Collagen Alignment via Electro-Compaction for Biofabrication Applications: A Review
Abstract
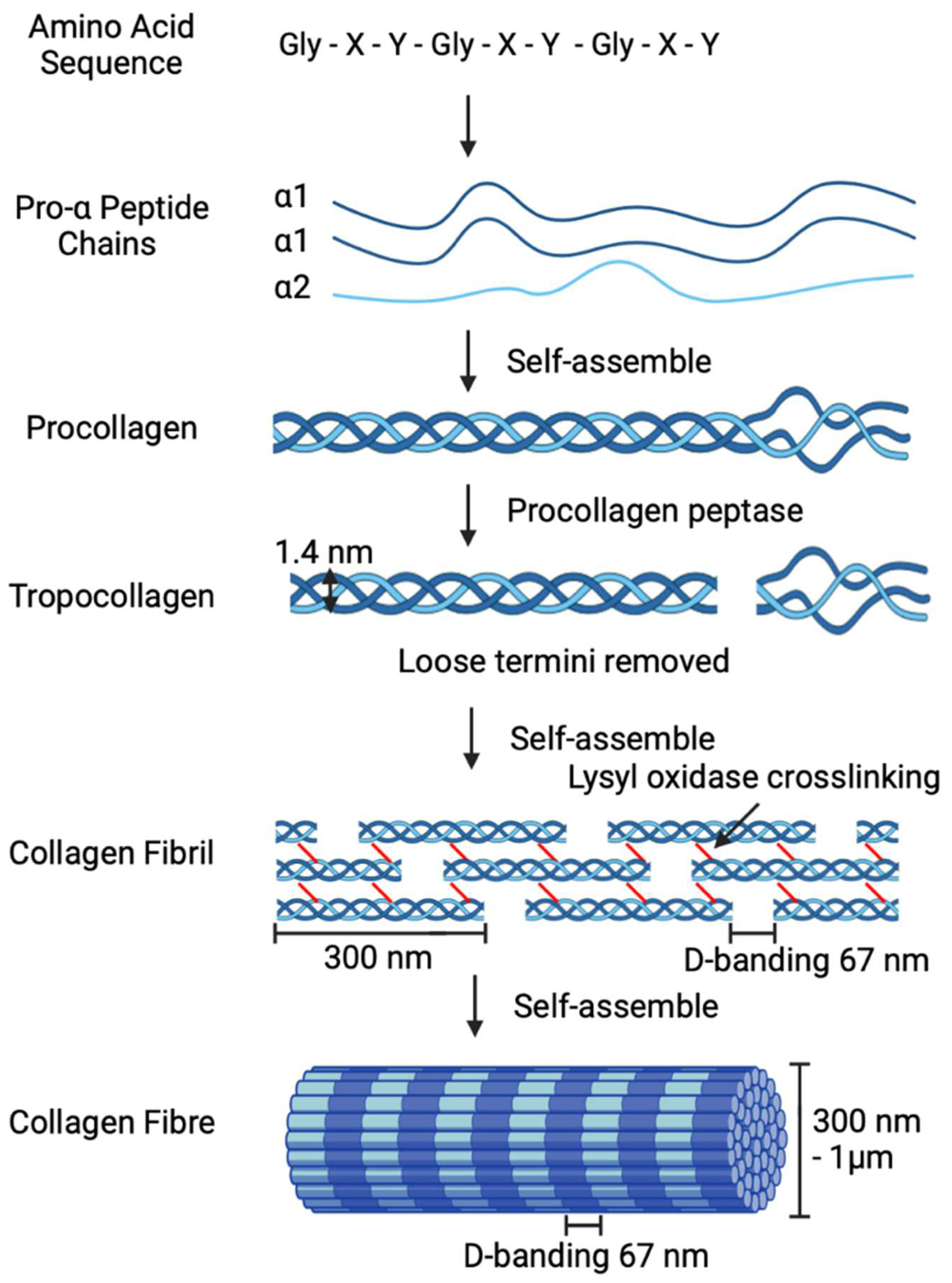
1. Collagen
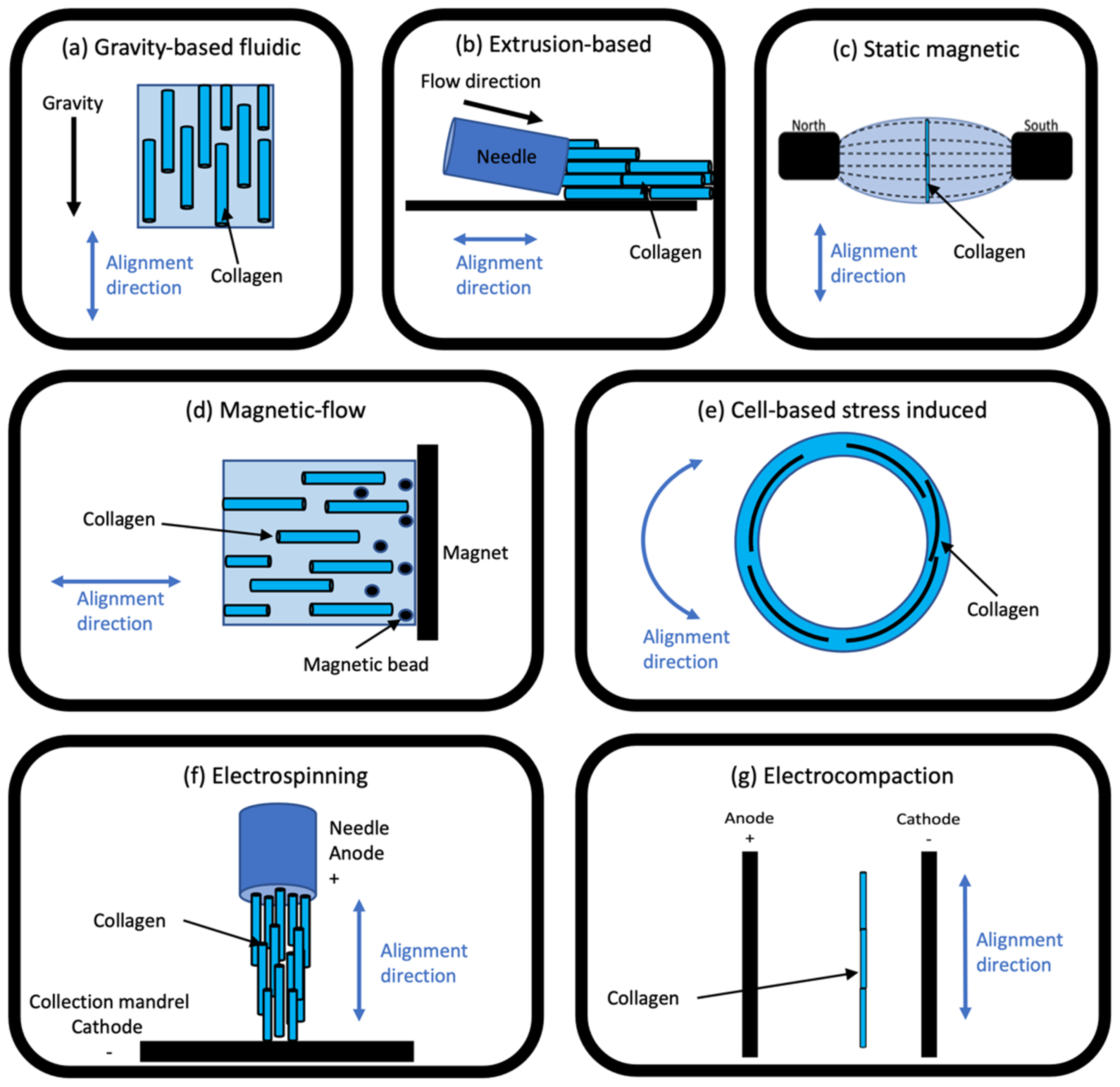
2. Overview of Collagen Alignment Techniques
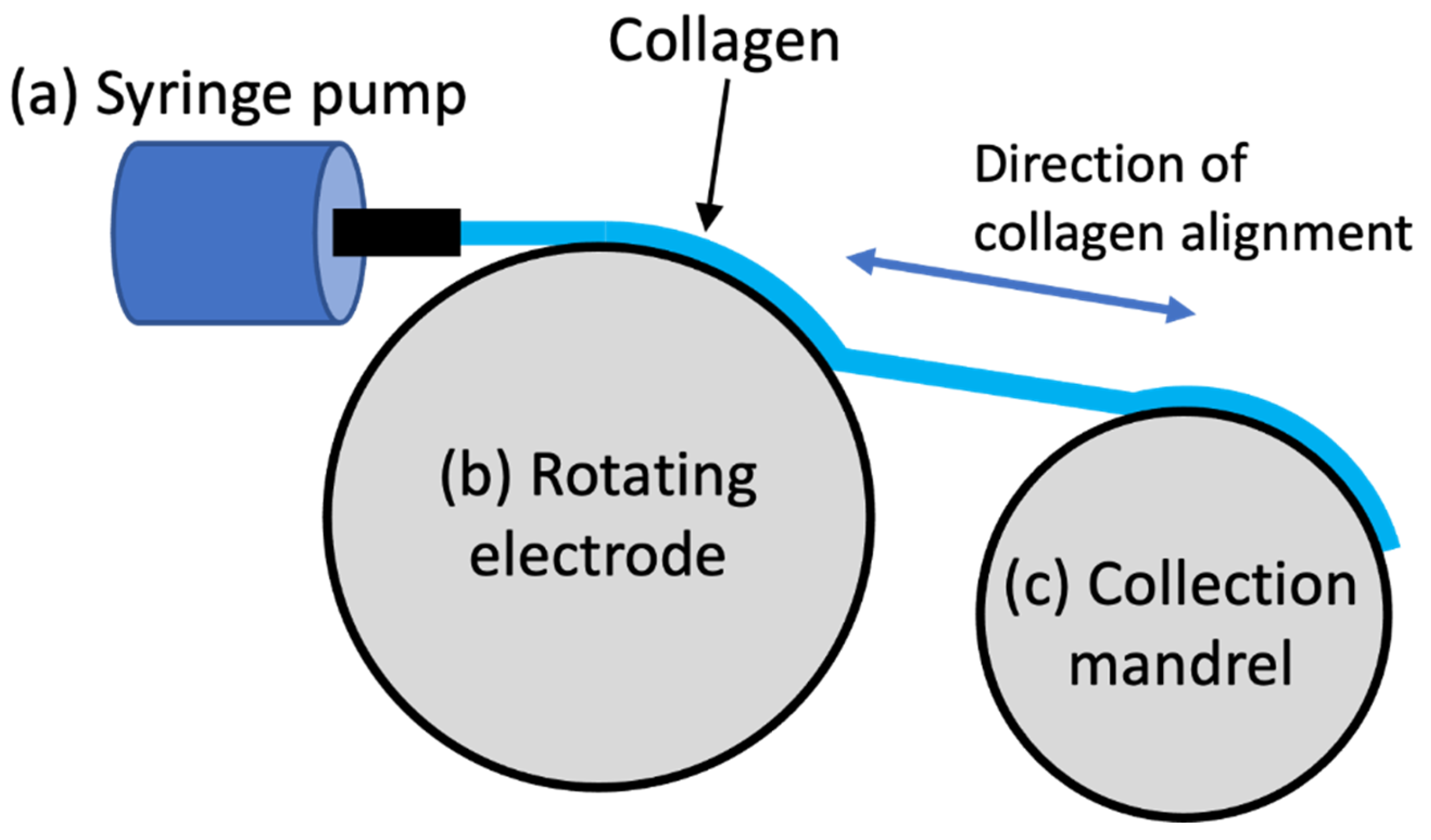
3. Electro-Compaction
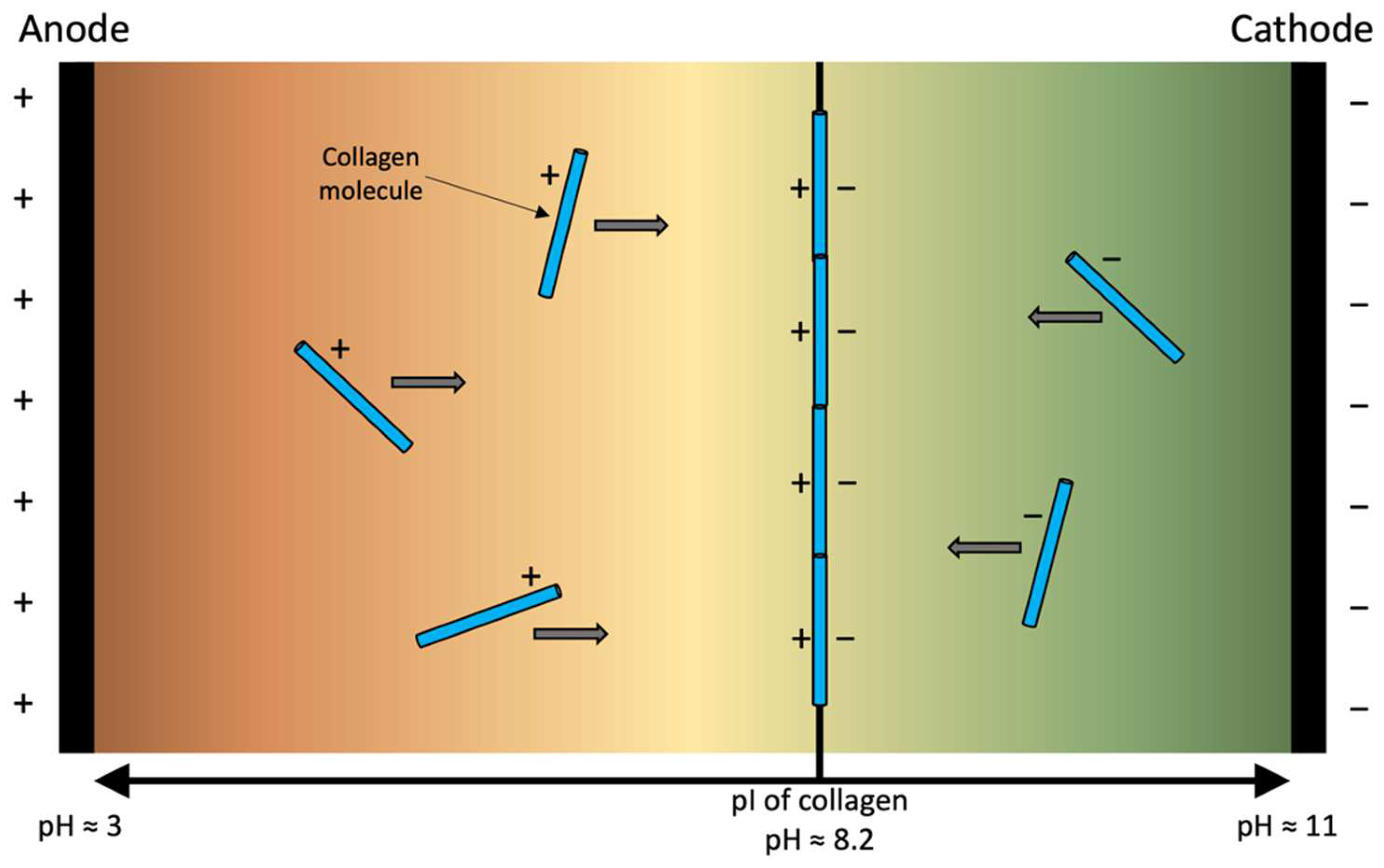
3.1. Sources of Collagen for Electro-Compaction
3.2. Electro-Compacted Scaffold Types
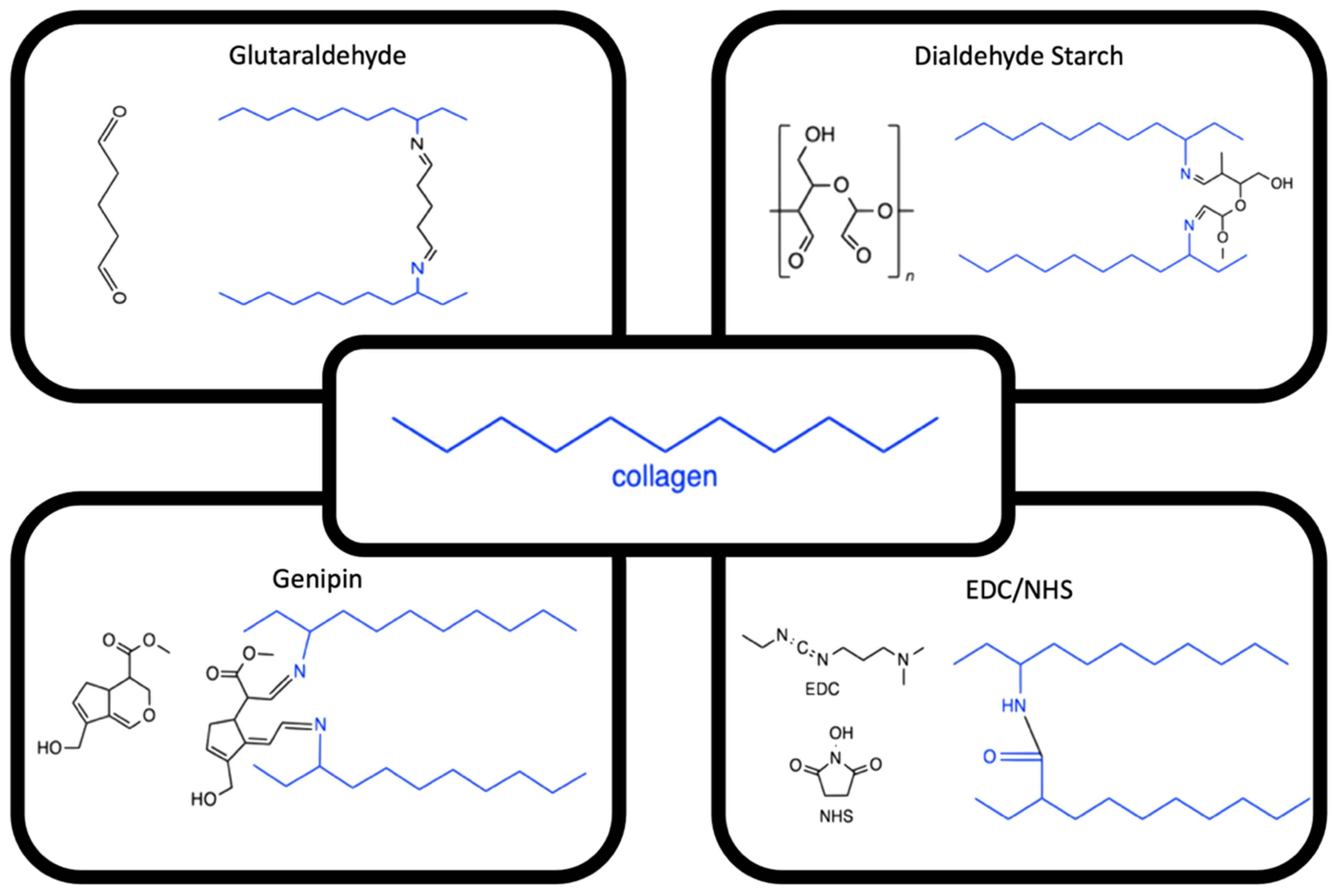
3.3. Enhancing Electro-Compacted Collagen Strength
3.4. Co-Electro-Compaction of Collagen with Fillers
3.5. Post-Alignment Fabrication Methods
3.6. Clinical Applications Using Electro-Compacted Collagen Scaffolds
4. Conclusion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorushanova, A.; Coentro, Q.; Pandit, A.; Zeugolis, D.I.; Raghunath, M. Collagen: Materials analysis and implant uses. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 332–350. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. BioMed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Blidi, O.E.; Omari, N.E.; Balahbib, A.; Ghchime, R.; Menyiy, N.E.; Ibrahimi, A.; Kaddour, K.B.; Bouyahya, A.; Chokairi, O.; Barkiyou, M. Extraction methods, characterization and biomedical applications of collagen: A review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. gelatine-based biomaterials and their biocompatibility: Review and perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: London, UK, 2011; pp. 17–52. [Google Scholar] [CrossRef]
- Krane, S.M. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 2008, 35, 703–710. [Google Scholar] [CrossRef]
- Gara, S.K.; Grumati, P.; Urciuolo, A.; Bonaldo, P.; Kobbe, B.; Koch, M.; Paulsson, M.; Wagener, R. Three novel collagen VI chains with high homology to the alpha3 chain. J. Biol. Chem. 2008, 283, 10658–10670. [Google Scholar] [CrossRef]
- Kruger, T.; Miller, A.; Wang, J. Collagen Scaffolds in Bone Sialoprotein-Mediated Bone Regeneration. Sci. World J. 2013, 2013, 812718. [Google Scholar] [CrossRef]
- Guo, C.; Kaufman, L.J. Flow and magnetic field induced collagen alignment. Biomaterials 2007, 28, 1105–1114. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A. best of both hydrogel worlds: Harnessing bioactivity and tunability by incorporating glycosaminoglycans in collagen hydrogels. Bioengineering 2020, 7, 156. [Google Scholar] [CrossRef]
- Oliveira, V.d.M.; Assis, C.R.D.; Costa, B.d.A.M.; Neri, R.C.d.A.; Monte, F.T.D.; Freitas, H.M.S.d.C.V.; França, R.C.P.; Santos, J.F.; Bezerra, R.d.S.; Porto, A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Minary-Jolandan, M.; Yu, M.-F. Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules 2009, 10, 2565–2570. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Janorkar, A.V. Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100253. [Google Scholar] [CrossRef]
- Sachlos, E.; Wahl, D.A.; Triffitt, J.T.; Czernuszka, J.T. The impact of critical point drying with liquid carbon dioxide on collagen-hydroxyapatite composite scaffolds. Acta Biomater. 2008, 4, 1322–1331. [Google Scholar] [CrossRef]
- Daamen, W.F.; Van Moerkerk, H.T.B.; Hafmans, T.; Buttafoco, L.; Poot, A.A.; Veerkamp, J.H.; Van Kuppevelt, T.H. Preparation and evaluation of molecularly-defined collagen-elastin-glycosaminoglycan scaffolds for tissue engineering. Biomaterials 2003, 24, 4001–4009. [Google Scholar] [CrossRef]
- Kishore, V.; Iyer, R.; Frandsen, A.; Nguyen, T.U. In vitro characterization of electrochemically compacted collagen matrices for corneal applications. Biomed. Mater. 2016, 11, 055008. [Google Scholar] [CrossRef]
- Łagan, S.; Liber-Kneć, A. Mechanical properties of porcine aorta—Influence of specimen taken orientation. Adv. Intell. Syst. 2020, 1033, 279–287. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowaka, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Karsdal, M.A. Type XXIV collagen. In Biochemistry of Collagens, Laminins and Elastin; Karsdal, M.A., Ed.; Academic Press: Washington, DC, USA, 2016; pp. 143–145. [Google Scholar] [CrossRef]
- Koch, M.; Schulze, J.; Hansen, U.; Ashwodt, T.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E.; Bruckner, P.; Bruckner-Tuderman, L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004, 279, 22514–22521. [Google Scholar] [CrossRef]
- Kehlet, S.N.; Karsdal, M.A. Type XXIII collagen. In Biochemistry of Collagens, Laminins and Elastin; Karsdal, M.A., Ed.; Academic Press: Washington, DC, USA, 2016; pp. 139–141. [Google Scholar] [CrossRef]
- Izzi, V.; Heljasvaara, R.; Heikkinen, A.; Karppinen, S.-M.; Koivunen, J.; Pihlajaniemi, T. Exploring the roles of MACIT and multiplexin collagens in stem cells and cancer. Semin. Cancer Biol. 2020, 62, 134–148. [Google Scholar] [CrossRef]
