstract
:1. Introduction
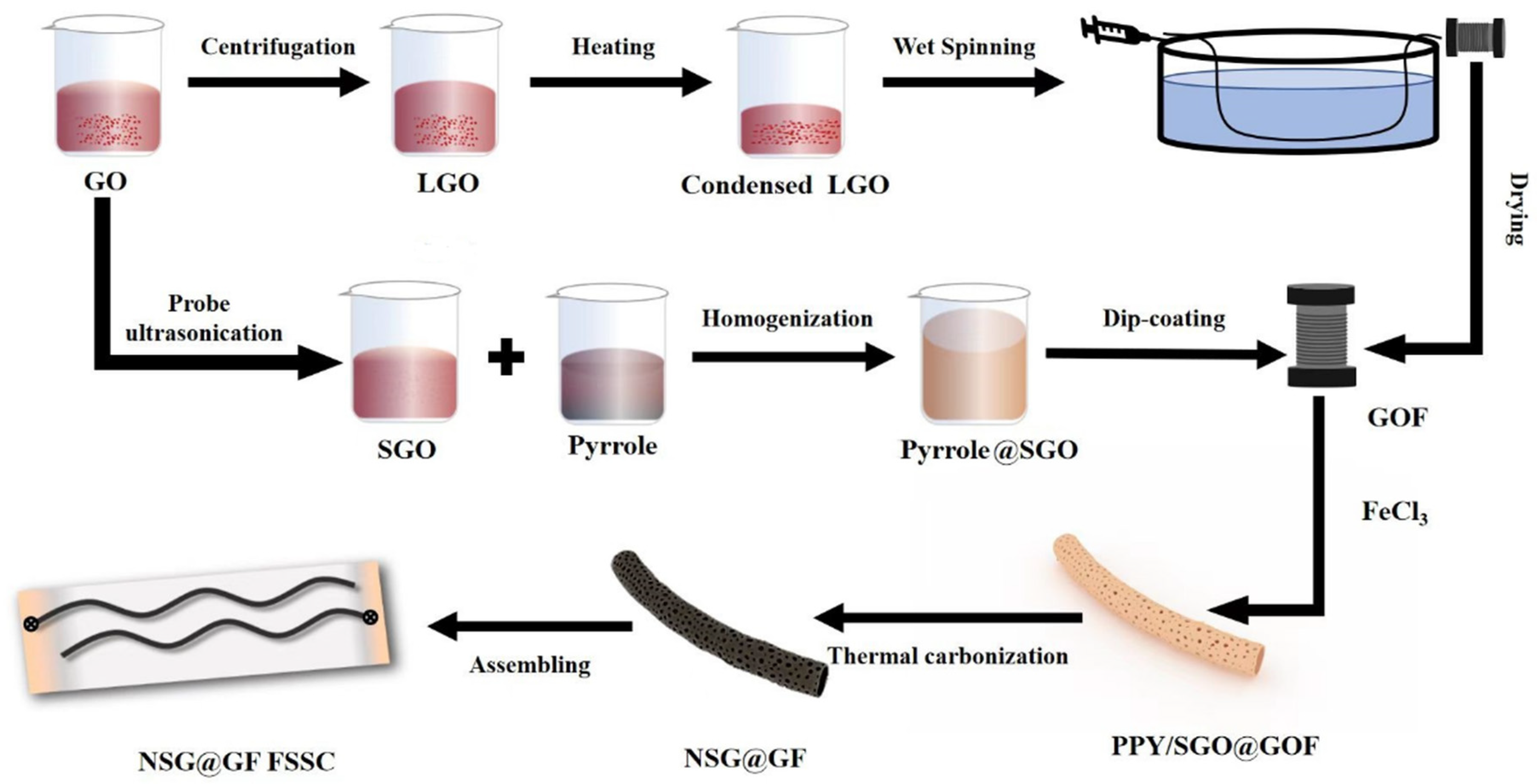
2. Materials and Methods
2.1. Materials
2.2. Preparation of Small-Sized Graphene Oxide (SGO)
2.3. Preparation of GF
2.4. Preparation of SG@GFs
2.5. Preparation of NSG@GFs
2.6. Assembly of NSG@GF-Based FSSCs
2.7. Characterization
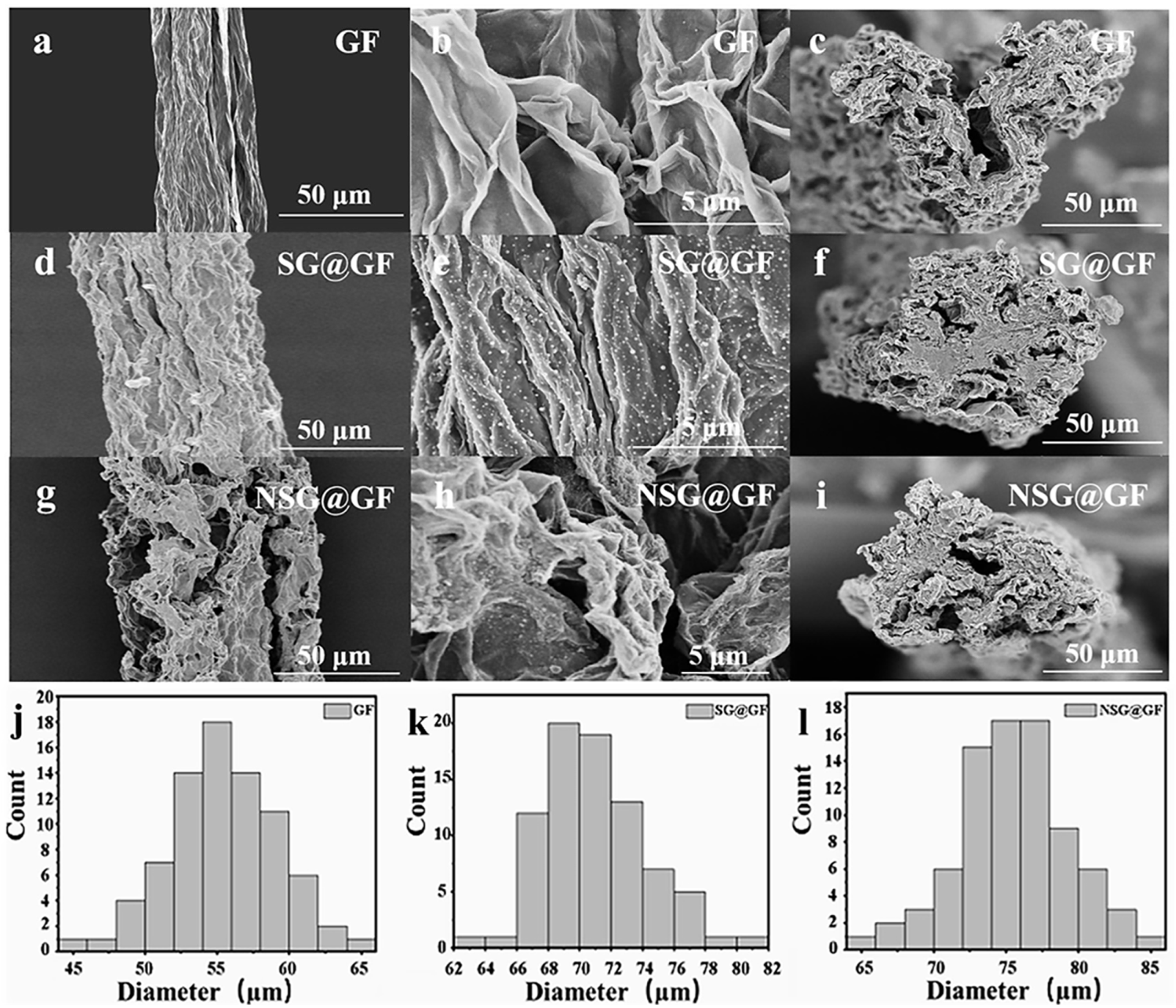
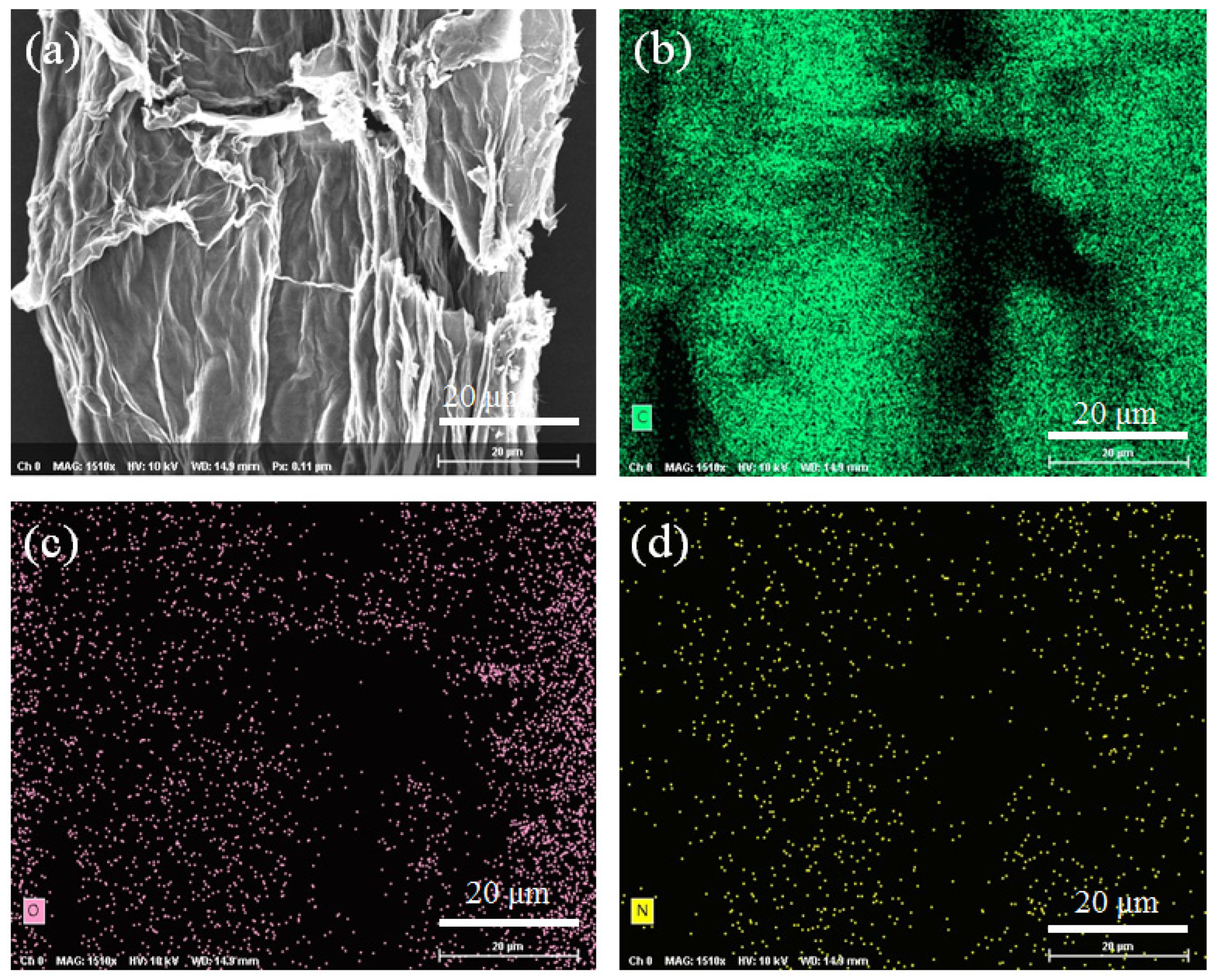
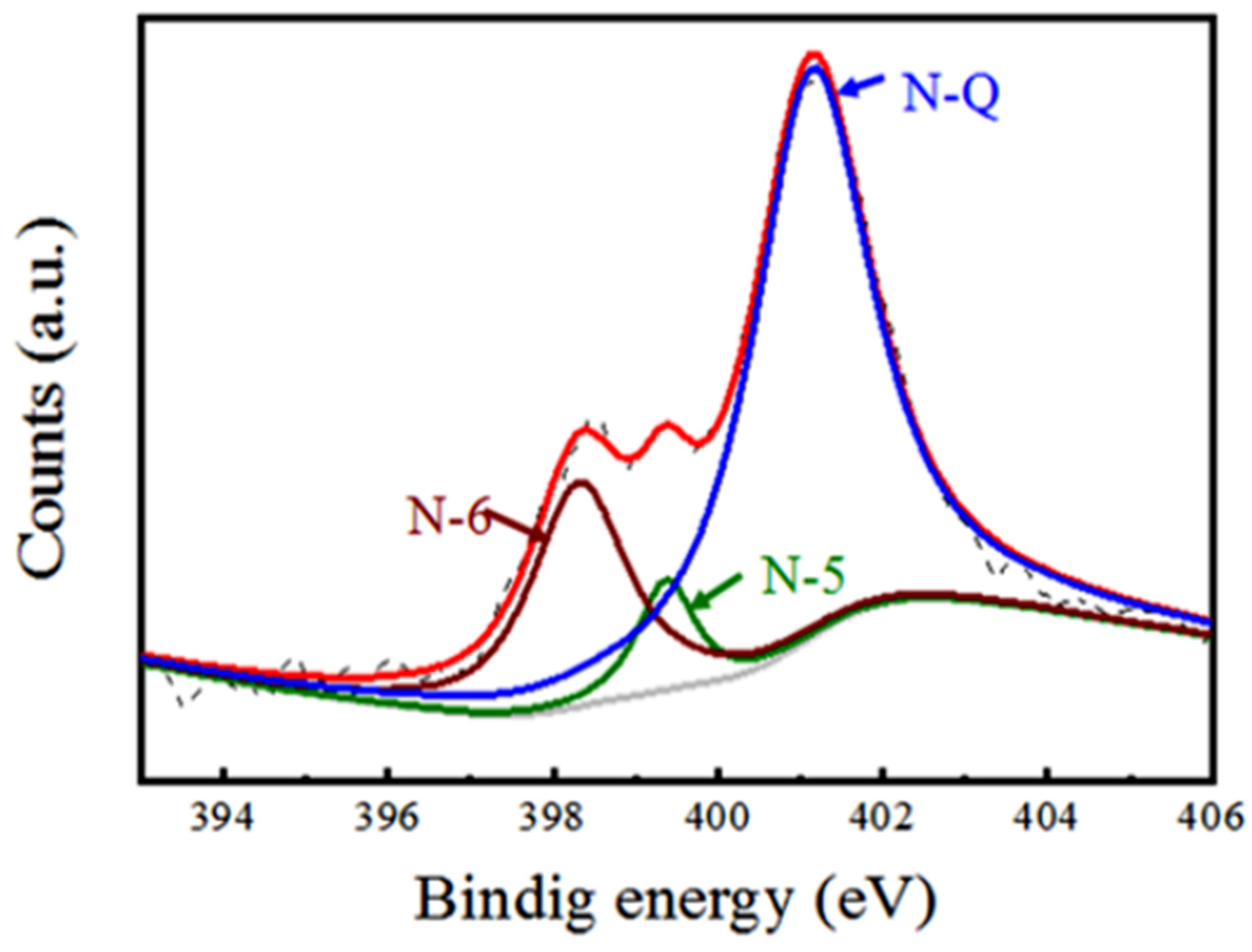
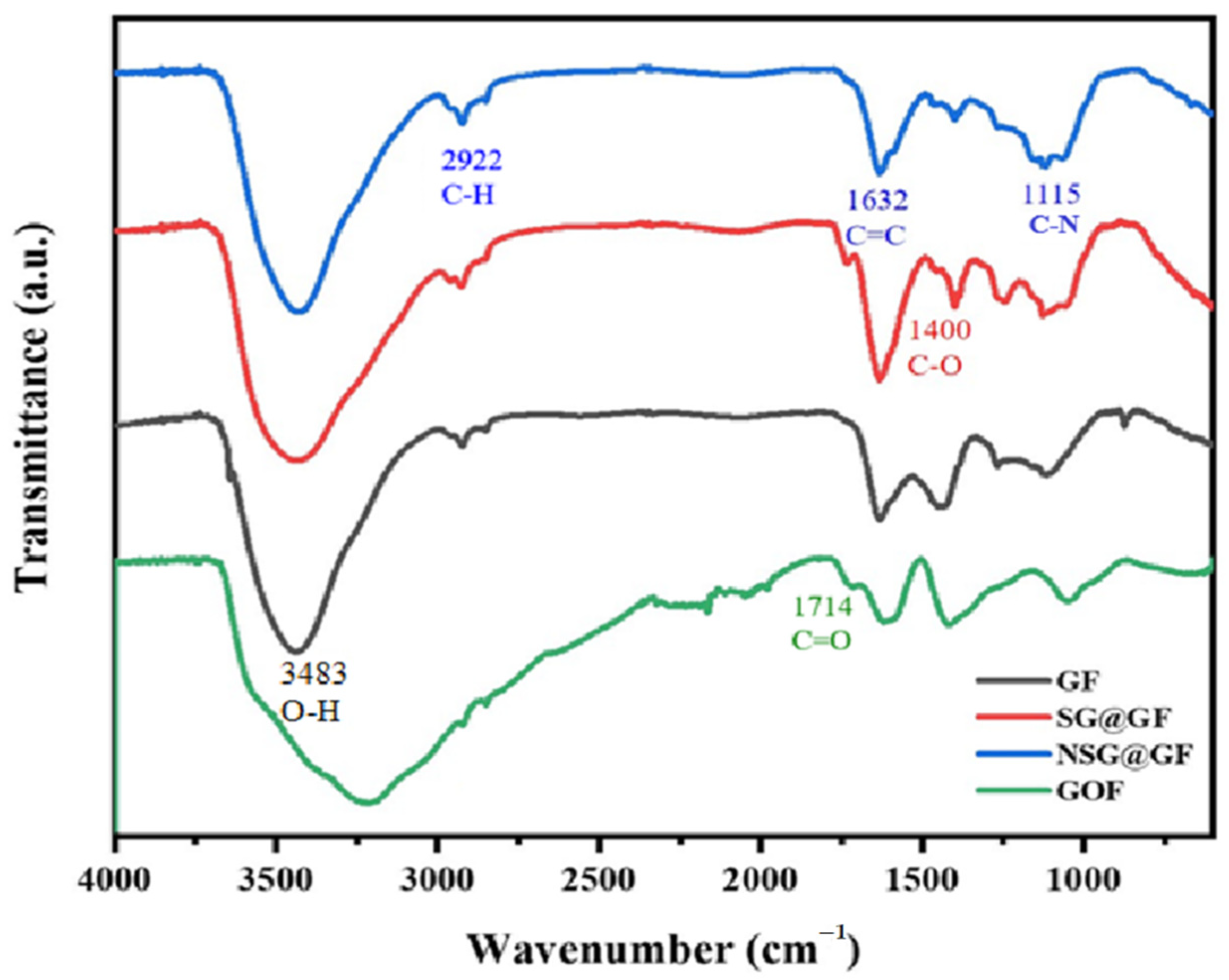
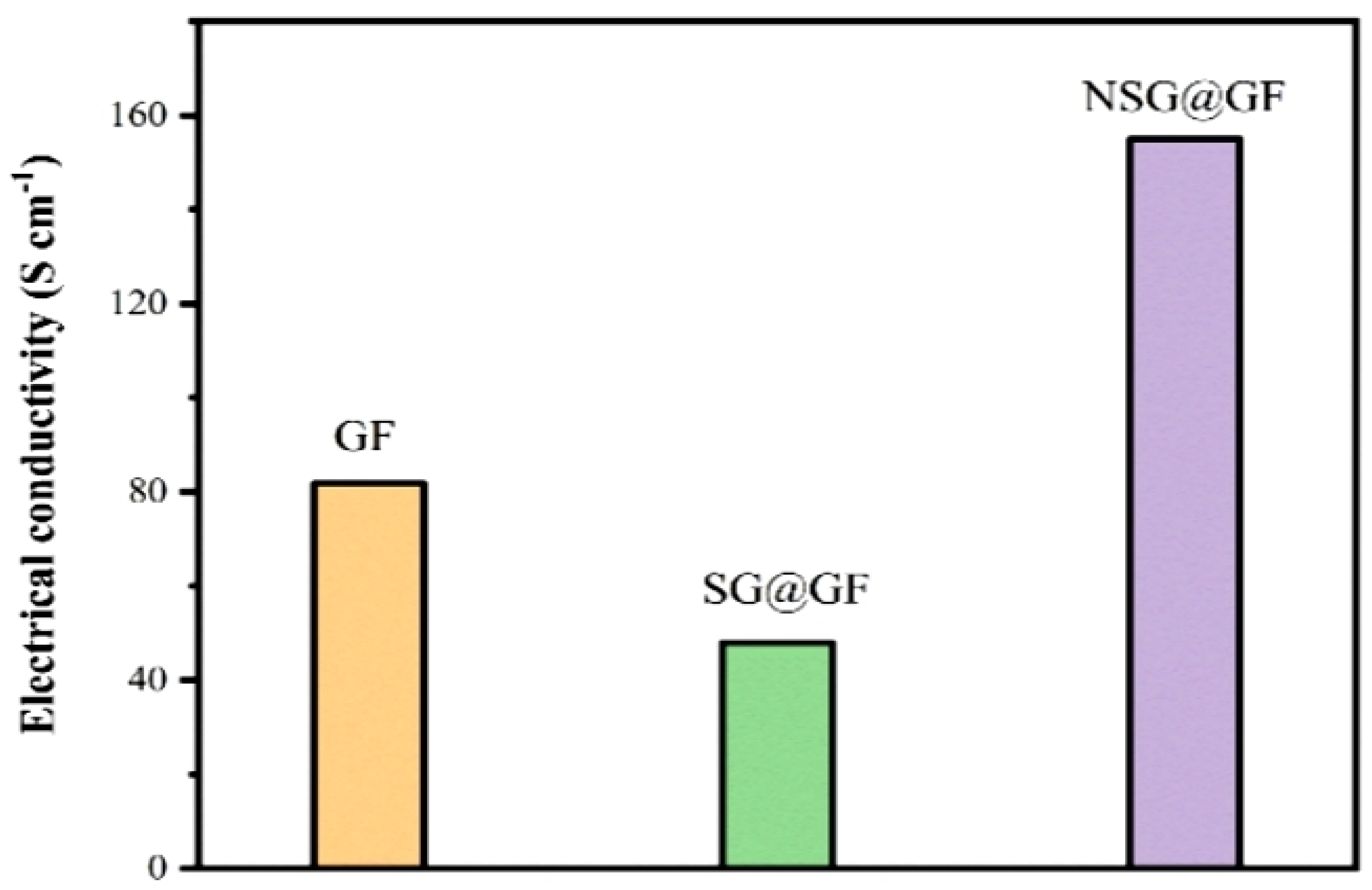
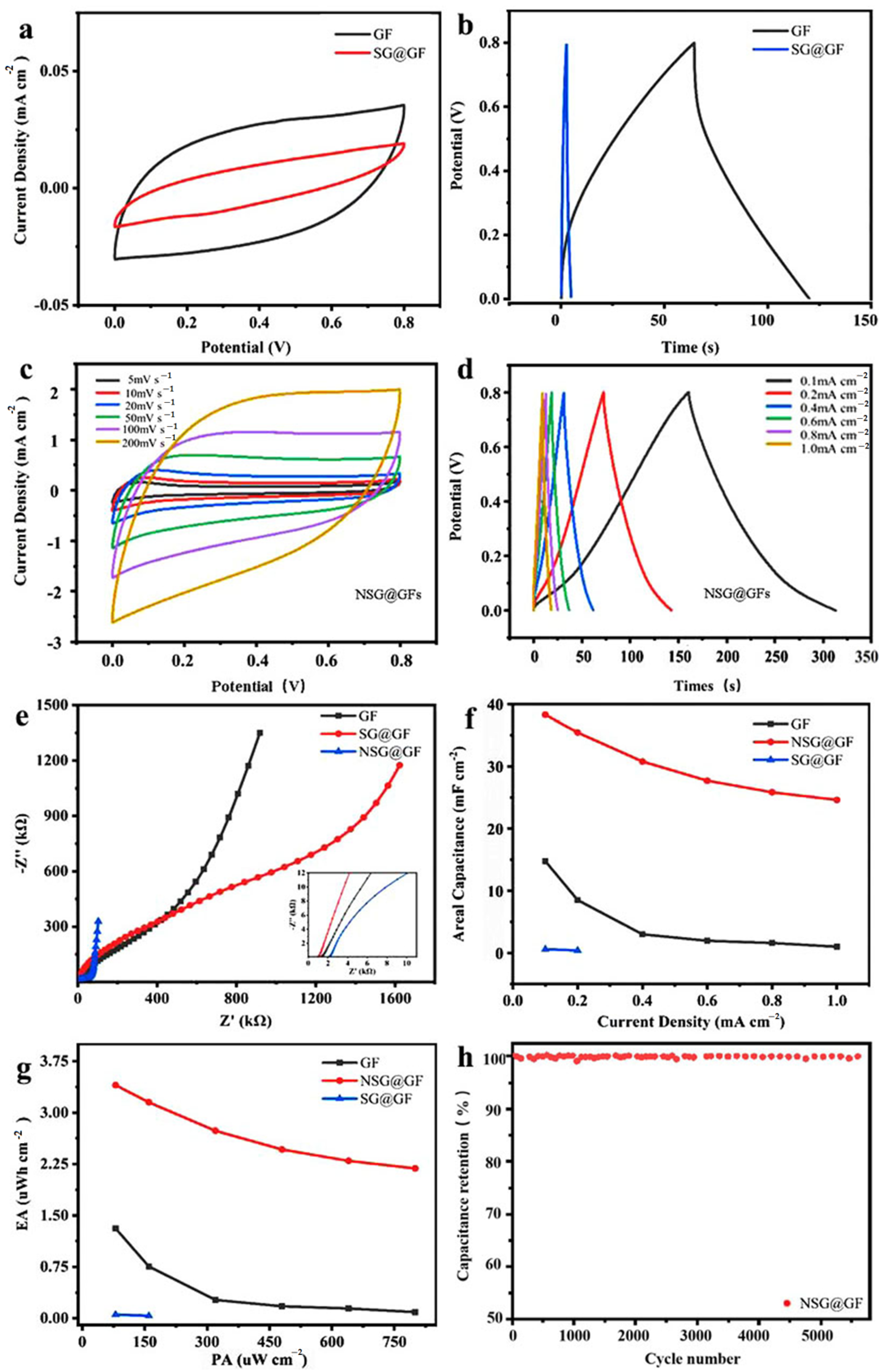
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.W.; Hall, A.S.; Kim, J.-D. A Facile and Template-Free Hydrothermal Synthesis of Mn3O4 Nanorods on Graphene Sheets for Supercapacitor Electrodes with Long Cycle Stability. Chem. Mater. A Publ. Am. Chem. Soc. 2012, 24, 1158–1164. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Shi, B.; Gao, S.; Meng, G.; Huang, K. Novel activated N-doped hollow microporous carbon nanospheres from pyrrole-based hyper-crosslinking polystyrene for supercapacitors. React. Funct. Polym. 2019, 143, 104326. [Google Scholar] [CrossRef]
- Meng, J.; Nie, W.; Zhang, K.; Xu, F.; Ding, X.; Wang, S.; Qiu, Y. Enhancing Electrochemical Performance of Graphene Fiber-Based Supercapacitors by Plasma Treatment. ACS Appl. Mater. Interfaces 2018, 10, 13652–13659. [Google Scholar] [CrossRef]
