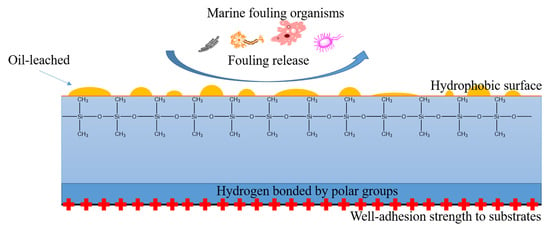
Fouling Release Coatings Based on Acrylate–MQ Silicone Copolymers Incorporated with Non-Reactive Phenylmethylsilicone Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
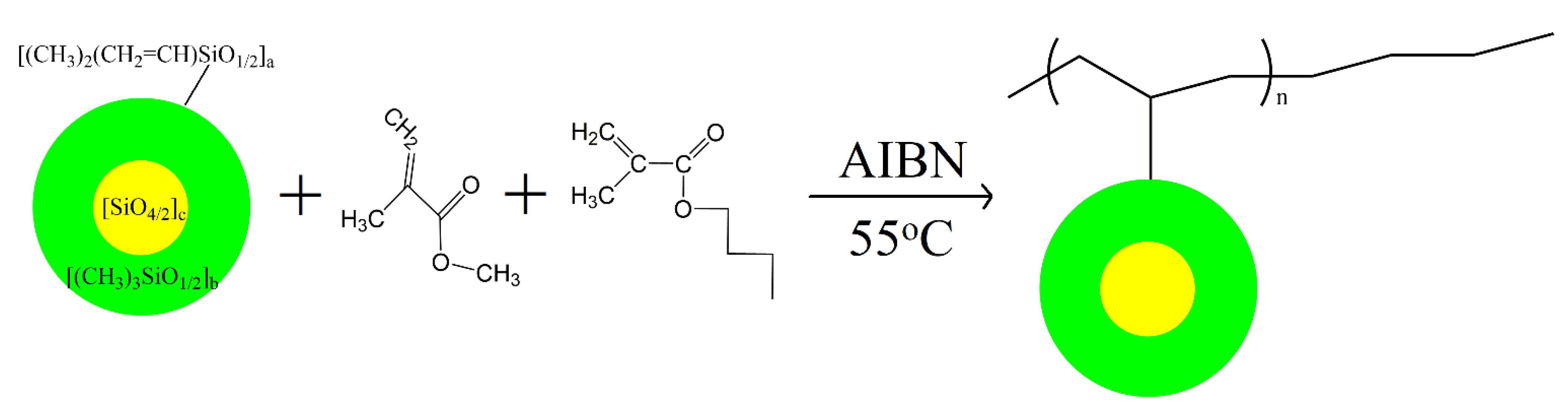
2.2. Synthesis of Acrylate–MVMQ Silicone Copolymer (AMQ)
2.3. Preparation of the FR Coating
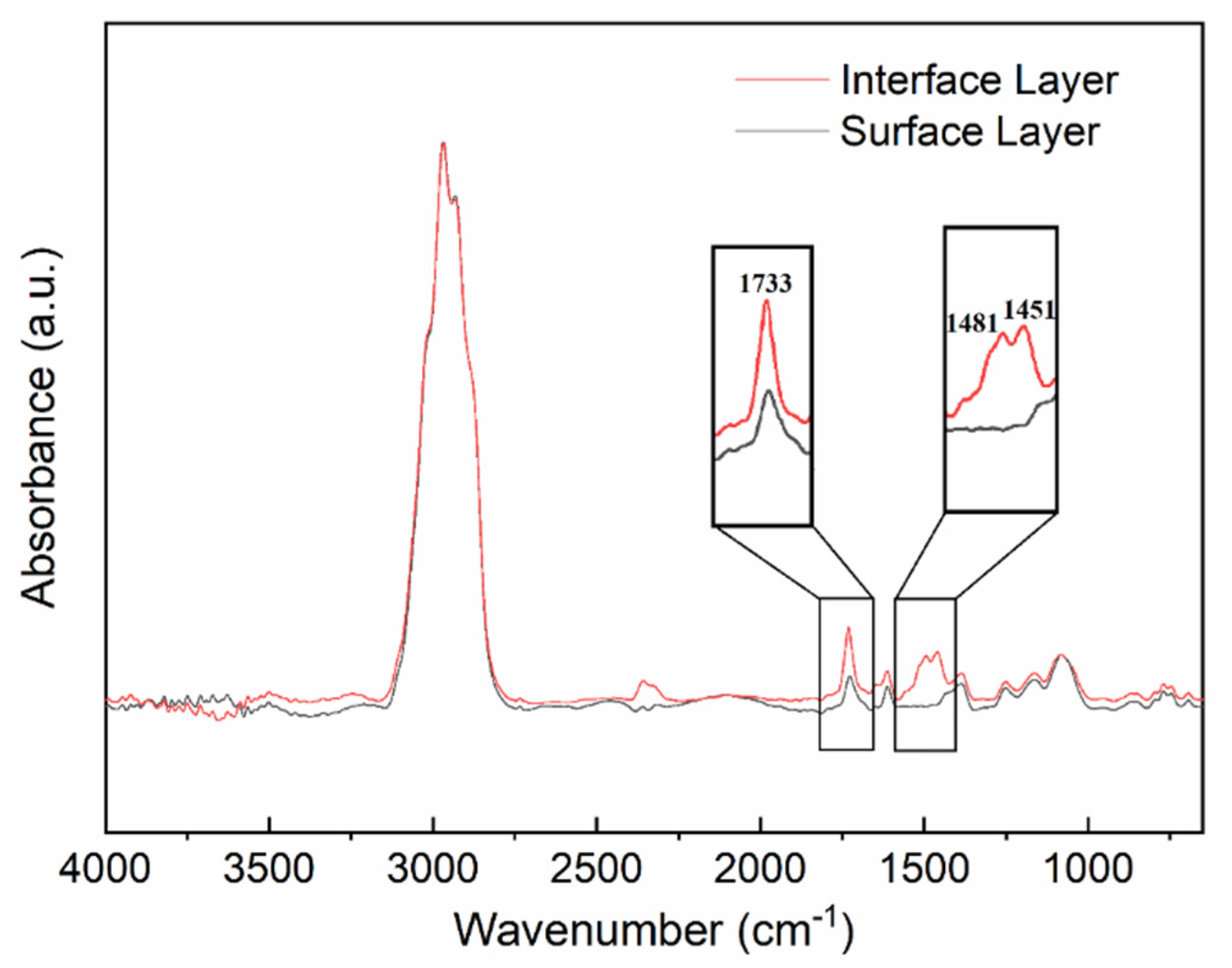
2.4. Analysis of the AMQ Copolymer
2.5. Surface Properties
2.6. Swelling Test
2.7. Mechanical Properties
2.8. Pull-Off Strength between the Coatings and Substrates
2.9. Observation of Leaching PSO
2.10. Biofilm Adhesion Assay
3. Results and Discussion
3.1. Synthesis and Characterization of AMQ
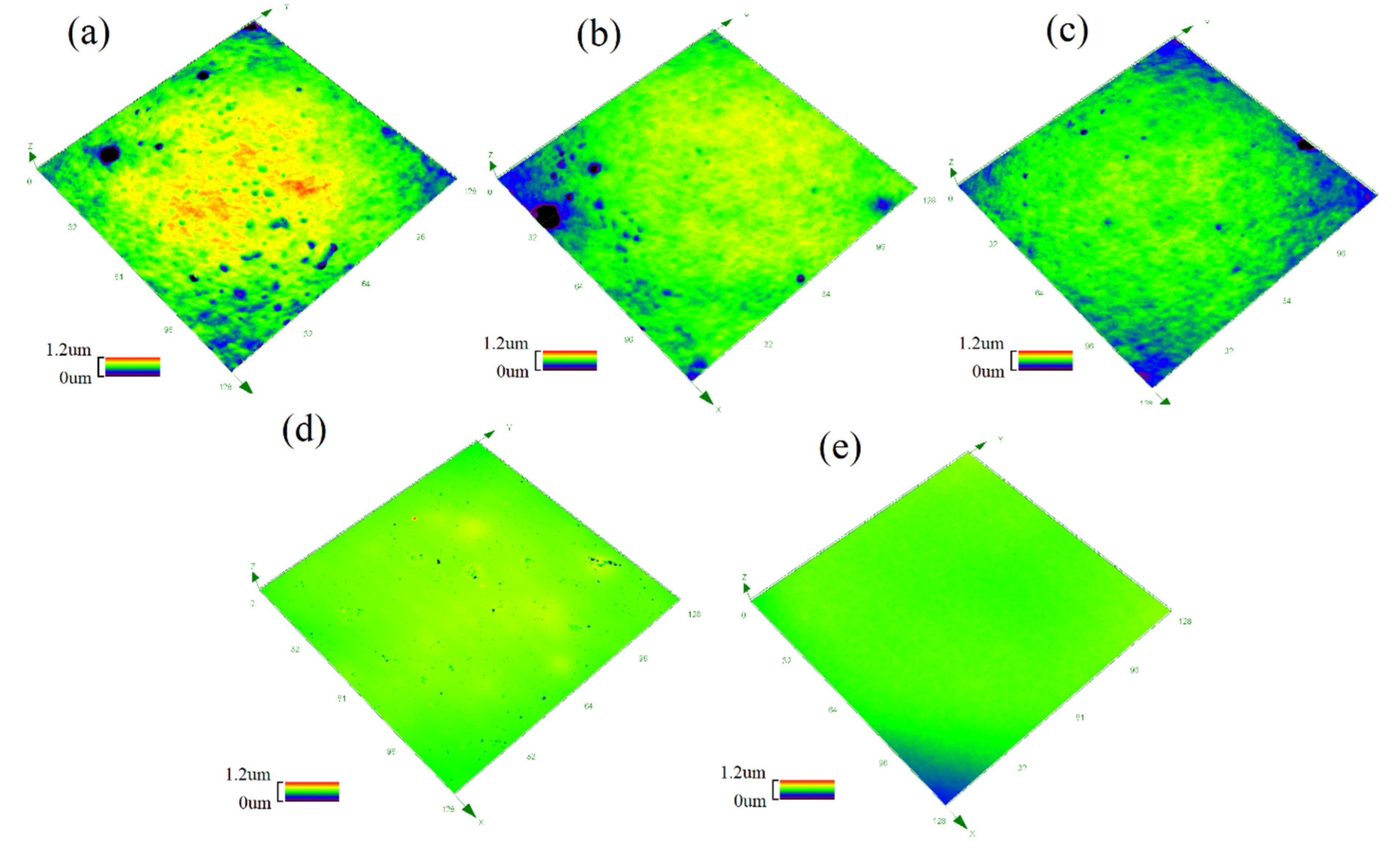
3.2. Surface Properties
3.3. Swelling Stability in Seawater
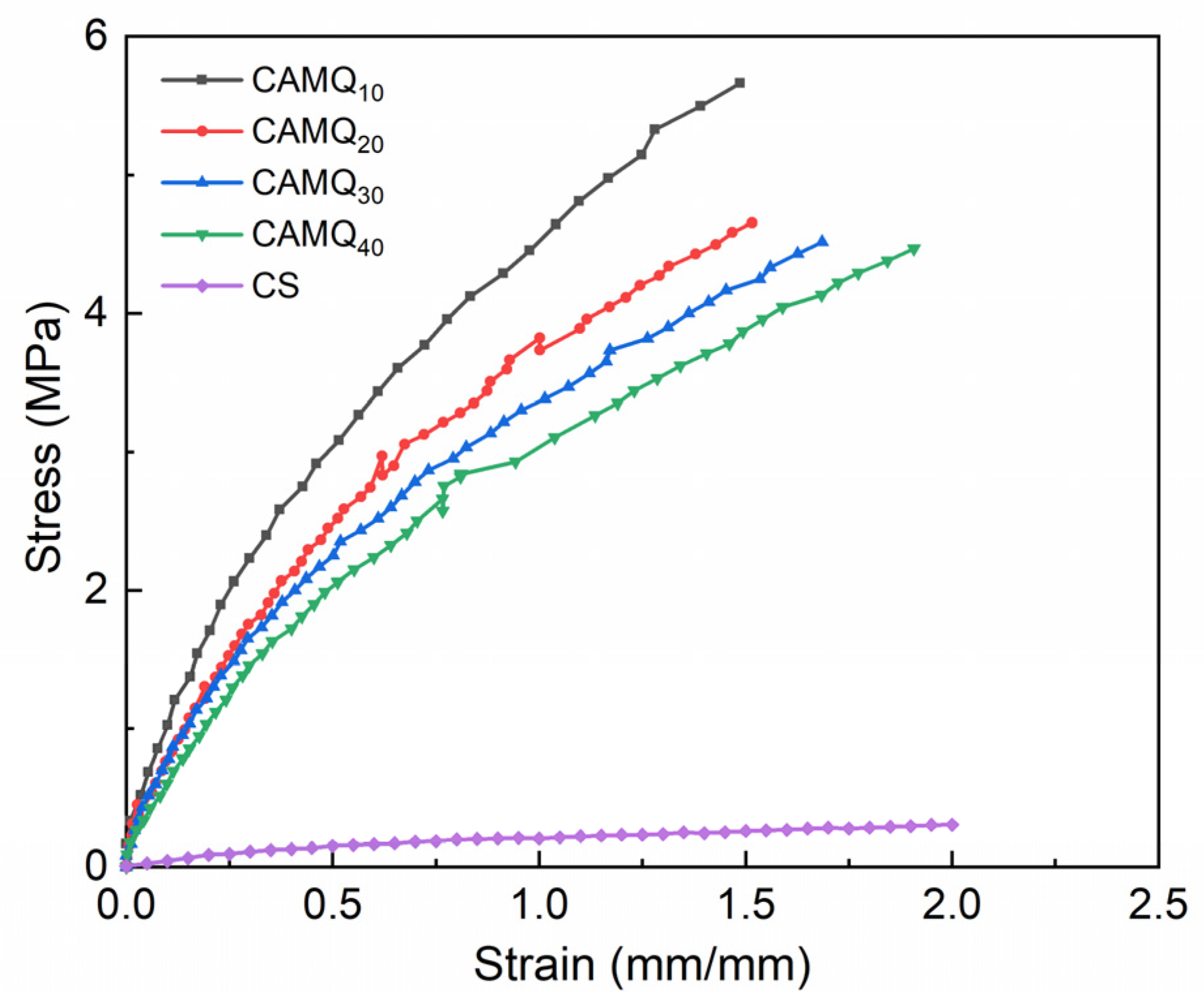
3.4. Mechanical Properties
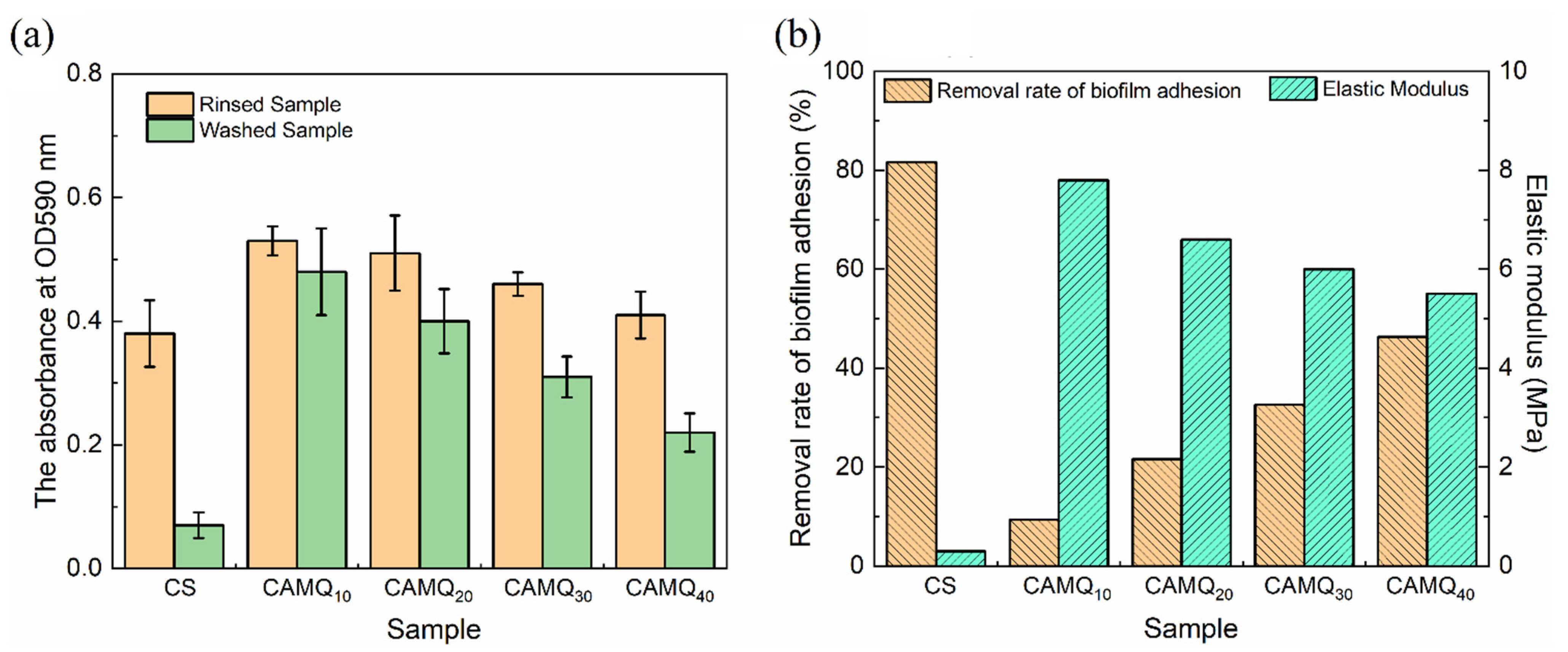
3.5. Pull-Off Strength Analysis
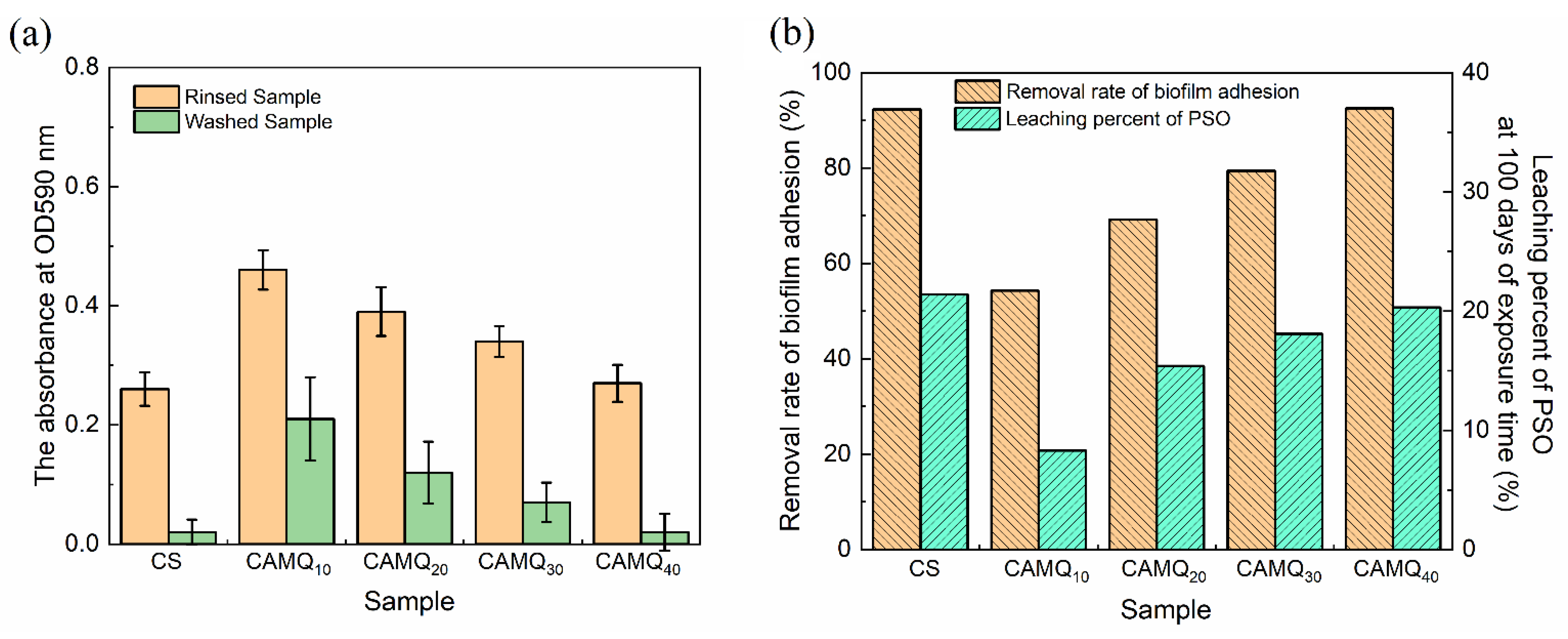
3.6. Leaching Behavior of PSO
3.7. Biofilm Adhesion Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, M.S.; Sun, Y.H.; Chen, G.M.; Wang, G.Y.; Lin, S.Z.; Sun, Z.Y. Preparation of a self-healing silicone coating for inhibiting adhesion of benthic diatoms. Mater. Lett. 2020, 268, 4. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Ware, C.S.; Smith-Palmer, T.; Peppou-Chapman, S.; Scarratt, L.R.J.; Humphries, E.M.; Balzer, D.; Neto, C. Marine Antifouling Behavior of Lubricant-Infused Nanowrinkled Polymeric Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 4173–4182. [Google Scholar] [CrossRef]
- Rasulev, B.; Jabeen, F.; Stafslien, S.; Chisholm, B.J.; Bahr, J.; Ossowski, M.; Boudjouk, P. Polymer Coating Materials and Their Fouling Release Activity: A Cheminformatics Approach to Predict Properties. ACS Appl. Mater. Interfaces 2017, 9, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Hu, P.; Xie, Q.Y.; Ma, C.F.; Zhang, G.Z. Silicone-Based Fouling-Release Coatings for Marine Antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef] [Green Version]
- Guazzelli, E.; Perondi, F.; Criscitiello, F.; Pretti, C.; Oliva, M.; Casu, V.; Maniero, F.; Gazzera, L.; Galli, G.; Martinelli, E. New amphiphilic copolymers for PDMS-based nanocomposite films with long-term marine antifouling performance. J. Mater. Chem. B 2020, 8, 9764–9776. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.Y.; Pan, J.S.; Ma, C.F.; Zhang, G.Z. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [Green Version]
- Azemar, F.; Fay, F.; Rehel, K.; Linossier, I. Ecofriendly silicon-poly(lactic acid) hybrid antifouling coatings. Prog. Org. Coat. 2020, 148, 105841. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.K.; Ober, C.K. Polymer-Based Marine Antifouling and Fouling Release Surfaces: Strategies for Synthesis and Modification. In Annual Review of Chemical and Biomolecular Engineering; Prausnitz, J.M., Ed.; Annual Reviews; Palo Alto: Santa Clara, CA, USA, 2019; Volume 10, pp. 241–264. [Google Scholar]
- Zhang, Z.-P.; Song, X.-F.; Cui, L.-Y.; Qi, Y.-H. Synthesis of Polydimethylsiloxane-Modified Polyurethane and the Structure and Properties of Its Antifouling Coatings. Coatings 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Silva, J.; Pedrosa, R. The marine invasive seaweeds Asparagopsis armata and Sargassum muticum as targets for greener antifouling solutions. Sci. Total. Environ. 2021, 750, 141372. [Google Scholar] [CrossRef]
- Leigh, B.L.; Cheng, E.; Xu, L.J.; Derk, A.; Hansen, M.R.; Guymon, C.A. Antifouling photograftable zwitterionic coatings on PDMS substrates. Langmuir 2019, 35, 1100–1110. [Google Scholar] [CrossRef]
- Selim, M.S.; Elmarakbi, A.; Azzam, A.M.; Shenashen, M.A.; El-Saeed, A.M.; El-Safty, S.A. Eco-friendly design of superhydrophobic nano-magnetite/silicone composites for marine foul-release paints. Prog. Org. Coat. 2018, 116, 21–34. [Google Scholar] [CrossRef]
- Su, C.M. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef]
