Study on the Influence of Nano-OvPOSS on the Compatibility, Molecular Structure, and Properties of SBS Modified Asphalt by Molecular Dynamics Simulation
Abstract
1. Introduction
2. Simulation Models
2.1. Molecular Model of Matrix Asphalt
2.2. Molecular Model of Nano-OvPOSS
2.3. Molecular Model of Linear SBS
2.4. Molecular Model of Nano-OvPOSS/SBS Modified Asphalt
3. MD Simulation Theory and Method
3.1. Simulation Task
3.1.1. Mixing Energy
3.1.2. Rg
3.1.3. RDF
3.1.4. RFV
3.2. Simulation Method
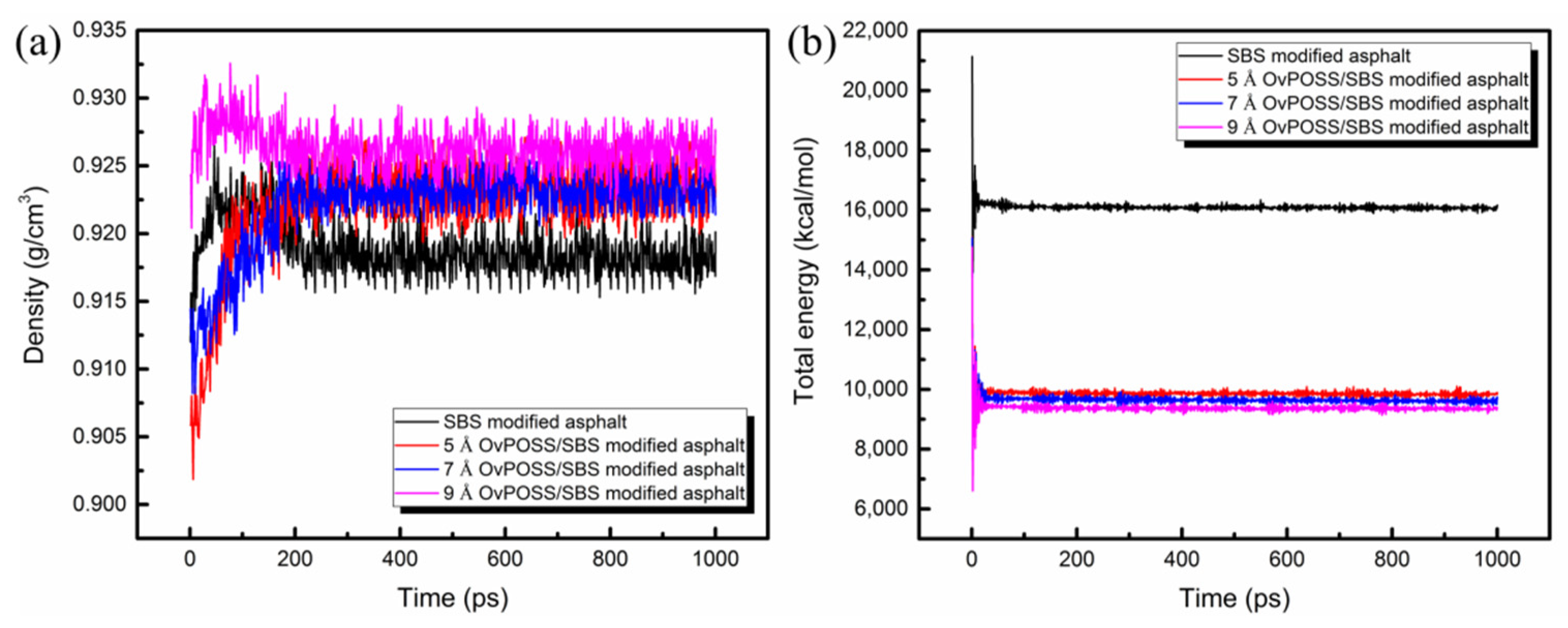
3.3. Model Validation
4. Results and Discussion
4.1. Compatibility Analysis
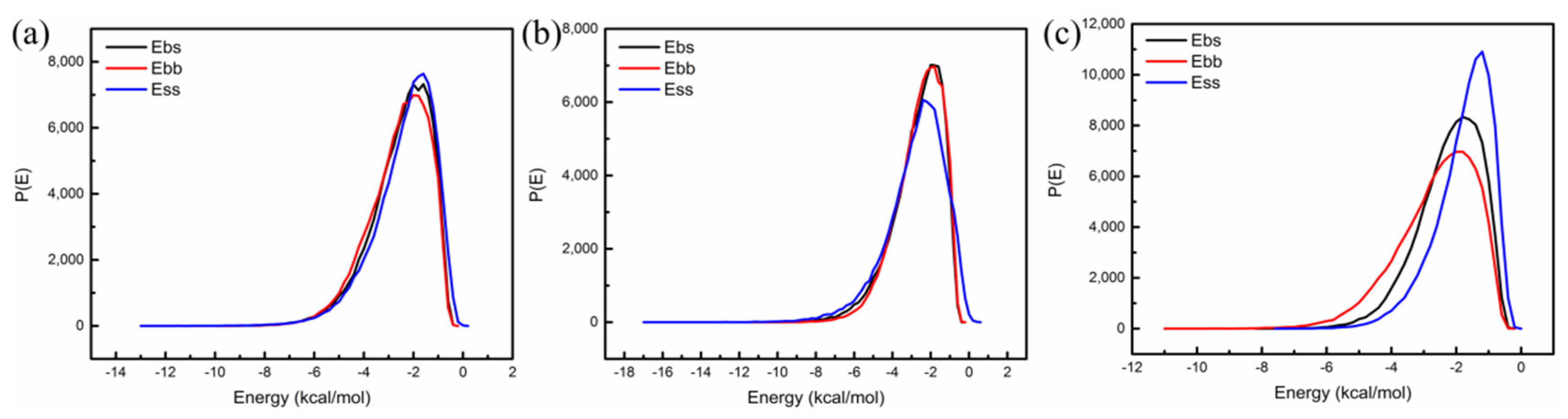
4.1.1. The Compatibility of Nano-OvPOSS with SBS
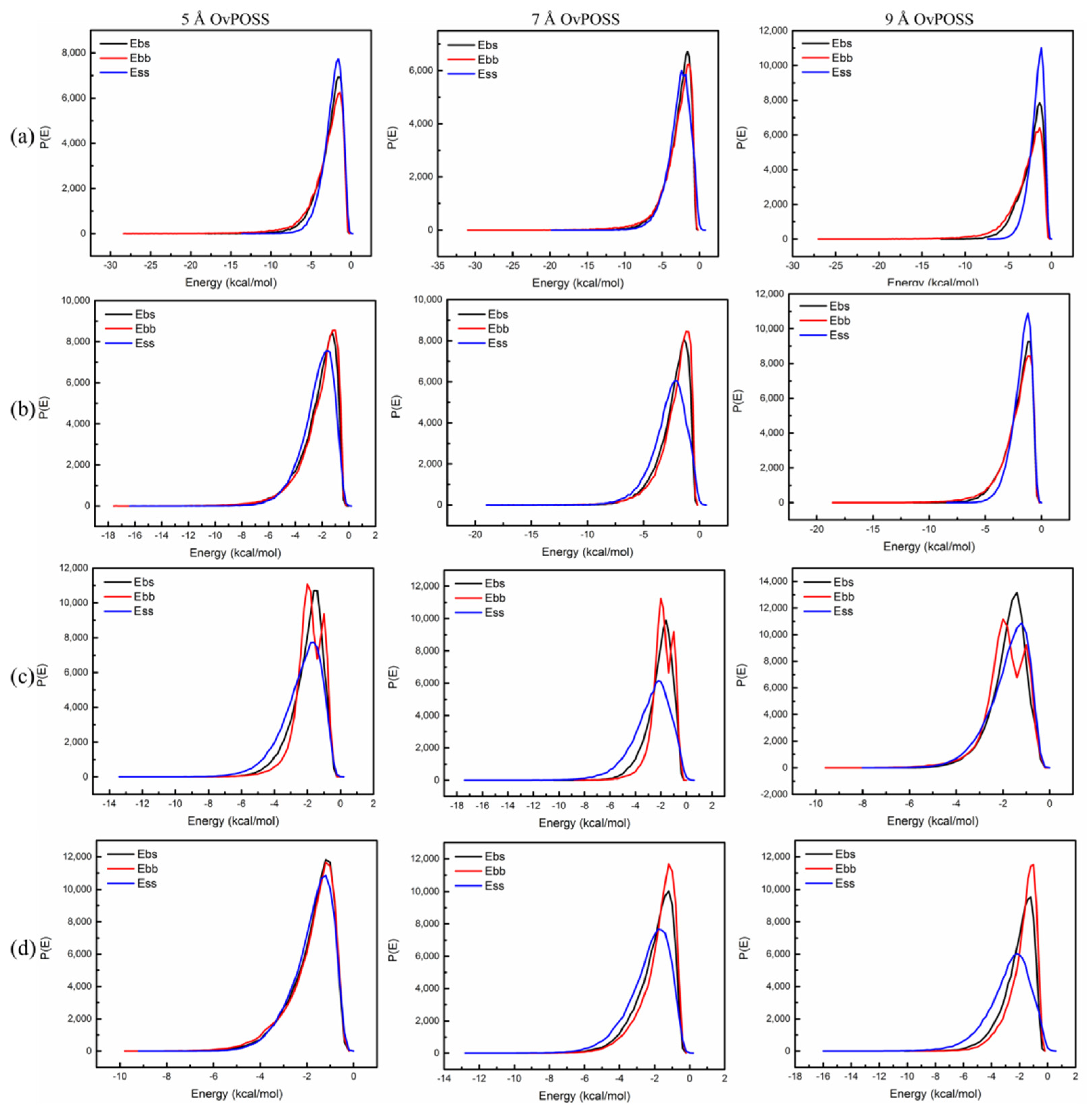
4.1.2. The Compatibility of Nano-OvPOSS with Four Asphalt Components
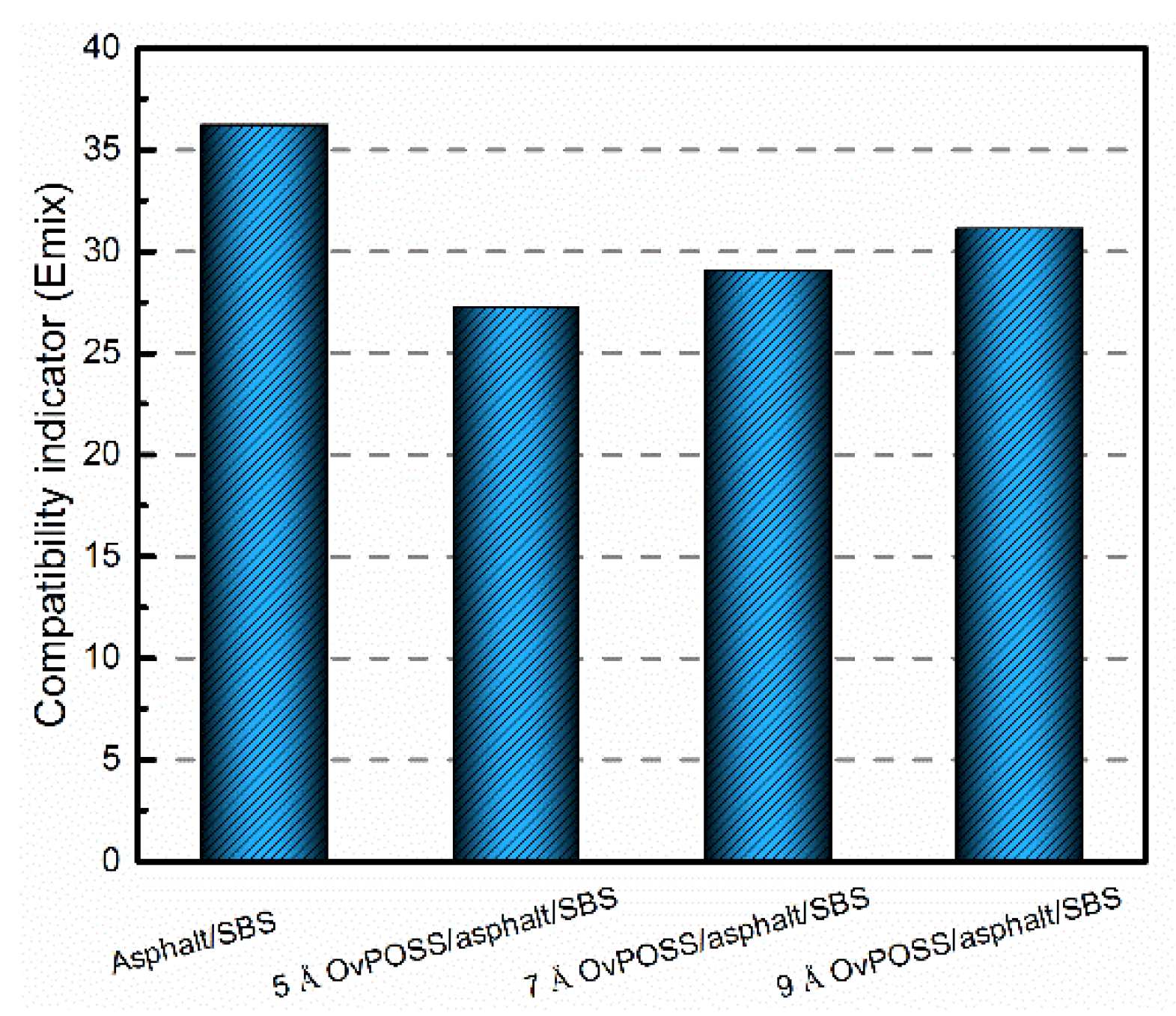
4.1.3. The Effect of Nano-OvPOSS on the Compatibility of SBS Modified Asphalt
4.2. Influence of Nano-OvPOSS on the Structure of SBS Modified Asphalt
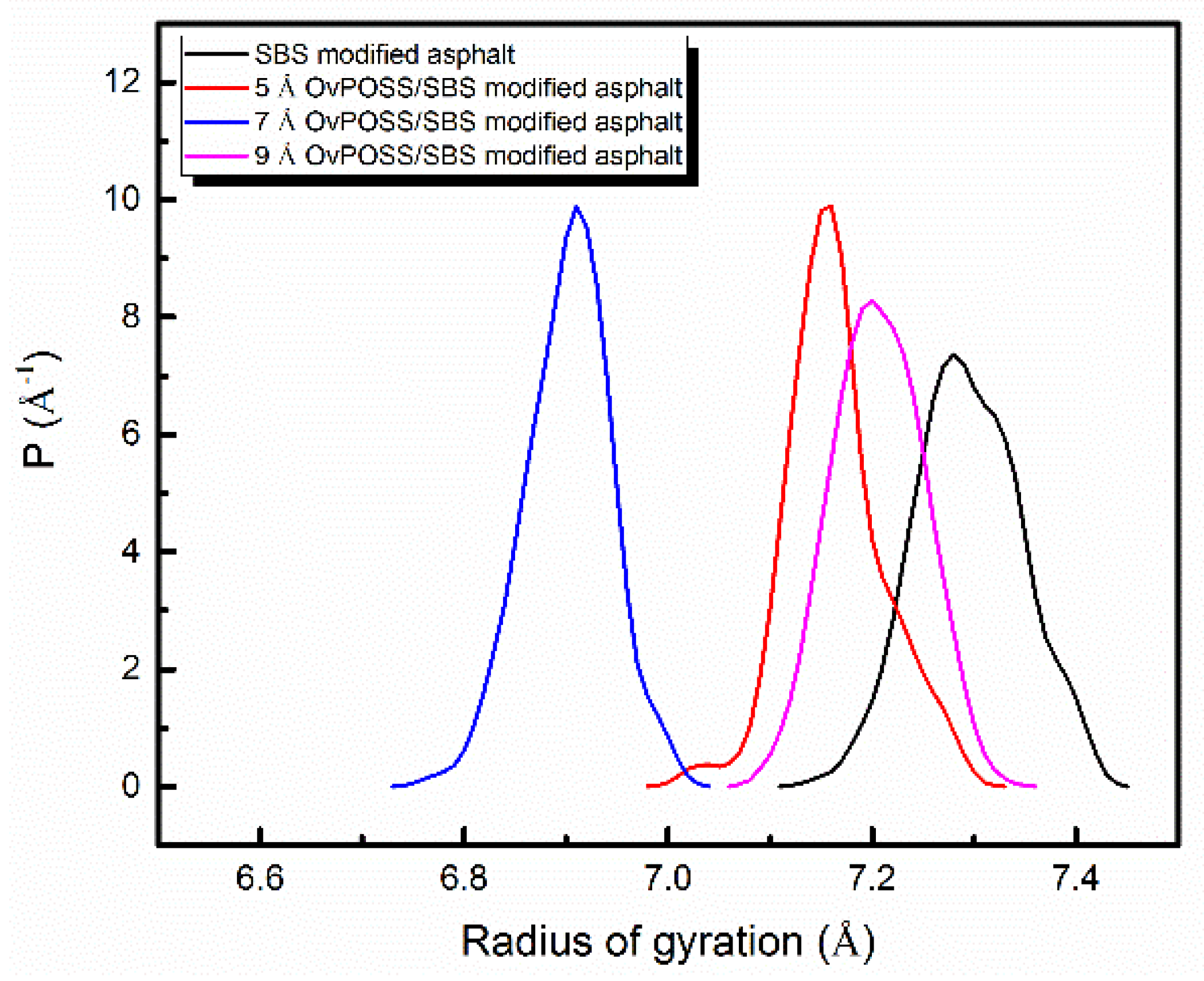
4.2.1. Influence of Nano-OvPOSS on Rg of SBS
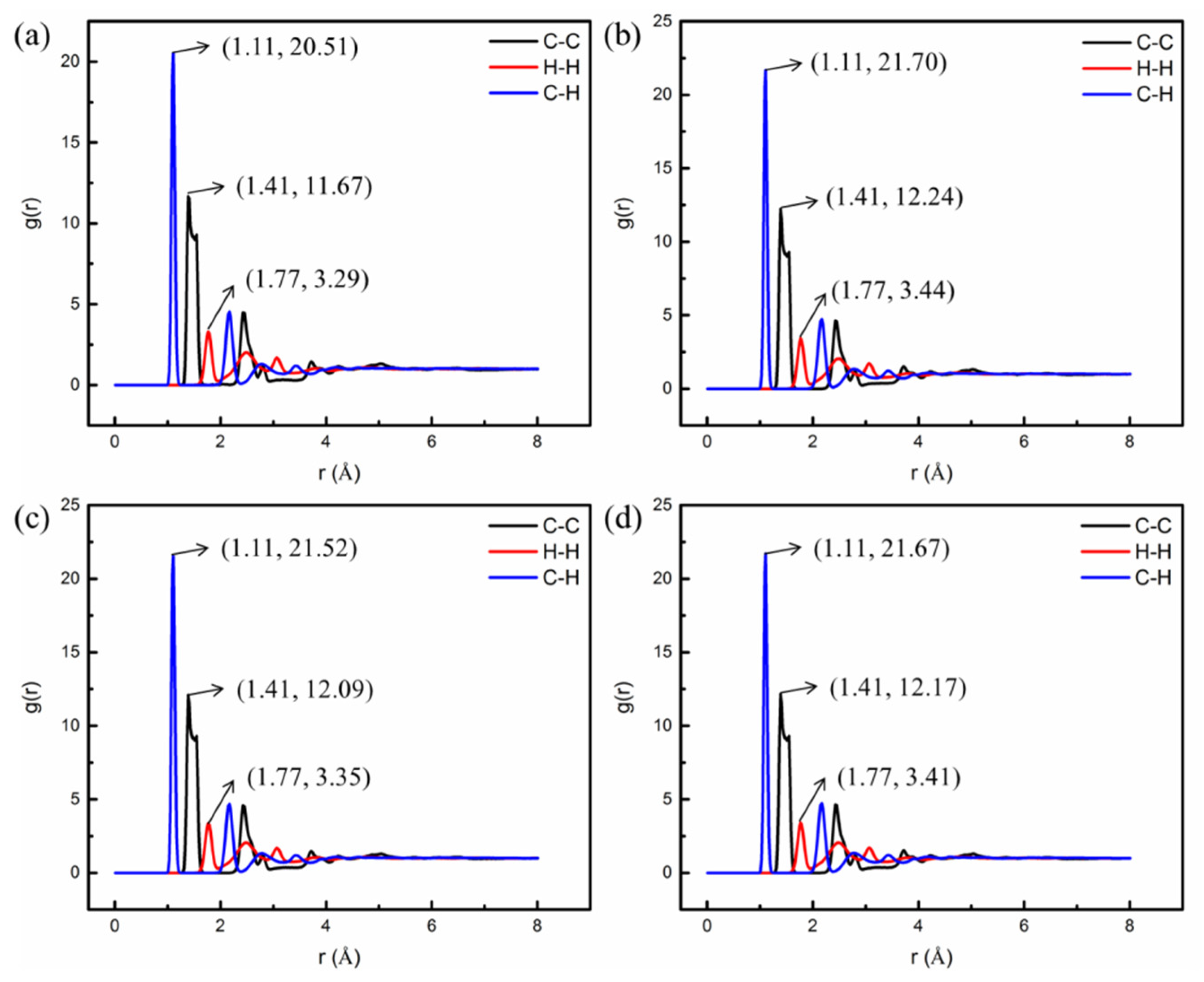
4.2.2. Influence of Nano-OvPOSS on RDF of SBS Modified Asphalt
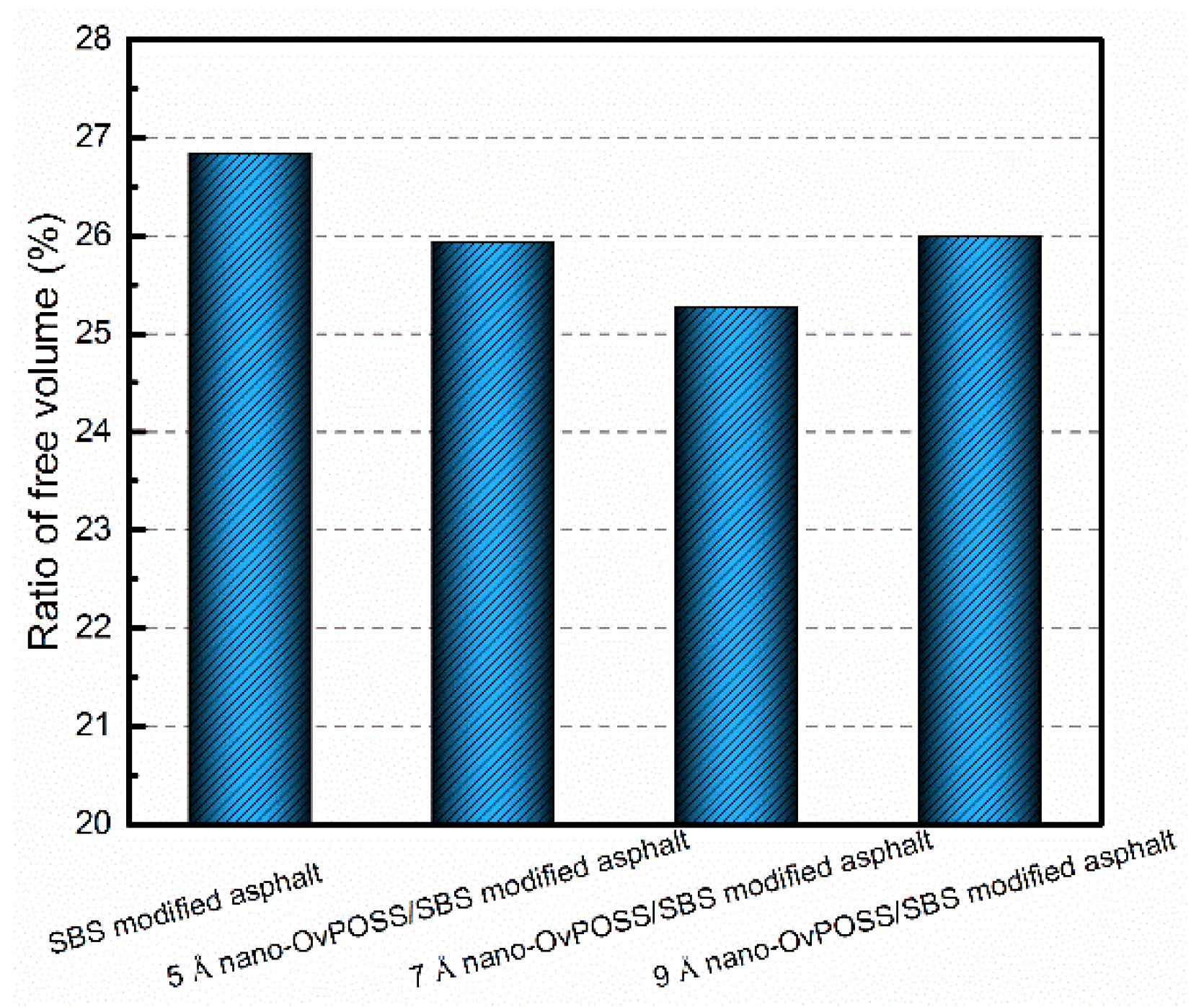
4.2.3. Influence of Nano-OvPOSS on RFV of SBS Modified Asphalt
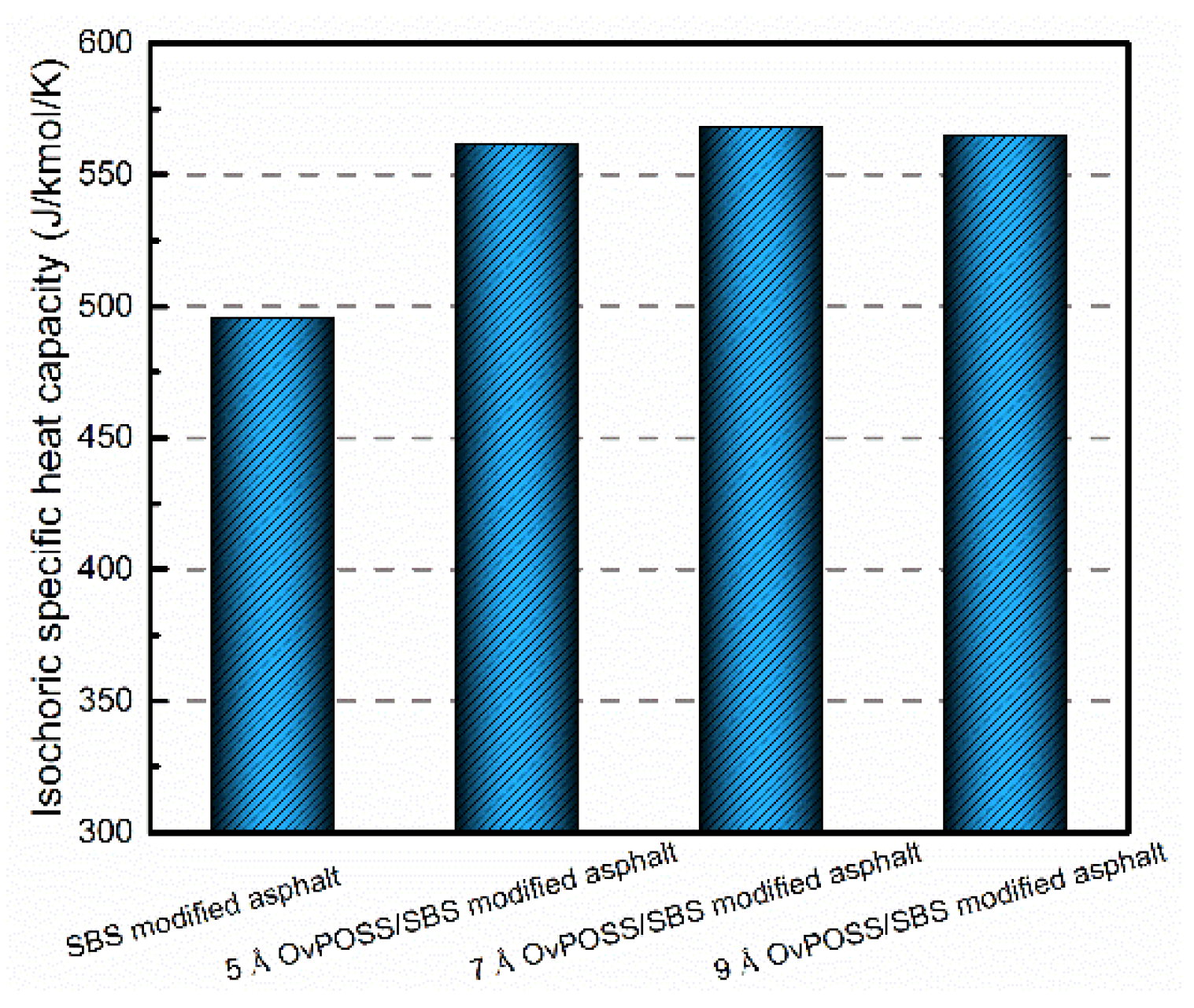
4.3. The Effect of Nano-OvPOSS on the Temperature Stability of SBS Modified Asphalt
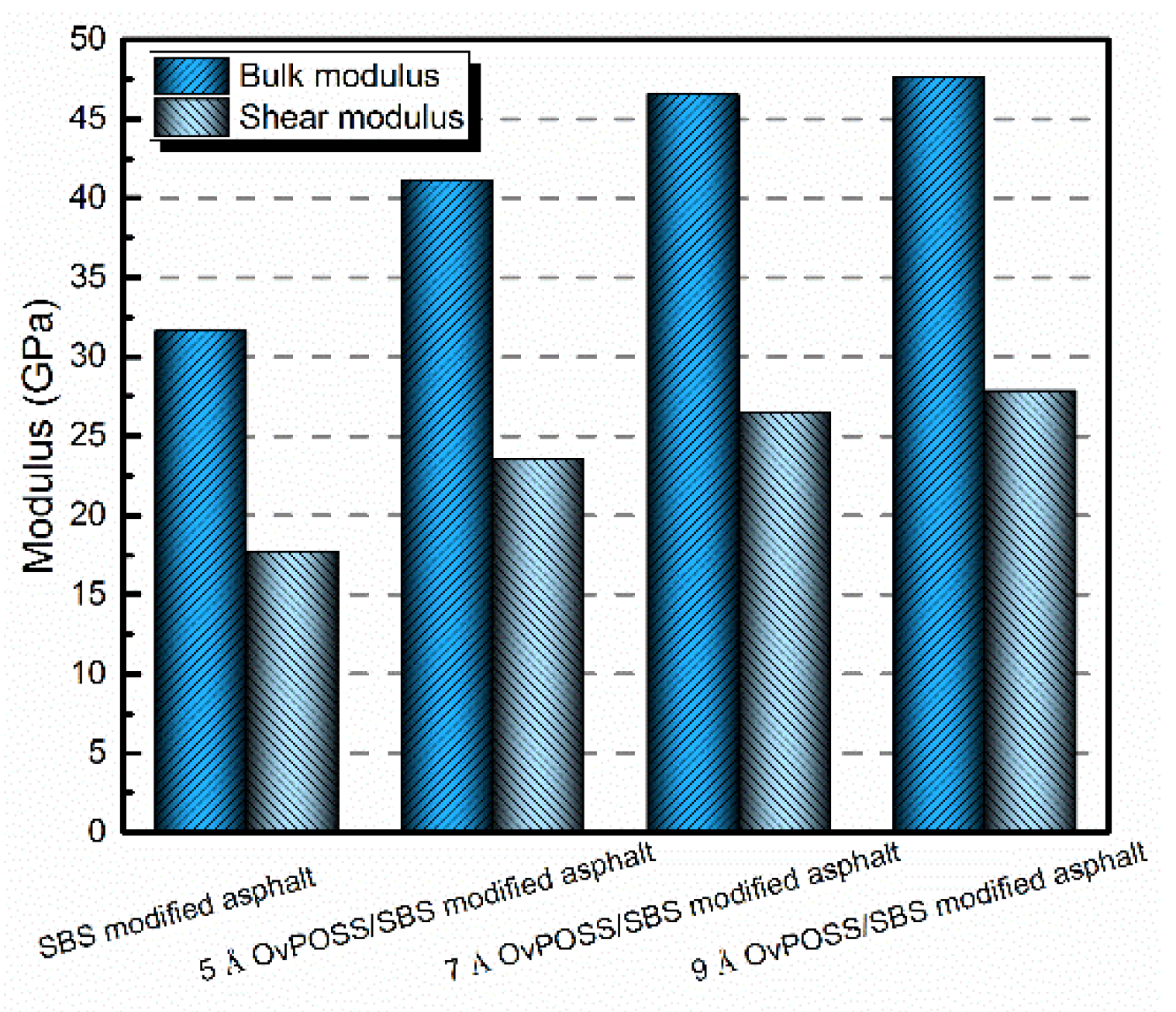
4.4. The Effect of Nano-OvPOSS on the Mechanical Properties of SBS Modified Asphalt
5. Conclusions
- Nano-OvPOSS is compatible with SBS as well as with four asphalt components, and is also able to improve the compatibility between SBS and asphalt. This is because the eight ethylene organic branches of nano-OvPOSS are capable of interacting with the active groups of polymers. Furthermore, 5 Å nano-OvPOSS has better ability to improve the compatibility between SBS and asphalt than 7 Å nano-OvPOSS and 9 Å nano-OvPOSS since it has the larger contact range with polymer molecules.
