Star-Shaped Polydimethylsiloxanes with Organocyclotetrasilsesquioxane Branching-Out Centers: Synthesis and Properties
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
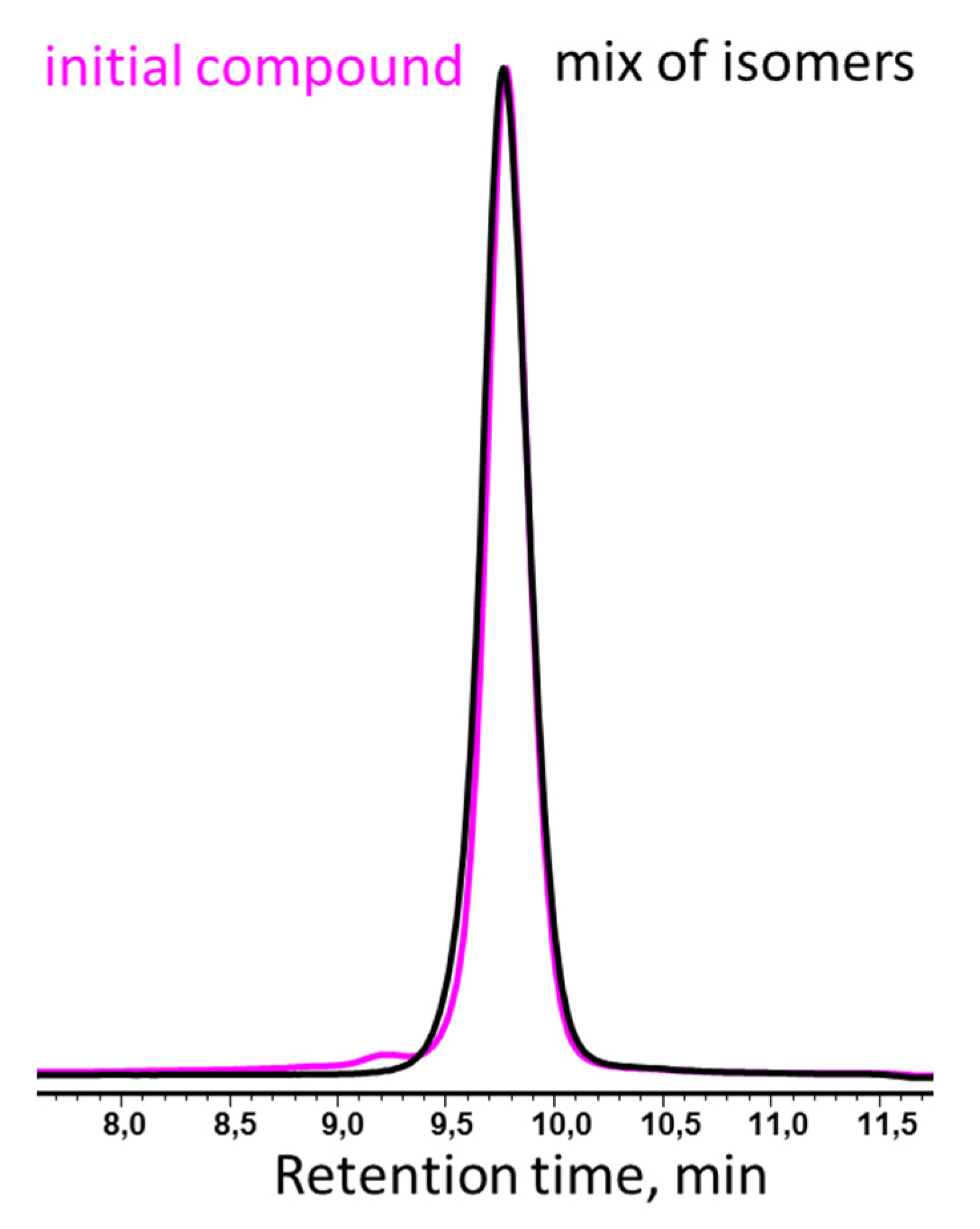
2.2.1. Reaction of Cis-Tetra[phenyl(dimethylsiloxy)cyclotetrasiloxane Isomerization
2.2.2. Synthesis of PDMS-15
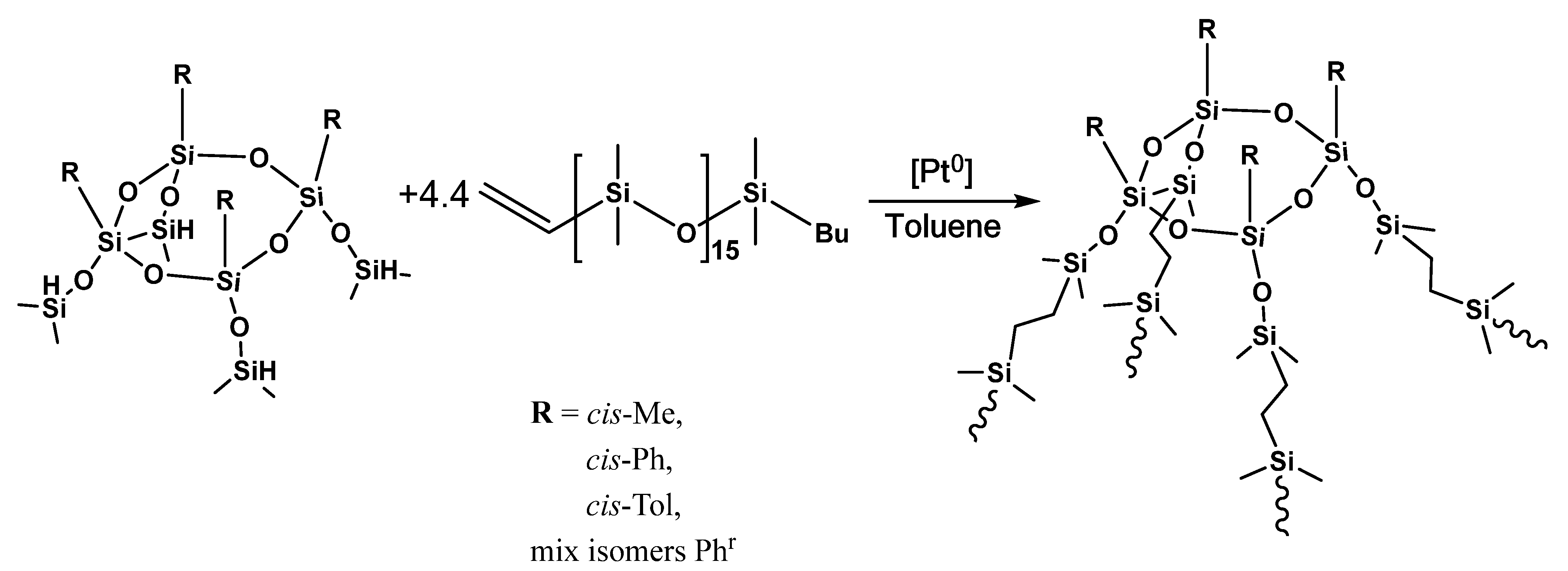
2.2.3. Synthesis of Star-Shaped Siloxane Polymers
General Synthesis Technique
2.2.4. Synthesis of Me4-15 Star-Shaped Polymer
2.2.5. Synthesis of Ph4-15 Star-Shaped Polymer
2.2.6. Synthesis of Phr4-15 Star-Shaped Polymer
2.2.7. Synthesis of Tol4-15 Star-Shaped Polymer
3. Results and their Discussion
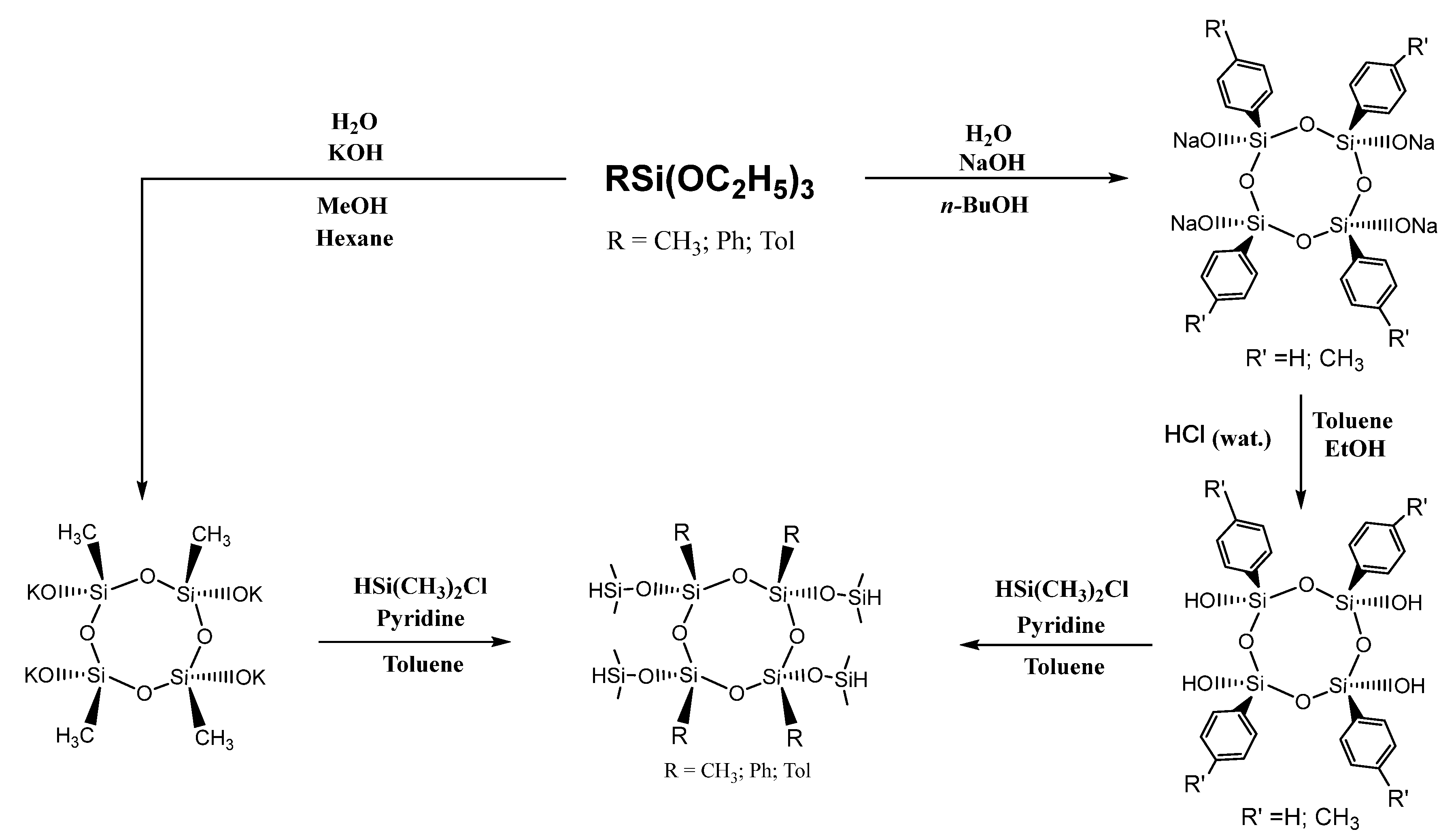
3.1. Cores Synthesis
3.2. Synthesis of Arm
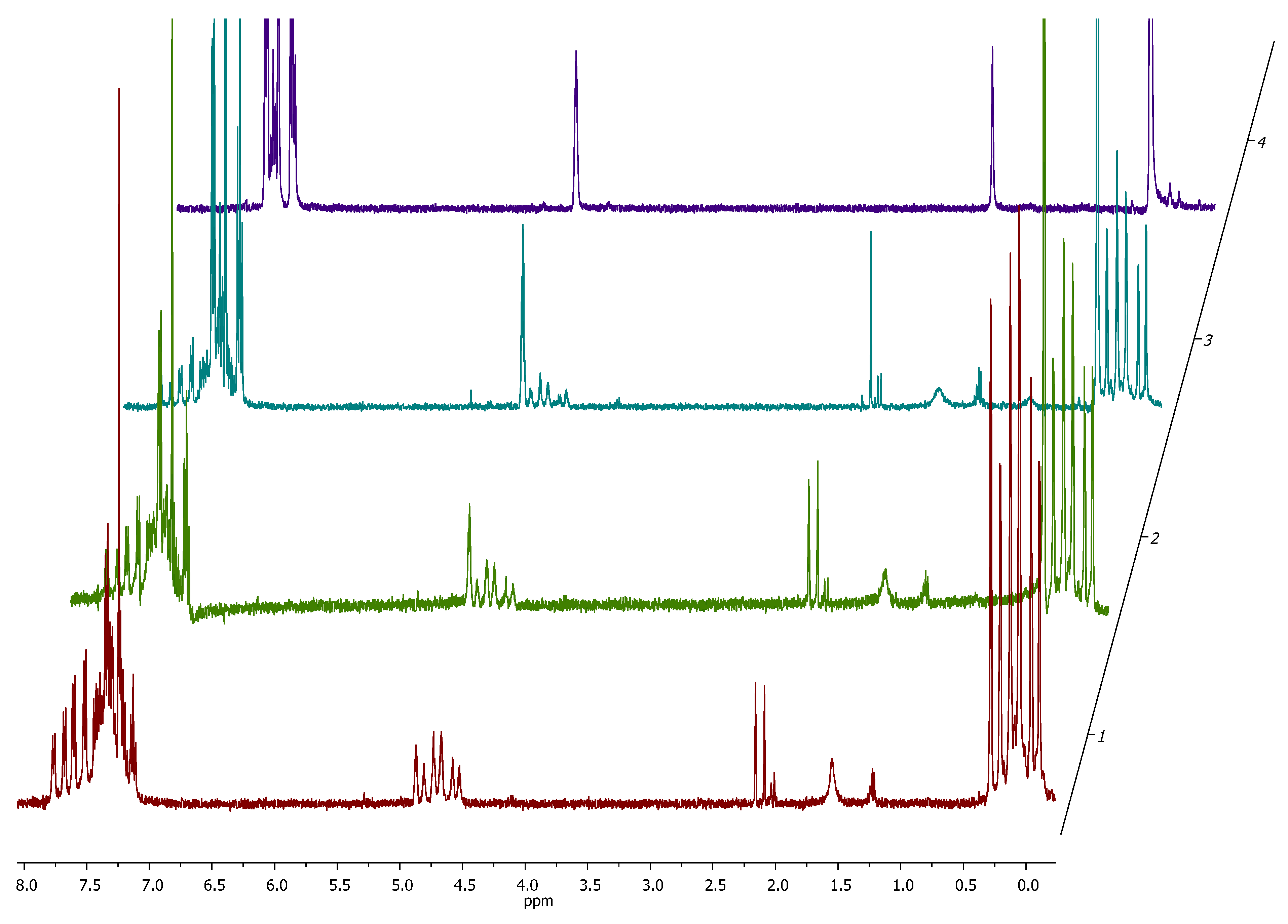
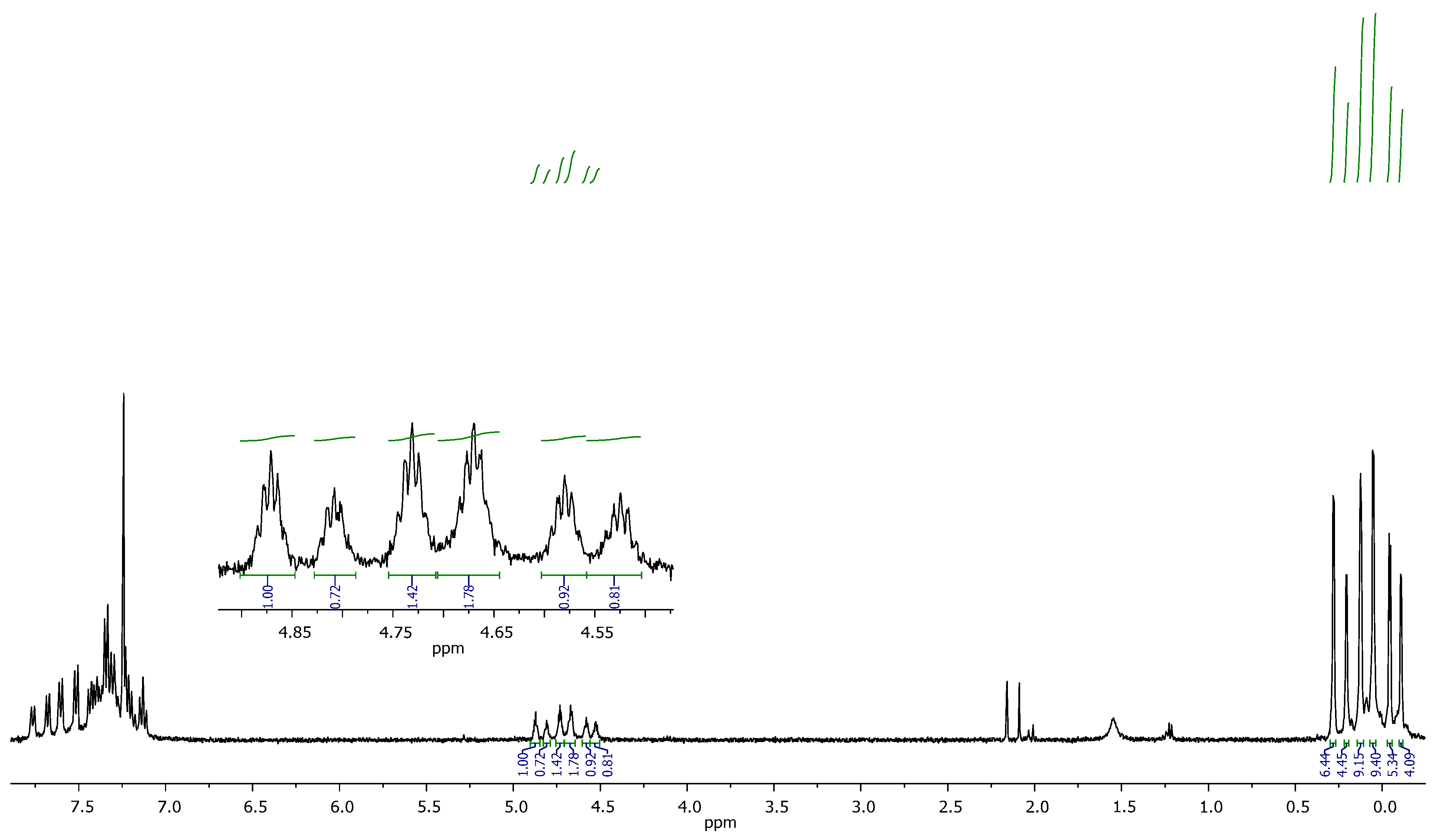
3.3. Assembly of Star-Shaped Polydimethylsiloxanes
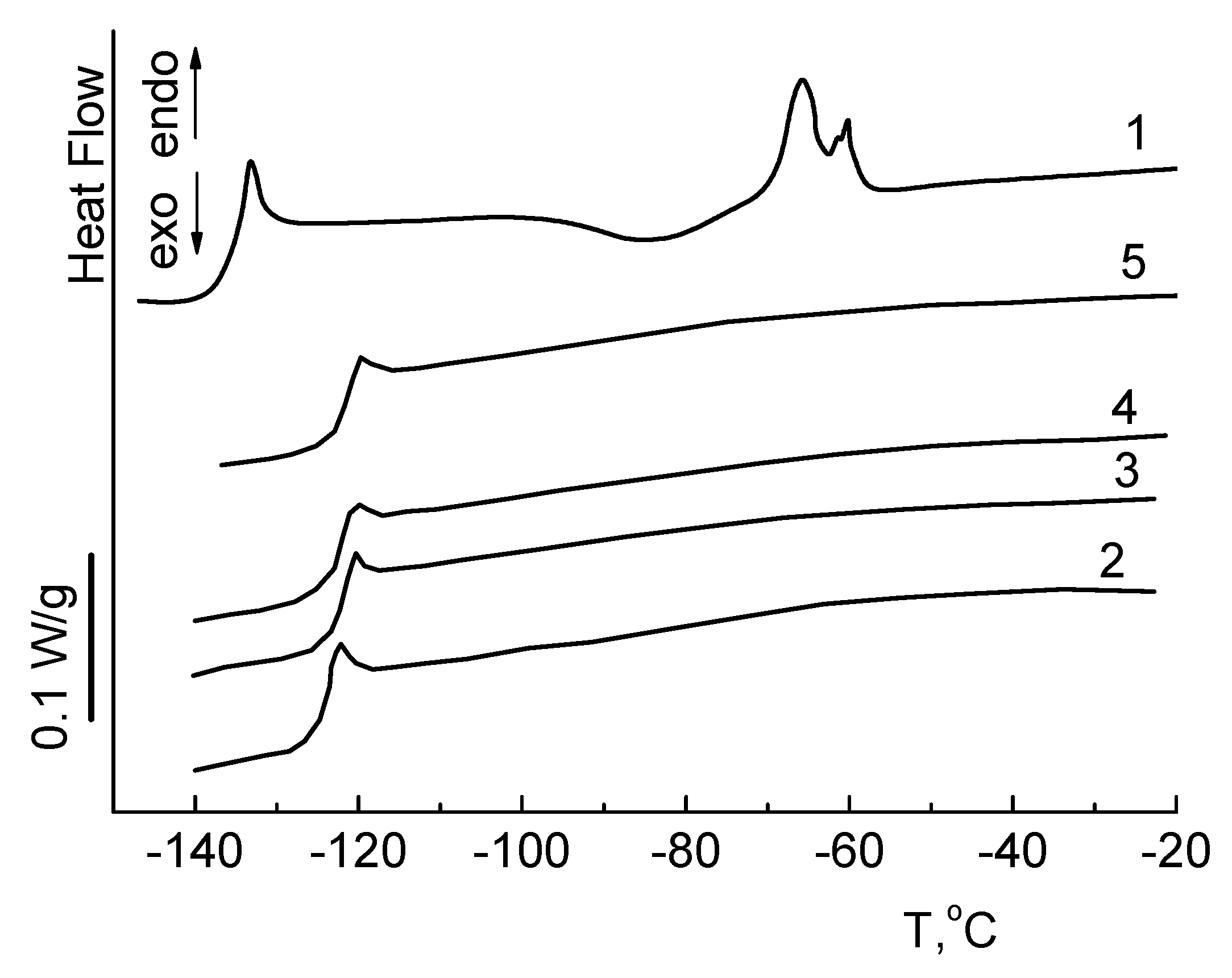
3.4. Thermal Properties
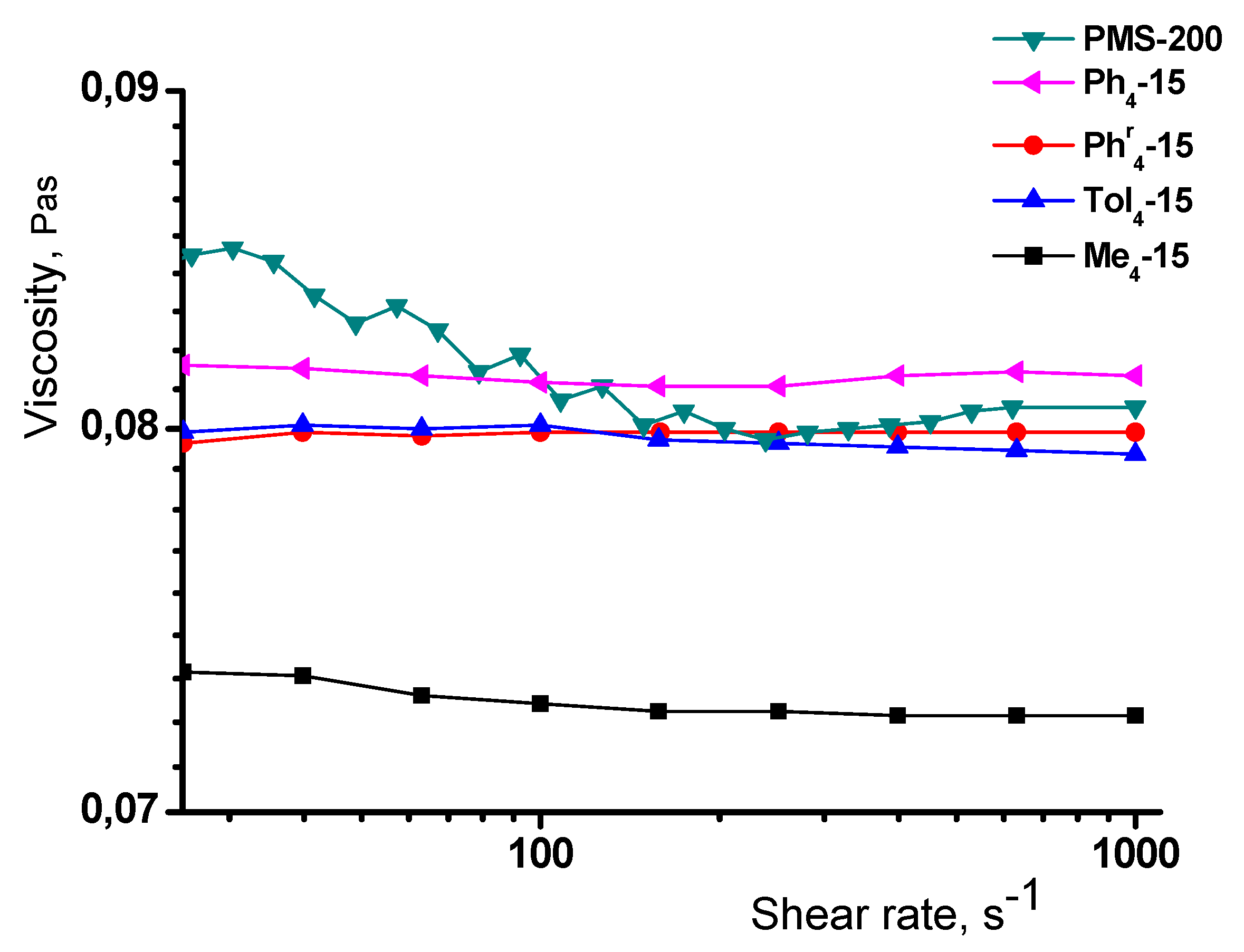
3.5. Rheological Properties
In Solution
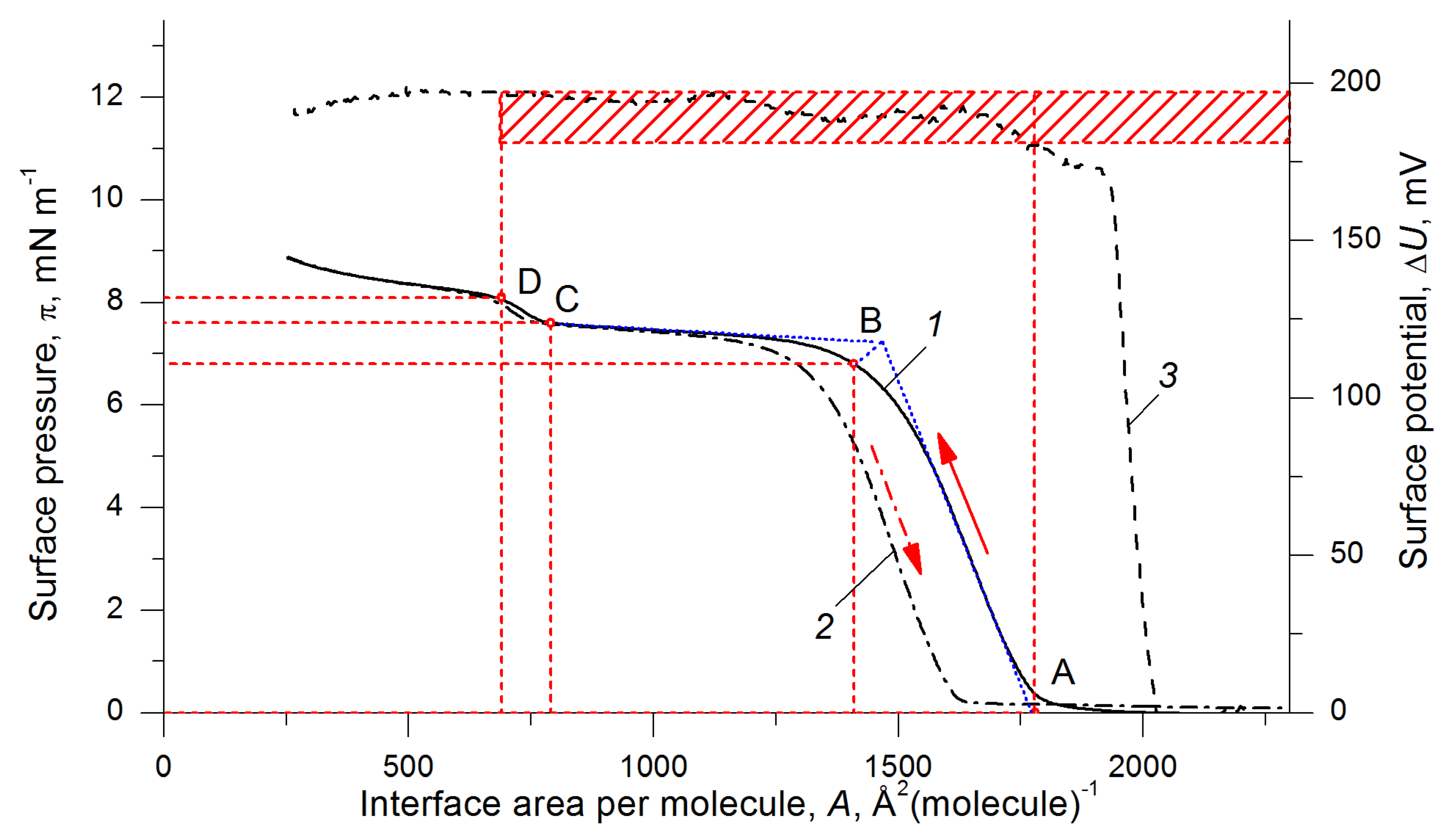
3.6. Langmuir Layers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Soldatov, M. Hybrid porous polymers based on cage-like organosiloxanes: Synthesis, properties and applications. Prog. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- Irzhak, V.I. Topological structure and relaxation properties of branched polymers. Russ. Chem. Rev. 2006, 75, 919–934. [Google Scholar] [CrossRef]
- Patil, R.A.; Aloorkar, N.H.; Kulkarni, A.S.; Ingale, D.J. Star Polymers: An Overview. Int. J. Pharm. Sci. Nanotech. 2012, 5, 1675–1684. [Google Scholar] [CrossRef]
- Polymeropoulos, G.; Zapsas, G.; Ntetsikas, K.; Bilalis, P.; Gnanou, Y.; Hadjichristidis, N. 50th Anniversary perspective: Polymers with complex architectures. Macromolecules 2017, 50, 1253–1290. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Mark, J.E. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef]
- Yoda, R. Elastomers for biomedical applications. J. Biomater. Sci. Polym. Ed. 1998, 9, 561–626. [Google Scholar] [CrossRef]
- Hron, P. Hydrophilisation of silicone rubber for medical applications. Polym. Int. 2003, 52, 1531–1539. [Google Scholar] [CrossRef]
