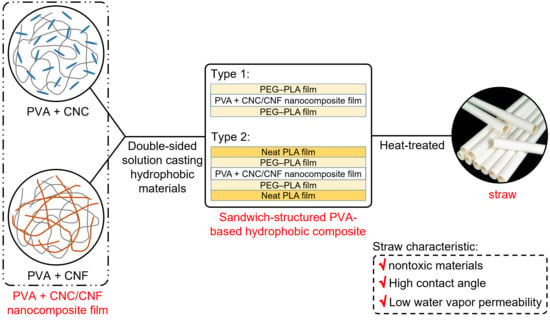
Sandwich-Structured, Hydrophobic, Nanocellulose-Reinforced Polyvinyl Alcohol as an Alternative Straw Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
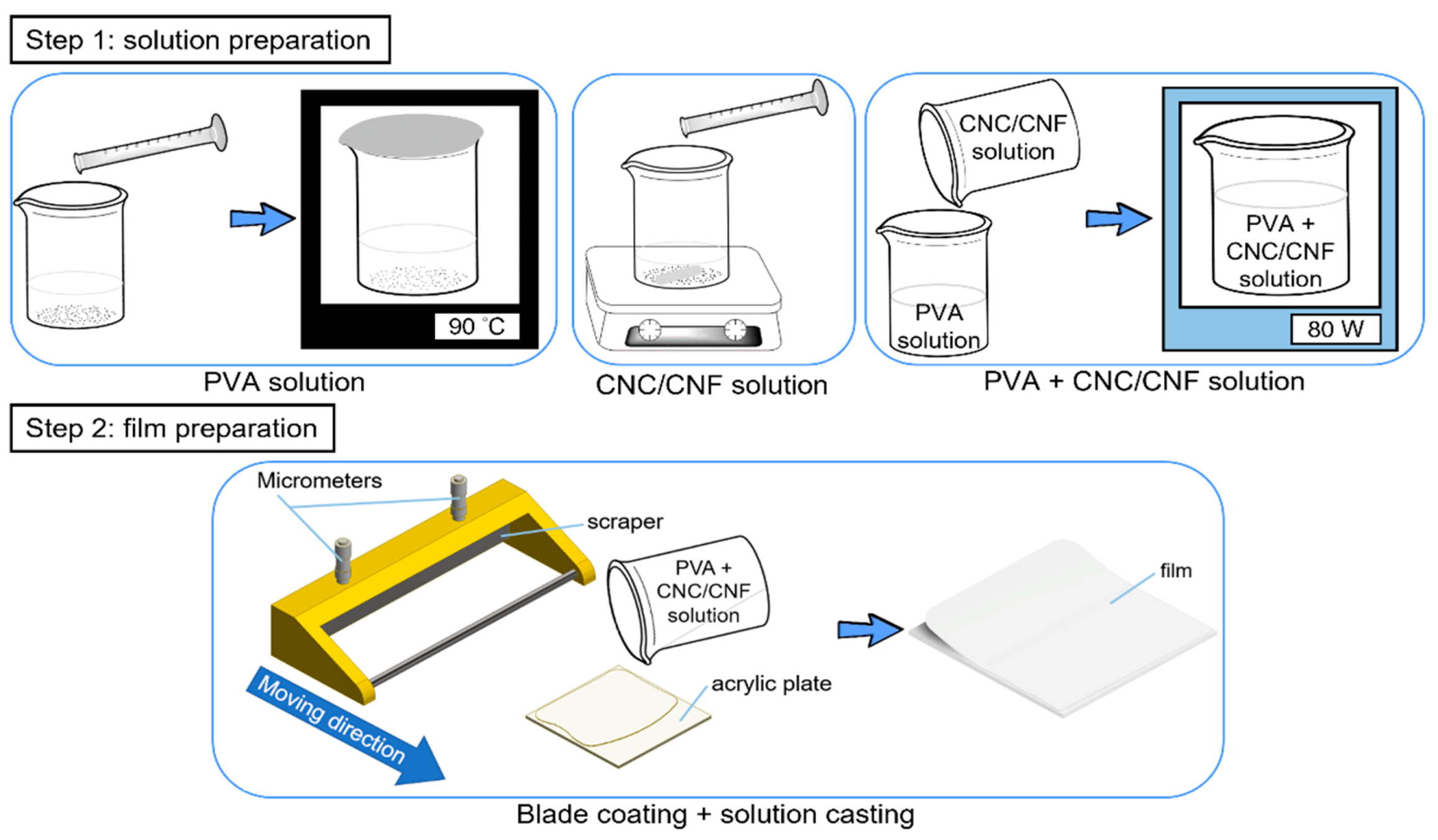
2.1.1. PVA + CNC/CNF Nanocomposite Films
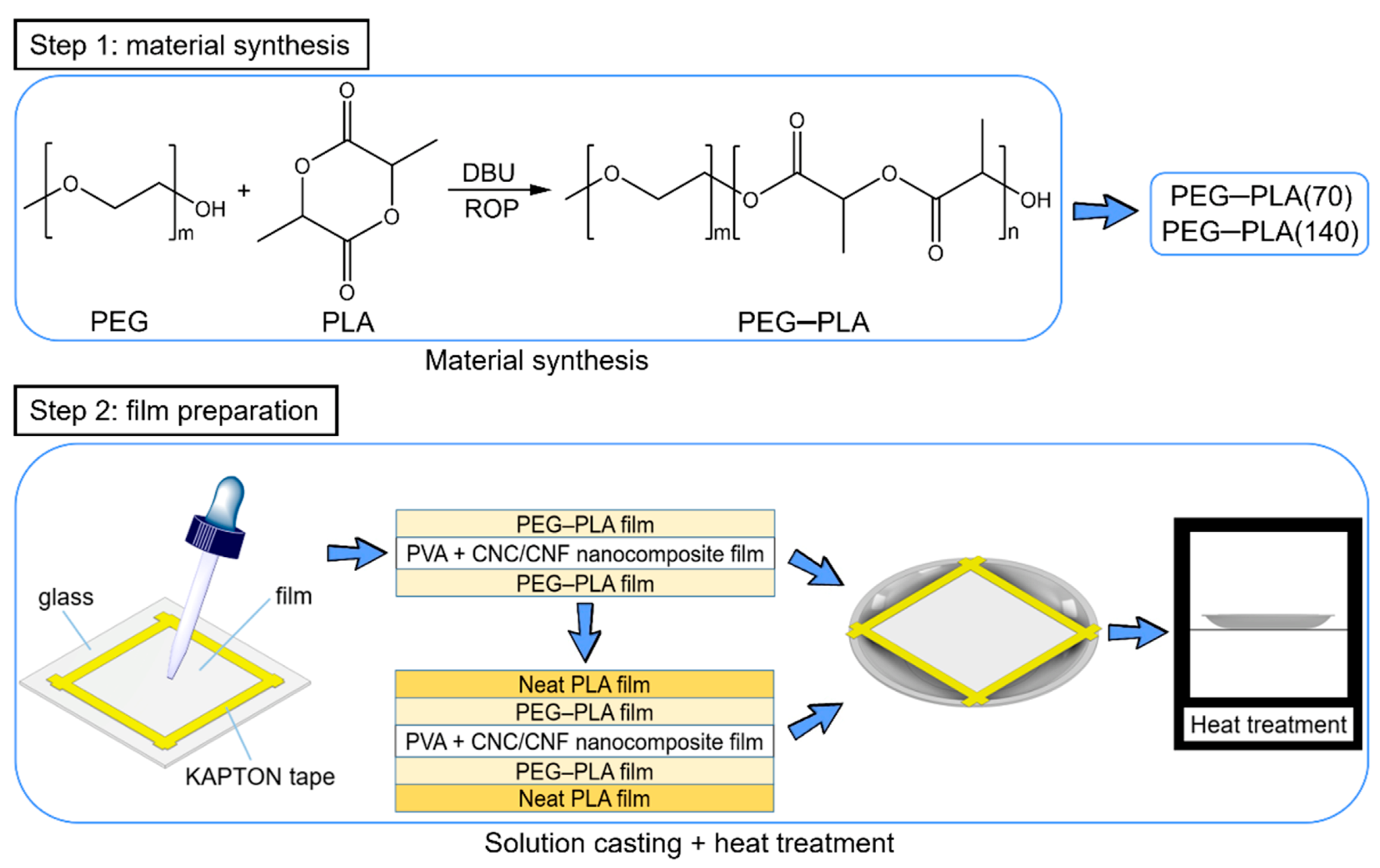
2.1.2. Hydrophobic Materials
2.2. Methods
2.2.1. Preparation of PVA + CNC/CNF Nanocomposite Films