- The addition of nano-OvPOSS enhances the inter atomic bonding strength of asphalt component molecules. Meanwhile, it can reinforce the tractility of branched chains of SBS and make SBS easier to wrap and adsorb the surrounding asphalt molecules. Moreover, the free movement space of molecules in the SBS modified asphalt system declines. All of these effects result in a more stable colloidal structure of asphalt.
- The improvement of the Cv of SBS modified asphalt suggests that nano-OvPOSS/SBS modified asphalt system has better temperature stability. The reason why the addition of nano-OvPOSS is able to alleviate the thermodynamic instability of SBS modified asphalt is that the inorganic body of nano-OvPOSS is capable of absorbing more heat.
- The nano-OvPOSS modifier has a positive effect on bulk modulus and shear modulus of SBS modified asphalt, which renders better mechanical properties and deformation resistance of SBS modified asphalt in asphalt pavement.
- The overall results of the study prove the feasibility of nano-OvPOSS as an ideal asphalt modifier to attain a well-rounded performance of conventional SBS modified asphalt. As a functional material, POSS is capable of changing its eight substituents to realize more functions, one of which is nano-OvPOSS. Hence, the complexity and diversity of the structures of POSS allow for quite a few possibilities of its application in the field of modified asphalt. It is earnestly anticipated that more and more modifiers taking the advantage of POSS can be explicitly explored by many a researcher in the near future.
Author Contributions
Funding
Conflicts of Interest
References
- Yildirim, Y. Polymer modified asphalt binders. Constr. Build. Mater. 2007, 21, 66–72. [Google Scholar] [CrossRef]
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef]
- Yut, I.; Zofka, A. Correlation between rheology and chemical composition of aged polymer-modified asphalts. Constr. Build. Mater. 2014, 62, 109–117. [Google Scholar] [CrossRef]