- Lu, C.; Meng, J.; Zhang, J.; Chen, X.; Du, M.; Chen, Y.; Hou, C.; Wang, J.; Ju, A.; Wang, X.; et al. Three-Dimensional Hierarchically Porous Graphene Fiber-Shaped Supercapacitors with High Specific Capacitance and Rate Capability. ACS Appl. Mater. Interfaces 2019, 11, 25205–25217. [Google Scholar] [CrossRef]
- Wu, G.; Yang, Z.; Zhang, Z.; Ji, B.; Hou, C.; Li, Y.; Jia, W.; Zhang, Q.; Wang, H. High performance stretchable fibrous supercapacitors and flexible strain sensors based on CNTs/MXene-TPU hybrid fibers. Electrochim. Acta 2021, 395, 139141. [Google Scholar] [CrossRef]
- Gopi, C.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.-J. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J. Energy Storage 2020, 27, 101035. [Google Scholar]
- Wei, W.; Aifang, Y.; Junyi, Z.; Lin, W.Z. Recent Progress of Functional Fiber and Textile Triboelectric Nanogenerators: Towards Electricity Power Generation and Intelligent Sensing. Adv. Fiber Mater. 2021, 3, 394–412. [Google Scholar]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Liu, Y.Q.; Hao, Y.L.; Han, D.D.; Zhang, Y.L.; You, Z. Laser Fabrication of Graphene-Based Flexible Electronics. Adv. Mater. 2020, 32, 1901981. [Google Scholar] [CrossRef]
- Xu, D.; Xuan, C.; Li, X.; Luo, Z.; Wang, Z.; Tang, T.; Wen, J.; Li, M.; Xiao, J. Novel helical carbon nanotubes-embedded reduced graphene oxide in three-dimensional architecture for high-performance flexible supercapacitors. Electrochim. Acta 2020, 339, 135912. [Google Scholar] [CrossRef]
- Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen, D.; Ruoff, R.S. Exfoliation of graphite oxide in propylene carbonate and thermal reduction of the resulting graphene oxide platelets. ACS Nano 2010, 4, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, G.; Cui, X.T.; Sheng, G.; Luo, X. Enhanced catalytic and dopamine sensing properties of electrochemically reduced conducting polymer nanocomposite doped with pure graphene oxide, Biosensors & Bioelectronics: The International Journal for the Professional Involved with Research. Technol. Appl. Biosensers Relat. Devices 2014, 58, 153–156. [Google Scholar]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Mankge, N.S.; Madito, M.J.; Hlongwa, N.W.; Kuvarega, A.T. Review of electrochemical production of doped graphene for energy storage applications. J. Energy Storage 2022, 46, 103527. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Li, B.; Yu, C.; Wang, H.; Li, Q. Construction of heteroatom-doped and three-dimensional graphene materials for the applications in supercapacitors: A review. J. Energy Storage 2021, 44, 103437. [Google Scholar] [CrossRef]
- Ding, D.; Ma, L.; Li, X.; Liu, Z.; Hui, L.; Zhang, F.; Zhao, Y. Porous Carbon Material Derived from Steam-Exploded Poplar for Supercapacitor: Insights into Synergistic Effect of KOH and Urea on the Structure and Electrochemical Properties. Materials 2022, 15, 2741. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Zhang, Y.; Xu, J.; Li, Z.; Yang, K.; Zhu, L.; Liu, Z. Three-dimensional functionalized graphenes with systematical control over the interconnected pores and surface functional groups for high energy performance supercapacitors. Carbon 2015, 85, 351–362. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Xiang, Y.; Zhang, Q. Enhanced electrochemical capacitance and oil-absorbability of N-doped graphene aerogel by using amino-functionalized silica as template and doping agent. J. Power Sources 2018, 379, 240–248. [Google Scholar] [CrossRef]
- Abbas, Q.; Raza, R.; Shabbir, I.; Olabi, A.G. Heteroatom doped high porosity carbon nanomaterials as electrodes for energy storage in electrochemical capacitors: A review. J. Sci. Adv. Mater. Devices 2019, 4, 341–352. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Wu, Y.; Zhao, S.; Wang, Z.; Wang, S. Nitrogen-doped graphene prepared by a millisecond photo-thermal process and its applications. Org. Electron. 2018, 56, 221–231. [Google Scholar] [CrossRef]
- Babel, K.; Jurewicz, K. KOH activated carbon fabrics as supercapacitor material. J. Phys. Chem. Solids 2004, 65, 275–280. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.; Sui, Z.; Zhang, X.; Hou, S.; Cao, G.; Yang, Y. What is the choice for supercapacitors: Graphene or graphene oxide? Energy Environ. Sci. EES 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhong, T.; Wang, Z. Plasma-Engineered N-CoOx Nanowire Array as a Bifunctional Electrode for Supercapacitor and Electrocatalysis. Nanomaterials 2022, 12, 2984. [Google Scholar] [CrossRef]
