Effects of Nanofillers Based on Cetyltrimethylammonium-Modified Clays in a Polypropylene Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Organo-Clays
2.3. Synthesis of Nanocomposites
2.4. Characterizations
3. Results
3.1. CTA-Modified Clay
3.1.1. XRD Characterization
3.1.2. Fourier-Transform Infrared Spectroscopy Analysis
3.2. CTA-Modified Clay/Nanocomposites
3.2.1. XRD Characterization
3.2.2. TEM Images
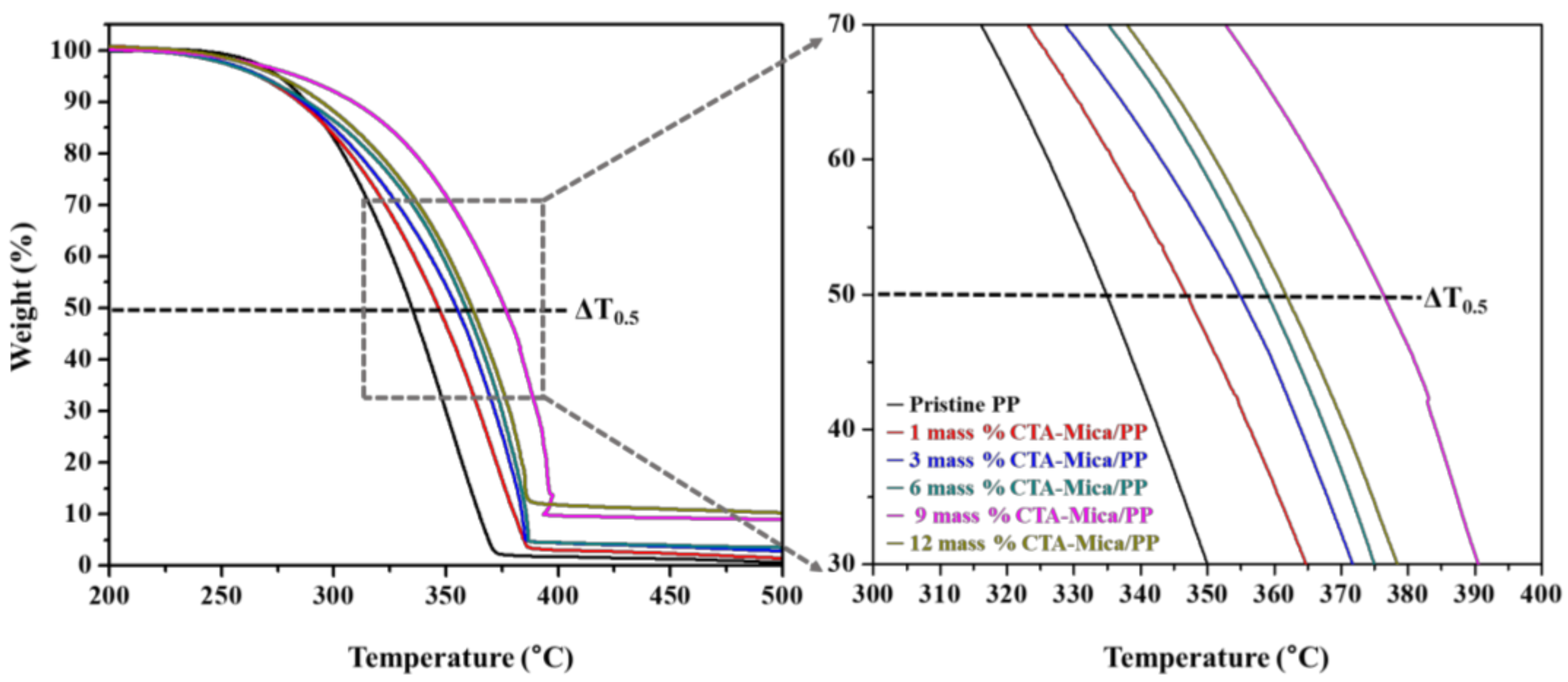
3.2.3. Thermal Stability
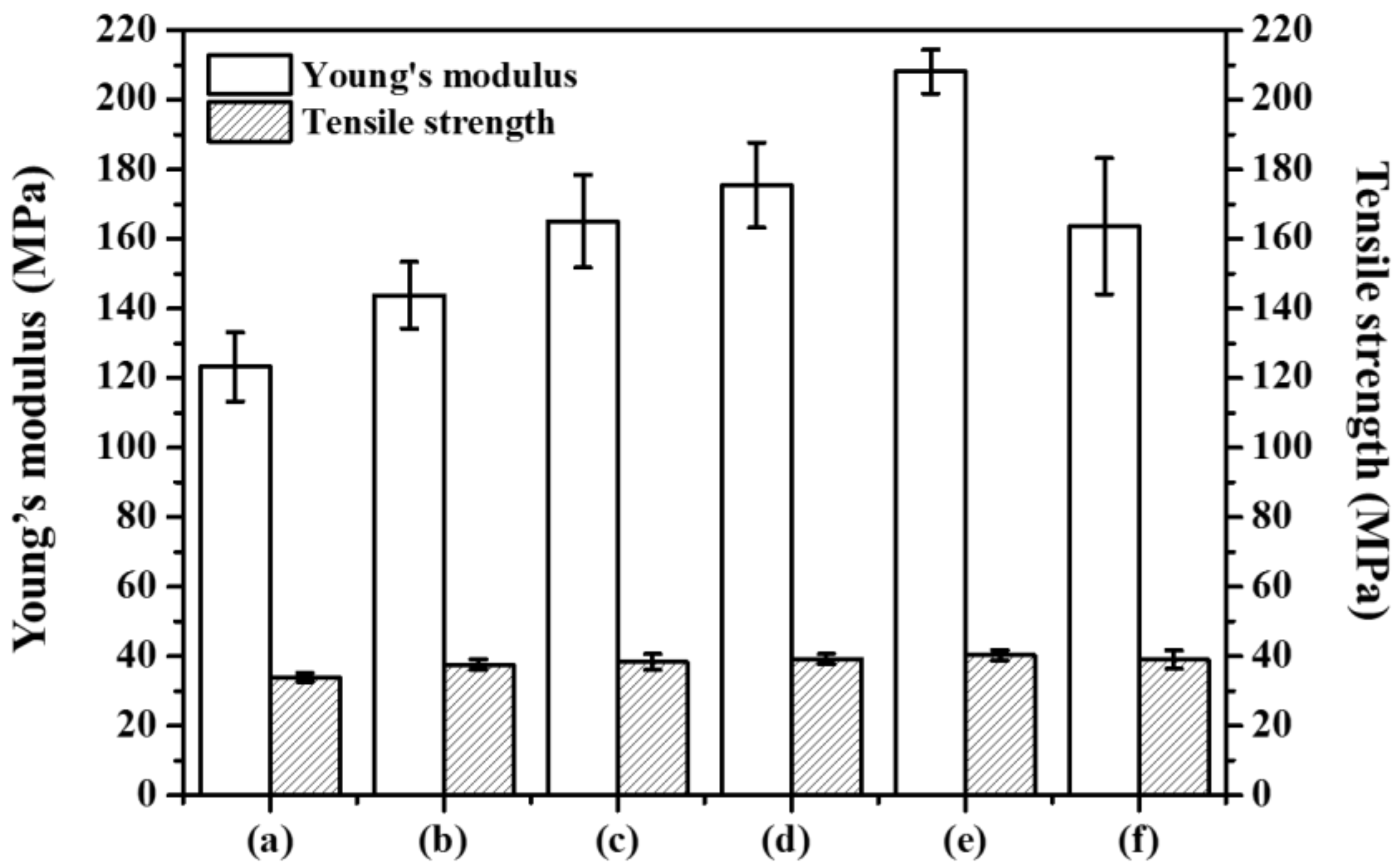
3.2.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, G.; Ling, Z.; Chunzhong, L. A new method combining modification of montmorillonite and crystal regulation to enhance the mechanical properties of polypropylene. Polym. Test. 2020, 82, 106236. [Google Scholar]
- Wissam, A.; Victor, T.; Musa, R.K. The Effect of Spray-Freeze Drying of Montmorillonite on the Morphology, Dispersion, and Crystallization in Polypropylene Nanocomposites. Polym. Eng. Sci. 2020, 60, 168–179. [Google Scholar]
- Mrah, L.; Meghabar., R. Infuence of clay modifcation process in polypyrrole-layered silicate nanocomposite. SN Appl. Sci. 2020, 2, 659. [Google Scholar] [CrossRef]
- Karimpour-Motlagh, N.; Khonakdar, H.A.; Jafari, S.H.; Panahi-Sarmad, M.; Javadi, A.; Shojaei, S.; Goodarz, V. An experimental and theoretical mechanistic analysis of thermal degradation of polypropylene/polylactic acid/clay nanocomposites. Polym. Adv. Technol. 2019, 30, 2695–2706. [Google Scholar] [CrossRef]
