Synthesis and Characterization of Zn–Organic Frameworks Containing Chitosan as a Low-Cost Inhibitor for Sulfuric-Acid-Induced Steel Corrosion: Practical and Computational Exploration
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
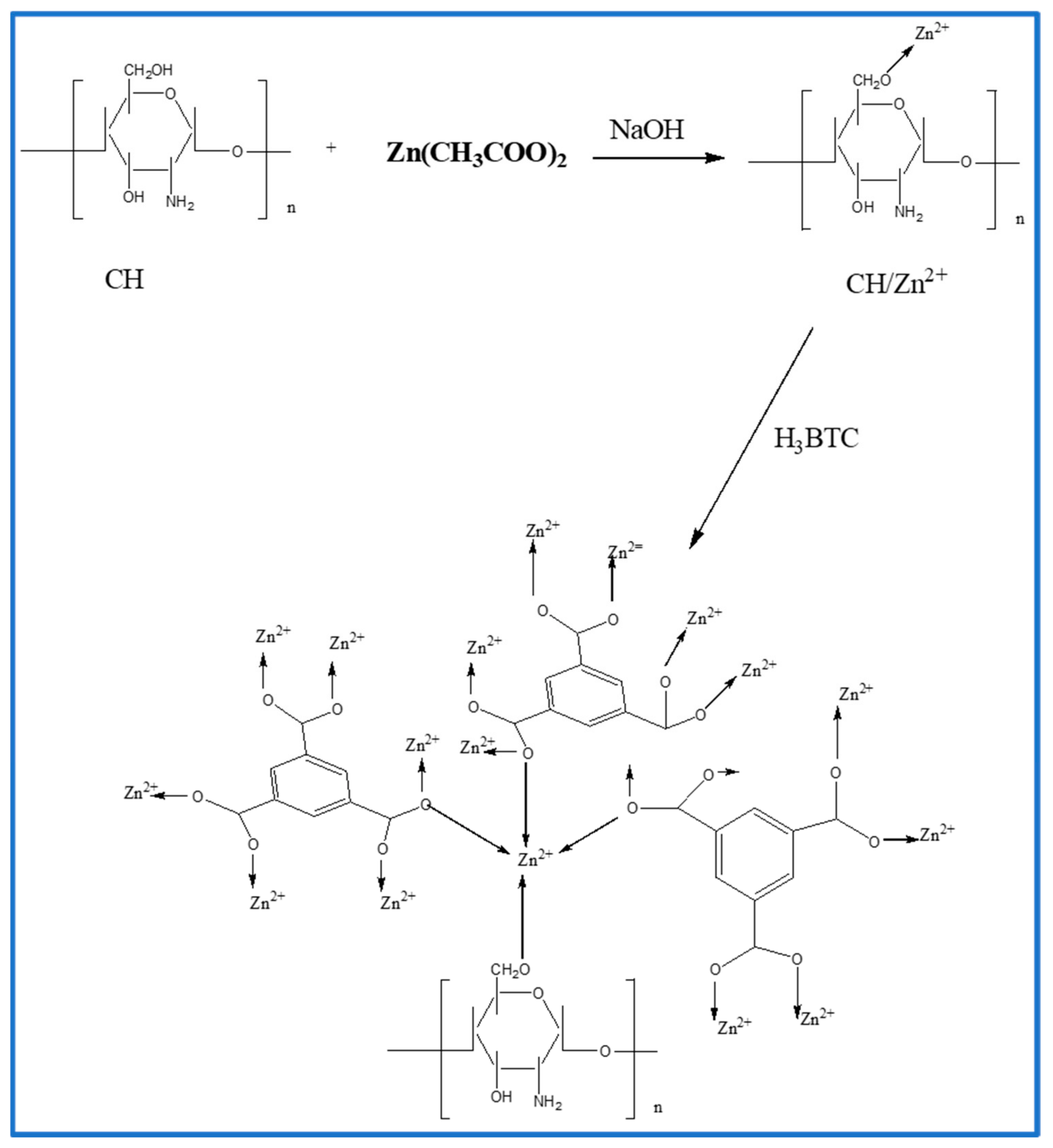
2.2. Preparation of CH/Zn@H3BTC Composite Beads
2.3. Materials’ Characterization
2.4. Specific Surface Area Studies
2.5. Corrosion Protection Evaluation
2.6. Surface Exploration
2.7. Theoretical Approaches
3. Results and Discussions
3.1. Characterization Analysis
3.1.1. FTIR
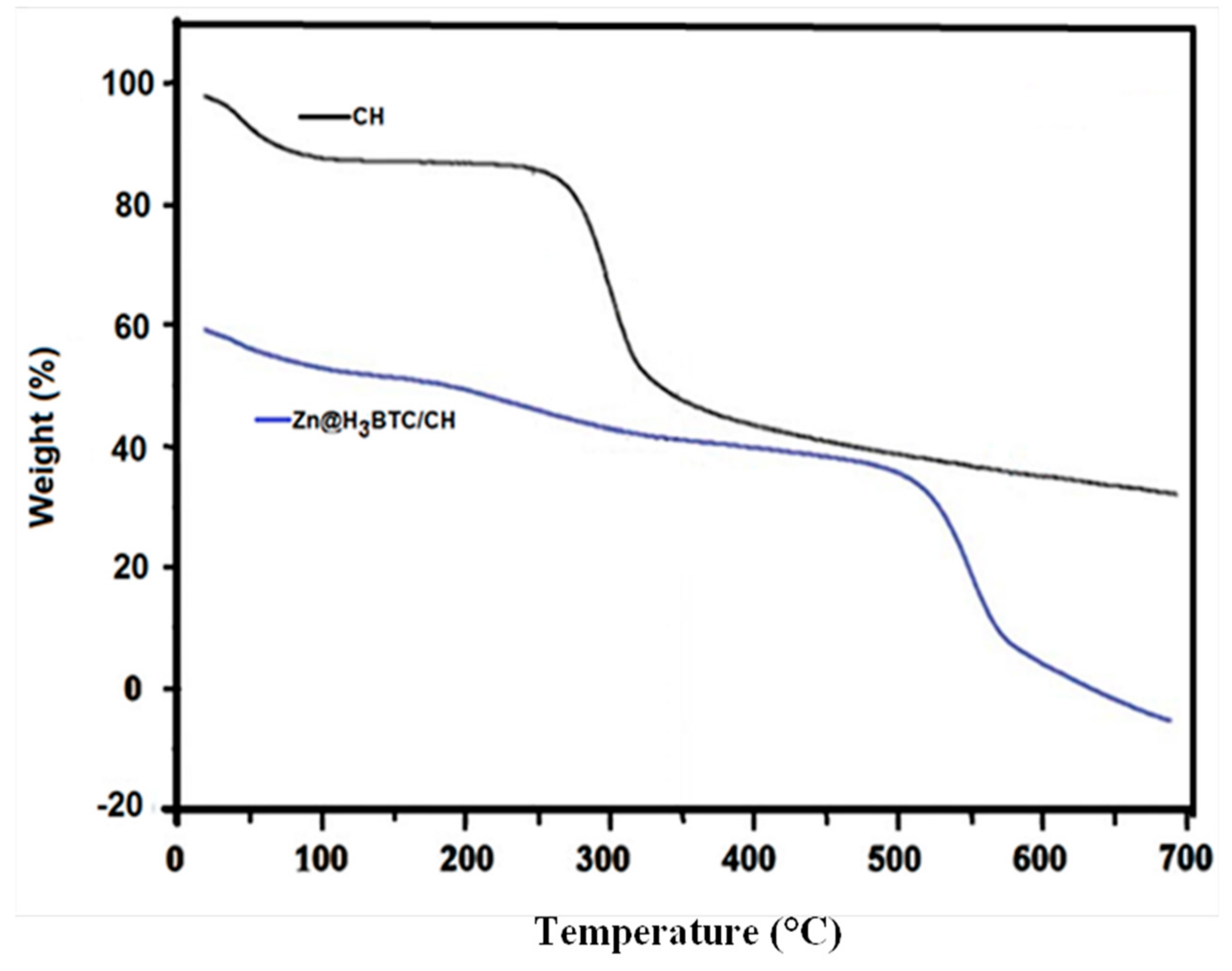
3.1.2. Thermal Analysis
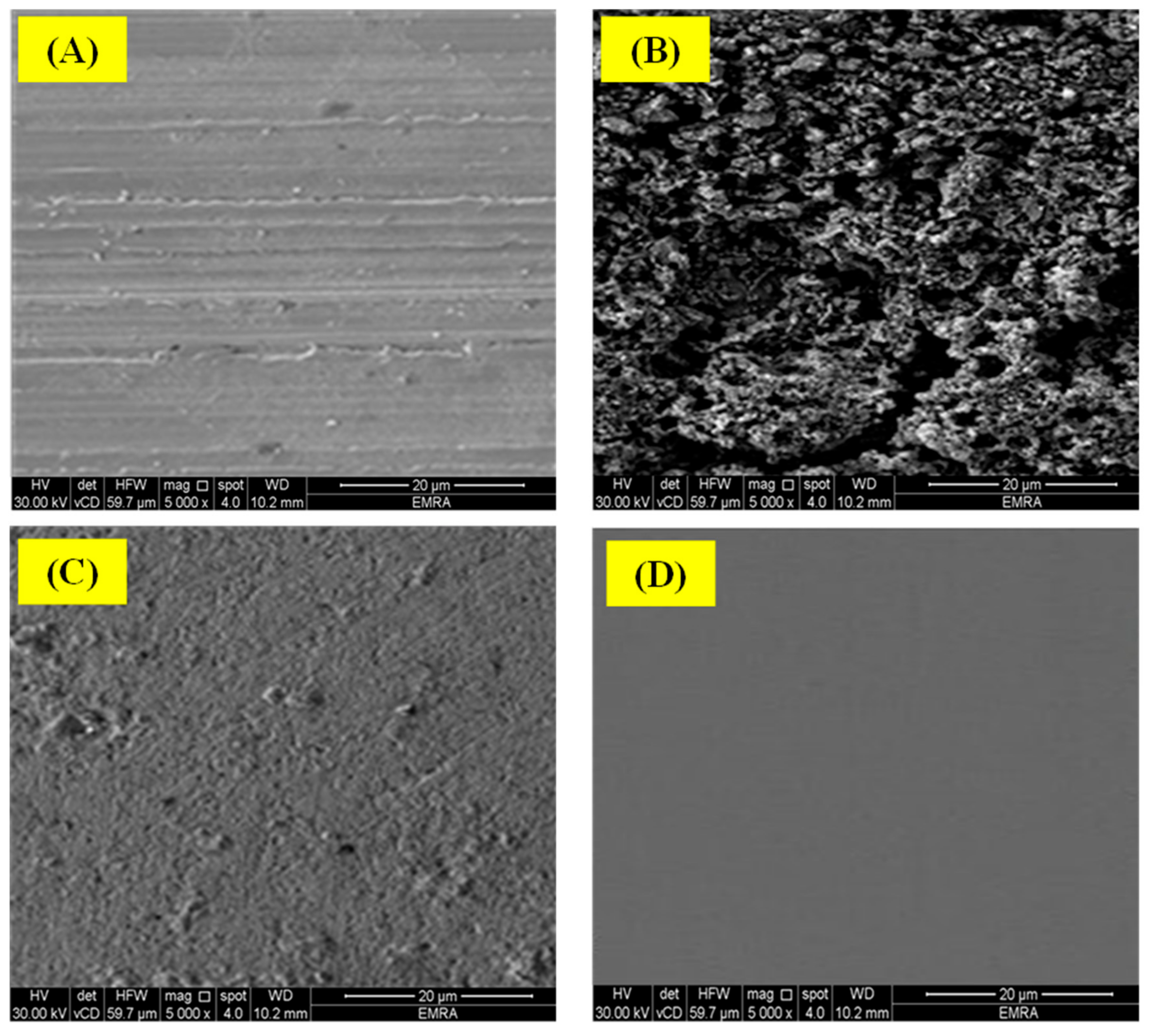
3.1.3. FESEM
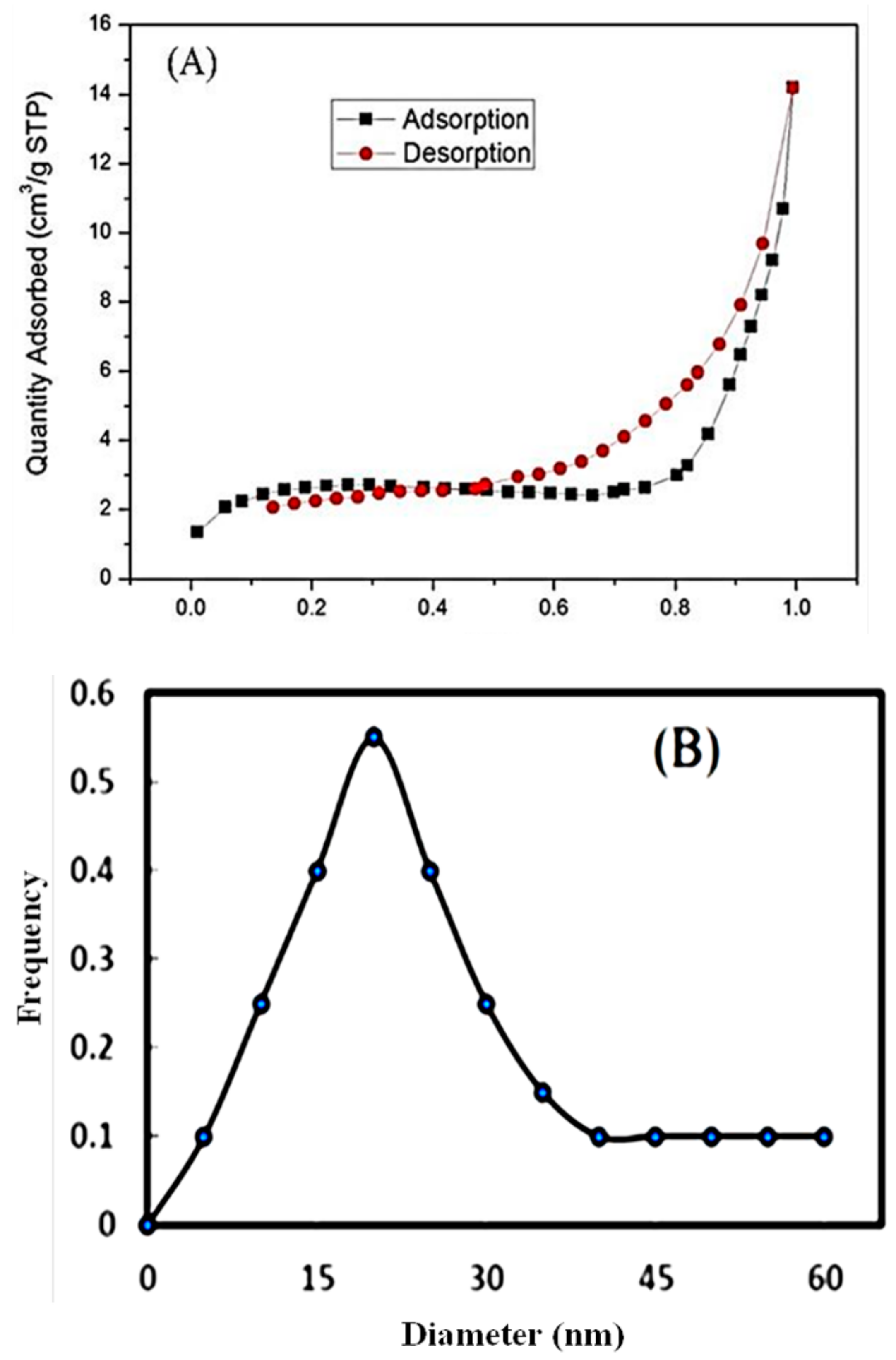
3.1.4. BET Analysis
3.2. Corrosion Protection Evaluation
3.2.1. EOCP−t Profiles
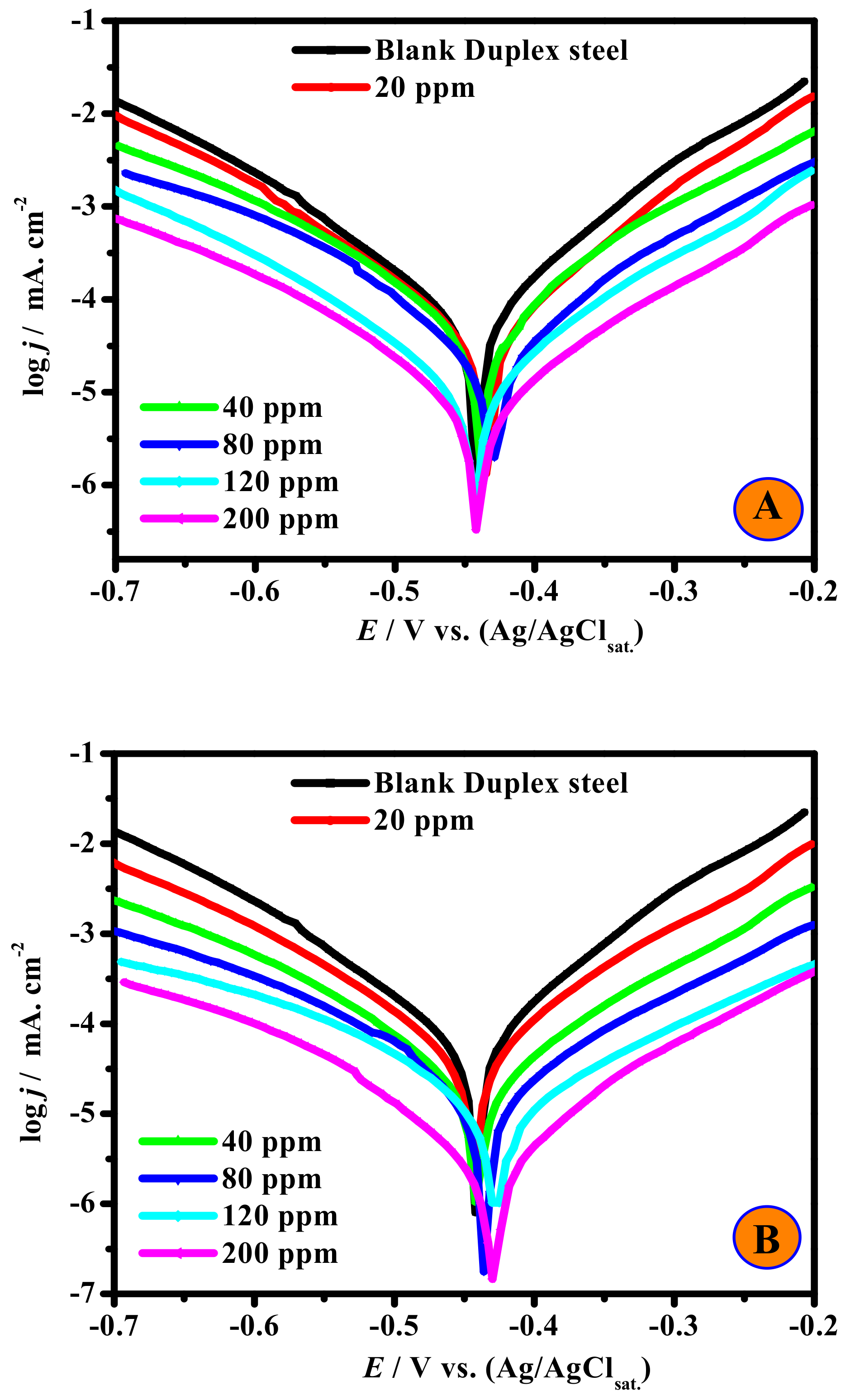
3.2.2. PDP Studies and Comparative Evaluation
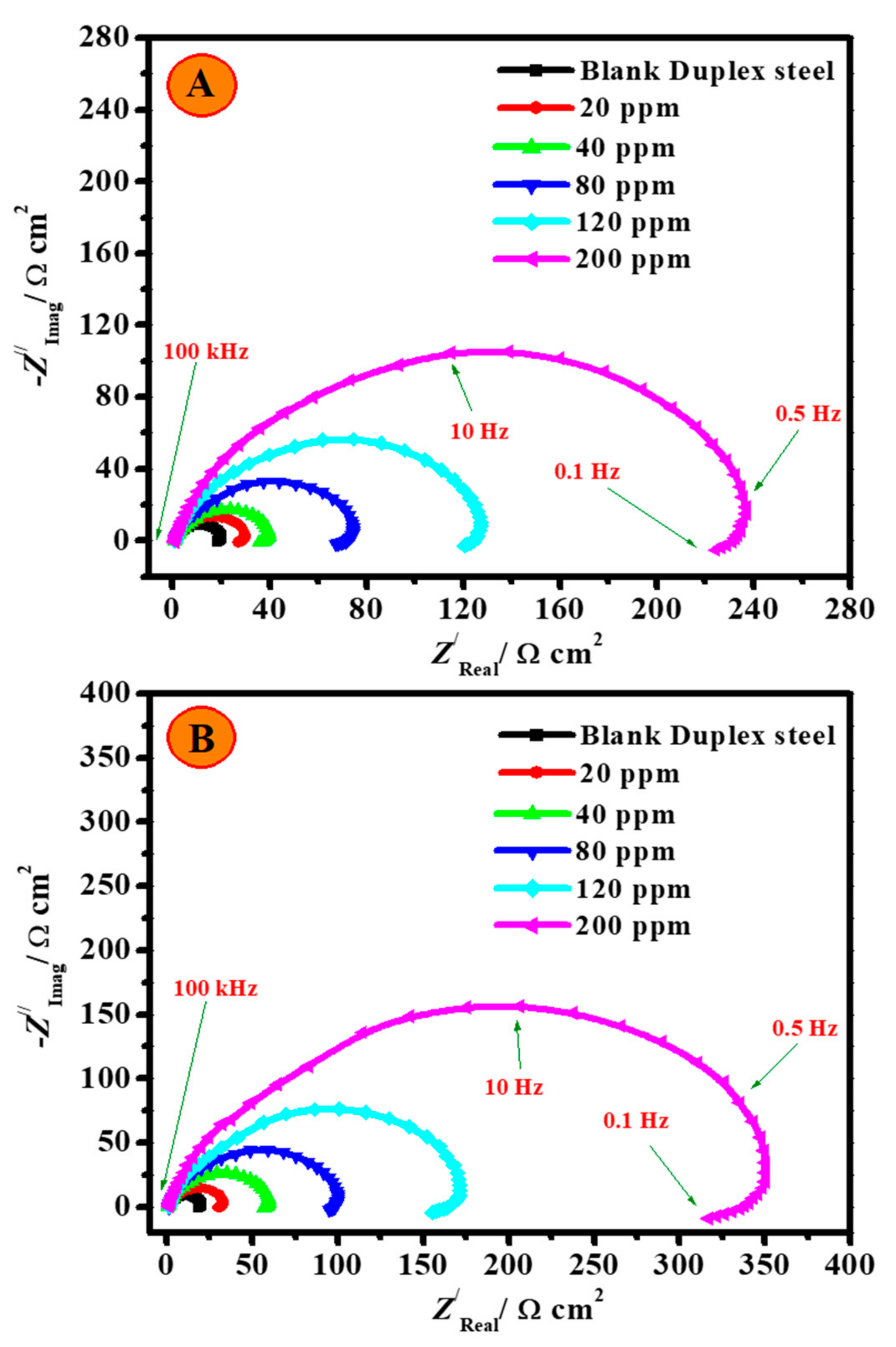
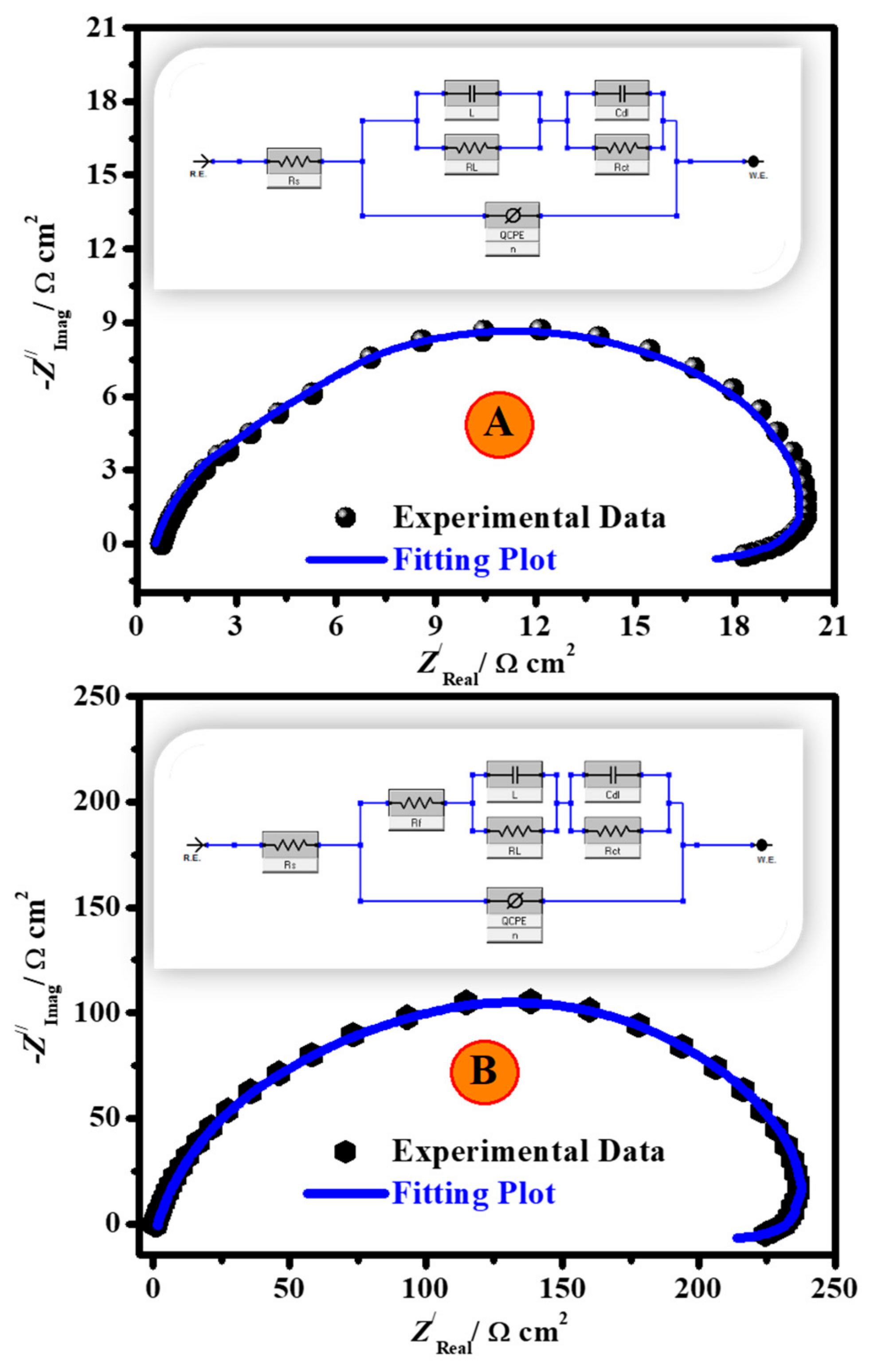
3.2.3. EIS Studies
3.3. Adsorption Considerations, Thermodynamic Research, and Corrosion Mitigation Mechanism
3.4. Surface Topography Exploration
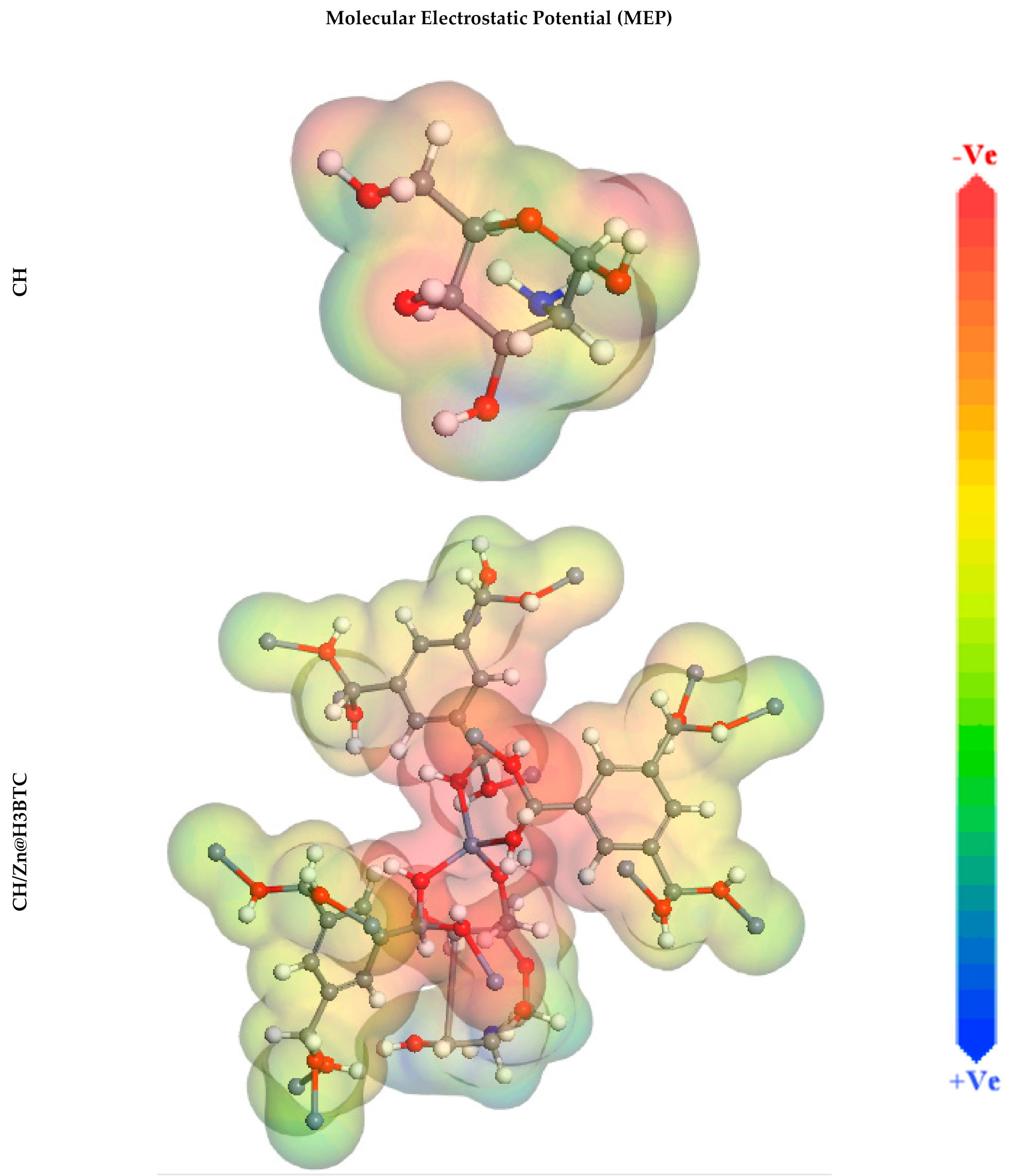
3.5. DFT Studies
3.6. MC Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Q.; Luo, C.; Liu, S.; Li, H.; Wang, P.; Liu, D.; Lei, Y. Investigation of arc stability, microstructure evolution and corrosion resistance in underwater wet FCAW of duplex stainless steel. J. Mater. Res. Technol. 2021, 15, 5482–5495. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Thakur, S.; Pani, B.; Pandey, A.K.; Lgaz, H.; Chung, I.-M.; Ebenso, E.E. An Exploration about the Interaction of Mild Steel with Hydrochloric Acid in the Presence of N-(Benzo[d]thiazole-2-yl)-1-phenylethan-1-imines. J. Phys. Chem. C 2019, 123, 22897–22917. [Google Scholar] [CrossRef]
- Singh, D.K.; Kumar, S.; Udayabhanu, G.; John, R.P. 4(N,N-dimethylamino) benzaldehyde nicotinic hydrazone as corrosion inhibitor for mild steel in 1 M HCl solution: An experimental and theoretical study. J. Mol. Liq. 2016, 216, 738–746. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Wang, Q.; Li, R.; Liu, H.; Jiang, H.; Liu, J. Inhibition effect and adsorption behavior of two pyrimidine derivatives as corrosion inhibitors for Q235 steel in CO2-saturated chloride solution. J. Electroanal. Chem. 2021, 903, 115827. [Google Scholar] [CrossRef]
- Laabaissi, T.; Rbaa, M.; Benhiba, F.; Rouifi, Z.; Kumar, U.P.; Bentiss, F.; Oudda, H.; Lakhrissi, B.; Warad, I.; Zarrouk, A. Insight into the corrosion inhibition of new benzodiazepine derivatives as highly efficient inhibitors for mild steel in 1 M HCl: Experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127428. [Google Scholar] [CrossRef]