- Ren, J.; Han, P.; Wei, H.; Jia, L. Fouling-resistant behavior of silver nanoparticle-modified surfaces against the bioadhesion of microalgae. ACS Appl. Mater. Interfaces 2014, 6, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-X.; Zheng, Y.-M.; Ba, M.; Wang, Y.-F.; Kong, J.-J.; Liu, J.-H.; Wu, Q. Long time super-hydrophobic fouling release coating with the incorporation of lubricant. Prog. Org. Coat. 2021, 152, 106136. [Google Scholar] [CrossRef]
- Shi, S.; Li, B.; Qian, Y.; Mei, P.; Wang, N. A simple and universal strategy to construct robust and anti-biofouling amidoxime aerogels for enhanced uranium extraction from seawater. Chem. Eng. J. 2020, 397, 125337. [Google Scholar] [CrossRef]
- Galhenage, T.P.; Webster, D.C.; Moreira, A.M.S.; Burgett, R.J.; Stafslien, S.J.; Vanderwal, L.; Finlay, J.A.; Franco, S.C.; Clare, A.S. Poly(ethylene) glycol-modified, amphiphilic, siloxane-polyurethane coatings and their performance as fouling-release surfaces. J. Coat. Technol. Res. 2017, 14, 307–322. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; El-Sockary, M.A.; Hashem, A.I.; Elenien, O.M.A.; El-Saeed, A.M.; Fatthallah, N.A. Smart photo-induced silicone/TiO2 nanocomposites with dominant 110 exposed surfaces for self-cleaning foul-release coatings of ship hulls. Mater. Des. 2016, 101, 218–225. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Q.; Ma, C.; Zhang, G. Fouling Release Property of Polydimethylsiloxane-Based Polyurea with Improved Adhesion to Substrate. Ind. Eng. Chem. Res. 2016, 55, 6671–6676. [Google Scholar] [CrossRef]