- Cai, G.; Weber, W.P. Synthesis of terminal Si–H irregular tetra-branched star polysiloxanes. Pt-catalyzed hydrosilylation with unsaturated epoxides. Polysiloxane films by photo-acid catalyzed crosslinking. Polymer 2004, 45, 2941–2948. [Google Scholar] [CrossRef]
- Chernyy, S.; Kirkensgaard, J.J.K.; Mahalik, J.P.; Kim, H.; Arras, M.M.L.; Kumar, R.; Sumpter, B.G.; Smith, G.S.; Mortensen, K.; Russell, T.P.; et al. Bulk and surface morphologies of ABC miktoarm star terpolymers composed of PDMS, PI, and PMMA arms. Macromolecules 2018, 51, 1041–1051. [Google Scholar] [CrossRef]
- Minehara, H.; Pitet, L.M.; Kim, S.; Zha, R.H.; Meijer, E.W.; Hawker, C.J. Branched block copolymers for tuning of morphology and feature size in thin film nanolithography. Macromolecules 2016, 49, 2318–2326. [Google Scholar] [CrossRef]
- Georgopanos, P.; Lo, T.-Y.; Ho, R.-M.; Avgeropoulos, A. Synthesis, molecular characterization and self-assembly of (PS-b-PDMS)n type linear (n = 1, 2) and star (n = 3, 4) block copolymers. Polym. Chem. 2017, 8, 843–850. [Google Scholar] [CrossRef]
- Fragouli, P.G.; Iatrou, H.; Hadjichristidis, N.; Sakurai, T.; Hirao, A. Synthesis and characterization of model 3-miktoarm star copolymers of poly (dimethylsiloxane) and poly (2-vinylpyridine). J. Polym. Sci. A Polym. Chem. 2005, 44, 614–619. [Google Scholar] [CrossRef]
- Tezuka, Y.; Iwase, T.; Shiomi, T. Tailored synthesis of star and network poly (dimethylsiloxane) s through electrostatic self-assembly and subsequent covalent fixation of telechelics having cyclic onium salt groups. Macromolecules 1997, 30, 5220–5226. [Google Scholar] [CrossRef]
- Chojnowski, J.; Cypryk, M.; Fortuniak, W.; Ścibiorek, M.; Rózga-Wijas, K. Synthesis of branched polysiloxanes with controlled branching and functionalization by anionic ring-opening polymerization. Macromolecules 2003, 36, 3890–3897. [Google Scholar] [CrossRef]
- Cypryk, M.; Pospiech, P.; Strzelec, K.; Wąsikowska, K.; Sobczak, J.W. Soluble polysiloxane-supported palladium catalysts for the Mizoroki–Heck reaction. J. Mol. Catal A Chem. 2010, 319, 30–38. [Google Scholar] [CrossRef]