- Tan, H.; Wang, L.; Wen, X.; Deng, L.; Mijowska, E.; Tang, T. Insight into the influence of polymer topological structure on the exfoliation of clay in polystyrene matrix via annealing process. Appl. Clay Sci. 2020, 194, 105708. [Google Scholar] [CrossRef]
- Bozkurt, Ö.Y.; Bulut, M.; Erkliğ, A.; Faydh, W.H. Axial and lateral buckling analysis of fiber reinforced S-glass/epoxy composites containing nano-clay particles. Compos. Part B Eng. 2019, 158, 82–91. [Google Scholar] [CrossRef]
- Lopes Alves, J.; de Tarso Vieira e Rosa, P.; Morales, A.R. Hybrid organo-montmorillonite produced by simultaneous intercalation of phosphonium and ammonium/amine based surfactants. Mater. Chem. Phys. 2018, 218, 279–288. [Google Scholar] [CrossRef]
- Chen, H.H.; Thirumavalavan, M.; Ma, Y.J.; Lee, J.F. The influence of structural and processing parameters of modifiers on the interlayer structure of modified montmorillonite. RSC Adv. 2015, 5, 83217–83224. [Google Scholar] [CrossRef]
- Stojšić, J.; Raos, P.; Milinović, A.; Damjanović, D. A Study of the Flexural Properties of PA12/Clay Nanocomposites. Polymers 2022, 14, 434. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, W.; Ryu, H.; Lee, J.H.; Park, D.H.; Choi, J.Y.; Vinu, A.; Elzatahryde, A.A.; Choy, H.H. Influence of anionic surface modifiers on the thermal stability and mechanical properties of layered double hydroxide/polypropylene nanocomposites. J. Mater. Chem. A 2015, 3, 22730–22738. [Google Scholar] [CrossRef]
- Smole, M.S.; Akleinschek, K.S. Nanofilled polypropylene fibres. In Nanofibers and Nanotechnology in Textiles; Brown, P.J., Stevens, K., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2007; Volume 3, pp. 154–196. [Google Scholar]
