Characterization and Bioactivity of Polysaccharides Separated through a (Sequential) Biorefinery Process from Fucus spiralis Brown Macroalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods of Extraction
2.1.1. Extracts Containing Polyphenols
2.1.2. Extracts Containing Polysaccharides
2.2. Chemical Characterization of Extracts
2.3. Determination of Extract Bioactivity
2.3.1. DNA Binding Studies
2.3.2. Superoxide Anion Radical Assay
2.3.3. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
3. Results
3.1. Biomass Proximate and Ultimate Analyses
3.2. A Biorefinery Approach for Fucus spiralis Macroalgal Biomass Processing
3.3. Extract Characterization
3.3.1. Yield and Chemical Characterization of Polyphenolic Extract (E1)
3.3.2. Yield and Chemical Characterization of Fucoidan Extract (E2)
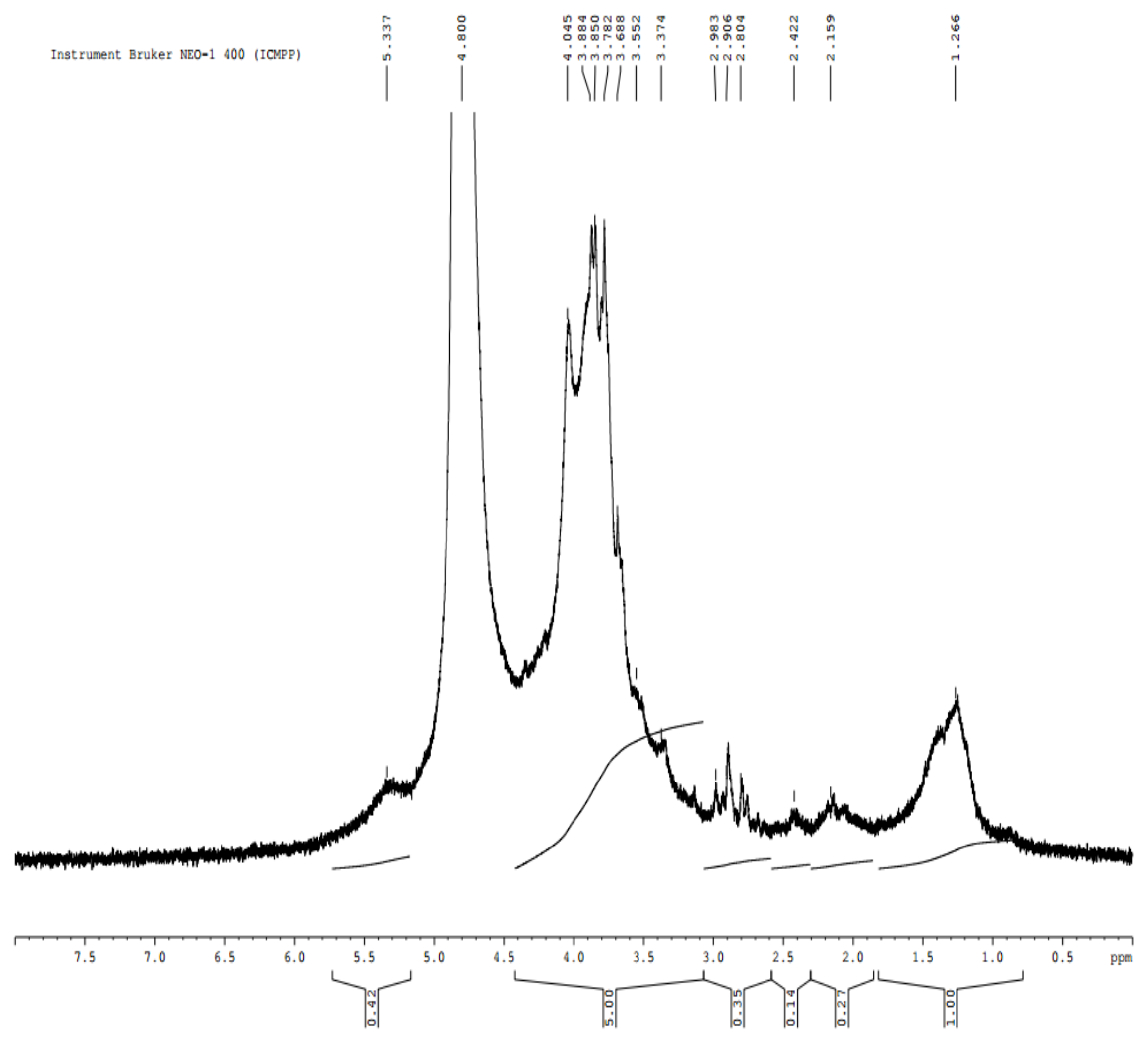
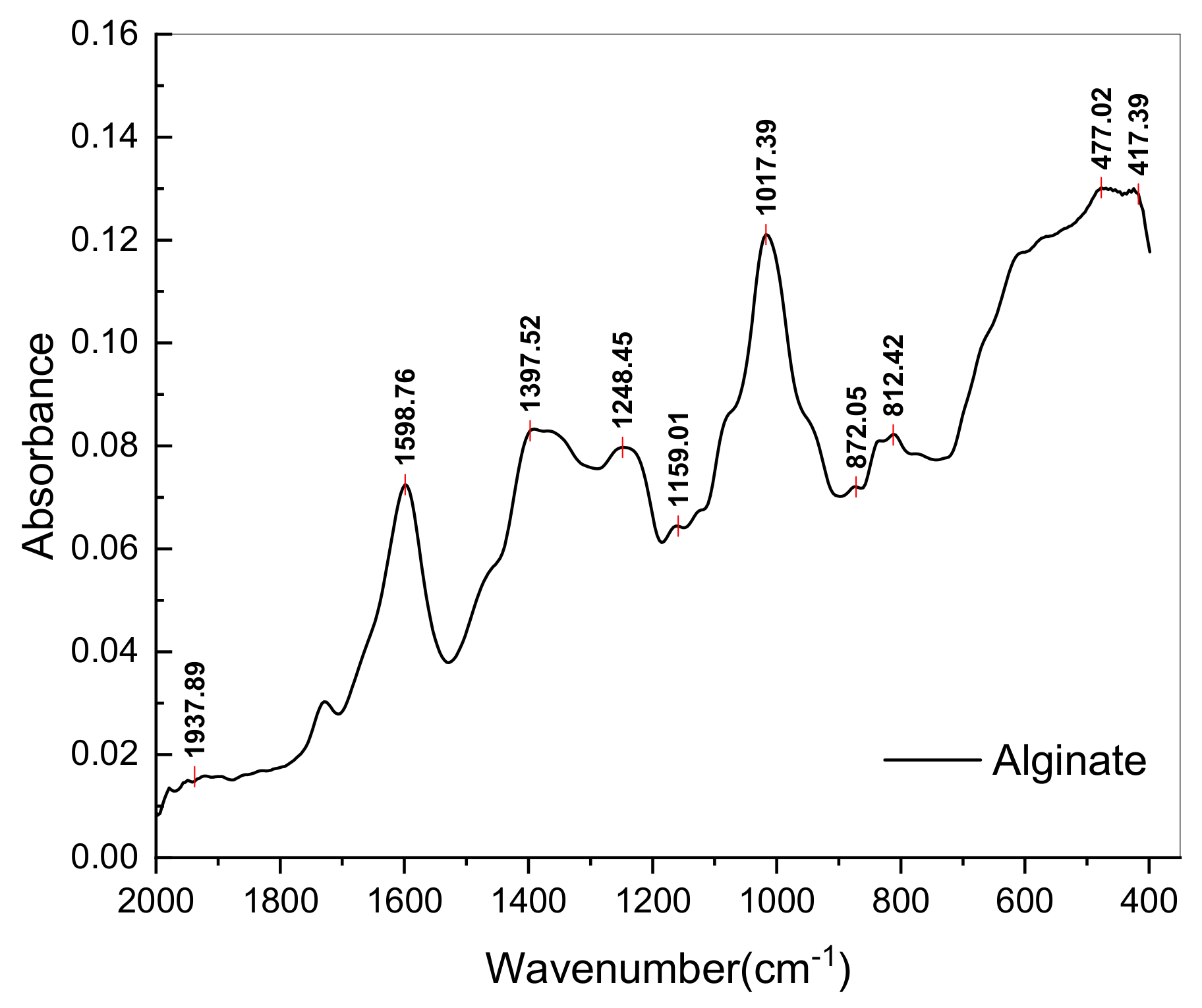
3.3.3. Yield and Chemical Characterization of Alginate Extract (E3)
3.4. Assessment of Targeted Molecule Bioactivity
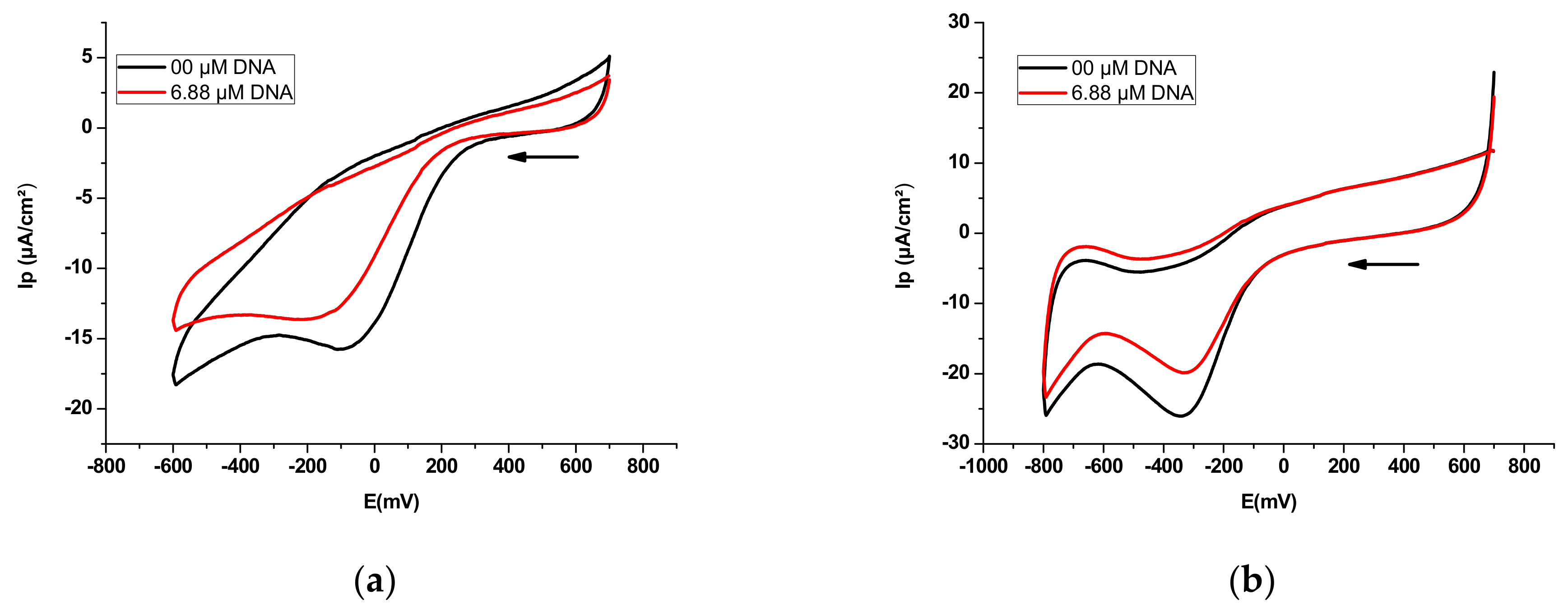
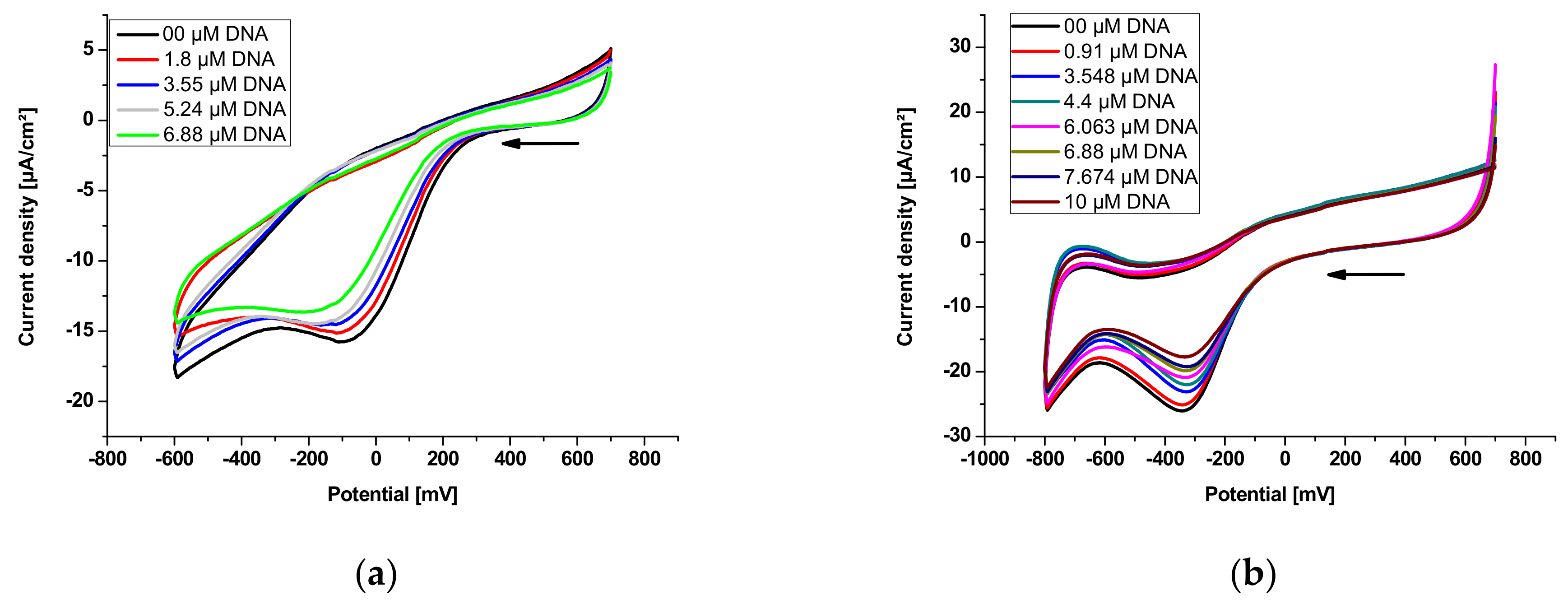
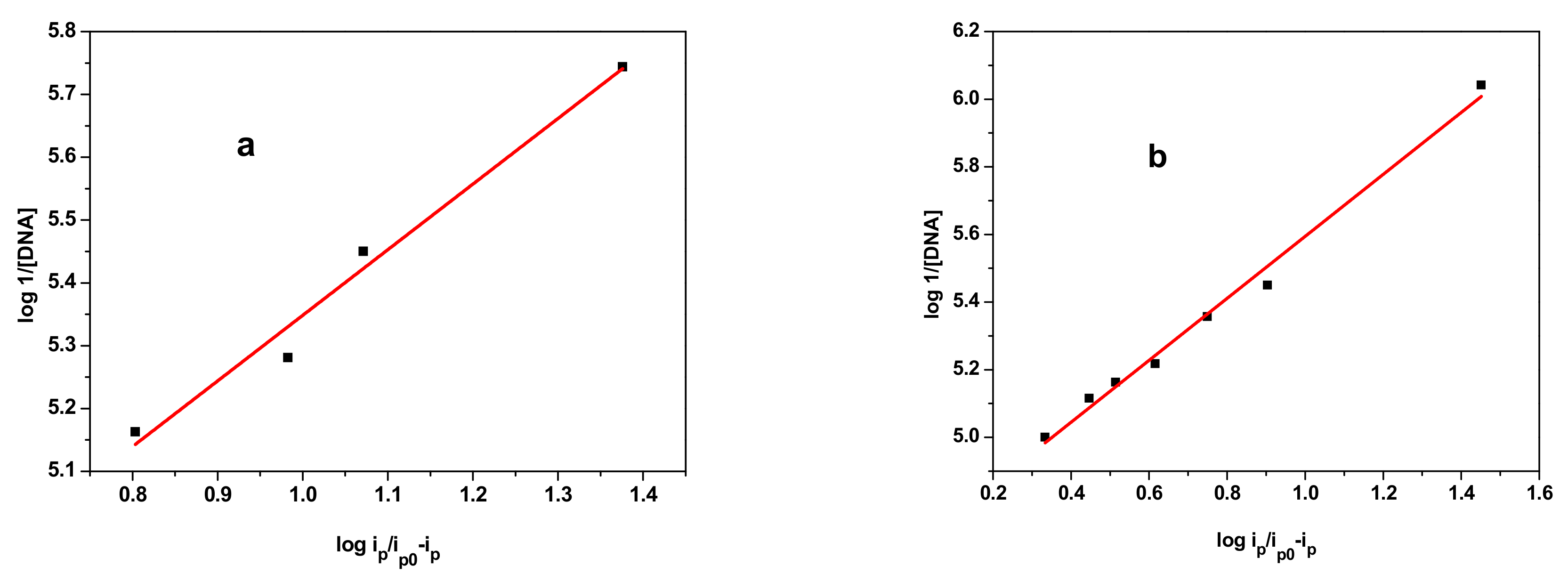
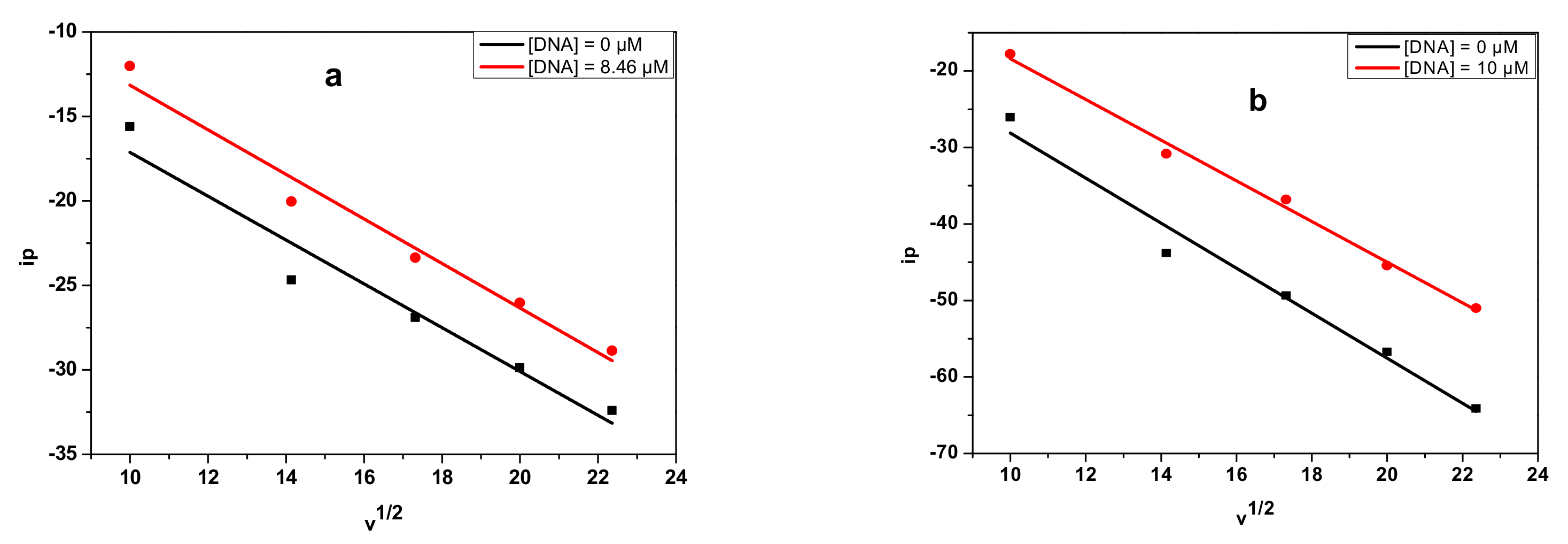
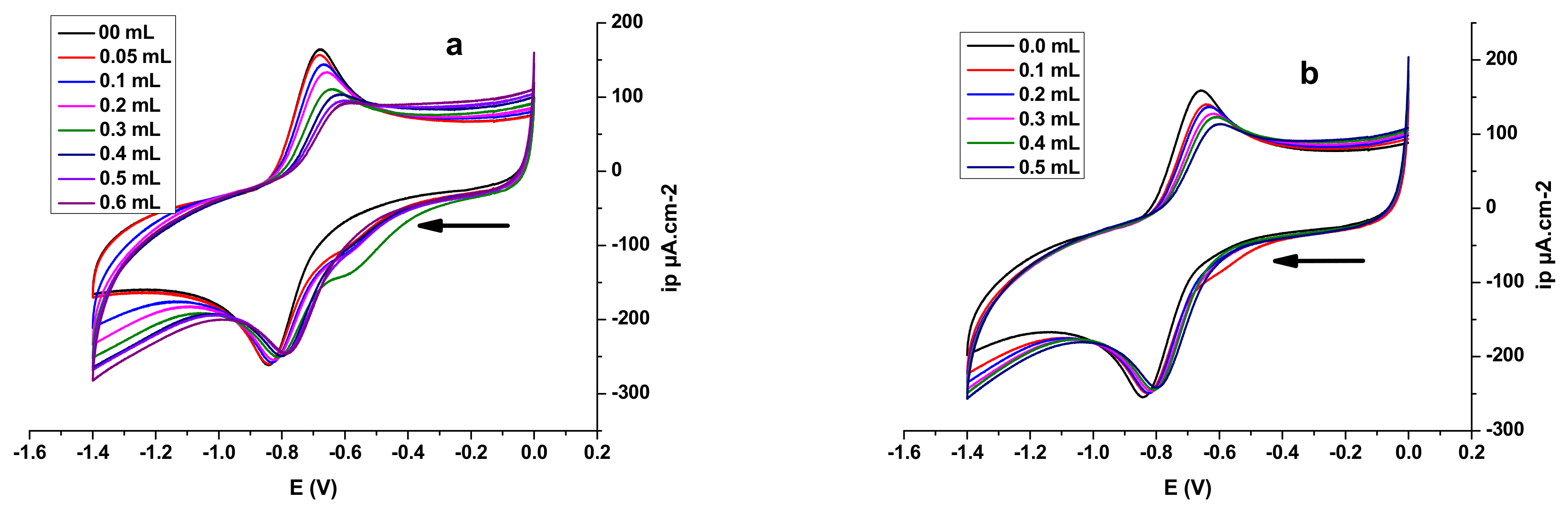
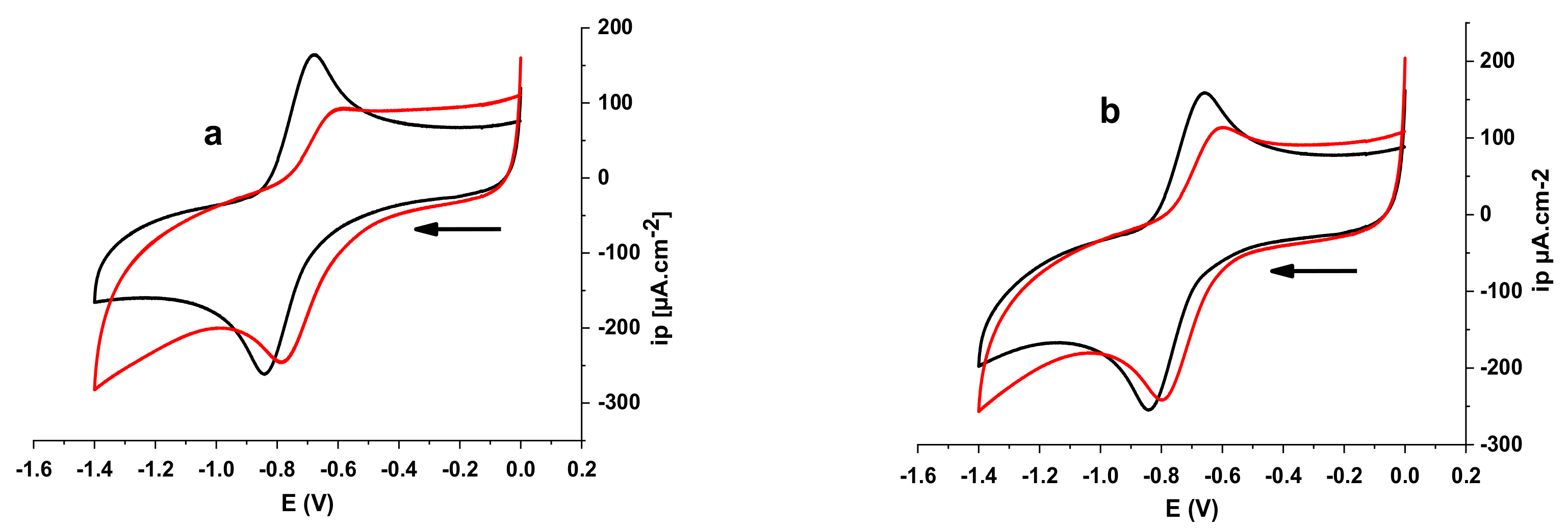
3.4.1. Electrochemical DNA Binding Study
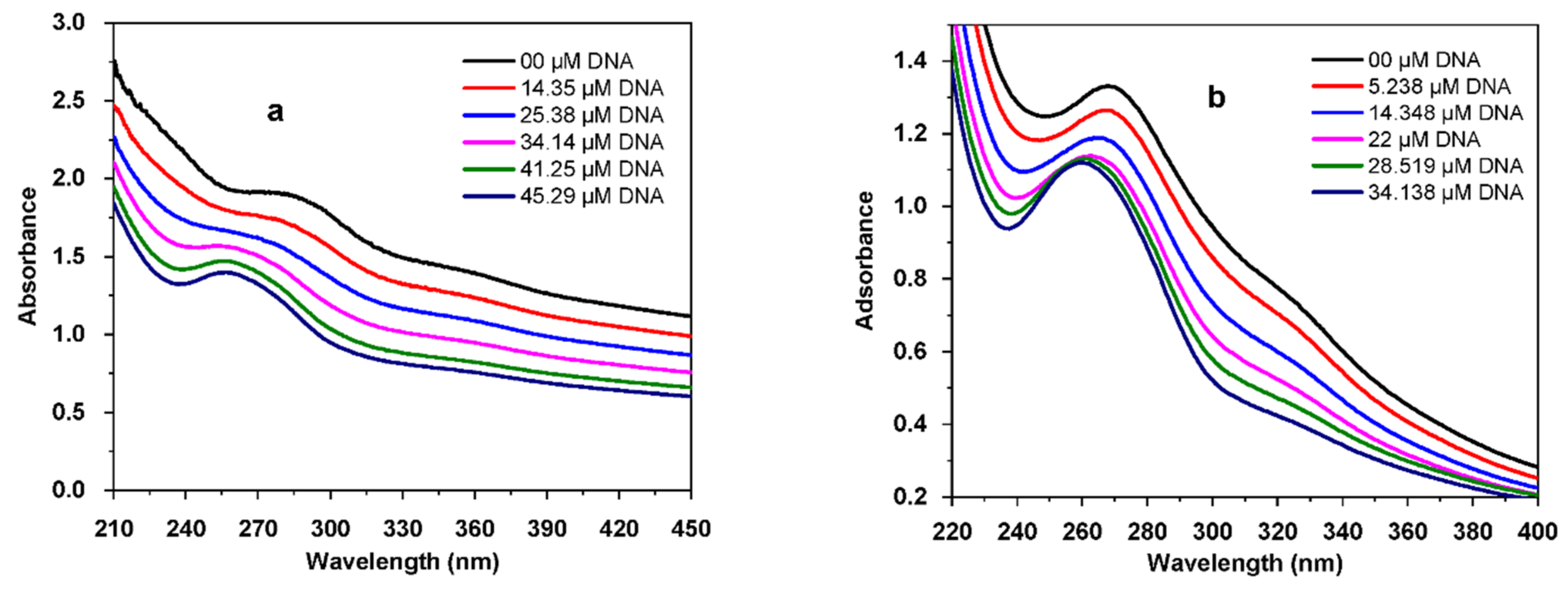
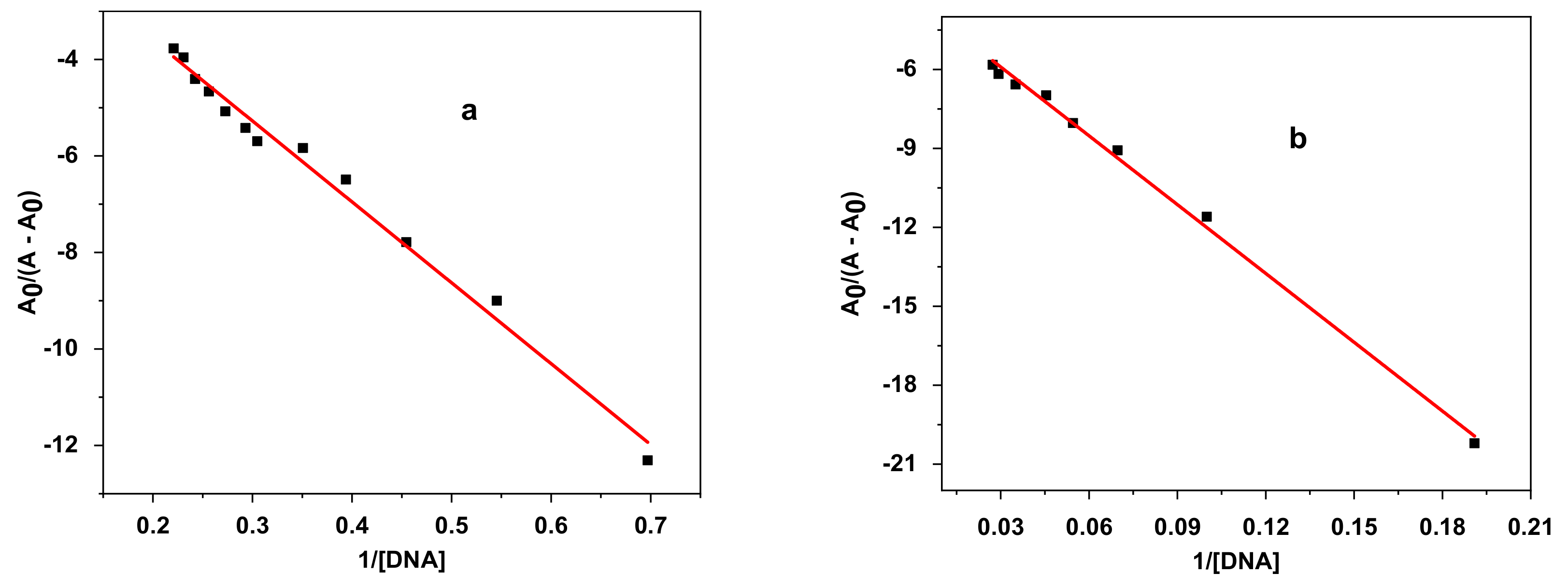
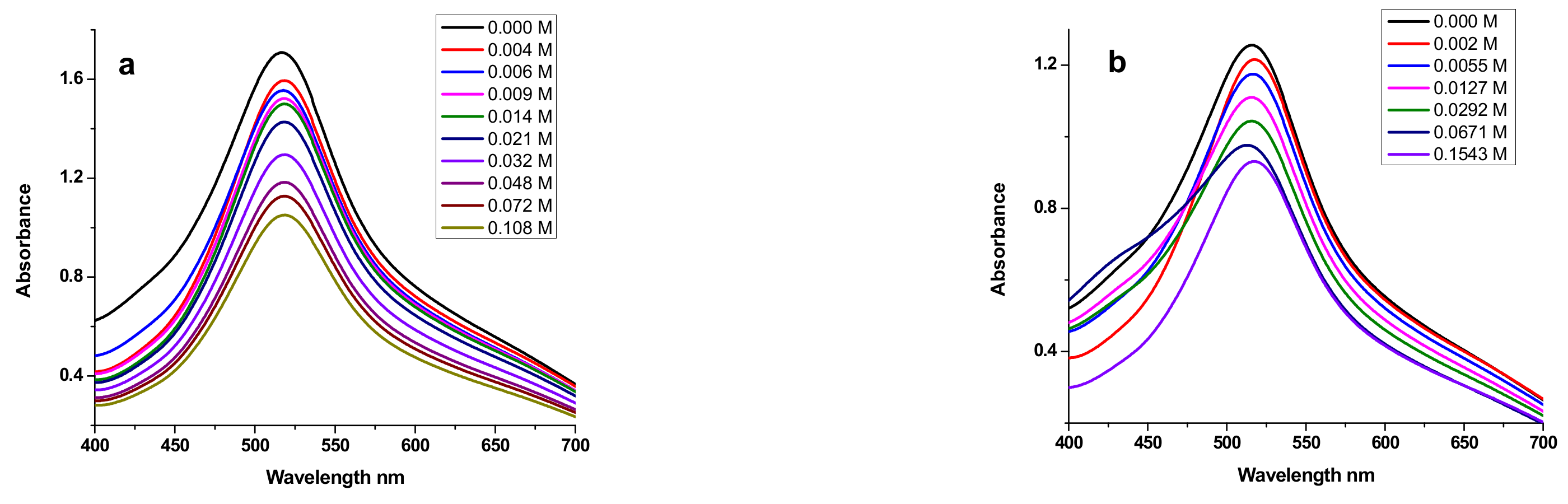
3.4.2. Electronic Spectroscopy DNA Binding Study
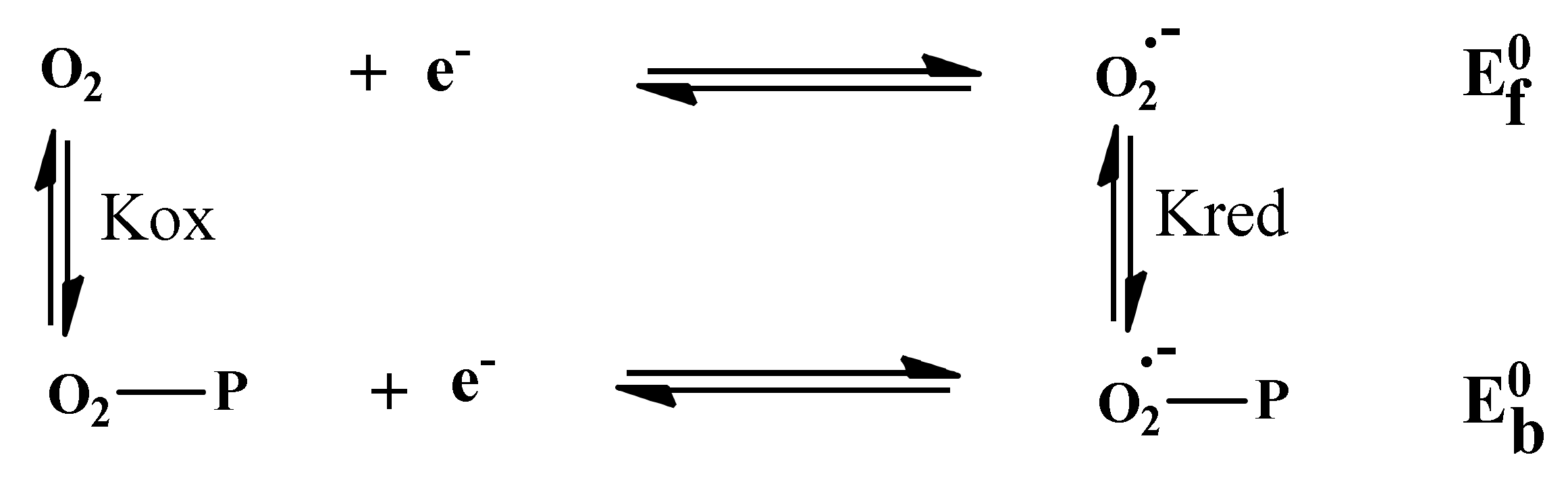
3.4.3. Superoxide Anion Radical Assay