- Tu, H.; Huhtala, P.; Lee, H.M.; Adams, J.C.; Pihlajaniemi, T. Membrane-associated collagens with interrupted triple-helices (MACITs): Evolution from a bilaterian common ancestor and functional conservation in C. elegans. BMC Evol. Biol. 2015, 15, 281. [Google Scholar] [CrossRef]
- Samad, N.A.B.A.; Sikarwar, A.S. Collagen: New dimension in cosmetic and healthcare. Int. J. Biochem. Res. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Lou, Y.Y.; Li, T.H.; Liu, B.Z.; Chen, K.; Zhang, D.; Li, T. Cross-linking methods of type I collagen-based scaffolds for cartilage tissue engineering. Am. J. Transl. Res. 2022, 14, 1146–1159. [Google Scholar]
- Zhang, T.; Yu, Z.; Ma, Y.; Chiou, B.-S.; Liu, F.; Zhong, F. Modulating physicochemical properties of collagen films by cross-linking with glutaraldehyde at varied pH values. Food Hydrocoll. 2022, 124, 107270. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, L.; Liu, Q.; Li, D.; Mu, C.; Zhao, L.; Li, X.; Ge, L. Crosslinking effect of dialdehyde cholesterol modified starch nanoparticles on collagen hydrogel. Carbohydr. Polym. 2022, 285, 119237. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-chemical characterization and biological tests of collagen/silk fibroin/chitosan scaffolds cross-linked by dialdehyde starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef]
- Cudjoe, E.; Younesi, M.; Cudjoe, E.; Akkus, O.; Rowan, S.J. Synthesis and fabrication of nanocomposite fibers of collagen-cellulose nanocrystals by coelectrocompaction. Biomacromolecules 2017, 18, 1259–1267. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Scialla, S.; Gullotta, F.; Izzo, D.; Palazzo, B.; Scalera, F.; Martin, I.; Sannino, A.; Gervaso, F. Genipin-crosslinked collagen scaffolds inducing chondrogenesis: A mechanical and biological characterization. J. Biomed. Mater. Res. A 2022, 110, 1372–1385. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Celik, H.; Akkus, O.; King, M.W. Evaluation of an electrochemically aligned collagen yarn for textile scaffold fabrication. Biomed. Mater. 2021, 16, 025001. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; You, J.; Song, Y.; Tomaskovic-Crook, E.; Sutton, G.; Crook, J.M.; Wallace, G.G. Biomimetic corneal stroma using electro-compacted collagen. Acta Biomater. 2020, 113, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, M.; Para, A. Structural and molecular properties of dialdehyde starch. Carbohydr. Polym. 2006, 63, 360–366. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Lukonina, O.; Darvish, D.; Nashchekin, A.; Elokhovskiy, V.; Yudin, V.; Mikhailova, N. Biological and rheological properties of collagen cross-linked with glutaraldehyde. Tech. Phys. 2020, 65, 1535–1540. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is dialdehyde starch a valuable cross-linking agent for collagen/elastin based materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef]
- Wegrzynowska-Drzymalska, K.; Mylkie, K.; Nowak, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Dialdehyde starch nanocrystals as a novel cross-linker for biomaterials able to interact with human serum proteins. Int. J. Mol. Sci. 2022, 23, 7652. [Google Scholar] [CrossRef]
- Ramos-de-la-Peña, A.M.; Renard, C.M.G.C.; Montañez, J.; de la Luz Reyes-Vega, M.; Contreras-Esquivel, J.C. A review through recovery, purification and identification of genipin. Phytochem. Rev. 2016, 15, 37–49. [Google Scholar] [CrossRef]
- Riacci, L.; Sorriento, A.; Ricotti, L. Genipin-based crosslinking of jellyfish collagen 3D hydrogels. Gels 2021, 7, 238. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Wissink, M.J.B.; Beernink, R.; Pieper, J.S.; Poot, A.A.; Engbers, G.H.M.; Beugeling, T.; van Aken, W.G.; Feijen, J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials 2001, 22, 151–163. [Google Scholar] [CrossRef]
- Jia, W.; Li, M.; Kang, L.; Gu, G.; Guo, Z.; Chen, Z. Fabrication and comprehensive characterization of biomimetic extracellular matrix electrospun scaffold for vascular tissue engineering applications. J. Mater. Sci. 2019, 54, 10871–10883. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Pietrucha, K.; Safandowska, M. Dialdehyde cellulose-crosslinked collagen and its physicochemical properties. Process. Biochem. 2015, 50, 2105–2111. [Google Scholar] [CrossRef]