- Galhenage, T.P.; Hoffman, D.; Silbert, S.D.; Stafslien, S.J.; Daniels, J.; Miljkovic, T.; Finlay, J.A.; Franco, S.C.; Clare, A.S.; Nedved, B.T.; et al. Fouling-Release Performance of Silicone Oil-Modified Siloxane-Polyurethane Coatings. ACS Appl. Mater. Interfaces 2016, 8, 29025–29036. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.; Zhang, Z.-P.; Qi, Y.-H. The leaching behavior of phenylmethylsilicone oil and antifouling performance in nano-zinc oxide reinforced phenylmethylsilicone oil-Polydimethylsiloxane blend coating. Prog. Org. Coat. 2018, 125, 167–176. [Google Scholar] [CrossRef]
- Ba, M.; Zhang, Z.; Qi, Y. Fouling Release Coatings Based on Polydimethylsiloxane with the Incorporation of Phenylmethylsilicone Oil. Coatings 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Selim, M.S.; Shenashen, M.A.; Elmarakbi, A.; El-Saeed, A.M.; Selim, M.M.; El-Safty, S.A. Sunflower oil-based hyperbranched alkyd/spherical ZnO nanocomposite modeling for mechanical and anticorrosive applications. RSC Adv. 2017, 7, 21796–21808. [Google Scholar] [CrossRef] [Green Version]