- Airey, G.D.; Singleton, T.M.; Collop, A.C. Properties of polymer modified bitumen after rubber-bitumen interaction. J. Mater. Civ. Eng. 2002, 14, 344–354. [Google Scholar] [CrossRef]
- Sengoz, B.; Isikyakar, G. Analysis of styrene-butadiene-styrene polymer modified bitumen using fluorescent microscopy and conventional test methods. J. Hazard. Mater. 2008, 150, 424–432. [Google Scholar] [CrossRef]
- Ye, F.; Yin, W.; Lu, H. A model for the quantitative relationship between temperature and microstructure of styrene-butadiene-styrene modified asphalt. Constr. Build. Mater. 2015, 79, 397–401. [Google Scholar] [CrossRef]
- Liang, M.; Liang, P.; Fan, W.; Qian, C.; Xin, X.; Shi, J.; Nan, G. Thermo-rheological behavior and compatibility of modified asphalt with various styrene-butadiene structures in SBS copolymers. Mater. Des. 2015, 88, 177–185. [Google Scholar] [CrossRef]
- Cong, P.; Xu, P.; Chen, S. Effects of carbon black on the anti aging, rheological and conductive properties of SBS/asphalt/carbon black composites. Constr. Build. Mater. 2014, 52, 306–313. [Google Scholar] [CrossRef]
- Chen, J.; Liao, M.; Shiah, M.S. Asphalt modified by styrene-butadiene-styrene triblock copolymer: Morphology and model. J. Mater. Civ. Eng. 2002, 14, 224–229. [Google Scholar] [CrossRef]
- Qian, C.; Fan, W.; Ming, L.; Nan, G.; Luo, H. Influence of compatibilizer composition on performance of SBS modified asphalt. Mater. Sci. Energy Technol. Power Eng. II 2018, 1971, 050011. [Google Scholar]
- Ouyang, C.; Gao, Q.; Shi, Y.; Shan, X. Compatibilizer in waste tire powder and low-density polyethylene blends and the blends modified asphalt. J. Appl. Polym. Sci. 2011, 123, 485–492. [Google Scholar] [CrossRef]
- Lu, X.; Isacsson, U.; Ekblad, J. Phase separation of SBS polymer modified bitumens. J. Mater. Civ. Eng. 1999, 11, 51–57. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, C.; Deng, J. Crumb tire rubber and polyethylene mutually stabilized in asphalt by screw extrusion. J. Appl. Polym. Sci. 2014, 131, 81–86. [Google Scholar] [CrossRef]