- Ganicz, T.; Pakula, T.; Fortuniak, W.; Białecka-Florjańczyk, E. Linear and hyperbranched liquid crystalline polysiloxanes. Polymer 2005, 46, 11380–11388. [Google Scholar] [CrossRef]
- Vasilenko, N.G.; Getmanova, E.V.; Myakushev, V.D.; Rebrov, E.A.; Mufazarov, A.M.; Moeller, M. Synthesis of polylithium-derivatives of carbosilane dendrimers. Vysokomol. Soedin. Seriya A 1997, 39, 1449–1455. Available online: http://polymsci.ru/static/Archive/1997/VMS_1997_T39_9/VMS_1997_T39_9_1449-1455.pdf (accessed on 6 December 2021).
- Vasilenko, N.G.; Rebrov, E.A.; Muzafarov, A.M.; Eßwein, B.; Striegel, B.; Möller, M. Preparation of multi-arm star polymers with polylithiated carbosilane dendrimers. Macromol. Chem. Phys. 1998, 199, 889–895. [Google Scholar] [CrossRef]
- Gnanasekaran, D.; Madhavan, K.; Reddy, B.S.R. Developments of polyhedral oligomeric silsesquioxanes (PaSS), pass nanocomposites and their applications: A review. J. Sci. Ind. Res. 2009, 68, 437–464. Available online: http://nopr.niscair.res.in/bitstream/123456789/4321/4/JSIR%2068%286%29%20437-464.pdf (accessed on 6 December 2021).
- Lee, A.S.S.; Choi, S.S.; Baek, K.Y.; Hwang, S.S. Thiol-ene photopolymerization of well-defined hybrid graft polymers from a ladder-like polysilsesquioxane. Macromol. Res. 2015, 23, 60–66. [Google Scholar] [CrossRef]
- Skaria, S.; Schricker, S.R. Synthesis and characterization of inorganic-organic hybrid materials derived from polysilsesquioxanes (POSS). J. Macromol. Sci. Part A Pure Appl. Chem. 2010, 47, 381–391. [Google Scholar] [CrossRef]
- Zhang, W.A.; Muller, A.H.E. Architecture, self-assembly and properties of well-defined hybrid polymers based on polyhedral oligomeric silsequioxane (POSS). Prog. Polym. Sci. 2013, 38, 1121–1162. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 2013, 22, 1733–1746. [Google Scholar] [CrossRef]
- Tanaka, K.; Adachi, S.; Chujo, Y. Structure–property relationship of octa-substituted POSS in thermal and mechanical reinforcements of conventional polymers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5690–5697. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Yu, Z.-W.; Gao, S.-X.; Xu, K.; Zhang, Y.-X.; Peng, J.; Chen, M.