- Shamsa, A.; Barmatov, E.; Hughes, T.L.; Hua, Y.; Neville, A.; Barker, R. Hydrolysis of imidazoline based corrosion inhibitor and effects on inhibition performance of X65 steel in CO2 saturated brine. J. Pet. Sci. Eng. 2022, 208, 109235. [Google Scholar] [CrossRef]
- Yang, H.M. Role of organic and eco-friendly inhibitors on the corrosion mitigation of steel in acidic environments—A state-of-art review. Molecules 2021, 26, 3473. [Google Scholar] [CrossRef]
- De Sousa Rodrigues, F.A.; Gonçalves, Y.M.H.; Horta, B.A.C.; da Silva Santos, I.; Silva, B.V.; D’Elia, E. Experimental and theoretical studies of isonitrosoacetanilides derivatives as corrosion inhibitors for mild steel in 1 mol L−1 HCl. J. Mol. Struct. 2021, 1245, 131256. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—Inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr. Polym. 2016, 136, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Solomon, M.M.; Udosoro, I.I.; Udoh, A.P. Synergistic and antagonistic effects between halide ions and carboxymethyl cellulose for the corrosion inhibition of mild steel in sulphuric acid solution. Cellulose 2010, 17, 635–648. [Google Scholar] [CrossRef]
- Hefni, H.H.H.; Azzam, E.M.; Badr, E.A.; Hussein, M.; Tawfik, S.M. Synthesis, characterization and anticorrosion potentials of chitosan-g-PEG assembled on silver nanoparticles. Int. J. Biol. Macromol. 2016, 83, 297–305. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic biomolecules as green corrosion inhibitors. J. Mol. Liq. 2021, 341, 117265. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Qurashi, A.; Chauhan, D.S.; Quraishi, M.A. Frontiers and advances in green and sustainable inhibitors for corrosion applications: A critical review. J. Mol. Liq. 2021, 321, 114385. [Google Scholar] [CrossRef]
- Al Obeidli, A.; Ben Salah, H.; Al Murisi, M.; Sabouni, R. Recent advancements in MOFs synthesis and their green applications. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Nguyen Thi, H.P.; Ninh, H.D.; Tran, C.; Van Le, B.T.; Bhosale, S.V.; La, D.D. Size-Control and Surface Modification of Flexible Metal-Organic Framework MIL-53(Fe) by Polyethyleneglycol for 5- Fluorouracil Anticancer Drug Delivery. ChemistrySelect 2019, 4, 2333–2338. [Google Scholar] [CrossRef]
- Zheng, J.; Cui, X.; Yang, Q.; Ren, Q.; Yang, Y.; Xing, H. Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage. Chem. Eng. J. 2018, 354, 1075–1082. [Google Scholar] [CrossRef]
- Barbosa, P.F.P.; Cumba, L.R.; Andrade, R.D.A.; do Carmo, D.R. Chemical Modifications of Cyclodextrin and Chitosan for Biological and Environmental Applications: Metals and Organic Pollutants Adsorption and Removal. J. Polym. Environ. 2019, 27, 1352–1366. [Google Scholar] [CrossRef]