- Sanusi, O.M.; Benelfellah, A. Clays and carbon nanotubes as hybrid nanofillers in thermoplastic-based nanocomposites—A review. Appl. Clay Sci. 2020, 185, 105408. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.W.; Lee, J.H.; Jang, S.W.; Kim, H.J.; Choi, Y.; Choy, J.H.; Yang, J.H. Clay-organic intumescent hybrid system for the synergetic flammability of polymer nanocomposites. J. Therm. Anal. Calorim. 2018, 132, 2009–2014. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Lee, J.H.; Zhang, W.; Ryu, H.J.; Choi, G.; Choi, J.Y.; Choy, J.H. Enhanced thermal stability and mechanical property of EVA nanocomposites upon addition of organo-intercalated LDH nanoparticles. Polymer 2019, 177, 274–281. [Google Scholar] [CrossRef]
- Ryu, H.J.; Hang, N.T.; Lee, J.H.; Choi, J.Y.; Choi, G.; Choy, J.H. Effect of organo-smectite clays on the mechanical properties and thermal stability of EVA nanocomposites. Appl. Clay Sci. 2020, 196, 105750. [Google Scholar] [CrossRef]
- Souza, D.H.S.; Andrade, C.T.; Dias, M.L. Rheological behavior of poly(lactic acid)/synthetic mica nanocomposites. Mater. Sci. Eng. C 2013, 33, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Jia, D.; Cai, C. Effects of organo-montmorillonite dispersion on thermal stability of epoxy resin nanocomposites. Eur. Polym. J. 2004, 40, 1743–1748. [Google Scholar] [CrossRef]
- Foungfung, D.; Phattanarudee, S.; Seetapan, N.; Kiatkamjornwong, S. Acrylamide–itaconic acid superabsorbent polymers and superabsorbent polymer/mica nanocomposites. Polym. Adv. Technol. 2011, 22, 635–647. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M. The Effect of Bi-Functionalized MMT on Morphology, Thermal Stability, Dynamic Mechanical, and Tensile Properties of Epoxy/Organoclay Nanocomposites. Polymers 2019, 11, 2012. [Google Scholar] [CrossRef]