- Sugano, M.; Kajita, J.; Ochiai, M.; Takagi, N.; Iwai, S.; Hirano, K. Mechanisms for chemical reactivity of two kinds of polymer modified asphalts during thermal degradation. Chem. Eng. J. 2011, 176, 231–236. [Google Scholar] [CrossRef]
- Li, J.; Jia, W.; Yuan, W. Effect of polyethylene grafted with maleic anhydride on asphalt properties. J. Perform. Constr. Facil. 2014, 28, 04014012. [Google Scholar] [CrossRef]
- Fang, C.; Yu, R.; Liu, S.; Li, Y. Nanomaterials applied in asphalt modification: A review. J. Mater. Sci. Technol. 2013, 29, 589–594. [Google Scholar] [CrossRef]
- Lu, H.; Ye, F.; Yuan, J.; Yin, W. Properties comparison and mechanism analysis of naphthenic oil/SBS and nano-MMT/SBS modified asphalt. Constr. Build. Mater. 2018, 187, 1147–1157. [Google Scholar] [CrossRef]
- Galooyak, S.S.; Dabir, B.; Nazarbeygi, A.E.; Moeini, A. Rheological properties and storage stability of bitumen/SBS/montmorillonite composites. Constr. Build. Mater. 2010, 24, 300–307. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Zeng, X.; Wu, S.; Li, B. Effect of montmorillonite on properties of styrene-butadiene-styrene copolymer modified bitumen. Polym. Eng. Sci. 2007, 47, 1289–1295. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Wu, S. Effect of montmorillonite organic modification on ultraviolet aging properties of SBS modified bitumen. Constr. Build. Mater. 2012, 27, 553–559. [Google Scholar] [CrossRef]
- Ouyang, C.; Wang, S.; Zhang, Y.; Zhang, Y. Preparation and properties of styrene-butadiene-styrene copolymer/Kaolinite clay compound and asphalt modified with the compound. Polym. Degrad. Stab. 2005, 87, 309–317. [Google Scholar] [CrossRef]
- Rezaei, S.; Ziari, H.; Nowbakht, S. High-temperature functional analysis of bitumen modified with composite of nano-SiO2 and styrene butadiene styrene polymer. Pet. Sci. Technol. 2016, 34, 1195–1203. [Google Scholar] [CrossRef]