3.4.4. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konda, N.V.S.N.M.; Singh, S.; Simmons, B.A.; Klein-Marcuschamer, D. An Investigation on the Economic Feasibility of Macroalgae as a Potential Feedstock for Biorefineries. Bioenergy Res. 2015, 8, 1046–1056. [Google Scholar] [CrossRef]
- Lehahn, Y.; Ingle, K.N.; Golberg, A. Global potential of offshore and shallow waters macroalgal biorefineries to provide for food, chemicals and energy: Feasibility and sustainability. Algal Res. 2016, 17, 150–160. [Google Scholar] [CrossRef]
- Kawai, S.; Murata, K. Biofuel production based on carbohydrates from both brown and red macroalgae: Recent developments in key biotechnologies. Int. J. Mol. Sci. 2016, 17, 145. [Google Scholar] [CrossRef]
- Ryu, H.J.; Oh, K.K. Combined De-Algination Process as a Fractionation Strategy for Valorization of Brown Macroalga Saccharina japonica. Appl. Biochem. Biotechnol. 2017, 182, 238–249. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity—The biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Marinho, G.; Nunes, C.; Sousa-Pinto, I.; Pereira, R.; Rema, P.; Valente, L.M.P. The IMTA-cultivated Chlorophyta Ulva spp. as a sustainable ingredient in Nile tilapia (Oreochromis niloticus) diets. J. Appl. Phycol. 2013, 25, 1359–1367. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-inflamatory effects of Fucoidan: A Review. Polymers 2020, 12, 2388. [Google Scholar] [CrossRef]
- Bodea, I.M.; Cătunescu, G.M.; Pop, C.R.; Fiț, N.I.; David, A.P.; Dudescu, M.C.; Stănilă, A.; Rotar, A.M.; Beteg, F.I. Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction. Polymers 2022, 14, 1435. [Google Scholar] [CrossRef]