2.2.2. Preparation of Hydrophobic Materials
2.3. Characterization
2.3.1. X-ray Diffraction
2.3.2. Contact Angle Analysis
2.3.3. Water Vapor Permeability
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Water Uptake
2.3.7. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. PVA + CNC/CNF Nanocomposite Films
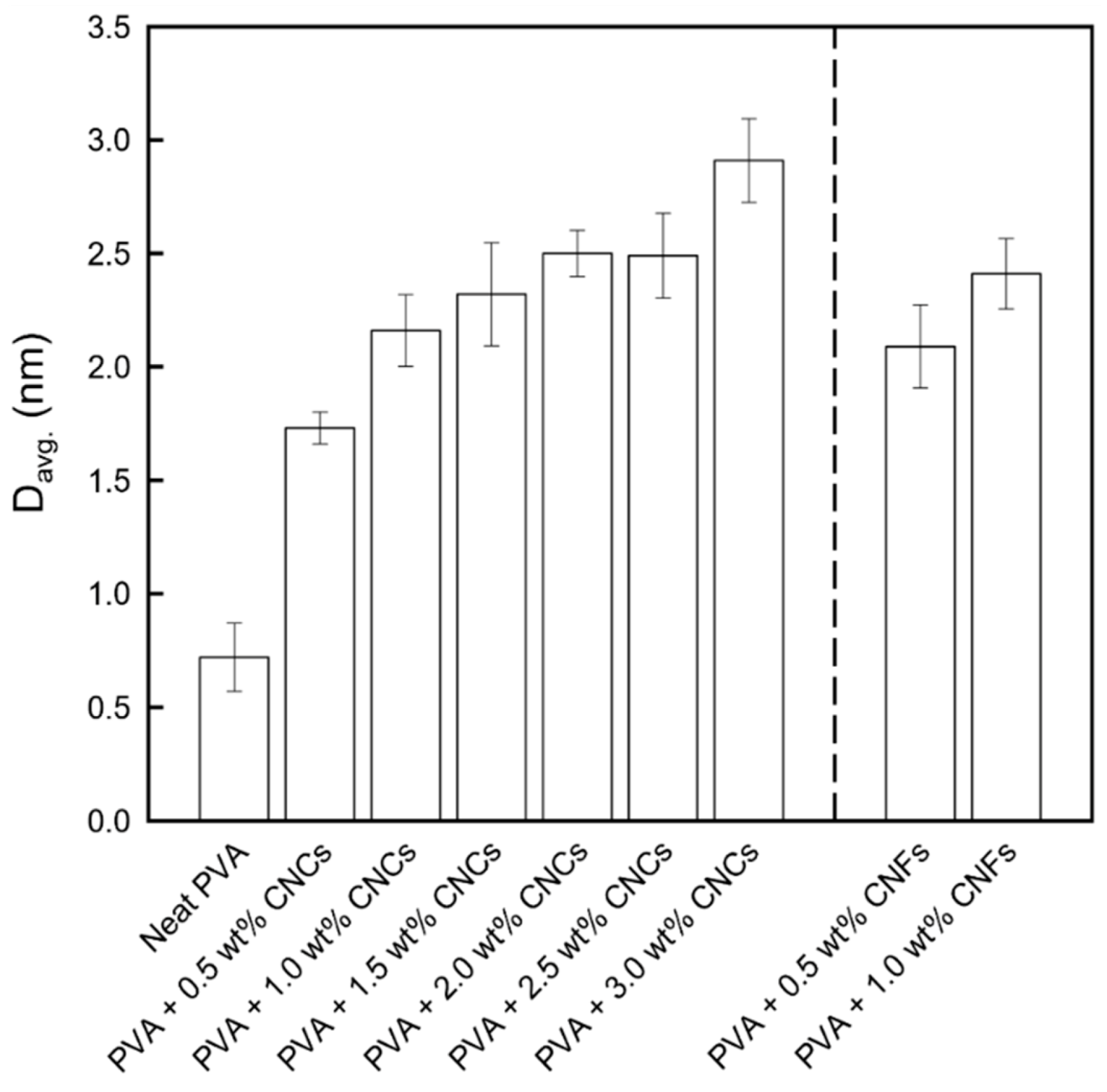
3.1.1. X-ray Diffraction
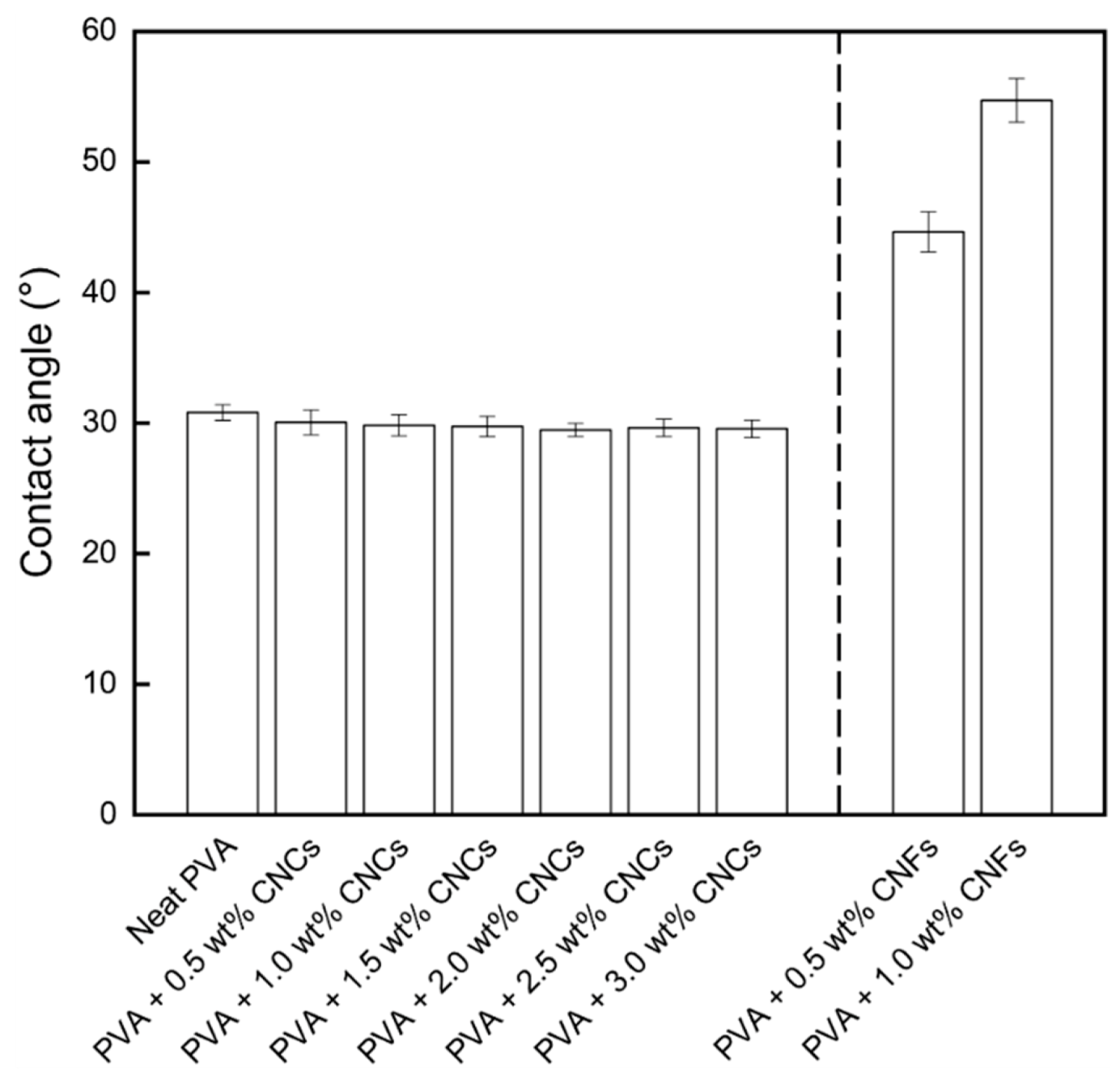