- Qin, X.; Huang, Y.; Wang, K.; Xu, T.; Wang, Y.; Liu, P.; Kang, Y.; Zhang, Y. Novel hierarchically porous Ti-MOFs/nitrogen-doped graphene nanocomposite served as high efficient oxygen reduction reaction catalyst for fuel cells application. Electrochim. Acta 2019, 297, 805–813. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, A.L.; Kim, K.-H.; Deep, A. A novel CdTe/Eu-MOF photoanode for application in quantum dot-sensitized solar cell to improve power conversion efficiency. J. Ind. Eng. Chem. 2017, 53, 77–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, H.-C. Synthesis of MOFs for heterogeneous catalysis via linker design. Polyhedron 2018, 154, 189–201. [Google Scholar] [CrossRef]
- Zhan, K.; Zhu, Y.; Yan, J.; Chen, Y. Enhanced-performance relative humidity sensor based on MOF-801 photonic crystals. Phys. Lett. A 2020, 384, 126678. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, Y.; Wang, C.; Guo, L. NiCo-MOF nanosheets wrapping polypyrrole nanotubes for high-performance supercapacitors. Appl. Surf. Sci. 2020, 507, 145089. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Eldin, M.S.M.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
- Omer, A.M.; Ziora, Z.M.; Tamer, T.M.; Khalifa, R.E.; Hassan, M.A.; Mohy-Eldin, M.S.; Blaskovich, M.A. Formulation of quaternized aminated chitosan nanoparticles for efficient encapsulation and slow release of curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Huang, X.; Lu, G.; Tang, Z. Research Progresses in the Preparation of Co-based Catalyst Derived from Co-MOFs and Application in the Catalytic Oxidation Reaction. Catal. Surv. Asia 2018, 23, 64–89. [Google Scholar] [CrossRef]
- Altaf, M.; Sohail, M.; Mansha, M.; Iqbal, N.; Sher, M.; Fazal, A.; Ullah, N.; Isab, A.A. Synthesis, Characterization, and Photoelectrochemical Catalytic Studies of a Water-Stable Zinc-Based Metal–Organic Framework. ChemSusChem 2018, 11, 542–546. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Z.; Yang, H.; Ma, X.; Liu, H. Synergistic interface phenomena between MOFs, NiPx for efficient hydrogen production. Mol. Catal. 2019, 467, 78–86. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Siddaiah, A.; Menezes, P.L. Synergistic wear-corrosion analysis and modelling of nanocomposite coatings. Tribol. Int. 2018, 121, 30–44. [Google Scholar] [CrossRef]
- Yang, D.; Yang, G.; Gai, S.; He, F.; Li, C.; Yang, P. Multifunctional Theranostics for Dual-Modal Photodynamic Synergistic Therapy via Stepwise Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 6829–6838. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Development of metal-organic framework (MOF) decorated graphene oxide nanoplatforms for anti-corrosion epoxy coatings. Carbon 2020, 161, 231–251. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Yin, D.; Chen, L.; Jiang, Y.; Zhu, L. Fabrication of BTA-MOF-TEOS-GO nanocomposite to endow coating systems with active inhibition and durable anticorrosion performances. Prog. Org. Coatings 2020, 143, 105629. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- ASTM License Agreement G3—89A, Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing. 2010. pp. 1–9. Available online: https://www.document-center.com/standards/show/ASTM-G3 (accessed on 5 January 2021).