- Shivapooja, P.; Cao, C.; Orihuela, B.; Levering, V.; Zhao, X.; Rittschof, D.; Lopez, G.P. Incorporation of silicone oil into elastomers enhances barnacle detachment by active surface strain. Biofouling 2016, 32, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Fernández Estarlich, F.M.; Eaton, P.J.; Fletcher, R.L.; Lewey, S.A.; Nevell, T.G.; Smith, J.R.; Tsibouklis, J. The Effects of Incorporated Silicone Oils and Calcium Carbonate on the Resistance to Settlement and the Antifouling Performance of a Silicone Elastomer. J. Adhes. Sci. Technol. 2012, 25, 2183–2198. [Google Scholar] [CrossRef]
- Nendza, M. Hazard assessment of silicone oils (polydimethylsiloxanes, PDMS) used in antifouling-/foul-release-products in the marine environment. Mar. Pollut. Bull. 2007, 54, 1190–1196. [Google Scholar] [CrossRef]
- Liang, W.J.; Ge, X.; Ge, J.F.; Li, T.H.; Zhao, T.K.; Chen, X.J.; Song, Y.Z.; Cui, Y.D.; Khan, M.; Ji, J.Y.; et al. Reduced Graphene Oxide Embedded with MQ Silicone Resin Nano-Aggregates for Silicone Rubber Composites with Enhanced Thermal Conductivity and Mechanical Performance. Polymers 2018, 10, 1254. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymer. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Baier, R.E. Surface behaviour of biomaterials: The theta surface for biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef]