- Su, M.; Si, C.; Zhang, Z.; Zhang, H. Molecular dynamics study on influence of nano-ZnO/SBS on physical properties and molecular structure of asphalt binder. Fuel 2020, 263, 116777.1–116777.13. [Google Scholar] [CrossRef]
- Scott, D.W. Thermal rearrangement of branched-chain methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, L.; Zhang, Y.; Zhang, Y.; Yin, N. Isothermal crystallization kinetics of polypropylene/POSS composites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1762–1772. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zeng, Z.; Zhang, Y. Properties of POSS-filled polypropylene: Comparison of physical blending and reactive blending. J. Appl. Polym. Sci. 2008, 110, 3745–3751. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, L.; Zhang, Y.; Zhang, Y.; Yin, N. Preparation and properties of POSS grafted polypropylene by reactive blending. Eur. Polym. J. 2008, 44, 3057–3066. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, Y.; Yin, N. Rheological behavior of polypropylene/octavinyl polyhedral oligomeric silsesquioxane composites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 526–533. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, S.; Cardoen, G. Polymer nanocomposites through controlled self-assembly of cubic silsesquioxane scaffolds. Macromolecules 2004, 37, 8606–8611. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.S.; Mather, P.T. Vertex group effects in entangled polystyrene-polyhedral oligosilsesquioxane (POSS) copolymers. Macromolecules 2009, 42, 1142–1152. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: A review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Phillips, S.H.; Haddad, T.S.; Tomczak, S.J. Developments in nanoscience: Polyhedral oligomeric silsesquioxane (POSS)-polymers. Curr. Opin. Solid State Mater. Sci. 2004, 8, 21–29. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Li, G.; Pittman, C.U. Polyhedral oligomeric silsesquioxane (POSS) polymers, copolymers, and resin nanocomposites. In Macromolecules Containing Metal and Metal-like Elements: Group IVA Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 79–131. [Google Scholar]