- Abd El-Lateef, H.M.; Sayed, A.R.; Shalabi, K. Synthesis and theoretical studies of novel conjugated polyazomethines and their application as efficient inhibitors for C1018 steel pickling corrosion behavior. Surf. Interfaces 2021, 23, 101037. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Abdelhamid, A.A. One-pot synthesis of novel triphenyl hexyl imidazole derivatives catalyzed by ionic liquid for acid corrosion inhibition of C1018 steel: Experimental and computational perspectives. J. Mol. Liq. 2021, 334, 116081. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Al-Hadeethi, M.R.; Ali, I.H.; Mohamed, S.K.; Toumiat, K.; Salghi, R. Naproxen-Based Hydrazones as Effective Corrosion Inhibitors for Mild Steel in 1.0 M HCl. Coatings 2020, 10, 700. [Google Scholar] [CrossRef]
- Khamaysa, O.M.A.; Selatnia, I.; Zeghache, H.; Lgaz, H.; Sid, A.; Chung, I.-M.; Benahmed, M.; Gherraf, N.; Mosset, P. Enhanced corrosion inhibition of carbon steel in HCl solution by a newly synthesized hydrazone derivative: Mechanism exploration from electrochemical, XPS, and computational studies. J. Mol. Liq. 2020, 315, 113805. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Lgaz, H.; Al-Hadeethi, M.R.; Ali, I.H.; Masroor, S.; Chung, I.-M. Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl. Coatings 2020, 10, 640. [Google Scholar] [CrossRef]
- Chai, C.; Xu, Y.; Shi, S.; Zhao, X.; Wu, Y.; Xu, Y.; Zhang, L. Functional polyaspartic acid derivatives as eco-friendly corrosion inhibitors for mild steel in 0.5 M H2SO4 solution. RSC Adv. 2018, 8, 24970–24981. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Peng, S.; Gong, Z.; Chen, J. A combination of experiment and theoretical methods to study the novel and low-cost corrosion inhibitor 1-hydroxy-7-azabenzotriazole for mild steel in 1 M sulfuric acid. RSC Adv. 2018, 8, 38506–38516. [Google Scholar] [CrossRef] [Green Version]
- Lgaz, H.; Salghi, R.; Masroor, S.; Kim, S.-H.; Kwon, C.; Kim, S.Y.; Yang, Y.-J.; Chung, I.-M. Assessing corrosion inhibition characteristics of hydrazone derivatives on mild steel in HCl: Insights from electronic-scale DFT and atomic-scale molecular dynamics. J. Mol. Liq. 2020, 308, 112998. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Corrosion inhibition characteristics of a novel salycilidene isatin hydrazine sodium sulfonate on carbon steel in HCl and a synergistic nickel ions additive: A combined experimental and theoretical perspective. Appl. Surf. Sci. 2020, 501, 144237. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Experimental and computational approaches of sustainable quaternary bisammonium fluorosurfactants for corrosion inhibition as protective films at mild steel/H2SO4 interface. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126141. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2-3.5%NaCl: DFT, Monte Carlo simulations and experimental approaches. J. Clean. Prod. 2020, 250, 119510. [Google Scholar] [CrossRef]
- Kaya, S.; Guo, L.; Kaya, C.; Tüzün, B.; Obot, I.B.; Touir, R.; Islam, N. Quantum chemical and molecular dynamic simulation studies for the prediction of inhibition efficiencies of some piperidine derivatives on the corrosion of iron. J. Taiwan Inst. Chem. Eng. 2016, 65, 522–529. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Cationic gemini-surfactants based on waste cooking oil as new ‘green’ inhibitors for N80-steel corrosion in sulphuric acid: A combined empirical and theoretical approaches. J. Mol. Struct. 2020, 1203, 127442. [Google Scholar] [CrossRef]
- Saleh, M.M.; Mahmoud, M.G.; Abd El-Lateef, H.M. Comparative study of synergistic inhibition of mild steel and pure iron by 1-hexadecylpyridinium chloride and bromide ions. Corros. Sci. 2019, 154, 70–79. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Mohamed, I.M.A.; Zhu, J.-H.; Khalaf, M.M. An efficient synthesis of electrospun TiO2-nanofibers/Schiff base phenylalanine composite and its inhibition behavior for C-steel corrosion in acidic chloride environments. J. Taiwan Inst. Chem. Eng. 2020, 112, 306–321. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: Experimental and computational investigations. New J. Chem. 2020, 44, 17791–17814. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A.; Ramkumar, S.; Obote, I.B. Corrosion inhibition of N80 steel in 15% HCl by pyrazolone derivatives: Electrochemical, surface and quantum chemical studies. RSC Adv. 2016, 6, 24130–24141. [Google Scholar] [CrossRef]
- Masroor, S.; Mobin, M.; Alam, M.J.; Ahmad, S. The novel iminium surfactant p-benzylidene benzyldodecyl iminium chloride as a corrosion inhibitor for plain carbon steel in 1 M HCl: Electrochemical and DFT evaluation. RSC Adv. 2017, 7, 23182–23196. [Google Scholar] [CrossRef] [Green Version]
- Gadow, H.S.; Motawea, M.M. Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv. 2017, 7, 24576–24588. [Google Scholar] [CrossRef] [Green Version]