- Nair, M.; Best, S.M.; Cameron, R.E. Crosslinking collagen constructs: Achieving cellular selectivity through modifications of physical and chemical properties. Appl. Sci. 2020, 10, 6911. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. A 2009, 89A, 363–369. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tobolsky, A.V. Cross-linking of gelatine by dehydration. Nature 1967, 215, 509–510. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Ashworth, J.C.; Cameron, R.E.; Oyen, M.L. Multi-scale mechanical response of freeze-dried collagen scaffolds for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2015, 42, 19–25. [Google Scholar] [CrossRef]
- Biazar, E.; Kamalvand, M.; Keshel, S.H.; Pourjabbar, B.; Rezaei-Tavirani, M. Cross-Linked Collagen Scaffold from Fish Skin as an Ideal Biopolymer for Tissue Engineering. Korean J. Mater. Res. 2022, 32, 186–192. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen- glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef]
- Siegel, R.C.; Pinnell, S.R.; Martin, G.R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry 1970, 9, 4486–4492. [Google Scholar] [CrossRef]
- Chen, R.-N.; Ho, H.-O.; Sheu, M.-T. Characterization of collagen matrices crosslinked using microbial transglutaminase. Biomaterials 2005, 26, 4229–4235. [Google Scholar] [CrossRef]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2015, 27, 14. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Islam, M.M.; Alarcon, E.I.; Samanta, A.; Wang, S.; Lundström, P.; Hilborn, J.; Griffith, M.; Phopase, J. Functionalised type-I collagen as a hydrogel building block for bio-orthogonal tissue engineering applications. J. Mater. Chem. B 2016, 4, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Daikos, O.; Naumov, S.; Knolle, W.; Heymann, K.; Scherzer, T. Photoinitiator-free radical photopolymerization using polybrominated and polychlorinated aromatic methacrylates: Investigations on the mechanisms of initiation. J. Photochem. Photobiol. 2022, 429, 113916. [Google Scholar] [CrossRef]
- Zhang, Y.; Conrad, A.H.; Conrad, G.W. Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking. J. Biol. Chem. 2011, 286, 13011–13022. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef]
- Dewle, A.; Pathak, N.; Rakshasmare, P.; Srivastava, A. Multifarious fabrication approaches of producing aligned collagen scaffolds for tissue engineering applications. ACS Biomater. Sci. Eng. 2020, 6, 779–797. [Google Scholar] [CrossRef]
- Nguyen, T.U.; Shojaee, M.; Bashur, C.A.; Kishore, V. Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering. Biofabrication 2019, 11, 015007. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Wang, T.; Chen, P.; Zheng, M.; Wang, A.; Lloyd, D.; Leys, T.; Zheng, Q.; Zheng, M.H. In vitro loading models for tendon mechanobiology. J. Orthop. Res. 2018, 36, 566–575. [Google Scholar] [CrossRef]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef]
- Kirkwood, J.E.; Fuller, G.G. Liquid crystalline collagen: A self-assembled morphology for the orientation of mammalian cells. Langmuir 2009, 25, 3200–3206. [Google Scholar] [CrossRef]
- Debons, N.; Matsumoto, K.; Hirota, N.; Coradin, T.; Ikoma, T.; Aimé, C. Magnetic field alignment, a perspective in the engineering of collagen-silica composite biomaterials. Biomolecules 2021, 11, 749. [Google Scholar] [CrossRef]
- Wilks, B.T.; Evans, E.B.; Nakhla, M.N.; Morgan, J.R. Directing fibroblast self-assembly to fabricate highly-aligned, collagen-rich matrices. Acta Biomater. 2018, 81, 70–79. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Gallentine, S.C.; Powell, H.M. Collagen-based electrospun materials for tissue engineering: A systematic review. Bioengineering 2021, 8, 39. [Google Scholar] [CrossRef]
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 2008, 29, 3278–3288. [Google Scholar] [CrossRef]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Lee, P.; Lin, R.; Moon, J.; Lee, L.P. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed. Microdevices 2006, 8, 35–41. [Google Scholar] [CrossRef]
- Matsugaki, A.; Isobe, Y.; Saku, T.; Nakano, T. Quantitative regulation of bone-mimetic, oriented collagen/apatite matrix structure depends on the degree of osteoblast alignment on oriented collagen substrates. J. Biomed. Mater. Res. A 2015, 103, 489–499. [Google Scholar] [CrossRef]
- Lai, E.S.; Huang, N.F.; Cooke, J.P.; Fuller, G.G. Aligned nanofibrillar collagen regulates endothelial organization and migration. Regen. Med. 2012, 7, 649–661. [Google Scholar] [CrossRef]
- Volkenstein, S.; Kirkwood, J.E.; Lai, E.; Dazert, S.; Fuller, G.G.; Heller, S. Oriented collagen as a potential cochlear implant electrode surface coating to achieve directed neurite outgrowth. Eur. Arch. Otorhinolaryngol. 2012, 269, 1111–1116. [Google Scholar] [CrossRef]