- Schiener, P.; Atack, T.; Wareing, R.A.; Kelly, M.S.; Hughes, A.D. The by-products from marine biofuels as a feed source for the aquaculture industry: A novel example of the biorefinery approach. Biomass Convers. Biorefin. 2016, 6, 281–287. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Assacute, L.; Romagnoli, F.; Cappelli, A.; Ciocci, C. Algae-based biorefinery concept: An LCI analysis for a theoretical plant. Energy Procedia 2018, 147, 15–24. [Google Scholar] [CrossRef]
- Offei, F.; Mensah, M.; Kemausuor, F.; Thygesen, A. A biorefinery approach to bioethanol and bioelectricity co-production from tropical seaweeds. J. Appl. Phycol. 2019, 31, 3899–3913. [Google Scholar] [CrossRef]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M.D. Marine algal carbohydrates as carbon sources for the production of biochemicals and biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef]
- Tedesco, S.; Stokes, J. Valorisation to biogas of macroalgal waste streams: A circular approach to bioproducts and bioenergy in Ireland. Chem. Pap. 2017, 71, 721–728. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Arunagiri, V.; Tsai, H.C.; Darge, H.F.; Hanurry, E.Y.; Lee, C.Y.; Lai, J.Y.; Wu, S.Y. Enhanced cellular uptake in an electrostatically interacting fucoidan–l-arginine fiber complex. Polymers 2021, 13, 1795. [Google Scholar] [CrossRef]