- Ding, X.; Bai, J.; Xu, T.; Li, C.; Zhang, H.-M.; Qu, L. A novel nitrogen-doped graphene fiber microelectrode with ultrahigh sensitivity for the detection of dopamine. Electrochem. Commun. 2016, 72, 122–125. [Google Scholar] [CrossRef]
- Wu, G.; Tan, P.; Wu, X.; Peng, L.; Cheng, H.; Wang, C.-F.; Chen, W.; Yu, Z.; Chen, S. High-Performance Wearable Micro-Supercapacitors Based on Microfluidic-Directed Nitrogen-Doped Graphene Fiber Electrodes. Adv. Funct. Mater. 2017, 27, 1702493. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, K.; Yao, L.; Qiu, Y.; Wang, S. Hierarchically porous sheath–core graphene-based fiber-shaped supercapacitors with high energy density. J. Mater. Chem. A 2018, 6, 896–907. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Cheng, H.; Xue, H.; Zhao, G.; Hong, C.; Zhang, X. Preparation, characterization, and properties of graphene-based composite aerogels via in situ polymerization and three-dimensional self-assembly from graphene oxide solution. RSC Adv. 2016, 6, 78538–78547. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.; Li, S.; Gu, Q.; Wang, D.; Jiang, C.; Liu, Y. N-doped honeycomb-like porous carbon derived from biomass as an efficient carbocatalyst for H2S selective oxidation. J. Hazard. Mater. 2021, 403, 123806. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Xu, Y.; Zhang, R. Catalytic performance of dioxide reforming of methane over Co/AC-N catalysts: Effect of nitrogen doping content and calcination temperature. Int. J. Hydrog. Energy 2019, 44, 16424–16435. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Qin, X.; Liu, J.; Li, G.; Xu, Y.; Lv, Y. Catalytic performance of CH4–CO2 reforming over metal free nitrogen-doped biomass carbon catalysts: Effect of different preparation methods. Int. J. Hydrog. Energy 2021, 46, 31586–31597. [Google Scholar] [CrossRef]
- Li, J.; Han, K.; Wang, D.; Teng, Z.; Cao, Y.; Qi, J.; Li, M.; Wang, M. Fabrication of high performance structural N-doped hierarchical porous carbon for supercapacitors. Carbon 2020, 164, 42–50. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Q.; Liu, Y. Hybrid of Polyoxometalate-Based Ionic Salt and N-Doped Carbon toward Reductant-Free Aerobic Hydroxylation of Benzene to Phenol. ACS Sustain. Chem. Eng. 2016, 4, 4986–4996. [Google Scholar] [CrossRef]
- Mehetre, S.S.; Maktedar, S.S.; Singh, M. Understanding the mechanism of surface modification through enhanced thermal and electrochemical stabilities of N-doped graphene oxide. Appl. Surf. Sci. A J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2016, 366, 514–522. [Google Scholar] [CrossRef]
- Han, F.; Jing, W.; Wu, Q.; Tian, B.; Lin, Q.; Wang, C.; Zhao, L.; Liu, J.; Sun, Y.; Jiang, Z. Nitrogen-doped graphene fiber electrodes with optimal micro-/meso-/macro-porosity ratios for high-performance flexible supercapacitors. J. Power Sources 2022, 520, 230866. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect Of Nitrogen- And Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Dumont, J.H.; Martinez, U.; Artyushkova, K.; Purdy, G.M.; Dattelbaum, A.M.; Zelenay, P.; Mohite, A.; Atanassov, P.; Gupta, G. Nitrogen-doped graphene oxide electrocatalysts for the oxygen reduction reaction. ACS Appl. Nano Mater. 