- Alam, R.; Mobin, M.; Aslam, J. Investigation of anti-corrosive properties of poly(aniline-co-2-pyridylamine-co-2,3-xylidine) and its nanocomposite poly(aniline-co-2-pyridylamine-co-2,3-xylidine)/ZnO on mild steel in 0.1 M HCl. Appl. Surf. Sci. 2016, 368, 360–367. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Haque, J.; Dohare, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Effect of electron donating functional groups on corrosion inhibition of mild steel in hydrochloric acid: Experimental and quantum chemical study. J. Taiwan Inst. Chem. Eng. 2018, 82, 233–251. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Nabavi-Amri, S.A. Polarization and impedance methods in corrosion inhibition study of carbon steel by amines in petroleum–water mixtures. Electrochim. Acta 2002, 47, 2239–2244. [Google Scholar] [CrossRef]
- Boulhaoua, M.; El Hafi, M.; Zehra, S.; Eddaif, L.; Alrashdi, A.A.; Lahmidi, S.; Guo, L.; Mague, J.T.; Lgaz, H. Synthesis, structural analysis and corrosion inhibition application of a new indazole derivative on mild steel surface in acidic media complemented with DFT and MD studies. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 617, 126373. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition and adsorption features of novel bioactive cationic surfactants bearing benzenesulphonamide on C1018-steel under sweet conditions: Combined modeling and experimental approaches. J. Mol. Liq. 2020, 320, 114564. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.E. Experimental and computational studies of graphene oxide covalently functionalized by octylamine: Electrochemical stability, hydrogen evolution, and corrosion inhibition of the AZ13 Mg alloy in 3.5% NaCl. RSC Adv. 2020, 10, 11426–11434. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Lateef, H.M.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, Experimental, and Computational Studies of Water Soluble Anthranilic Organoselenium Compounds as Safe Corrosion Inhibitors for J55 Pipeline Steel in Acidic Oilfield Formation Water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126894. [Google Scholar] [CrossRef]
- Upadhyay, A.; Purohit, A.K.; Mahakur, G.; Dash, S.; Kar, P.K. Verification of corrosion inhibition of Mild steel by some 4-Aminoantipyrine-based Schiff bases—Impact of adsorbate substituent and cross-conjugation. J. Mol. Liq. 2021, 333, 115960. [Google Scholar] [CrossRef]
- Oyebamiji, A.K.; Adeleke, B.B. Quantum chemical studies on inhibition activities of 2,3-dihydroxypropyl-sulfanyl derivative on carbon steel in acidic media. Int. J. Corros. Scale Inhib. 2018, 7, 498–508. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Kaya, S. Theoretically and experimentally exploring the corrosion inhibition of N80 steel by pyrazol derivatives in simulated acidizing environment. J. Mol. Struct. 2020, 1206, 127685. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Hegazy, M.M.; Awad, M.K.; Shawky, M. Chemical, electrochemical, theoretical (DFT & MEP), thermodynamics and surface morphology studies of carbon steel during gas and oil production using three novel di-cationic amphiphilies as corrosion inhibitors in acidic medium. J. Mol. Liq. 2021, 337, 116541. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Sayed, A.R.; Gomha, S.M.; Bakir, E.M. The novel polythiadiazole polymer and its composite with α-Al(OH)3 as inhibitors for steel alloy corrosion in molar H2SO4: Experimental and computational evaluations. J. Ind. Eng. Chem. 2022, 105, 238–250. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.M.; El-Askalany, A.H.; Shahba, M.M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480. [Google Scholar] [CrossRef]
- El Aadad, H.; Galai, M.; Ouakki, M.; Elgendy, A.; Touhami, M.E.; Chahine, A. Improvement of the corrosion resistance of mild steel in sulfuric acid by new organic-inorganic hybrids of Benzimidazole-Pyrophosphate: Facile synthesis, characterization, experimental and theoretical calculations (DFT and MC). Surf. Interfaces 2021, 24, 101084. [Google Scholar] [CrossRef]
- Dehghani, A.; Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B. A detailed study on the synergistic corrosion inhibition impact of the Quercetin molecules and trivalent europium salt on mild steel; electrochemical/surface studies, DFT modeling, and MC/MD computer simulation. J. Mol. Liq. 2020, 316, 113914. [Google Scholar] [CrossRef]










| Inhibitors Code | Cinh ppm by weight | Ecor V vs. Ag/AgCl | jcor µA cm−2 ±SD | βa mV dec −1 ±SD | −βc mV dec −1 ±SD | θ | ɀPP % |
|---|---|---|---|---|---|---|---|
| Blank | 0.0 | −0.441 | 1137.7 ± 71 | 70.7 ± 5.2 | 138.8 ± 11.6 | - | - |
| CH | 20 | −0.433 | 713.33 ± 51.4 | 81.5 ± 3.4 | 146.4 ± 10.1 | 0.373 | 37.3 |
| 40 | −0.437 | 550.68 ± 32.2 | 78.1 ± 5.7 | 147.9 ± 9.7 | 0.516 | 51.6 | |
| 80 | −0.429 | 293.52 ± 19.3 | 79.8 ± 4.1 | 143.7 ± 6.8 | 0.742 | 74.2 | |
| 120 | −0.442 | 120.59 ± 9.8 | 81.2 ± 7.1 | 140.3 ± 8.5 | 0.894 | 89.4 | |
| 200 | −0.437 | 67.12 ± 5.3 | 83.9 ± 2.9 | 153.4 ± 6.3 | 0.941 | 94.1 | |
| CH/Zn@H3BTC | 20 | −0.442 | 613.22 ± 45.6 | 84.6 ± 3.8 | 152.5 ± 7.6 | 0.461 | 46.1 |
| 40 | −0.441 | 427.77 ± 23.9 | 86.1 ± 4.8 | 153.6 ± 12.2 | 0.624 | 62.4 | |
| 80 | −0.434 | 145.62 ± 10.2 | 79.2 ± 3.9 | 149.2 ± 13.4 | 0.872 | 87.2 | |
| 120 | −0.428 | 71.67 ± 6.4 | 87.5 ± 7.5 | 150.4 ± 9.8 | 0.937 | 93.7 | |
| 200 | −0.427 | 25.02 ± 1.5 | 82.3 ± 7.1 | 153.7 ± 7.4 | 0.978 | 97.8 |
| Additive Codes | Cinh. ppm | Re Ω cm2 | Rct Ω cm2 ±SD | Cdl F cm−2 × 10−6 | Rp Ω cm2 ±SD | RL Ω cm2 | L H cm2 | QCPE | χ2 × 10−4 | ζE % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0 μΩ−1 sn cm−2 | n | ||||||||||
| 2.0 M H2SO4 | 0.0 | 0.69 | 20.6 ± 1.6 | 540.5 | 9.6 | 18.2 | 11.82 | 133.93 | 0.739 | 3.91 | -- |
| CH | 20 | 0.83 | 30.4 ± 2.1 | 161.8 | 14.2 | 26.9 | 29.91 | 38.27 | 0.829 | 4.93 | 32.2 |
| 40 | 0.99 | 41.1 ± 3.2 | 134.3 | 19.1 | 35.7 | 36.25 | 32.42 | 0.845 | 4.96 | 49.8 | |
| 80 | 1.21 | 75.4 ± 4.7 | 84.8 | 35.3 | 66.7 | 57.15 | 20.13 | 0.841 | 4.99 | 72.6 | |