- Guido, S.; Tranquillo, R.T. A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J. Cell Sci. 1993, 105, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Iwasaka, M.; Ueno, S.; Curtis, A. Magnetic orientation of collagen and bone mixture. J. Appl. Phys. 2000, 87, 6191–6193. [Google Scholar] [CrossRef]
- Dubey, N.; Letourneau, P.C.; Tranquillo, R.T. Neuronal contact guidance in magnetically aligned fibrin gels: Effect of variation in gel mechano-structural properties. Biomaterials 2001, 22, 1065–1075. [Google Scholar] [CrossRef]
- Worcester, D.L. Structural origins of diamagnetic anisotropy in proteins. Proc. Natl. Acad. Sci. USA 1978, 75, 5475–5477. [Google Scholar] [CrossRef]
- Dickinson, R.B.; Guido, S.; Tranquillo, R.T. Biased cell migration of fibroblasts exhibiting contact guidance in oriented collagen gels. Ann. Biomed. Eng. 1994, 22, 342–356. [Google Scholar] [CrossRef]
- Torbet, J.; Ronziere, M.C. Magnetic alignment of collagen during self-assembly. Biochem. J. 1984, 219, 1057–1059. [Google Scholar] [CrossRef]
- Wilson, S.; Guilbert, M.; Sulé-Suso, J.; Torbet, J.; Jeannesson, P.; Sockalingum, G. A microscopic and macroscopic study of aging collagen on its molecular structure, mechanical properties, and cellular response. FASEB J. 2013, 28, 14–25. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D.J. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Builles, N.; Janin-Manificat, H.; Malbouyres, M.; Justin, V.; Rovère, M.R.; Pellegrini, G.; Torbet, J.; Hulmes, D.J.S.; Burillon, C.; Damour, O.; et al. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 2010, 31, 8313–8322. [Google Scholar] [CrossRef]
- Hiraki, H.L.; Matera, D.L.; Rose, M.J.; Kent, R.N.; Todd, C.W.; Stout, M.E.; Wank, A.E.; Schiavone, M.C.; DePalma, S.J.; Zarouk, A.A.; et al. Magnetic alignment of electrospun fiber segments within a hydrogel composite guides cell spreading and migration phenotype switching. Front. Bioeng. Biotechnol. 2021, 9, 679165. [Google Scholar] [CrossRef]
- Eguchi, Y.; Ohtori, S.; Sekino, M.; Ueno, S. Effectiveness of magnetically aligned collagen for neural regeneration in vitro and in vivo. Bioelectromagnetics 2015, 36, 233–243. [Google Scholar] [CrossRef]
- Ambrock, K.; Grohe, B.; Mittler, S. Oriented type I collagen—A review on artificial alignment strategies. Int. J. Surf. Eng. Interdiscip. Mater. Sci. 2021, 9, 96–123. [Google Scholar] [CrossRef]
- Xu, B.; Chow, M.-J.; Zhang, Y. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int. J. Biomater. 2011, 2011, 172389. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Schell, J.Y.; Wilks, B.T.; Patel, M.; Franck, C.; Chalivendra, V.; Cao, X.; Shenoy, V.B.; Morgan, J.R. Harnessing cellular-derived forces in self-assembled microtissues to control the synthesis and alignment of ECM. Biomaterials 2016, 77, 120–129. [Google Scholar] [CrossRef]
- Zdraveva, E.; Fang, J.; Mijovic, B.; Lin, T. Electrospun nanofibers. In Structure and Properties of High-Performance Fibers; Bhat, G., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 267–300. [Google Scholar]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Depeigne, L.; Zdraveva, E. Electrospun biomaterials’ applications and processing. J. Biomim. Biomater. Biomed. Eng. 2021, 49, 91–100. [Google Scholar] [CrossRef]
- Ameer, J.M.; PR, A.K.; Kasoju, N. Strategies to Tune Electrospun Scaffold Porosity for Effective Cell Response in Tissue Engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef]
- Yang, L.; Fitié, C.F.C.; van der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials 2008, 29, 955–962. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Malara, M.M.; Baumann, M.E.; McFarland, K.L.; Supp, D.M.; Powell, H.M. Fractional CO2 laser micropatterning of cell-seeded electrospun collagen scaffolds enables rete ridge formation in 3D engineered skin. Acta Biomater. 2020, 102, 287–297. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Ramakrishna, S. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta Biomater. 2011, 7, 3113–3122. [Google Scholar] [CrossRef]
- Powell, H.M.; Supp, D.M.; Boyce, S.T. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials 2008, 29, 834–843. [Google Scholar] [CrossRef]
- Willard, J.J.; Drexler, J.W.; Das, A.; Roy, S.; Shilo, S.; Shoseyov, O.; Powell, H.M. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng. Part A 2013, 19, 1507–1518. [Google Scholar] [CrossRef]
- Huang, G.P.; Shanmugasundaram, S.; Masih, P.; Pandya, D.; Amara, S.; Collins, G.; Arinzeh, T.L. An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds. J. Biomed. Mater. Res. A 2015, 103, 762–771. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Sun, J.; Zhuang, Y.; Shi, J.; Guan, D.; Chen, Y.; Dai, J. Radially aligned electrospun fibers with continuous gradient of SDF1α for the guidance of neural stem cells. Small 2016, 12, 5009–5018. [Google Scholar] [CrossRef]