3.1.2. Contact Angle Analysis
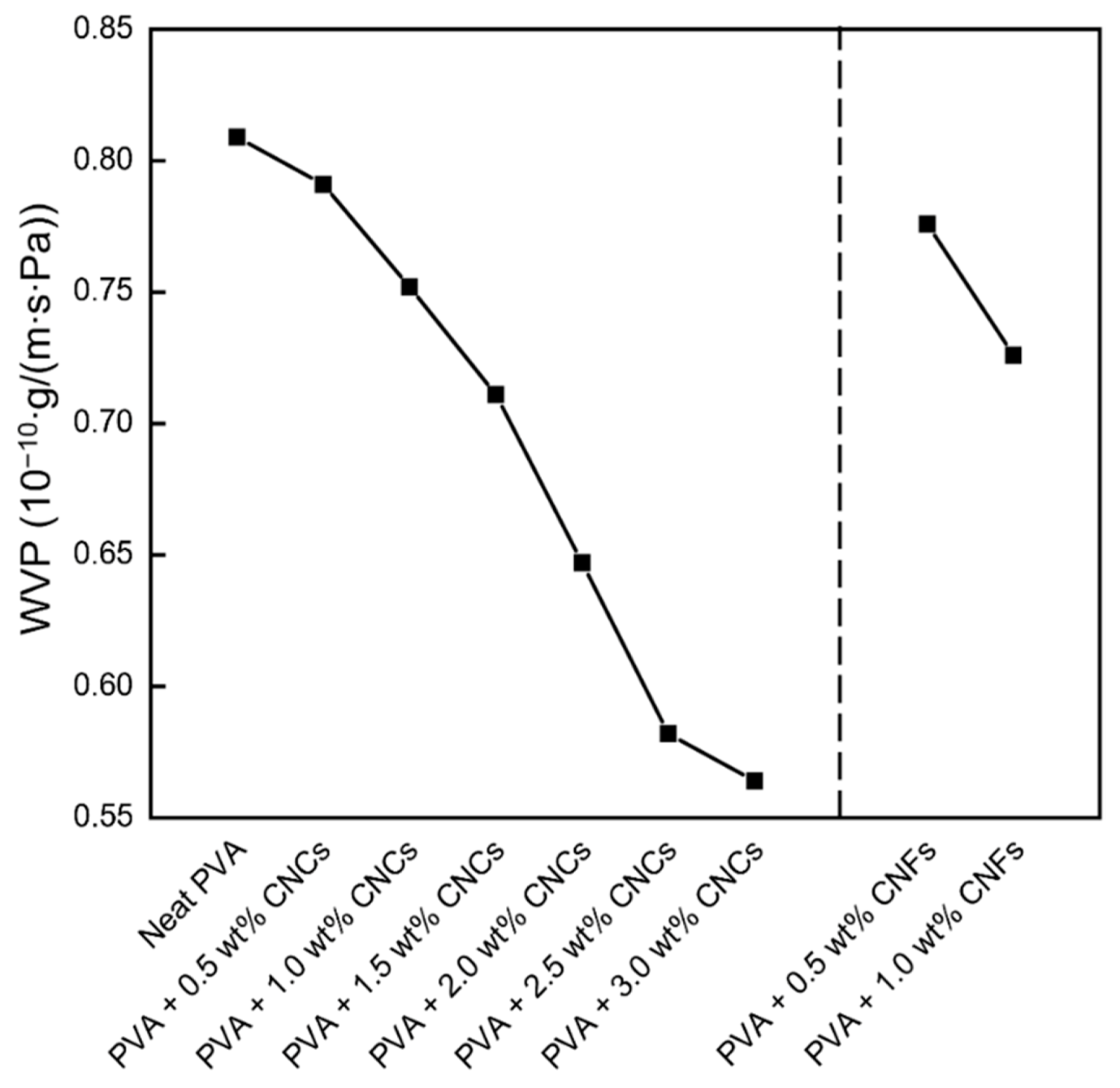
3.1.3. Water Vapor Permeability
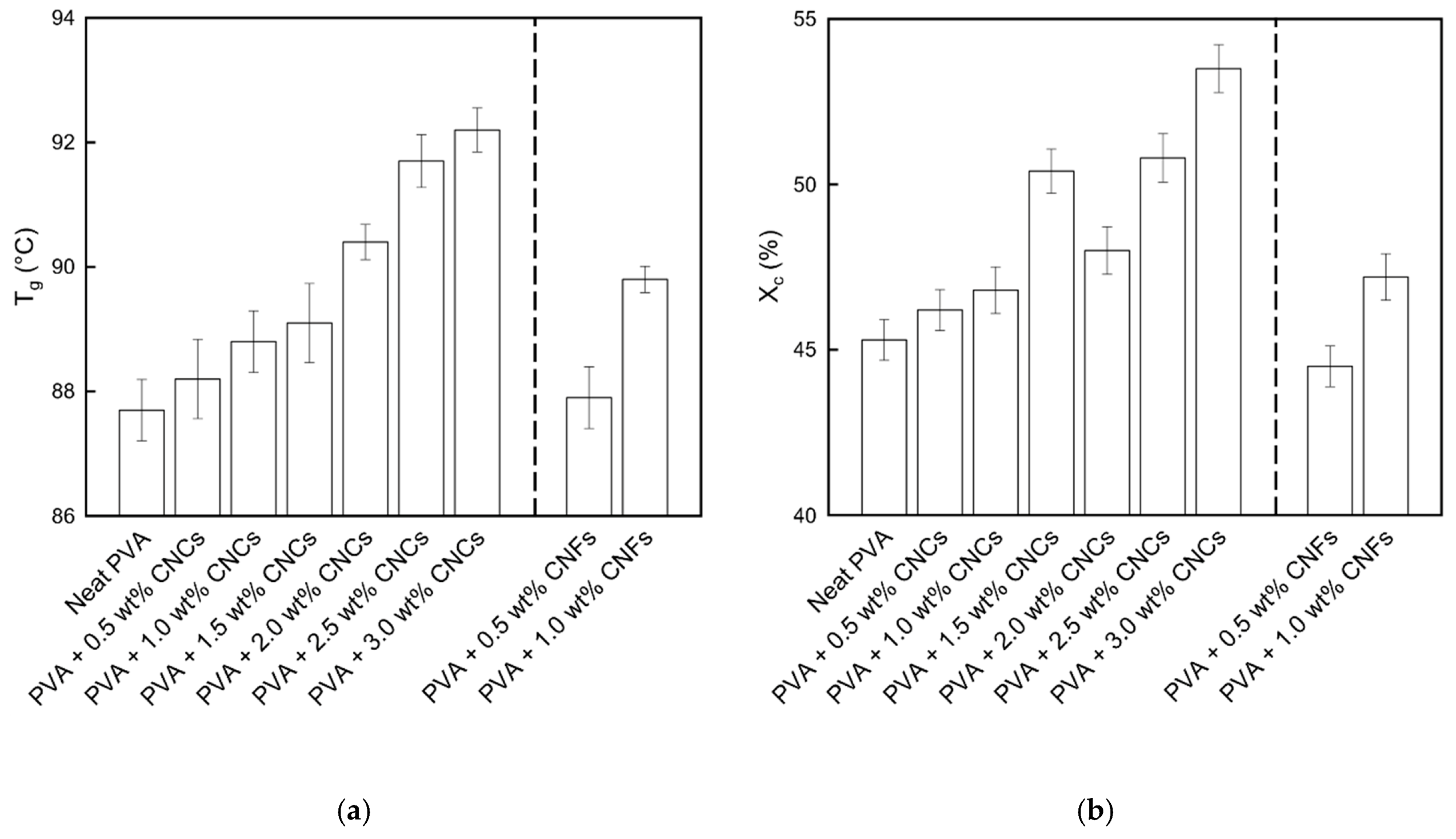
3.1.4. Differential Scanning Calorimetry
3.1.5. Thermogravimetric Analysis
3.2. Hydrophobic Materials
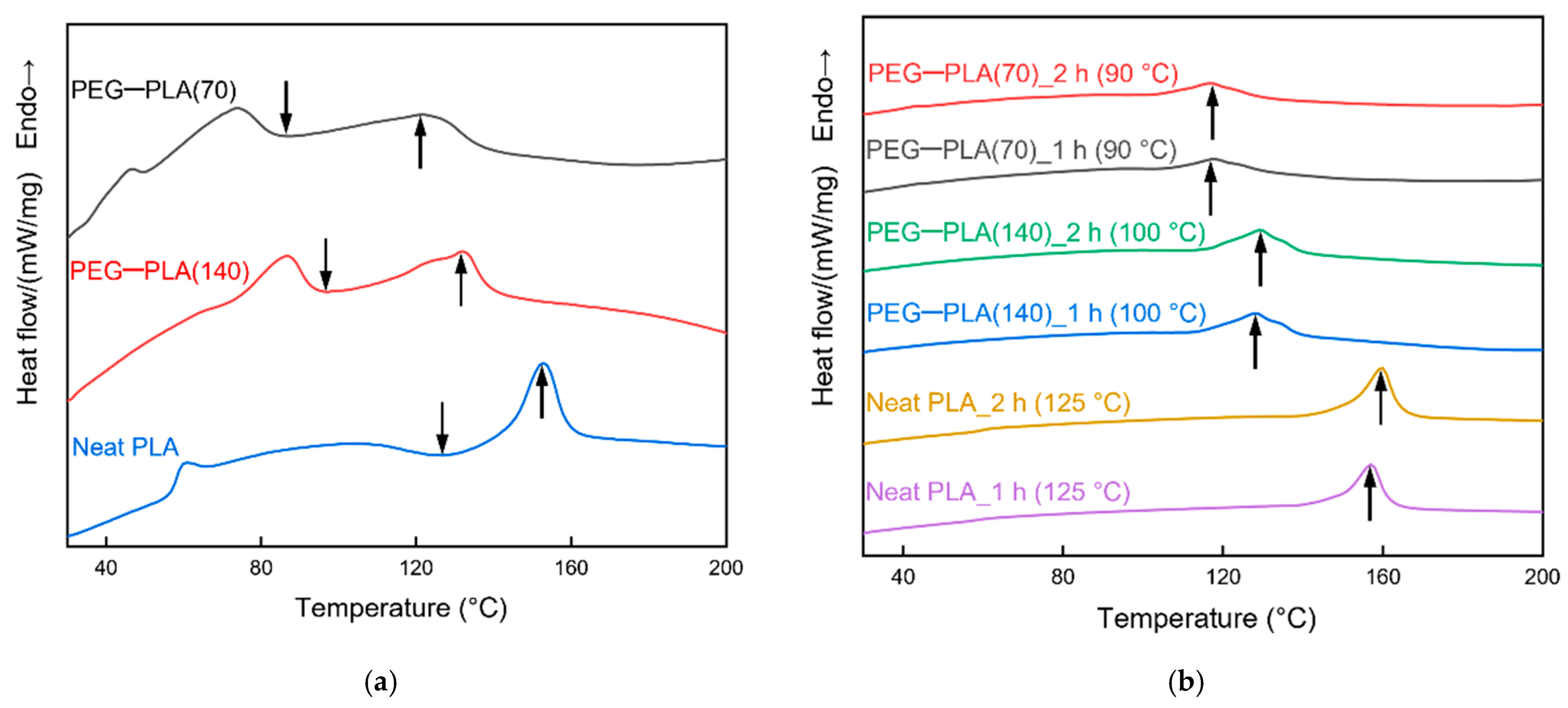
3.2.1. Differential Scanning Calorimetry
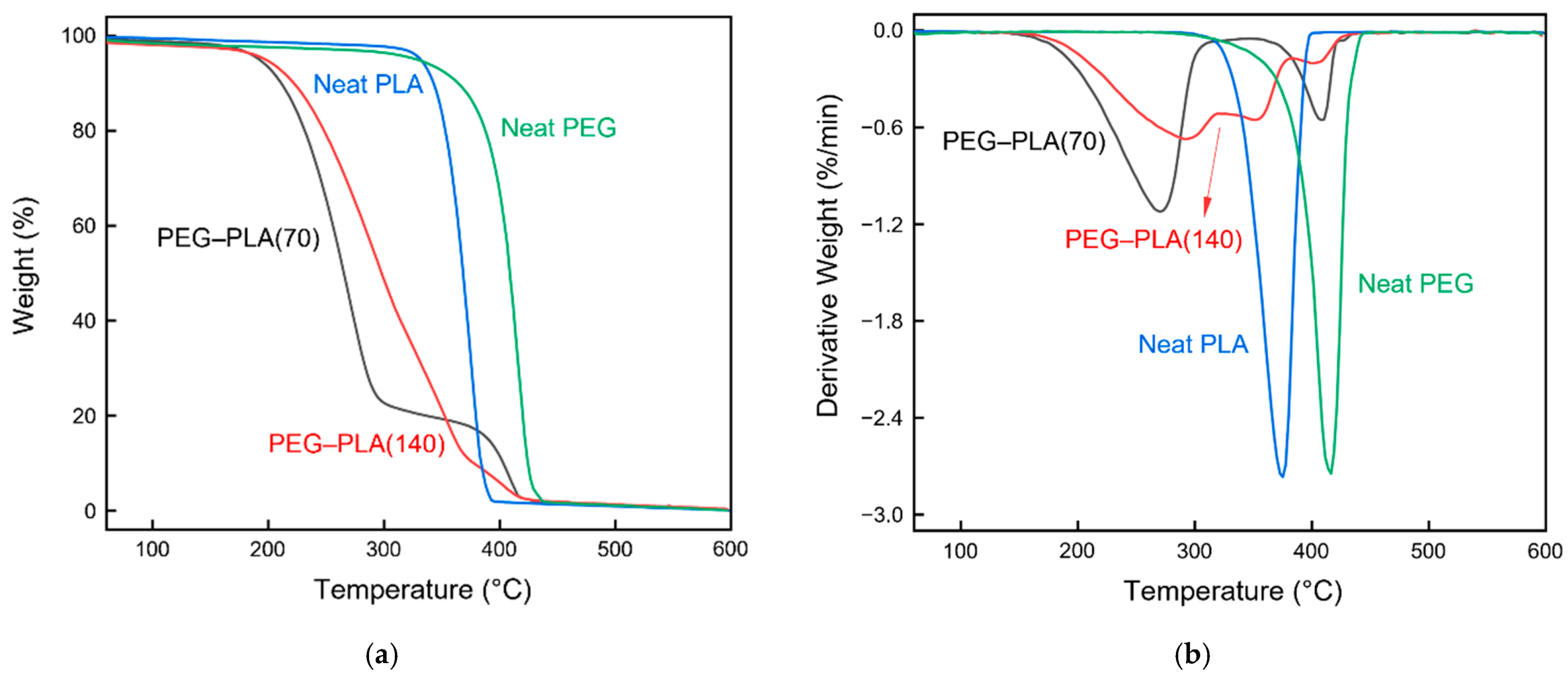
3.2.2. Thermogravimetric Analysis