-C. Synthesis and characterization of silsesquioxane-cored star-shaped hybrid polymer via “grafting from” RAFT polymerization. Chin. Chem. Lett. 2016, 27, 1696–1700. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Costa, R.O.R.; Vasconcelos, W.L.; Tamaki, R.; Laine, R.M. Organic/inorganic nanocomposite star polymers via atom transfer radical polymerization of methyl methacrylate using octafunctional silsesquioxane cores. Macromolecules 2001, 34, 5398–5407. [Google Scholar] [CrossRef]
- Pan, Q.; Gao, L.; Chen, X.; Fan, X.; Zhou, Q. Star mesogen-jacketed liquid crystalline polymers with silsesquioxane core: synthesis and characterization. Macromolecules 2007, 40, 4887–4894. [Google Scholar] [CrossRef]
- Uner, A.; Doganci, E.; Tasdelen, M.A.; Yilmaz, F.; Gürek, A.G. Synthesis, characterization and surface properties of star-shaped polymeric surfactants with polyhedral oligomeric silsesquioxane core. Polym. Int. 2017, 66, 1610–1616. [Google Scholar] [CrossRef]
- He, Z.; Zhong, M.; Yang, Y.; Wu, C.; Yang, J. Synthesis of POSS-based star-shaped poly(ionic liquid)s and its application in supercritical CO2 microcellular foaming of polystyrene. J. Polym. Res. 2016, 23, 243. [Google Scholar] [CrossRef]
- Omura, N.; Kennedy, J.P. Synthesis, characterization, and properties of stars consisting of many polyisobutylene arms radiating from a core of condensed cyclosiloxanes. Macromolecules. 1997, 30, 3204–3214. [Google Scholar] [CrossRef]
- Asadul Hoque, M.; Komiyama, H.; Nishiyama, H.; Nagai, K.; Kawauchi, T.; Iyoda, T. Amphiphilic liquid-crystalline 4-miktoarm star copolymers with a siloxane junction leading to cylindrically nanostructured templates for a siloxane-based nanodot array. J Polym. Sci. A Polym. Chem. 2015, 54, 1175–1188. [Google Scholar] [CrossRef]
- Hurduc, N.; Sandu, V.; Ibanescu, C.; Nor, I. Synthesis and characterization of star and brush grafted polysiloxanes, obtained by atom transfer radical polymerization. E-Polymers 2008, 8, 138. [Google Scholar] [CrossRef][Green Version]
- Kang, Y.; Lee, J.; Lee, J.; Lee, C. Ionic conductivity and electrochemical properties of cross-linked solid polymer electrolyte using star-shaped siloxane acrylate. J. Power Sources 2007, 165, 92–96. [Google Scholar] [CrossRef]