- Chen, G.; Ma, Y.; Qi, Z. Preparation and morphological study of an exfoliated polystyrene/montmorillonite nanocomposite. Scr. Mater. 2001, 44, 125–128. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, D.S.; Li, J.; Mamba, B.B. Critical review of montmorillonite/polymer mixed-matrix filtration membranes: Possibilities and challenges. Appl. Clay Sci. 2019, 168, 21–30. [Google Scholar] [CrossRef]
- Bunekar, N.; Tsai, T.Y.; Huang, J.Y.; Chen, S.J. Investigation of thermal, mechanical and gas barrier properties of polypropylene-modified clay nanocomposites by micro-compounding Process. J. Taiwan Inst. Chem. Eng. 2018, 88, 252–260. [Google Scholar] [CrossRef]
- Madaleno, L.; Schjødt-Thomsen, J.; Pinto, J.C. Morphology, thermal and mechanical properties of PVC/MMT nanocomposites prepared by solution blending and solution blending + melt compounding. Compos. Sci. Technol. 2010, 70, 804–814. [Google Scholar] [CrossRef]
- Pospíšil, M.; Čapková, P.; Měříınská, D.; Maláč, Z.; Šimoník, J. Structure Analysis of Montmorillonite Intercalated with Cetylpyridinium and Cetyltrimethylammonium: Molecular Simulations and XRD Analysis. J. Colloid Interface Sci. 2001, 236, 127–131. [Google Scholar] [CrossRef]
- Yu, W.H.; Ren, Q.Q.; Tong, D.S.; Zhou, C.H.; Wang, H. Clean production of CTAB-montmorillonite: Formation mechanism and swelling behavior in xylene. Appl. Clay Sci. 2014, 97, 222–234. [Google Scholar] [CrossRef]