- Liu, T.; Houle, J.D.; Xu, J.; Chan, B.P.; Chew, S.Y. Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng. Part A 2012, 18, 1057–1066. [Google Scholar] [CrossRef]
- Ouyang, Y.; Huang, C.; Zhu, Y.; Fan, C.; Ke, Q. Fabrication of seamless electrospun collagen/PLGA conduits whose walls comprise highly longitudinal aligned nanofibers for nerve regeneration. J. Biomed. Nanotechnol. 2013, 9, 931–943. [Google Scholar] [CrossRef]
- Boland, E.D.; Matthews, J.A.; Pawlowski, K.J.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Electrospinning collagen and elastin: Preliminary vascular tissue engineering. Front. Biosci. 2004, 9, 1422–1432. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Yang, J.Q.; Heydarkhan, S.; Xu, Y.; Zuk, P.A.; MacLellan, W.R.; Beygui, R.E. Human adipose stem cells: A potential cell source for cardiovascular tissue engineering. Cells Tissues Organs 2008, 187, 263–274. [Google Scholar] [CrossRef]
- Jha, B.S.; Ayres, C.E.; Bowman, J.R.; Telemeco, T.A.; Sell, S.A.; Bowlin, G.L.; Simpson, D.G. Electrospun collagen: A tissue engineering scaffold with unique functional properties in a wide variety of applications. J. Nanomater. 2011, 2011, 348268. [Google Scholar] [CrossRef]
- Zhao, W.; Ju, Y.M.; Christ, G.; Atala, A.; Yoo, J.J.; Lee, S.J. Diaphragmatic muscle reconstruction with an aligned electrospun poly(ε-caprolactone)/collagen hybrid scaffold. Biomaterials 2013, 34, 8235–8240. [Google Scholar] [CrossRef]
- Lotfi, G.; Shokrgozar, M.A.; Mofid, R.; Abbas, F.M.; Ghanavati, F.; Baghban, A.A.; Yavari, S.K.; Pajoumshariati, S. Biological evaluation (in vitro and in vivo) of bilayered collagenous coated (nano electrospun and solid wall) chitosan membrane for periodontal guided bone regeneration. Ann. Biomed. Eng. 2016, 44, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Russell, S.J.; Yang, X.; Tronci, G.; Wood, D.J. Compositional and in vitro evaluation of Nonwoven Type I Collagen/Poly-dl-lactic Acid scaffolds for bone regeneration. J. Funct. Biomater. 2015, 6, 667. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.R.; Merschrod, S.E.F.; Poduska, K.M. Electrochemically controlled growth and positioning of suspended collagen membranes. Langmuir 2008, 24, 2970–2972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marino, A.A.; Becker, R.O. The effect of electric current on rat tail tendon collagen in solution. Calcif. Tissue Res. 1970, 4, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Jones, S. Effects of small electrical currents on collagen in solution. S. Afr. J. Sci. 1976, 72, 114–116. [Google Scholar]
- Webster, V.A.; Hawley, E.L.; Akkus, O.; Chiel, H.J.; Quinn, R.D. Effect of actuating cell source on locomotion of organic living machines with electrocompacted collagen skeleton. Bioinspir. Biomim. 2016, 11, 036012. [Google Scholar] [CrossRef]
- Abu-Rub, M.T.; Billiar, K.L.; van Es, M.H.; Knight, A.; Rodriguez, B.J.; Zeugolis, D.I.; McMahon, S.; Windebank, A.J.; Pandit, A. Nano-textured self-assembled aligned collagen hydrogels promote directional neurite guidance and overcome inhibition by myelin associated glycoprotein. Soft Matter 2011, 7, 2770–2781. [Google Scholar] [CrossRef]
- Kang, L.; Liu, X.; Yue, Z.; Chen, Z.; Baker, C.; Winberg, P.C.; Wallace, G.G. Fabrication and in vitro characterization of electrochemically compacted collagen/sulfated xylorhamnoglycuronan matrix for wound healing applications. Polymers 2018, 10, 415. [Google Scholar] [CrossRef]
- Nguyen, T.U.; Bashur, C.A.; Kishore, V. Impact of elastin incorporation into electrochemically aligned collagen fibers on mechanical properties and smooth muscle cell phenotype. Biomed. Mater. 2016, 11, 025008. [Google Scholar] [CrossRef]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef]
- Luo, X.; Guo, Z.; He, P.; Chen, T.; Li, L.; Ding, S.; Li, H. Study on structure, mechanical property and cell cytocompatibility of electrospun collagen nanofibers crosslinked by common agents. Int. J. Biol. Macromol. 2018, 113, 476–486. [Google Scholar] [CrossRef]
- Younesi, M.; Islam, A.; Kishore, V.; Panit, S.; Akkus, O. Fabrication of compositionally and topographically complex robust tissue forms by 3D-electrochemical compaction of collagen. Biofabrication 2015, 7, 035001. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Uquillas, J.A.; Kishore, V.; Akkus, O. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads. Biomed. Mater. 2011, 6, 035008. [Google Scholar] [CrossRef]