- Brady, R.F.; Singer, I.L. Mechanical factors favoring release from fouling release coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.; Zhang, Z.P.; Qi, Y.H. The influence of MWCNTs-OH on the properties of the fouling release coatings based on polydimethylsiloxane with the incorporation of phenylmethylsilicone oil. Prog. Org. Coat. 2019, 130, 132–143. [Google Scholar] [CrossRef]




| Chemicals and Reagents | AMQ10 | AMQ20 | AMQ30 | AMQ40 |
|---|---|---|---|---|
| Methyl methacrylate (g) | 60 | 50 | 40 | 20 |
| Butyl methacrylate (g) | 30 | 30 | 30 | 30 |
| MVMQ (g) | 10 | 20 | 30 | 40 |
| AIBN (g) | 1 | 1 | 1 | 1 |
| Mixed solvents (mL) | 100 | 100 | 120 | 140 |
| Sample | Static Contact Angles (°) | Surface Free Energy (mJ/m2) | Surface Roughness (Sa, μm) | |
|---|---|---|---|---|
| Water | Diiodomethane | |||
| CAMQ10 | 100.7 ± 0.33 | 60.1 ± 0.70 | 28.6 ± 0.56 | 0.137 |
| CAMQ20 | 103.8 ± 0.27 | 63.1 ± 0.63 | 26.9 ± 0.58 | 0.119 |
| CAMQ30 | 105.5 ± 0.71 | 64.8 ± 0.54 | 26.0 ± 0.77 | 0.057 |
| CAMQ40 | 107.8 ± 0.64 | 67.9 ± 0.38 | 24.3 ± 0.67 | 0.027 |
| CS | 109.8 ± 0.55 | 70.3 ± 0.52 | 22.9 ± 0.60 | 0.016 |
| Scheme 100 | Elastic Modulus (MPa) | Tensile Stress at 100% (MPa) | Breaking Elongation (%) | Shore Hardness (HA) | Pencil Hardness |
|---|---|---|---|---|---|
| CAMQ10 | 7.8 ± 1.01 | 4.45 | 148 | 60.7 ± 1.27 | 5H |
| CAMQ20 | 6.6 ± 0.77 | 3.82 | 151 | 54.5 ± 3.32 | 4H |