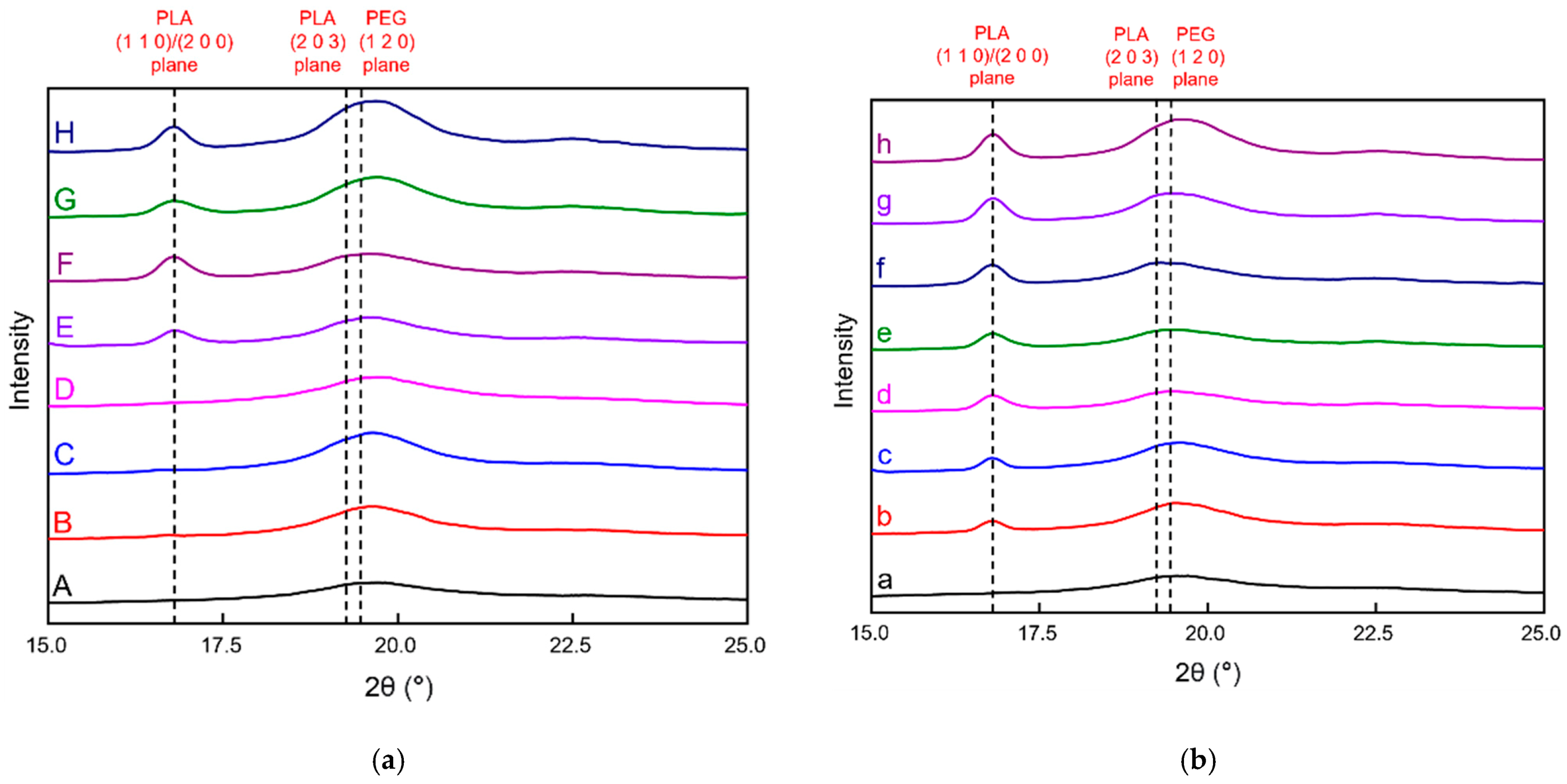
3.2.3. X-ray Diffraction
3.2.4. Contact Angle Analysis
3.2.5. Water Vapor Permeability
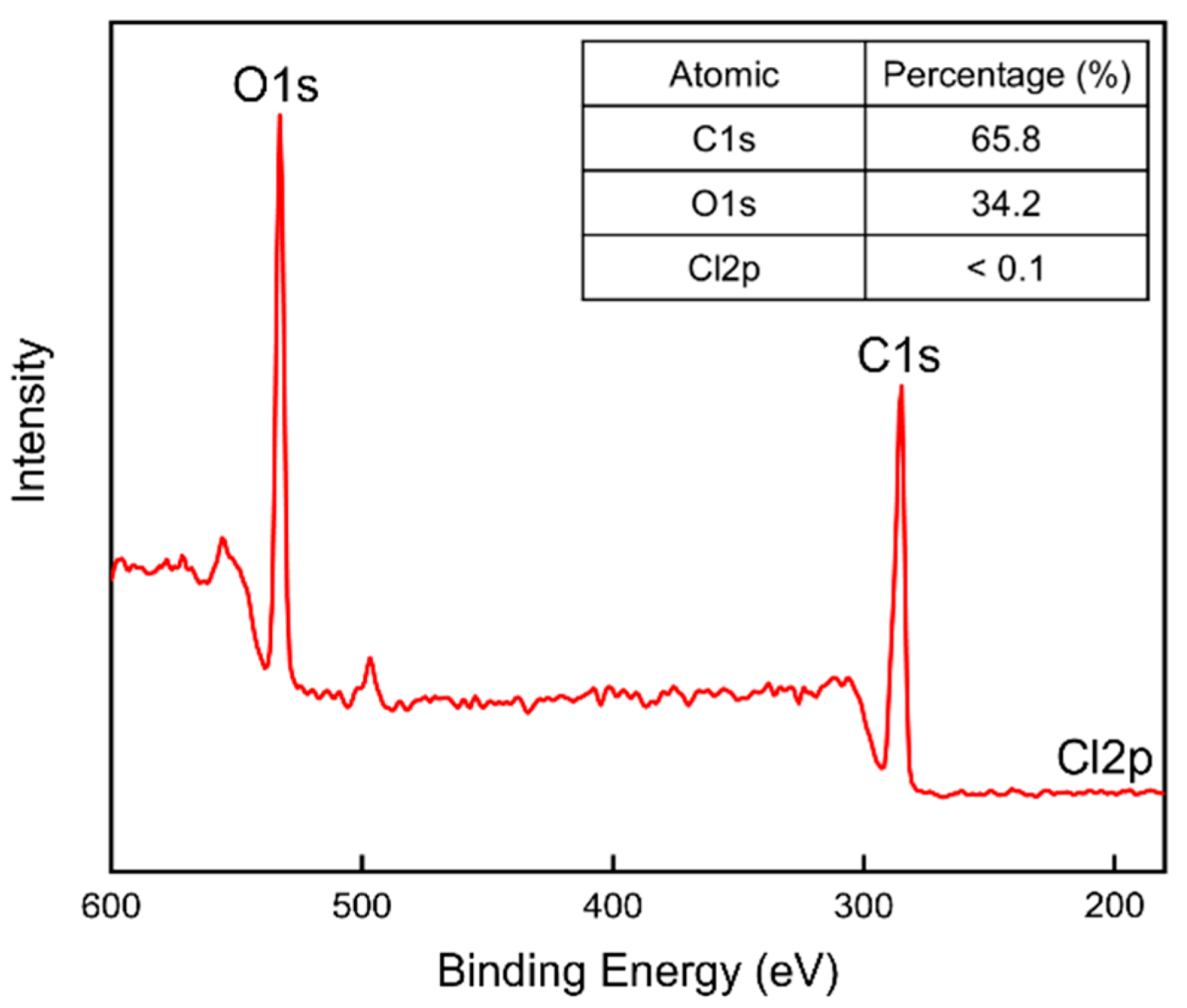
3.2.6. X-ray Photoelectron Spectroscopy
3.3. Heat-Treated Sandwich-Structured PVA-Based Hydrophobic Composite Film
3.3.1. Contact Angle Analysis
3.3.2. Water Vapor Permeability
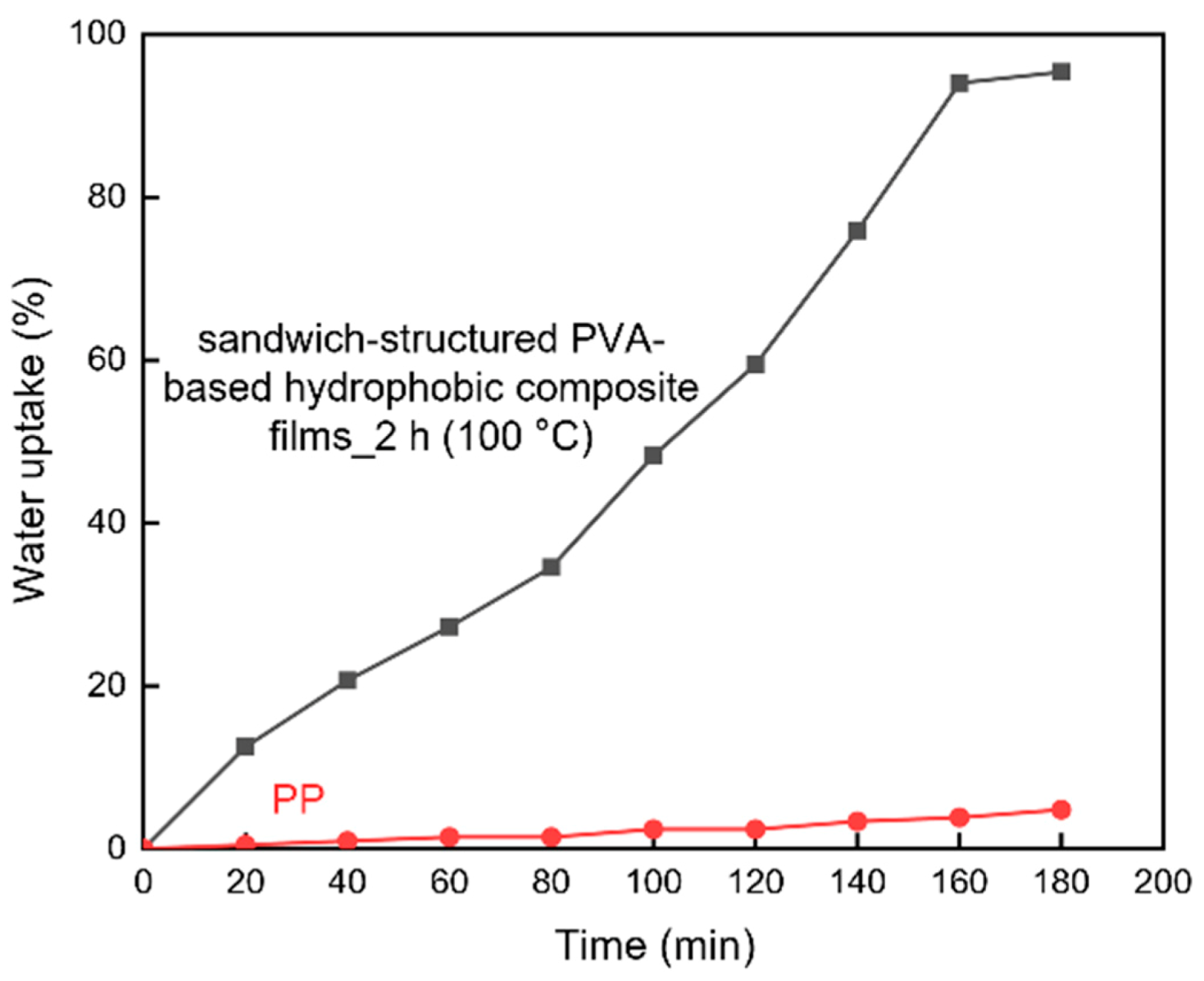
3.3.3. Water Uptake
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Definition | Abbreviation | Definition |
|---|---|---|---|
| CNCs | Cellulose nanocrystals | PEG–PLA | Polyethylene-glycol–poly(lactic acid) |
| CNFs | Cellulose nanofibers | PP | Polypropylene |
| DCM | Dichloromethane | PVA | Polyvinyl alcohol |
| DSC | Differential scanning calorimetry | ROP | Ring-opening polymerization |
| DTG | Derivative thermogravimetry | TGA | Thermogravimetric Analysis |
| EA | Ethyl acetate | WVP | Water vapor permeability |
| GO | Graphene oxide | WVTR | Water vapor transmission rate |
| LA | Lactide | XPS | X-ray photoelectron spectroscopy |
| LDPE | Low-density polyethylene | XRD | X-ray diffraction |
References
- Plastic Pollution. Available online: https://ourworldindata.org/plastic-pollution (accessed on 10 November 2021).