- Cross, V.L.; Zheng, Y.; Choi, N.W.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef]
- Giraud Guille, M.M.; Helary, C.; Vigier, S.; Nassif, N. Dense fibrillar collagen matrices for tissue repair. Soft Matter 2010, 6, 4963–4967. [Google Scholar] [CrossRef]
- Islam, A.; Chapin, K.; Younesi, M.; Akkus, O. Computer aided biomanufacturing of mechanically robust pure collagen meshes with controlled macroporosity. Biofabrication 2015, 7, 035005. [Google Scholar] [CrossRef]
- Kumar, M.R.; Merschrod, S.E.F.; Poduska, K.M. Correlating mechanical properties with aggregation processes in electrochemically fabricated collagen membranes. Biomacromolecules 2009, 10, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Paulovich, J.; Webster-Wood, V. Tuning the mechanical and geometric properties of electrochemically aligned collagen threads toward applications in biohybrid robotics. J. Biomech. Eng. 2021, 143, 051005. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Tebyanian, H.; Soufdoost, R.S.; Motavallian, E.; Barkhordari, A.; Nourani, M.R. Extraction and characterization of collagen with cost-effective method from human placenta for biomedical applications. World J. Plast. Surg. 2019, 8, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, Z.; Li, Z.; Zhang, C.; Zhang, D. The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep). J. Integr. Agric. 2018, 17, 704–711. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An insight on type I collagen from horse tendon for the manufacture of implantable devices. Int. J. Biol. Macromol. 2020, 154, 291–306. [Google Scholar] [CrossRef]
- Akram, A.N.; Zhang, C. Extraction of collagen-II with pepsin and ultrasound treatment from chicken sternal cartilage; physicochemical and functional properties. Ultrason. Sonochem. 2020, 64, 105053. [Google Scholar] [CrossRef]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater 2021, 10, 100098. [Google Scholar] [CrossRef]
- Maher, M.K.; White, J.F.; Glattauer, V.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Variation in hydrogel formation and network structure for Telo-, Atelo- and Methacrylated collagens. Polymers 2022, 14, 1775. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed collagen-sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen extraction from animal skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef]
- Pan, B.S.; En Chen, H.O.A.; Sung, W.C. Molecular and thermal characteristics of acid-soluble collagen from orbicular batfish: Effects of deep-sea water culturing. Int. J. Food Prop. 2018, 21, 1080–1090. [Google Scholar] [CrossRef]
- Kezwoń, A.; Chromińska, I.; Frączyk, T.; Wojciechowski, K. Effect of enzymatic hydrolysis on surface activity and surface rheology of type I collagen. Colloids Surf. B 2016, 137, 60–69. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, A.; Li, W.; Liu, T.; Su, Z. Mass spectrometric analysis of enzymatic digestion of denatured collagen for identification of collagen type. J. Chromatogr. A 2006, 1114, 274–277. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Younesi, M.; Islam, A.; Kishore, V.; Anderson, J.M.; Akkus, O. Tenogenic induction of human MSCs by anisotropically aligned collagen biotextiles. Adv. Funct. Mater. 2014, 24, 5762–5770. [Google Scholar] [CrossRef]
- Zhang, F.; Bambharoliya, T.; Xie, Y.; Liu, L.; Celik, H.; Wang, L.; Akkus, O.; King, M.W. A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments. Mater. Sci. Eng. C 2021, 118, 111418. [Google Scholar] [CrossRef]
- Singh, G.; Chanda, A. Mechanical properties of whole-body soft human tissues: A review. Biomed. Mater. 2021, 16, 062004. [Google Scholar] [CrossRef]
- Vogt, L.; Rivera, L.R.; Liverani, L.; Piegat, A.; El Fray, M.; Boccaccini, A.R. Poly(ε-caprolactone)/poly(glycerol sebacate) electrospun scaffolds for cardiac tissue engineering using benign solvents. Mater. Sci. Eng. C 2019, 103, 109712. [Google Scholar] [CrossRef]
- Jawad, H.; Ali, N.N.; Lyon, A.R.; Chen, Q.Z.; Harding, S.E.; Boccaccini, A.R. Myocardial tissue engineering: A review. J. Tissue Eng. Regen. Med. 2007, 1, 327–342. [Google Scholar] [CrossRef]
- Uquillas, J.A.; Kishore, V.; Akkus, O. Genipin crosslinking elevates the strength of electrochemically aligned collagen to the level of tendons. J. Mech. Behav. Biomed. Mater. 2012, 15, 176–189. [Google Scholar] [CrossRef]
- Kishore, V.; Paderi, J.E.; Akkus, A.; Smith, K.M.; Balachandran, D.; Beaudoin, S.; Panitch, A.; Akkus, O. Incorporation of a decorin biomimetic enhances the mechanical properties of electrochemically aligned collagen threads. Acta Biomater. 2011, 7, 2428–2436. [Google Scholar] [CrossRef]