| CAMQ30 | 6.0 ± 0.69 | 3.38 | 168 | 48.8 ± 4.01 | 4H |
| CAMQ40 | 5.5 ± 1.22 | 3.05 | 191 | 41.1 ± 2.17 | 4H |
| CS | 0.3 ± 0.04 | 0.21 | 200 | 12.5 ± 0.55 | 5B |
| Sample | CS | CAMQ10 | CAMQ20 | CAMQ30 | CAMQ40 |
|---|---|---|---|---|---|
| (day) | 0.4 | 3.1 | 1.6 | 1.1 | 0.8 |
| (%) | 21.4 | 8.3 | 15.4 | 18.1 | 20.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zheng, Y.; Li, M.; Ba, M.; Wang, Y. Fouling Release Coatings Based on Acrylate–MQ Silicone Copolymers Incorporated with Non-Reactive Phenylmethylsilicone Oil. Polymers 2021, 13, 3156. https://doi.org/10.3390/polym13183156
Zhou H, Zheng Y, Li M, Ba M, Wang Y. Fouling Release Coatings Based on Acrylate–MQ Silicone Copolymers Incorporated with Non-Reactive Phenylmethylsilicone Oil. Polymers. 2021; 13(18):3156. https://doi.org/10.3390/polym13183156
Chicago/Turabian StyleZhou, Hongwei, Yiming Zheng, Mengyu Li, Miao Ba, and Yufeng Wang. 2021. "Fouling Release Coatings Based on Acrylate–MQ Silicone Copolymers Incorporated with Non-Reactive Phenylmethylsilicone Oil" Polymers 13, no. 18: 3156. https://doi.org/10.3390/polym13183156
APA StyleZhou, H., Zheng, Y., Li, M., Ba, M., & Wang, Y. (2021). Fouling Release Coatings Based on Acrylate–MQ Silicone Copolymers Incorporated with Non-Reactive Phenylmethylsilicone Oil. Polymers, 13(18), 3156. https://doi.org/10.3390/polym13183156