- Dickstein, W.H.; Lillya, C.P. Telechelic star poly (dimethylsiloxane) s of highly defined structure. Macromolecules 1989, 22, 3886–3888. [Google Scholar] [CrossRef]
- Oulad Hammouch, S.; Beinert, G.J.; Herz, J. Contribution to a better knowledge of the crosslinking reaction of polydimethylsiloxane (PDMS) by end-linking: The formation of star-branched PDMS by the hydrosilylation reaction. Polymer 1996, 37, 3353–3360. [Google Scholar] [CrossRef]
- Cheesman, B.T.; Gates, P.J.; Castle, T.C.; Cosgrove, T.; Prescott, S.W. Linear and star architecture methacrylate-functionalised PDMS. Mater. Today Commun. 2015, 3, 122–129. [Google Scholar] [CrossRef]
- Grunlan, M.A.; Lee, N.S.; Mansfeld, F.; Kus, E.; Finlay, J.A.; Callow, J.A.; Callow, M.E.; Weber, W.P. Minimally adhesive polymer surfaces prepared from star oligosiloxanes and star oligofluorosiloxanes. J. Polym. Sci. A Polym. Chem. 2006, 44, 2551–2566. [Google Scholar] [CrossRef]
- Jin, P.-F.; Shao, Y.; Yin, G.-Z.; Yang, S.; He, J.; Ni, P.; Zhang, W.-B. Janus [3:5] polystyrene–polydimethylsiloxane star polymers with a cubic core. Macromolecules 2018, 51, 419–427. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Gorodov, V.V.; Anisimov, A.A.; Boldyrev, K.L.; Buzin, M.I.; Naumkin, A.V.; Maslakov, K.I.; Peregudov, A.S.; Shchegolikhina, O.I.; Muzafarov, A.M. New star-like polydimethylsiloxanes: Synthesis, properties, and application. Russ. Chem. Bull. 2017, 66, 10941098. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Anisimov, A.A.; Peregudov, A.S.; Dubovik, A.S.; Orlov, V.N.; Malakhova, Y.N.; Stupnikov, A.A.; Buzin, M.I.; Nikiforova, G.G.; Vasil’ev, V.G.; et al. Star-shapped siloxane polymers with various cyclic cores: Synthesis and properties. J. Polym. Sci. A Polym. Chem. 2019, 57, 1233–1246. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References; Wiley: New York, NY, USA, 1972. [Google Scholar]
- Anisimov, A.A.; Kononevich, Y.N.; Zhemchugov, P.V.; Milenin, S.A.; Korlyukov, A.A.; Tsareva, U.S.; Peregudov, A.S.; Derivatovskii, P.; Molodtsova, Y.A.; Takazova, R.U.; et al. Synthesis and structure of new polyhedral Ni, Na- and Cu, Na-metallasiloxanes with tolyl substituent at the silicon atom. RSC Adv. 2016, 6, 22052–22060. [Google Scholar] [CrossRef]