- Hansen, J.S.; Lemarchand, C.A.; Nielsen, E.; Dyre, J.C.; Schroder, T. Four-component united-atom model of bitumen. J. Chem. Phys. 2013, 138, 94508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Su, M.M.; Zhao, S.F.; Zhang, Y.P.; Zhang, Z.P. High and low temperature properties of nano-particles/polymer modified asphalt. Constr. Build. Mater. 2016, 114, 323–332. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Rigby, D.; Sun, H.; Eichinger, B.E. Computer simulations of poly (ethylene oxide): Force field, pvt diagram and cyclization behaviour. Polym. Int. 1997, 44, 311–330. [Google Scholar] [CrossRef]
- Nose, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.P.; Postma, J.; Gunsteren, W.; Dinola, A.D.; Haak, J.R. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684. [Google Scholar] [CrossRef]
- Huggins, M.L. Principles of polymer chemistry. J. Am. Chem. Soc. 1954, 76, 2854. [Google Scholar] [CrossRef]
- Bawendi, M.G.; Freed, K.F. A lattice model for self- and mutually avoiding semiflexible polymer chains. J. Chem. Phys. 1987, 86, 3720–3730. [Google Scholar] [CrossRef]
- Fan, C.F.; Olafson, B.D.; Blanco, M.; Hsu, S.L. Application of molecular simulation to derive phase diagrams of binary mixtures. Macromolecules 1992, 25, 3667–3676. [Google Scholar] [CrossRef]
- Freed, K.F.; Bawendi, M.G. Lattice theories of polymeric fluids. J. Phys. Chem. 1989, 93, 2194–2203. [Google Scholar] [CrossRef]
- Pesci, A.I.; Freed, K.F. Lattice models of polymer fluids: Monomers occupying several lattice sites. II. Interaction energies. J. Chem. Phys. 1989, 90, 2003–2016. [Google Scholar] [CrossRef]
- Choi, W.S.; Lee, H. Nanostructured materials for water purification: Adsorption of heavy metal ions and organic dyes. Polymers 2022, 14, 2183. [Google Scholar] [CrossRef]