- Yang, J.H.; Han, Y.S.; Choy, J.H.; Tateyama, H. Intercalation of alkylammonium cations into expandable fluorine mica and its application for the evaluation of heterogeneous charge distribution. J. Mater. Chem. 2001, 11, 1305–1312. [Google Scholar] [CrossRef]
- Faucheu, J.; Gauthier, C.; Chazeau, L.; Cavaillé, J.Y.; Mellon, V.; Pardal, F.; Lami, E.B. Properties of polymer/clay interphase in nanoparticles synthesized through in-situ polymerization processes. Polymer 2010, 51, 4462–4471. [Google Scholar] [CrossRef]
- Jung, H.; Kim, H.M.; Choy, Y.B.; Hwang, S.J.; Choy., J.H. Itraconazole–Laponite: Kinetics and mechanism of drug release. Appl. Clay Sci. 2008, 40, 99–107. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.Y.; Yang, J.H.; Choy, J.H. Photophysical Properties of Hemicyanine Dyes Intercalated in Na-Fluorine Mica. J. Phys. Chem. A 2000, 104, 1388–1392. [Google Scholar] [CrossRef]
- Park, D.H.; Yang, J.H.; Vinu, A.; Elzatahry, A.; Choy, J.H. X-ray diffraction and X-ray absorption spectroscopic analyses for intercalative nanohybrids with low crystallinity. Arab. J. Chem. 2016, 9, 190–205. [Google Scholar] [CrossRef]
- Young, D.A.; Smith, D.E. Simulations of clay mineral swelling and hydration: Dependence upon interlayer ion size and charge. J. Phys. Chem. B 2000, 104, 9163–9170. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Bao, C.; Yi, Y.; Lei, M.; Ke, C.; Zhang, W.; Lin, G. Synthesis and assessment of CTAB and NPE modified organo-montmorillonite for the fabrication of organo-montmorillonite/alginate based hydrophobic pharmaceutical controlled-release formulation. Colloids Surf. B Biointerfaces 2020, 191, 110983. [Google Scholar] [CrossRef]