- Gendron, R.; Kumar, M.R.; Paradis, H.; Martin, D.; Ho, N.; Gardiner, D.; Merschrod, S.E.F.; Poduska, K.M. Controlled cell proliferation on an electrochemically engineered collagen scaffold: Controlled cell proliferation on an electrochemically. Macromol. Biosci. 2012, 12, 360–366. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, F.; Akkus, O.; King, M.W. A collagen/PLA hybrid scaffold supports tendon-derived cell growth for tendon repair and regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, U.A.; Cheng, X.; Kishore, V.; Uquillas, J.A.; Akkus, O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J. Biomed. Mater. Res. A 2010, 94A, 1070–1079. [Google Scholar] [CrossRef]
- Okamoto, O.; Fujiwara, S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connect Tissue Res. 2006, 47, 177–189. [Google Scholar] [CrossRef]
- Paderi, J.E.; Panitch, A. Design of a synthetic collagen-binding peptidoglycan that modulates collagen fibrillogenesis. Biomacromolecules 2008, 9, 2562–2566. [Google Scholar] [CrossRef] [PubMed]
- Pergande, M.R.; Cologna, S.M. Isoelectric point separations of peptides and proteins. Proteomes 2017, 5, 4. [Google Scholar] [CrossRef]
- Vindin, H.; Mithieux, S.M.; Weiss, A.S. Elastin architecture. Matrix Biol. 2019, 84, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and nanocellulose in polymer composite materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Chen, L.; Hong, F.F. Homogeneous and efficient production of a bacterial nanocellulose-lactoferrin-collagen composite under an electric field as a matrix to promote wound healing. Biomater. Sci. 2021, 9, 930–941. [Google Scholar] [CrossRef]
- Xu, C.; Zhang Molino, B.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar] [CrossRef]
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Textile technologies and tissue engineering: A path toward organ weaving. Adv. Healthc. Mater. 2016, 5, 751–766. [Google Scholar] [CrossRef]
- Kishore, V.; Uquillas, J.A.; Dubikovsky, A.; Alshehabat, M.A.; Snyder, P.W.; Breur, G.J.; Akkus, O. In vivo response to electrochemically aligned collagen bioscaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 400–408. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- McClellan, P.; Ina, J.G.; Knapik, D.M.; Isali, I.; Learn, G.; Valente, A.; Wen, Y.; Wen, R.; Anderson, J.M.; Gillespie, R.J.; et al. Mesenchymal stem cell delivery via topographically tenoinductive collagen biotextile enhances regeneration of segmental tendon defects. Am. J. Sports Med. 2022, 50, 2281–2291. [Google Scholar] [CrossRef]
- Park, H.; Nazhat, S.N.; Rosenzweig, D.H. Mechanical activation drives tenogenic differentiation of human mesenchymal stem cells in aligned dense collagen hydrogels. Biomaterials 2022, 286, 121606. [Google Scholar] [CrossRef]
| Collagen Classification | Collagen Type | Distribution |
|---|---|---|
| Fibril-forming | I | Bone, skin, tendon, ligament, cornea |
| II | Cartilage, vitreous humour | |
| III | Skin, blood vessel | |
| V | Bone, dermis | |
| XI | Cartilage, intervertebral disc | |
| XXIV | Bone, cornea | |
| XXVII | Cartilage | |
| FACIT 1 | VII | Bladder, dermis |
| IX | Cartilage, cornea | |
| XII | Tendon, dermis | |
| XIV | Bone, dermis, cartilage | |
| XVI | Kidney, dermis | |
| XIX | Human rhabdomyosarcoma | |
| XX | Cornea of chick | |
| XXI | Kidney, stomach | |
| XXII | Muscle-tendon junction | |
| XXVI | Ovary, testis | |
| Network forming | IV | Basement membrane |
| VI | Muscle, dermis, cornea, cartilage | |
| VIII | Brain, skin, kidney, heart | |
| X | Hypertrophic cartilage | |
| XXVIII | Dermis, sciatic nerve | |
| MACIT 2 | XIII | Dermis, eye, endothelial cell |
| XVII | Hemi desmosomes in epithelia | |
| XXIII | Heart, retina | |
| XXV | Heart, testis, brain | |
| MULTIPLEXINs 3 | XV | Capillaries, testis, kidney, heart |
| XVIII | Liver, basement membrane |
| Method | Alignment | Collagen Concentration (mg.mL−1) | Effect on Collagen Structure | Mechanical Properties | Ease of Processing |
|---|---|---|---|---|---|
| Gravity-based Fluidic | ++ | 6–14 | + | Not reported | +++ |
| Extrusion-based Fluidic | ++ | >15 | + | Not reported | ++ |
| Stress-induced self-alignment | + | Not applicable | + | Not reported | + |
| Static magnet | +++ | <5 | + | MPa | ++ |
| Flow-magnetic | ++ | <5 | - | MPa | ++ |
| Electrospinning | ++ | >50 | - | MPa | ++ |
| Electro-compaction | +++ | <5 | + | MPa | +++ |
| Sample State | Testing Method | Load Cell | Strain Rate | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Ultimate Tensile Strain (%) | Ref |
|---|---|---|---|---|---|---|---|
| Thread | |||||||