- Vu, D.; Ahn, K. Triboelectric enhancement of polyvinylidene fluoride membrane using magnetic nanoparticle for water-based energy harvesting. Polymers 2022, 14, 1547. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Quan, Y.; Ma, R.; Pentzer, E.B.; Green, M.J.; Wang, Q. Thermal stability and flammability studies of mxene-organic hybrid polystyrene nanocomposites. Polymers 2022, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Fanourakis, S.K.; Pea-Bahamonde, J.; Rodrigues, D.F. Inorganic salts and organic matter effects on nanorod, nanowire, and nanoplate MoO3 aggregation, dissolution, and photocatalysis. Environ. Sci. Nano 2020, 7, 3794–3804. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Wei, C.; Duan, H.; Yu, J. Application of functionalized nanomaterials in asphalt road construction materials. In Handbook of Functionalized Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 865–907. [Google Scholar]
- Zhang, H.; Zhu, C.; Yan, K.; Yu, J. Effect of rectorite and its organic modification on properties of bitumen. J. Mater. Civ. Eng. 2015, 27, C4014002.1–C4014002.5. [Google Scholar] [CrossRef]
- Chen, A.; Zhao, N. Comparative study of the crowding-induced collapse effect in hard-sphere, flexible polymer and rod-like polymer systems. Phys. Chem. Chem. Phys. 2019, 21, 12335–12345. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L.; Ling, H.C. Free-volume theories for self-diffusion in polymer-solvent systems. I. Conceptual differences in theories. J. Polym. Sci. Part B Polym. Phys. Ed. 1985, 23, 275–288. [Google Scholar] [CrossRef]
- Rad, F.Y.; Sefidmazgi, N.R.; Bahia, H. Application of diffusion mechanism. Transp. Res. Rec. J. Transp. Res. Board 2014, 2444, 71–77. [Google Scholar] [CrossRef]
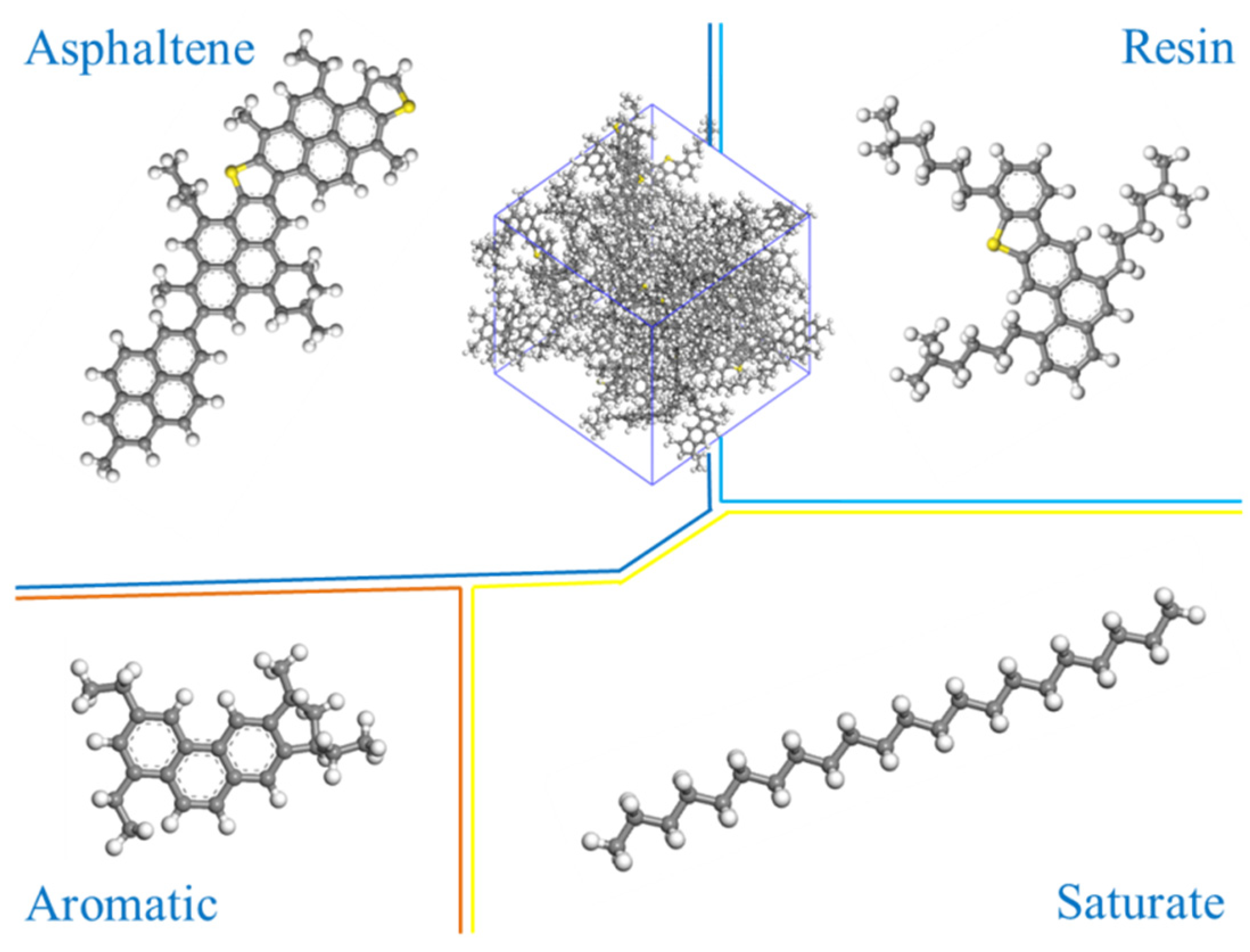

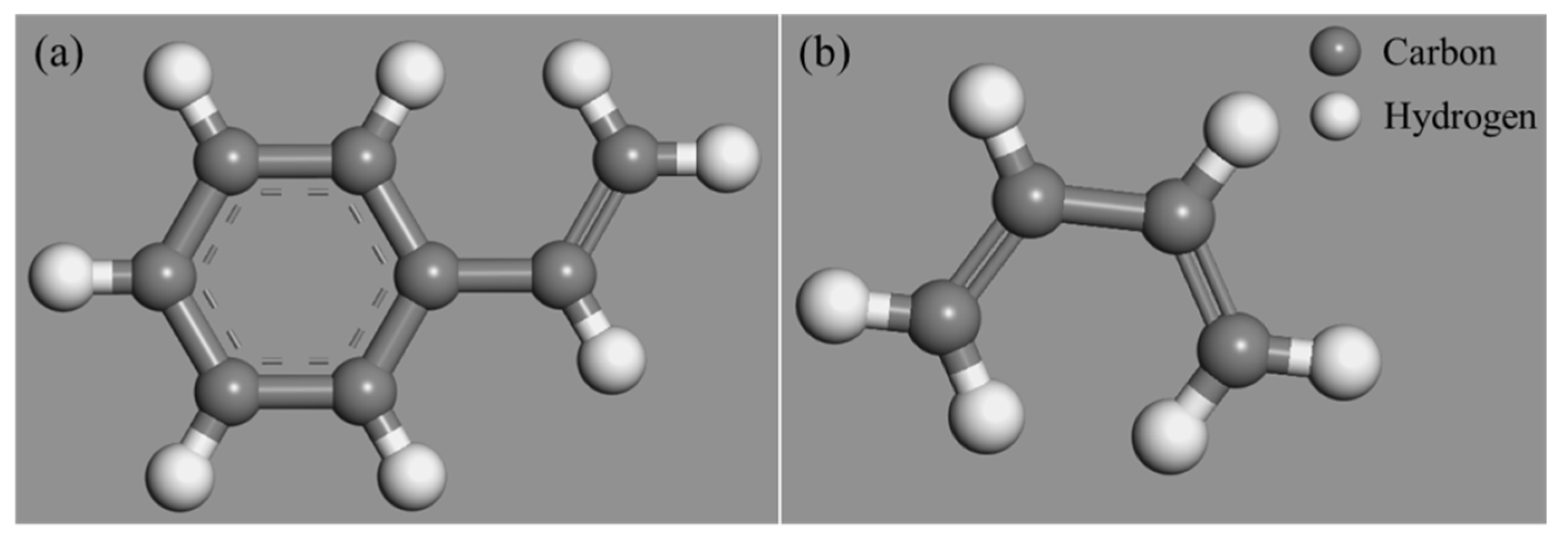
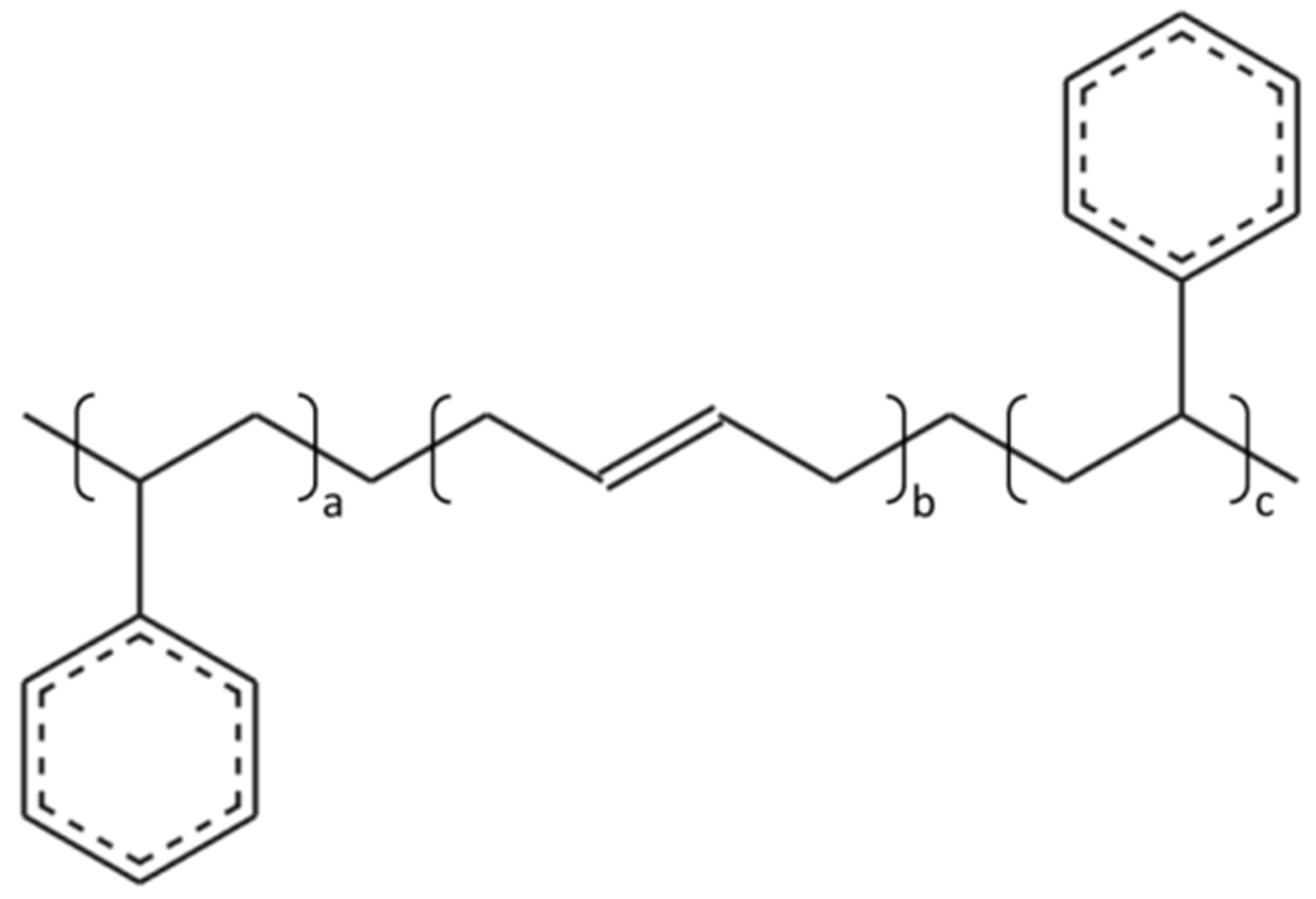

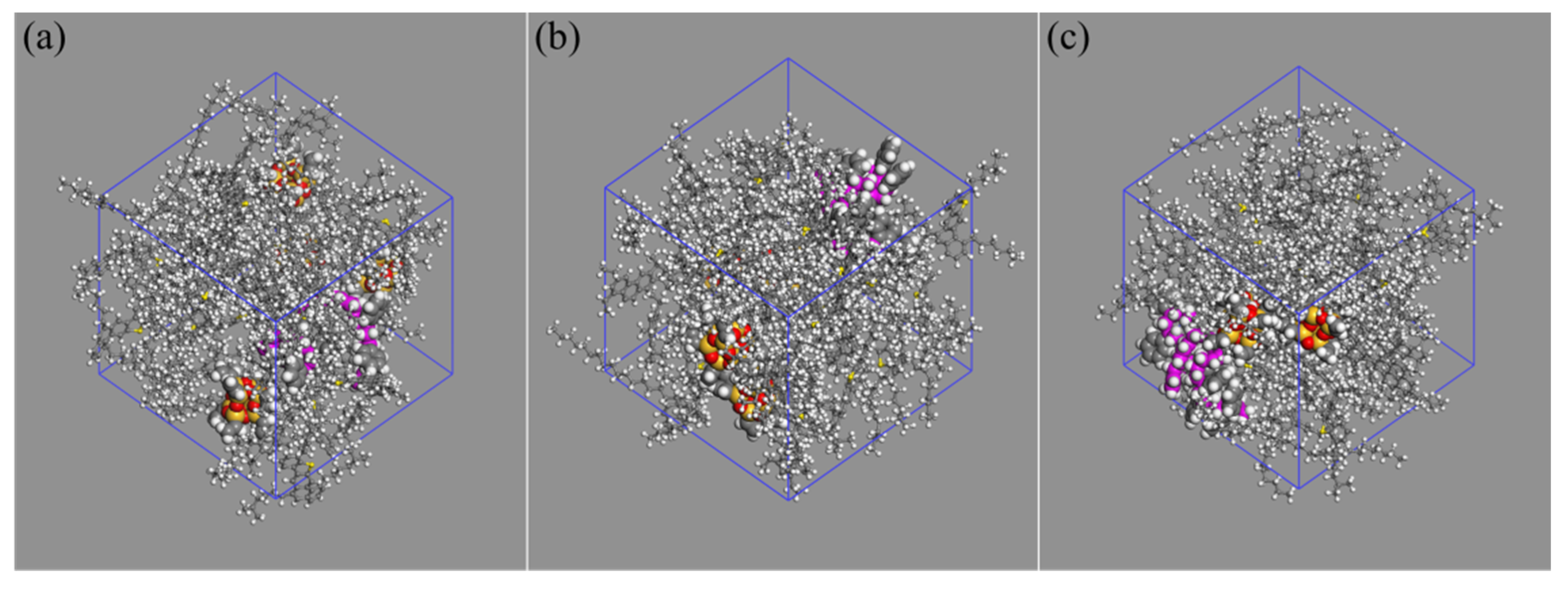



| Properties | Test Results | Test Standard |
|---|---|---|
| Penetration (0.1 mm) (25 °C, 100 g, 5 s) | 73.3 | ASTM D5-06 |
| Softening point (°C) | 47.5 | ASTM D36-06 |
| Ductility (cm) (5 cm/min, 10 °C) | 67.2 | ASTM D113-07 |