- Pozdniakova, Y.A.; Lyssenko, K.A.; Korlyukov, A.A.; Blagodatskikh, I.V.; Auner, N.; Katsoulis, D.; Shchegolikhina, O.I. Alkali-metal-directed hydrolytic condensation of trifunctional phenylalkoxysilanes. Eur. J. Inorg. Chem. 2004, 2004, 1253–1261. [Google Scholar] [CrossRef]
- Shchegolikhina, O.I.; Pozdnyakova, Y.A.; Chetverikov, A.A.; Peregudov, A.S.; Buzin, M.I.; Matukhina, E.V. cis-Tetra[(organo)(trimethylsiloxy)]cyclotetrasiloxanes: Synthesis and mesomorphic properties. Russ. Chem. Bull. 2007, 1, 80–86. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Anisimov, A.A.; Milenin, S.A.; Korlyukov, A.A.; Dolgushin, F.M.; Kononova, E.G.; Peregudov, A.S.; Buzin, M.I.; Shchegolikhina, O.I.; Muzafarov, A.M. New all-cis-tetra(p-tolyl)cyclotetrasiloxanetetraol and its functionalization. Mendeleev Commun. 2018, 28, 418–420. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Kononevich, Y.N.; Buzin, M.I.; Peregudov, A.S.; Shchegolikhina, O.I.; Muzafarov, A.M. Convenient synthesis of new si-h and si-vinyl functionalized stereospecific 8-, 12- and 24-membered cyclosiloxanes. Macroheterocycles 2016, 9, 442–452. [Google Scholar] [CrossRef]
- Mark, J.E. Polymer Data Handbook; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Herz, J.; Munch, J.P.; Candau, S. Experimental investigation of the role of trapped entanglements in swollen polydimethylsiloxane networks. J. Macromolec. Sci. Part B Phys. 1980, 18, 267–279. [Google Scholar] [CrossRef]
- Pertsova, N.V.; Andrianov, K.A.; Tverdokhlebova, I.I.; Pavlova, S.A. Changes in molecular-weight distribution of polyoctamethylcyclotetrasiloxane obtained with initiators of different structure. Vysokomol. Soedin. Seriya A 1970, 12, 1001–1006. [Google Scholar]
- Malakhova, Y.N.; Buzin, A.I.; Chvalun, S.N. Linear and cyclolinear polysiloxanes in the bulk and thin films on liquid and solid substrate surfaces. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2018, 12, 321–331. [Google Scholar] [CrossRef]