| 120 | 1.42 | 129.3 ± 9.4 | 44.4 | 62.2 | 120.1 | 107.53 | 10.62 | 0.849 | 5.25 | 84.1 | |
| 200 | 1.78 | 252.4 ± 16.8 | 26.9 | 117.8 | 220.9 | 178.45 | 6.37 | 0.865 | 5.06 | 91.8 | |
| CH/Zn@H3BTC | 20 | 0.83 | 33.3 ± 1.4 | 121.5 | 15.7 | 29.9 | 40.38 | 28.77 | 0.849 | 5.48 | 38.1 |
| 40 | 0.91 | 61.9 ± 3.6 | 82.8 | 29.5 | 56.6 | 58.26 | 19.61 | 0.862 | 5.44 | 66.7 | |
| 80 | 1.26 | 102.8 ± 6.2 | 67.7 | 49.3 | 94.8 | 71.79 | 15.84 | 0.897 | 5.18 | 79.9 | |
| 120 | 1.85 | 177.1 ± 16.9 | 37.6 | 82.7 | 155.5 | 130.75 | 8.92 | 0.856 | 5.14 | 88.3 | |
| 200 | 2.24 | 364.8 ± 23.1 | 14.7 | 168.3 | 312.7 | 244.02 | 3.72 | 0.889 | 5.32 | 94.3 | |
| Inhibitor | CH | CH/Zn@H3BTC |
|---|---|---|
| EHOMO, eV | −5.00 | −9.11 |
| ELUMO, eV | 0.81 | −8.57 |
| ∆E, eV | 5.80 | 0.55 |
| I | 5.00 | 9.11 |
| A | −0.81 | 8.57 |
| χ | 2.09 | 8.84 |
| η | 2.90 | 0.27 |
| σ | 0.34 | 3.66 |
| Dipole moment value, Debye | 6.09 | 53.16 |
| Molecular surface area, Å2 | 193.50 | 1188.75 |
| Structures | Adsorption Energy kcal mol−1 | Rigid Adsorption Energy kcal mol−1 | Deformation Energy kcal mol−1 | dEads/dNi: Inhibitor kcal mol−1 | dEads/dNi: Water kcal mol−1 |
|---|---|---|---|---|---|
| Fe (110) | −3045.58 | −2908.63 | −136.94 | −103.40 | −14.31 |
| CH | |||||
| water | |||||
| Fe (110) | −9676.99 | −4675.19 | −5001.80 | −1388.29 | −14.28 |
| CH/Zn@H3BTC | |||||
| water |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, M.; Khalaf, M.M.; Shalabi, K.; Al-Omair, M.A.; El-Lateef, H.M.A. Synthesis and Characterization of Zn–Organic Frameworks Containing Chitosan as a Low-Cost Inhibitor for Sulfuric-Acid-Induced Steel Corrosion: Practical and Computational Exploration. Polymers 2022, 14, 228. https://doi.org/10.3390/polym14020228
Gouda M, Khalaf MM, Shalabi K, Al-Omair MA, El-Lateef HMA. Synthesis and Characterization of Zn–Organic Frameworks Containing Chitosan as a Low-Cost Inhibitor for Sulfuric-Acid-Induced Steel Corrosion: Practical and Computational Exploration. Polymers. 2022; 14(2):228. https://doi.org/10.3390/polym14020228
Chicago/Turabian StyleGouda, Mohamed, Mai M. Khalaf, Kamal Shalabi, Mohammed A. Al-Omair, and Hany M. Abd El-Lateef. 2022. "Synthesis and Characterization of Zn–Organic Frameworks Containing Chitosan as a Low-Cost Inhibitor for Sulfuric-Acid-Induced Steel Corrosion: Practical and Computational Exploration" Polymers 14, no. 2: 228. https://doi.org/10.3390/polym14020228
APA StyleGouda, M., Khalaf, M. M., Shalabi, K., Al-Omair, M. A., & El-Lateef, H. M. A. (2022). Synthesis and Characterization of Zn–Organic Frameworks Containing Chitosan as a Low-Cost Inhibitor for Sulfuric-Acid-Induced Steel Corrosion: Practical and Computational Exploration. Polymers, 14(2), 228. https://doi.org/10.3390/polym14020228