- Camacho, W.; Karlsson, S. NIR, DSC, and FTIR as quantitative methods for compositional analysis of blends of polymers obtained from recycled mixed plastic waste. Polym. Eng. Sci. 2001, 41, 1626–1635. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Haweel, C.K.; Ammar, S.H. Preparation of polyvinyl alcohol from local raw material. Iraqi J. Chem. Pet. Eng. 2008, 9, 15–21. [Google Scholar] [CrossRef]
- Tubbs, R.K. Melting point and heat of fusion of poly (vinyl alcohol). J. Polym. Sci. Part A Gen. Pap. 1965, 3, 4181–4189. [Google Scholar] [CrossRef]
- Srinivasa, P.; Ramesh, M.; Kumar, K.; Tharanathan, R. Properties and sorption studies of chitosan–polyvinyl alcohol blend films. Carbohydr. Polym. 2003, 53, 431–438. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Shang, S.; Tao, X.-m. Synthesis and characterization of layer-aligned poly (vinyl alcohol)/graphene nanocomposites. Polymer 2010, 51, 3431–3435. [Google Scholar] [CrossRef]
- Mbhele, Z.; Salemane, M.; Van Sittert, C.; Nedeljković, J.; Djoković, V.; Luyt, A. Fabrication and characterization of silver–polyvinyl alcohol nanocomposites. Chem. Mater. 2003, 15, 5019–5024. [Google Scholar] [CrossRef]
- Mohanty, J.R.; Das, S.N.; Das, H.C.; Swain, S.K. Effective mechanical properties of polyvinylalcohol biocomposites with reinforcement of date palm leaf fibers. Polym. Compos. 2013, 34, 959–966. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Wu, X.; Moon, R.J.; Martini, A. Crystalline cellulose elastic modulus predicted by atomistic models of uniform deformation and nanoscale indentation. Cellulose 2013, 20, 43–55. [Google Scholar] [CrossRef]
- Dri, F.L.; Hector, L.G.; Moon, R.J.; Zavattieri, P.D. Anisotropy of the elastic properties of crystalline cellulose I β from first principles density functional theory with Van der Waals interactions. Cellulose 2013, 20, 2703–2718. [Google Scholar] [CrossRef]
- Sakurada, I.; Nukushina, Y.; Ito, T. Experimental determination of the elastic modulus of crystalline regions in oriented polymers. J. Polym. Sci. 1962, 57, 651–660. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromolecules 2009, 10, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Zhu, J.; Deng, Y.; Ragusa’s, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Process. 2014, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wu, Q. Recent development in applications of cellulose nanocrystals for advanced polymer-based nanocomposites by novel fabrication strategies. In Nanocrystals Synthesis, Characterization and Applications; Neralla, S., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 103–120. [Google Scholar]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Chaabouni, O.; Boufi, S. Cellulose nanofibrils/polyvinyl acetate nanocomposite adhesives with improved mechanical properties. Carbohydr. Polym. 2017, 156, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kord, B.; Malekian, B.; Yousefi, H.; Najafi, A. Preparation and characterization of nanofibrillated Cellulose/Poly (Vinyl Alcohol) composite films. Maderas Ciencia y Tecnología 2016, 18, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Ghahari, S.; Assi, L.N.; Alsalman, A.; Alyamaç, K.E. Fracture properties evaluation of cellulose nanocrystals cement paste. Materials 2020, 13, 2507. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.S.; do Nascimento, D.M.; Morais, J.P.S.; Vasconcelos, N.F.; Feitosa, J.P.; Brígida, A.I.S.; Rosa, M.d.F. Improvement of polyvinyl alcohol properties by adding nanocrystalline cellulose isolated from banana pseudostems. Carbohydr. Polym. 2014, 112, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, X.; Tian, H.; Maiti, S.; Ma, Z. Effects of cellulose nanofibrils on the structure and properties on PVA nanocomposites. Cellulose 2013, 20, 2981–2989. [Google Scholar] [CrossRef]
- Li, W.; Wu, Q.; Zhao, X.; Huang, Z.; Cao, J.; Li, J.; Liu, S. Enhanced thermal and mechanical properties of PVA composites formed with filamentous nanocellulose fibrils. Carbohydr. Polym. 2014, 113, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Voronova, M.I.; Surov, O.V.; Guseinov, S.S.; Barannikov, V.P.; Zakharov, A.G. Thermal stability of polyvinyl alcohol/nanocrystalline cellulose composites. Carbohydr. Polym. 2015, 130, 440–447. [Google Scholar] [CrossRef]
- Rowe, A.A.; Tajvidi, M.; Gardner, D.J. Thermal stability of cellulose nanomaterials and their composites with polyvinyl alcohol (PVA). J. Therm. Anal. Calorim. 2016, 126, 1371–1386. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking reaction of poly (vinyl alcohol) with glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly (vinyl alcohol) and nanocellulose from sugarcane bagasse. J. Ind. Eng. Chem. 2014, 20, 462–473. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Lee, H.; You, J.; Jin, H.-J.; Kwak, H.W. Chemical and physical reinforcement behavior of dialdehyde nanocellulose in PVA composite film: A comparison of nanofiber and nanocrystal. Carbohydr. Polym. 2020, 232, 115771. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG–PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shabat, S.; Kumar, N.; Domb, A.J. PEG-PLA block copolymer as potential drug carrier: Preparation and characterization. Macromol. Biosci. 2006, 6, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yu, M.; Zong, X.; Chiu, J.; Fang, D.; Seo, Y.-S.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Control of degradation rate and hydrophilicity in electrospun non-woven poly (D, L-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 2003, 24, 4977–4985. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.-L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Sun, X.; Lu, C.; Liu, Y.; Zhang, W.; Zhang, X. Melt-processed poly (vinyl alcohol) composites filled with microcrystalline cellulose from waste cotton fabrics. Carbohydr. Polym. 2014, 101, 642–649. [Google Scholar] [CrossRef]