- Kumar, H.; Katal, A.; Rawat, P. FT-IR spectroscopic and micellization studies of cetyltrimethylammonium bromide in aqueous and aqueous solution of ionic liquid (1-butyl-3-methylimidazolium bromide) at different temperatures. J. Mol. Liq. 2018, 249, 227–232. [Google Scholar] [CrossRef]
- Phothitontimongkol, T.; Siebers, N.; Sukpirom, N.; Unob, F. Preparation and characterization of novel organo-clay minerals for Hg(II) ions adsorption from aqueous solution. Appl. Clay Sci. 2009, 43, 343–349. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; Adelnia, H.; Naderi, N.; Moazeni, N.; Mohamad, Z. Ethylene Vinyl Acetate Copolymer Nanocomposites Based on (Un)Modified Sepiolite: Flame Retardancy, Thermal, and Mechanical Properties. Polym. Compos. 2017, 38, 1302–1310. [Google Scholar] [CrossRef]
- Alvi, M.U.; Zulflqar, S.; Sarwar, M.I.; Kidwa, A.A. Preparation and Properties of Nanocomposites Derived from Aromatic Polyamide and Surface Functionalized Nanoclay. Chem. Eng. Commun. 2016, 203, 242–250. [Google Scholar] [CrossRef]
- Morgana, A.B.; Harris, J.D. Effects of organoclay Soxhlet extraction on mechanical properties, flammability properties and organoclay dispersion of polypropylene nanocomposites. Polymer 2003, 44, 2313–2320. [Google Scholar] [CrossRef]
- George, J.; Ishida, H. A review on the very high nanofiller-content nanocomposites: Their preparation methods and properties with high aspect ratio fillers. Prog. Polym. Sci. 2018, 86, 1–39. [Google Scholar] [CrossRef]