2019, 2, 1675–1682. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, H.; Xiao, Y.; Hu, H.; Cai, Y.; Liang, Y.; Sun, L.; Liu, Y.; Zheng, M. Three-dimensional Nitrogen-doped graphene as binder-free electrode materials for supercapacitors with high volumetric capacitance and the synergistic effect between nitrogen configuration and supercapacitive performance. Electrochim. Acta 2016, 218, 32–40. [Google Scholar] [CrossRef]
- Obodo, R.M.; Onah, E.O.; Nsude, H.E.; Agbogu, A.; Nwanya, A.C.; Ahmad, I.; Zhao, T.; Ejikeme, P.M.; Maaza, M.; Ezema, F.I. Performance evaluation of graphene oxide based Co3O4@ GO, MnO2@ GO and Co3O4/MnO2@ GO electrodes for supercapacitors. Electroanalysis 2020, 32, 2786–2794. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Ndipingwi, M.M.; Ikpo, C.O.; Obodo, R.M.; Nwanya, S.C.; Botha, S.; Ezema, F.I.; Iwuoha, E.I.; Maaza, M. Zea mays lea silk extract mediated synthesis of nickel oxide nanoparticles as positive electrode material for asymmetric supercabattery. J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid State Chem. Phys. 2020, 822, 153581. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Gao, W.; Gao, A.; Qasim, A.M.; Zhang, F.; Wang, J.; Ding, K.; Wu, G.; Chu, P.K. Nickel plasma modification of graphene for high-performance non-enzymatic glucose sensing. Sens. Actuators B Chem. 2017, 251, 842–850. [Google Scholar] [CrossRef]