- Uddin, A.J.; Fujie, M.; Sembo, S.; Gotoh, Y. Outstanding reinforcing effect of highly oriented chitin whiskers in PVA nanocomposites. Carbohydr. Polym. 2012, 87, 799–805. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Li, C.; Zhang, X.; Lu, C.; Zhang, X.; Deng, Y. High performance poly (vinyl alcohol)/cellulose nanocrystals nanocomposites manufactured by injection molding. Cellulose 2014, 21, 485–494. [Google Scholar] [CrossRef]
- Schyrr, B.; Pasche, S.p.; Voirin, G.; Weder, C.; Simon, Y.C.; Foster, E.J. Biosensors based on porous cellulose nanocrystal–poly (vinyl alcohol) scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 12674–12683. [Google Scholar] [CrossRef]
- Roohani, M.; Habibi, Y.; Belgacem, N.M.; Ebrahim, G.; Karimi, A.N.; Dufresne, A. Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites. Eur. Polym. J. 2008, 44, 2489–2498. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Gimenez, V.; Mantecon, A.; Cadiz, V. Modification of poly (vinyl alcohol) with acid chlorides and crosslinking with difunctional hardeners. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 925–934. [Google Scholar] [CrossRef]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; You, M.; Shin, J.; Ha, S.; Kim, D.; Heo, M.H.; Nah, J.; Kim, Y.A.; Seol, J.H. Thermal conductivity enhancement in electrospun poly (vinyl alcohol) and poly (vinyl alcohol)/cellulose nanocrystal composite nanofibers. Sci. Rep. 2019, 9, 3026. [Google Scholar] [CrossRef]
- Yao, B.; Zhu, Q.; Liu, H.; Qiao, L.; Hao, J.; Qi, F. Conformation and aggregation behavior of poly (ethylene glycol)-b-poly (lactic acid) amphiphilic copolymer chains in dilute/semidilute THF solutions. J. Appl. Polym. Sci. 2012, 125, E223–E230. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Vestena, M.; Gross, I.P.; Müller, C.M.; Pires, A.T. Nanocomposite of poly (lactic acid)/cellulose nanocrystals: Effect of CNC content on the polymer crystallization kinetics. J. Braz. Chem. Soc. 2016, 27, 905–911. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, S.; Wang, R.; Zhou, Z.; Peng, C.; Zhu, M. Synthesis and characterization of an environmentally friendly PHBV/PEG copolymer network as a phase change material. Sci. China Chem. 2013, 56, 716–723. [Google Scholar] [CrossRef]
- Dai, X.; Cao, Y.; Shi, X.; Wang, X. Non-isothermal crystallization kinetics, thermal degradation behavior and mechanical properties of poly (lactic acid)/MOF composites prepared by melt-blending methods. RSC Adv. 2016, 6, 71461–71471. [Google Scholar] [CrossRef]
- Zhao, L.; Kong, J.; Tian, X.; Zhang, J.; Qin, S. Isothermal crystallization of poly (L-lactide) and poly (butylene adipate) crystalline/crystalline blends. Polym. J. 2014, 46, 323–329. [Google Scholar] [CrossRef]
- Jiao, Y.-P.; Cui, F.-Z. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2007, 2, R24–R37. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crop. Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]






| PEG–PLA(70) | |||
| Material | Molecular Weight | Content | Equivalent |
| PEG | 2000 | 200 mg/0.1 mmol | 1 |
| LA | 144.13 | 1008.91 mg/7 mmol | 70 |
| DBU | 152.24 | 38.06 mg/0.25 mmol | 2.5 |
| PEG–PLA(140) | |||
| Material | Molecular Weight | Content | Equivalent |
| PEG | 2000 | 200 mg/0.1 mmol | 1 |
| LA | 144.13 | 2017.82 mg/14 mmol | 140 |
| DBU | 152.24 | 38.06 mg/0.25 mmol | 2.5 |
| Hydrophobic Film Material | Heat Treatment | Notation | ||
|---|---|---|---|---|
| Temperature (°C) | Time (h) | |||
| PEG–PLA(70) | - | No Heat Treatment | A | |
| 90 | 1 | B | ||
| 2 | C | |||
| Neat PLA | No Heat Treatment | D | ||
| 90 | 1 | E | ||
| 2 | F | |||
| 125 | 1 | G | ||
| 2 | H | |||
| PEG–PLA(140) | - | No Heat Treatment | a | |
| 100 | 1 | b | ||
| 2 | c | |||
| Neat PLA | No Heat Treatment | d | ||
| 100 | 1 | e | ||
| 2 | f | |||
| 125 | 1 | g | ||
| 2 | h | |||
| Sample | Second Region | Third Region | ||||||
|---|---|---|---|---|---|---|---|---|
| Tonset (°C) | Toffset (°C) | Tmax (°C) | Mass Loss (%) | Tonset (°C) | Toffset (°C) | Tmax (°C) | Mass Loss (%) | |
| Neat PVA | 248.8 | 292.5 | 271.2 | 64.1 | 419.4 | 460.4 | 432.1 | 17.9 |
| PVA + 0.5 wt% CNCs | 249.4 | 293.5 | 274.0 | 66.8 | 422.2 | 456.3 | 433.0 | 16.0 |
| PVA + 1.0 wt% CNCs | 250.6 | 301.1 | 277.0 | 67.5 | 423.3 | 450.1 | 432.0 | 15.5 |
| PVA + 1.5 wt% CNCs | 252.1 | 300.2 | 275.1 | 67.6 | 422.9 | 449.3 | 431.1 | 15.5 |
| PVA + 2.0 wt% CNCs | 253.1 | 300.9 | 276.1 | 66.7 | 422.1 | 448.9 | 430.1 | 16.4 |
| PVA + 2.5 wt% CNCs | 253.3 | 308.2 | 277.1 | 69.7 | 424.4 | 452.4 | 433.1 | 14.2 |
| PVA + 3.0 wt% CNCs | 256.4 | 309.2 | 280.0 | 66.1 | 418.9 | 462.4 | 433.0 | 13.5 |
| Neat CNCs | 297.3 | 312.8 | 304.2 | 52.8 | 322.0 | 404.4 | 357.2 | 11.2 |
| PVA + 0.5 wt% CNFs | 250.7 | 297.5 | 275.1 | 64.6 | 418.3 | 447.8 | 428.1 | 16.4 |
| PVA + 1.0 wt% CNFs | 252.9 | 306.8 | 280.1 | 67.0 | 421.4 | 448.1 | 430.1 | 15.8 |
| Neat CNFs | 284.6 | 310.8 | 299.2 | 43.6 | 324.5 | 503.6 | 419.2 | 9.4 |
| Hydrophobic Materials | Heat Treatment | Xc (PLA) (%) |
|---|---|---|
| PEG–PLA(70) | - | 5.1 |
| 1 h (90 °C) | 24.1 | |
| 2 h (90 °C) | 28.9 | |
| PEG–PLA(140) | - | 1.1 |
| 1 h (100 °C) | 37.9 | |
| 2 h (100 °C) | 39.0 | |
| Neat PLA | - | 16.5 |
| 1 h (125 °C) | 52.2 | |
| 2 h (125 °C) | 62.4 |
| Material | WVP (10−10 g/(m·s·Pa)) |
|---|---|
| PP | 0.005 |
| sandwich-structured PVA-based hydrophobic composite film | 0.292 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-T.; Shi, S.-C.; Chen, C.-K. Sandwich-Structured, Hydrophobic, Nanocellulose-Reinforced Polyvinyl Alcohol as an Alternative Straw Material. Polymers 2021, 13, 4447. https://doi.org/10.3390/polym13244447
Chou C-T, Shi S-C, Chen C-K. Sandwich-Structured, Hydrophobic, Nanocellulose-Reinforced Polyvinyl Alcohol as an Alternative Straw Material. Polymers. 2021; 13(24):4447. https://doi.org/10.3390/polym13244447
Chicago/Turabian StyleChou, Chun-Tu, Shih-Chen Shi, and Chih-Kuang Chen. 2021. "Sandwich-Structured, Hydrophobic, Nanocellulose-Reinforced Polyvinyl Alcohol as an Alternative Straw Material" Polymers 13, no. 24: 4447. https://doi.org/10.3390/polym13244447
APA StyleChou, C.-T., Shi, S.-C., & Chen, C.-K. (2021). Sandwich-Structured, Hydrophobic, Nanocellulose-Reinforced Polyvinyl Alcohol as an Alternative Straw Material. Polymers, 13(24), 4447. https://doi.org/10.3390/polym13244447