- Fischer, B.; Ziadeh, M.; Pfaff, A.; Breu, J.; Altstädt, V. Impact of large aspect ratio, shear-stiff, mica-like clay on mechanical behaviour of PMMA/clay nanocomposites. Polymer 2012, 53, 3230–3237. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Kakati, K.; Pugazhenthi, G.; Iyer, P.K. Effect of Organomodified Ni-Al Layered Double Hydroxide (OLDH) on the Properties of Polypropylene (PP)/LDH Nanocomposites. Int. J. Polym. Mater. 2012, 61, 931–948. [Google Scholar] [CrossRef]
- Zhang, J.; Wilkie, C.A. Polyethylene and polypropylene nanocomposites based on polymerically-modified clay containing alkylstyrene units. Polymer 2006, 47, 5736–5743. [Google Scholar] [CrossRef]
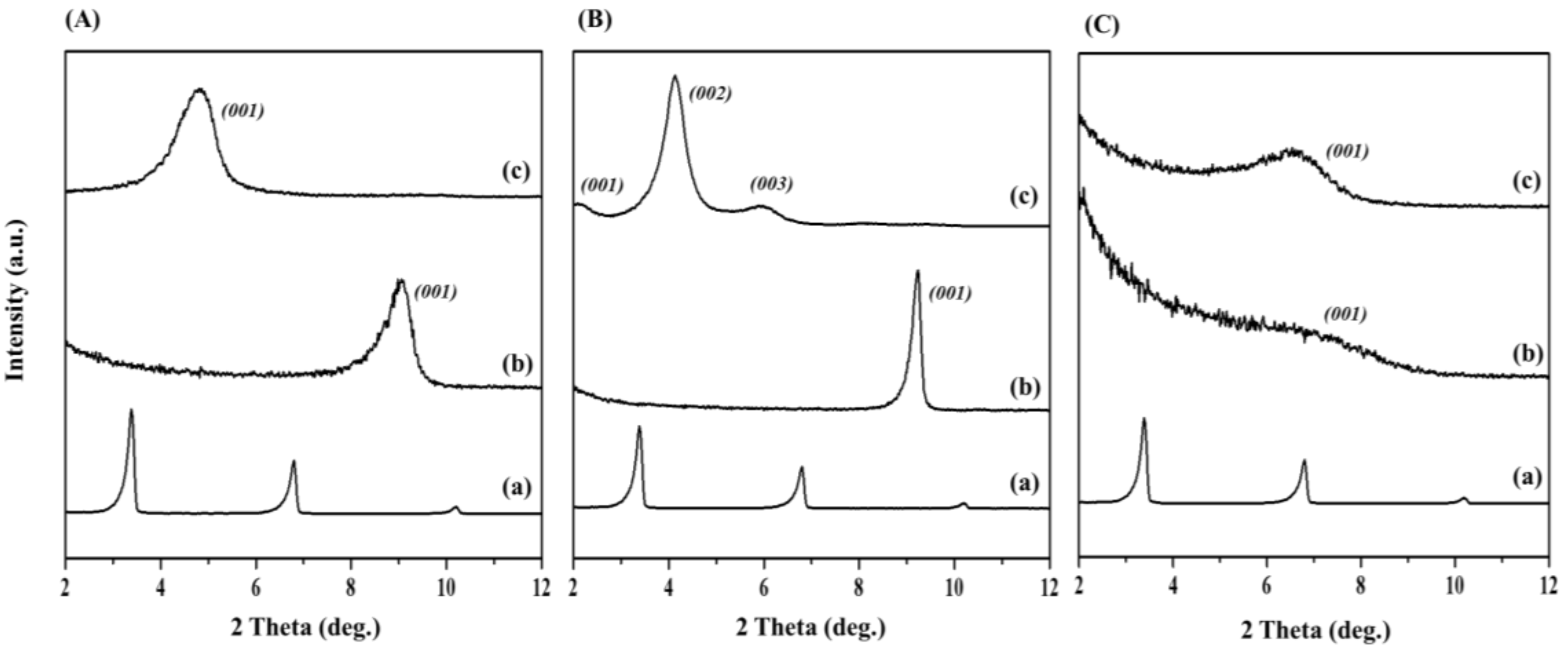
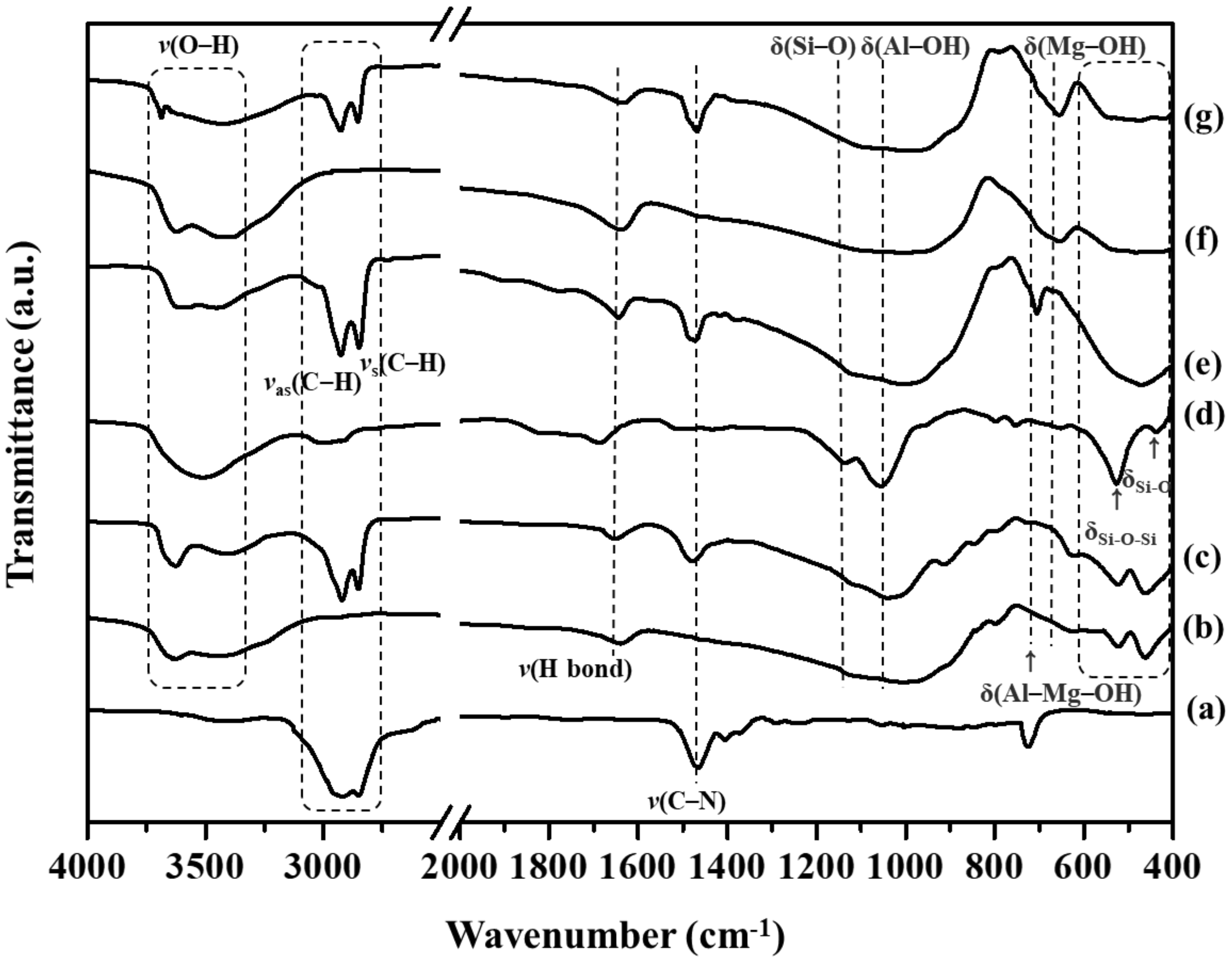
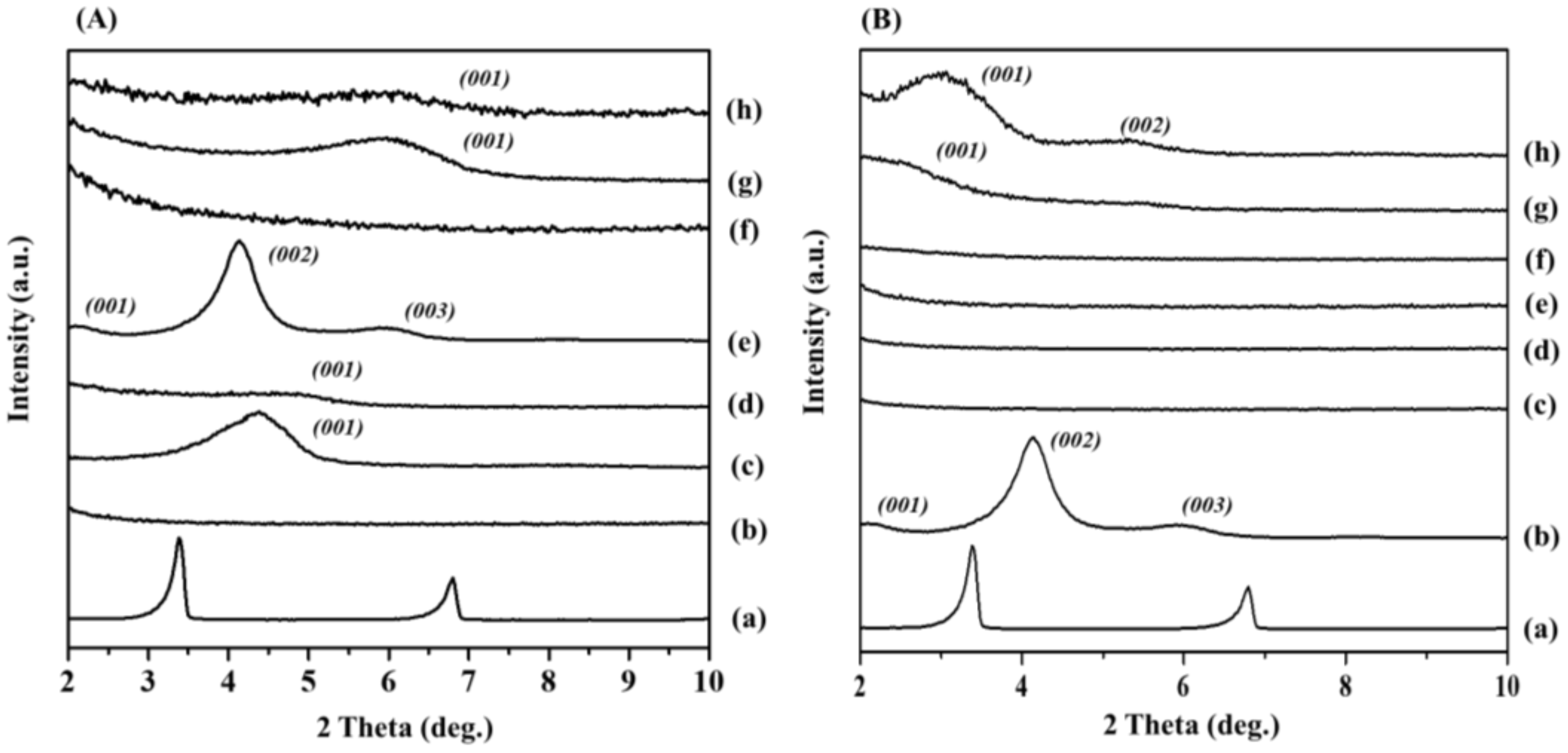


| Compound | Band Position (cm−1) | Assignments | ||
|---|---|---|---|---|
| CTA | 2930–2840 1470 | ν(C-H) ν(C-N) | ||
| Mt | Mica | Ht | ||
| Unmodified clays | 3630 | 3500 | 3625 | ν(Al-OH-Al) |
| 3416 | 3000 | 3421 | ν(H2O) | |
| 1640 | 1688 | 1640 | H-bonds | |
| 1120 | 1137 | 1091 | ν(Si-O) | |
| 1000 | 1055 | 956 | δ(Al-OH) | |
| 796 | 797 | 787 | δ(Al-Mg-OH) | |
| 625 | 655 | 651 | δ(Mg-OH) | |
| 523 | 526 | 542 | δ(Si-O-Al) | |
| 459 | 436 | 433 | δ(Si-O, Si-O-Si) | |
| CTA Clays | CTA-Mt | CTA-Mica | CTA-Ht | |
| 3637 | 3608 | 3693 | ν(Al-OH-Al) | |
| 3403 | 3441 | 3434 | ν(N-H) | |
| 2930–2840 | 2935–2840 | 2927–2853 | ν(C-H) | |
| 1652 | 1646 | 1639 | H-bonds | |
| 1480 | 1474 | 1470 | ν(C-N) | |
| 1122 | 1126 | 1111 | ν(Si-O) | |