| Parameter | Asphaltene | Resin | Saturate | Aromatic |
|---|---|---|---|---|
| Weight (g) | 0.074 | 0.278 | 0.222 | 0.386 |
| Percent (%) | 7.71 | 28.96 | 23.13 | 40.20 |
| Name | Chemical Formula | Number of Molecules | Number of Atoms | Content (%) |
|---|---|---|---|---|
| Asphaltene | C64H52S2 | 2 | 236 | 7.34 |
| Resin | C41H54S | 12 | 1152 | 28.81 |
| Saturate | C22H46 | 18 | 1224 | 23.17 |
| Aromatic | C24H28 | 31 | 1612 | 40.68 |
| Parameter | Results |
|---|---|
| Molecular formula | C16H24O12Si8 |
| Molecular weight | 633 |
| Density (g/cm3) | 1.22 |
| Melting point (°C) | >350 |
| Flash point (°C) | 148.4 |
| Toxicity | Non-toxic |
| Name | Chemical Formula | Content (%) |
|---|---|---|
| Asphaltene | C64H52S2 | 6.331 |
| Resin | C41H54S | 24.842 |
| Saturate | C22H46 | 19.992 |
| Aromatic | C24H28 | 35.082 |
| OvPOSS | C16H24O12Si8 | 9.054 |
| SBS | C100H112 | 4.699 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Zhao, P.; Chen, T.; Jing, M. Study on the Influence of Nano-OvPOSS on the Compatibility, Molecular Structure, and Properties of SBS Modified Asphalt by Molecular Dynamics Simulation. Polymers 2022, 14, 4121. https://doi.org/10.3390/polym14194121
Feng L, Zhao P, Chen T, Jing M. Study on the Influence of Nano-OvPOSS on the Compatibility, Molecular Structure, and Properties of SBS Modified Asphalt by Molecular Dynamics Simulation. Polymers. 2022; 14(19):4121. https://doi.org/10.3390/polym14194121
Chicago/Turabian StyleFeng, Lei, Peng Zhao, Tongdan Chen, and Minghai Jing. 2022. "Study on the Influence of Nano-OvPOSS on the Compatibility, Molecular Structure, and Properties of SBS Modified Asphalt by Molecular Dynamics Simulation" Polymers 14, no. 19: 4121. https://doi.org/10.3390/polym14194121
APA StyleFeng, L., Zhao, P., Chen, T., & Jing, M. (2022). Study on the Influence of Nano-OvPOSS on the Compatibility, Molecular Structure, and Properties of SBS Modified Asphalt by Molecular Dynamics Simulation. Polymers, 14(19), 4121. https://doi.org/10.3390/polym14194121