| Hydrated | Monotonic Tensile | 250 N | 10 mm.min−1 | 0.1–750 | 0.1–55 | 11–100 | [122,143,148,149] |
| Dehydrated | Monotonic Tensile | 250 g | 10 mm.min−1 | 200–1000 | 10–70 | 3–15 | [148] |
| Membrane | |||||||
| Hydrated | Monotonic Tensile | 0.1 N.min−1 | 4 kPa–2 | 10–200 kPa | 10–70 | [18] | |
| Hydrated | Monotonic Tensile | 10 N | 10 mm.min−1 | 0.25 | 3.5 | 0.2 | [116] |
| Hydrated | Compression | 1%.s−1 | 100 kPa | 35 kPa | 30 | [120] | |
| Hydrated | Tensile | 1%.s−1 | 30 | 3 | 30 | [120] | |
| Dehydrated | Nanoindentation | 0.10–0.22 GPa | [150] | ||||
| Hydrated | Rheology | Strain 1%, frequency 0.01–100 Hz, strain sweep | G′ 200–500 G″ 50–70 | [35] | |||
| Hydrated | Microindentation | 10 N | 0.1 mm.min−1 | 0.23 kPa | [35] | ||
| Dehydrated | Hertzian Model | 180–240 | [126] | ||||
| Dehydrated | Oliver-Pharr Model | 80–130 | [126] | ||||
| Tube | |||||||
| Hydrated | Monotonic Tensile Static ring | 0.1 N.min−1 | 0.05–0.18 | 0.05–0.18 | 20–25 | [61] | |
| Hydrated | Rheology Cyclic | Strain 8%, frequency 1 hz, 6 sweeps, five oscillations per cycle | G′ 0.06 G″ 0.01 | [61] | |||
| Crosslinker | Solvent | Exposure Concentration | Time | Temperature | Ref |
|---|---|---|---|---|---|
| EDC/NHS | 50 mM MES | 20 mM EDC, 20 mM NHS | 4 h | Room | [116] |
| EDC/NHS | 50 mM MES in Ethanol 70% (pH = 5.5) | 10 mM EDC, 5 mM NHS | 4 h | Room | [18,117] |
| EDC/NHS | Ethanol 80% | 1:25:50 (Col:EDC:NHS) | 2 h | [34,151] | |
| EDC/NHS | Ethanol 80% | 1:100:250 (Col:EDC:NHS) | 15 min | [114] | |
| Genipin | Ethanol 0, 70, 80, 80, and 100% | 0, 0.1, 0.625, 2.00 and 6.00% | 6, 12, 24 and 72 h | 37 °C | [148] |
| Genipin | 1 × PBS | 0.625% | 72 h | 37 °C | [111,152] |
| Genipin | Ethanol 90% | 0.625% | 24 h | Room | [18] |
| Genipin | Ethanol 90% | 0.625% | 72 h | 37 °C | [120,127,143] |
| Genipin | Ethanol 90% | 0.625% | 72 h | [31,64] |
| Scaffold Shape | Scaffold Filler | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Ultimate Tensile Strain (%) | In Vitro Response | Ref |
|---|---|---|---|---|---|---|
| Thread | Collagen only | 10 | 0.4 | 65 | [117] | |
| Thread | Soluble elastin | 3 | 0.2 | 60 | + | |
| Thread | Insoluble elastin | 4 | 0.2 | 45 | + | |
| Thread | t-CNC | 91.5–231.9 | 10.1–22.4 | 10.7–15.1 | Nil | [31] |
| Key: t-CNC TEMPO oxidised cellulose nanocrystals, + Enhanced response compared to collagen only | ||||||
| Fabrication Method | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Ultimate Tensile Strain (%) | Ref |
|---|---|---|---|---|
| Yarn | 520 | 65 | 20 | [143] |
| Braid | 277–671 | 24–88 | 7–24 | [152] |
| Lumen and cir thread | 0.282 | 0.047 | 51.2 | [61] |
| Lumen and long thread | 0.114 | 0.024 | 38.3 |
| Fabrication Method | Max Load (N) | Extension (mm) | Stiffness (N.mm−1) | Ref |
|---|---|---|---|---|
| Weave | 100–350 | 5–10 | 25–89 | [143,151] |
| Knit | 1.4 | 3.1 | 1.8 | [34] |
| Application | Cell | In Vivo | Ref | |
|---|---|---|---|---|
| Source | Type | |||
| Cornea | Human | Corneal Stromal | [35] | |
| Human | Keratocyte | [18] | ||
| Mouse | Corneal Stromal | [150] | ||
| Muscle | Chicken | Cardiomyocyte Skeletal Muscle | [114] | |
| Tendon | Human | Mesenchymal Stem | [64,148] | |
| Rat | Mesenchymal Stem | [152] | ||
| Tendon fibroblast | [69,152] | |||
| Rotator cuff and Achilles tendon | [151] | |||
| Rabbit White New Zealand | [162,164] | |||
| [149] | ||||
| Nerve | Rat | Pheochromocytoma | [115] | |
| Blood Vessel | Rat | Aortic Smooth Muscle | [117] | |
| Human | Umbilical Vein Endothelial | [61,117,144] | ||
| Skin | Human | Dermal Fibroblast | [116] | |
| Mouse | Dermal Fibroblast | Rat Sprague-Dawley | [159] | |
| Myocardium | Human | Cardiosphere-derived | [34] | |
| Tissue engineering | Human | Mesenchymal Stem | [120] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carr, B.P.; Chen, Z.; Chung, J.H.Y.; Wallace, G.G. Collagen Alignment via Electro-Compaction for Biofabrication Applications: A Review. Polymers 2022, 14, 4270. https://doi.org/10.3390/polym14204270
Carr BP, Chen Z, Chung JHY, Wallace GG. Collagen Alignment via Electro-Compaction for Biofabrication Applications: A Review. Polymers. 2022; 14(20):4270. https://doi.org/10.3390/polym14204270
Chicago/Turabian StyleCarr, Benjamin P., Zhi Chen, Johnson H. Y. Chung, and Gordon G. Wallace. 2022. "Collagen Alignment via Electro-Compaction for Biofabrication Applications: A Review" Polymers 14, no. 20: 4270. https://doi.org/10.3390/polym14204270
APA StyleCarr, B. P., Chen, Z., Chung, J. H. Y., & Wallace, G. G. (2022). Collagen Alignment via Electro-Compaction for Biofabrication Applications: A Review. Polymers, 14(20), 4270. https://doi.org/10.3390/polym14204270