- Malakhova, Y.N.; Stupnikov, A.A.; Chekusova, V.P.; Kuznetsov, N.M.; Belousov, S.I. Rheological behavior of polydimethylsiloxane Langmuir layers at the air-water interface. Bionanoscience 2020, 10, 403–408. [Google Scholar] [CrossRef]
- Owen, M.J. Silicone surface fundamentals. Macromol. Rapid Commun. 2021, 42, 2000360. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gura, M.C.; Cremer, P.S.; Yu, H. Chain conformation of poly (dimethyl siloxane) at the air/water interface by sum frequency generation. Langmuir 2008, 24, 10155–10160. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, V.L. Langmuir monolayers of polydimethylsiloxane with controllable surface potential. Thin Solid Film. 1998, 327, 816–821. [Google Scholar] [CrossRef]








| Sample | MnNMR, kDa | MnGPC, kDa | MwGPC, kDa | PDI | Output, % |
|---|---|---|---|---|---|
| PDMS-15 | 1.3 | 2.4 | 2.7 | 1.13 | 95 |
| Ph4-15 | 5.9 | 6.7 | 7.6 | 1.14 | 96 |
| Phr4-15 | 5.9 | 6 | 6.7 | 1.11 | 91 |
| Tol4-15 | 5.9 | 7.9 | 8.9 | 1.13 | 98 |
| Me4-15 | 5.5 | 6.6 | 7.1 | 1.08 | 91 |
| Sample | Tg, C | Tcc, C | Tm, C | Td5%, C | M, Mas. % | ||
|---|---|---|---|---|---|---|---|
| Air | Argon | Air | Argon | ||||
| PDMS-15 | −133 | −83 | −60 | 234 | 281 | 43 | 4 |
| Ph4-15 | −123 | - | - | 331 | 410 | 43 | 11 |
| Phr4-15 | −122 | - | - | 337 | 420 | 50 | 13 |
| Tol4-15 | −124 | - | - | 357 | 439 | 20 | 16 |
| Me4-15 | −123 | - | - | 322 | 413 | 46 | 9 |
| Sample | Star/ Linear Polymer | Pa*s Star | Ea, kJ/mol |
|---|---|---|---|
| Ph4-15 | 0.049/0.091 | 0.081 | 16.3 |
| Phr4-15 | 0.044/0.083 | 0.080 | 16.6 |
| Tol4-15 | 0.047/0.100 | 0.080 | 16.3 |
| Me4-15 | 0.054/0.086 | 0.073 | 15.8 |
| A | B | C | D | ||
|---|---|---|---|---|---|
| PDMS [57,58] | Area per (OSi(CH3)2) unit, Å2 | 19.0 | 15.0 | 8.0 | 7.0 |
| Surface pressure, mN m−1 | 0 | 7.0 | 8.5 | 9.0 | |
| Surface potential range, mV | 190–200 | ||||
| Me4-15 | Area per a molecule, Å2 | 1780 | 1410 | 790 | 690 |
| Area per (OSi(CH3)2) unit, Å2 | 18.5 | 14.6 | 8.2 | 7.2 | |
| Surface pressure, mNm−1 | 0 | 6.8 | 7.6 | 8.1 | |
| Surface potential range, mV | 180–195 | ||||
| Ph4-15 | Area per a molecule, Å2 | 1900 | 1530 | 850 | 700 |