- Zheng, Y.; Lia, Z.; Xu, J.; Wang, T.; Liu, X.; Duan, X.; Ma, Y.; Zhou, Y.; Pei, C. Multi-channeled hierarchical porous carbon incorporated Co3O4 nanopillar arrays as 3D binder-free electrode for high performance supercapacitors. Nano Energy 2016, 20, 94–107. [Google Scholar] [CrossRef]
- Iessa, K.H.S.; Zhang, Y.; Zhang, G.; Xiao, F.; Wang, S. Conductive porous sponge-like ionic liquid-graphene assembly decorated with nanosized polyaniline as active electrode material for supercapacitor. J. Power Sources 2016, 302, 92–97. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Q.; Liu, Y.; Xiang, R.; Zhang, Y.; Du, M.; Li, Z.; Wei, Y.; Zhang, K. Nitrogen-Doped Porous Core-Sheath Graphene Fiber-Shaped Supercapacitors. Polymers 2022, 14, 4300. https://doi.org/10.3390/polym14204300
Ke Q, Liu Y, Xiang R, Zhang Y, Du M, Li Z, Wei Y, Zhang K. Nitrogen-Doped Porous Core-Sheath Graphene Fiber-Shaped Supercapacitors. Polymers. 2022; 14(20):4300. https://doi.org/10.3390/polym14204300
Chicago/Turabian StyleKe, Qianlan, Yan Liu, Ruifang Xiang, Yuhui Zhang, Minzhi Du, Zhongxiu Li, Yi Wei, and Kun Zhang. 2022. "Nitrogen-Doped Porous Core-Sheath Graphene Fiber-Shaped Supercapacitors" Polymers 14, no. 20: 4300. https://doi.org/10.3390/polym14204300
APA StyleKe, Q., Liu, Y., Xiang, R., Zhang, Y., Du, M., Li, Z., Wei, Y., & Zhang, K. (2022). Nitrogen-Doped Porous Core-Sheath Graphene Fiber-Shaped Supercapacitors. Polymers, 14(20), 4300. https://doi.org/10.3390/polym14204300