| 1033 | 994 | 970 | δ(Al-OH) | |
| 900–790 | 707 | 787 | δ(Al-Mg-OH) | |
| 624 | 674 | 657 | δ(Mg-OH) | |
| 525 | 537 | 531 | δ(Si-O-Al) | |
| 462 | 472 | 479 | δ(Si-O, Si-O-Si) | |
| Sample | CTA Content (mass%) | T0.5 (°C) a | ΔT0.5 (°C) b |
|---|---|---|---|
| Pristine PP | − | 334 | − |
| CTA-Mt/PP | 1 | 333 | −1 |
| 3 | 355 | 21 | |
| 6 | 359 | 25 | |
| 9 | 352 | 18 | |
| 12 | 351 | 17 | |
| CTA-Mica/PP | 1 | 347 | 13 |
| 3 | 358 | 24 | |
| 6 | 361 | 27 | |
| 9 | 376 | 42 | |
| 12 | 362 | 28 | |
| CTA-Ht/PP | 6 | 328 | −6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.-J.; Hang, N.T.; Rejinold. N, S.; Jeong, B.; Choi, G.; Choy, J.-H. Effects of Nanofillers Based on Cetyltrimethylammonium-Modified Clays in a Polypropylene Nanocomposite. Polymers 2022, 14, 4110. https://doi.org/10.3390/polym14194110
Ryu H-J, Hang NT, Rejinold. N S, Jeong B, Choi G, Choy J-H. Effects of Nanofillers Based on Cetyltrimethylammonium-Modified Clays in a Polypropylene Nanocomposite. Polymers. 2022; 14(19):4110. https://doi.org/10.3390/polym14194110
Chicago/Turabian StyleRyu, Hyeon-Ju, Nguyen Thu Hang, Sanoj Rejinold. N, Byeongmoon Jeong, Goeun Choi, and Jin-Ho Choy. 2022. "Effects of Nanofillers Based on Cetyltrimethylammonium-Modified Clays in a Polypropylene Nanocomposite" Polymers 14, no. 19: 4110. https://doi.org/10.3390/polym14194110
APA StyleRyu, H.-J., Hang, N. T., Rejinold. N, S., Jeong, B., Choi, G., & Choy, J.-H. (2022). Effects of Nanofillers Based on Cetyltrimethylammonium-Modified Clays in a Polypropylene Nanocomposite. Polymers, 14(19), 4110. https://doi.org/10.3390/polym14194110