| Area per (OSi(CH3)2) unit, Å2 | 18.5 | 14.9 | 8.3 | 6.8 | |
| Surface pressure, mN m−1 | 0 | 6.2 | 7.2 | 8.6 | |
| Surface potential range, mV | 150–175 | ||||
| Phr4-15 | Area per a molecule, Å2 | 1680 | 1340 | 750 | 610 |
| Area per (OSi(CH3)2) unit, Å2 | 18.6 | 14.8 | 8.3 | 6.7 | |
| Surface pressure, mN m−1 | 0 | 6.1 | 7.0 | 8.4 | |
| Surface potential interval, mV | 160–175 | ||||
| Tol4-15 | Area per a molecule, Å2 | 2230 | 1800 | 1010 | 800 |
| Area per (OSi(CH3)2) unit, Å2 | 18.6 | 14.9 | 8.4 | 6.6 | |
| Surface pressure, mN m−1 | 0 | 6 | 7.0 | 8.4 | |
| Surface potential interval, mV | 155–180 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyuzhikova, Y.S.; Anisimov, A.A.; Peregudov, A.S.; Buzin, M.I.; Nikiforova, G.G.; Vasil’ev, V.G.; Kostrov, S.A.; Buzin, A.I.; Stupnikov, A.A.; Malakhova, Y.N.; et al. Star-Shaped Polydimethylsiloxanes with Organocyclotetrasilsesquioxane Branching-Out Centers: Synthesis and Properties. Polymers 2022, 14, 285. https://doi.org/10.3390/polym14020285
Dyuzhikova YS, Anisimov AA, Peregudov AS, Buzin MI, Nikiforova GG, Vasil’ev VG, Kostrov SA, Buzin AI, Stupnikov AA, Malakhova YN, et al. Star-Shaped Polydimethylsiloxanes with Organocyclotetrasilsesquioxane Branching-Out Centers: Synthesis and Properties. Polymers. 2022; 14(2):285. https://doi.org/10.3390/polym14020285
Chicago/Turabian StyleDyuzhikova, Yulia S., Anton A. Anisimov, Alexander S. Peregudov, Mikhail I. Buzin, Galina G. Nikiforova, Viktor G. Vasil’ev, Sergey A. Kostrov, Alexander I. Buzin, Alexei A. Stupnikov, Yulia N. Malakhova, and et al. 2022. "Star-Shaped Polydimethylsiloxanes with Organocyclotetrasilsesquioxane Branching-Out Centers: Synthesis and Properties" Polymers 14, no. 2: 285. https://doi.org/10.3390/polym14020285
APA StyleDyuzhikova, Y. S., Anisimov, A. A., Peregudov, A. S., Buzin, M. I., Nikiforova, G. G., Vasil’ev, V. G., Kostrov, S. A., Buzin, A. I., Stupnikov, A. A., Malakhova, Y. N., Shchegolikhina, O. I., & Muzafarov, A. M. (2022). Star-Shaped Polydimethylsiloxanes with Organocyclotetrasilsesquioxane Branching-Out Centers: Synthesis and Properties. Polymers, 14(2), 285. https://doi.org/10.3390/polym14020285