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of alginate from Sargassum muticum: Process optimization and study of its functional activities. J. Appl. Phycol. 2016, 28, 3625–3634. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Ravanal, M.C.; Salazar, O.; Lienqueo, M.E. Saccharification of brown macroalgae using an arsenal of recombinant alginate lyases: Potential application in the biorefinery process. J. Microbiol. Biotechnol. 2018, 28, 1671–1682. [Google Scholar] [CrossRef]
- Osman, M.M.M.; Shao, X.; Zhao, D.; Basheer, A.K.; Jin, H.; Zhang, Y. Methane production from alginate-extracted and non-extracted waste of Laminaria japonica: Anaerobic mono- and synergetic co-digestion effects on yield. Sustainability 2019, 11, 1269. [Google Scholar] [CrossRef]
- Peptu, C.A.; Băcăiță, E.S.; Logigan, C.L.S.; Luțcanu, M.; Agop, M. Hydrogels based on alginates and carboxymethyl cellulose with modulated drug release—An experimental and theoretical study. Polymers 2021, 13, 4461. [Google Scholar] [CrossRef] [PubMed]
- Gorroñogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers 2022, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Volf, I.; Popa, V. Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780444637741. [Google Scholar]
- Pérez-Larrán, P.; Torres, M.D.; Flórez-Fernández, N.; Balboa, E.M.; Moure, A.; Domínguez, H. Green technologies for cascade extraction of Sargassum muticum bioactives. J. Appl. Phycol. 2019, 31, 2481–2495. [Google Scholar] [CrossRef]
- Ramos, E.; Puente, A.; Juanes, J.A.; Neto, J.M.; Pedersen, A.; Bartsch, I.; Scanlan, C.; Wilkes, R.; Van den Bergh, E.; Ar Gall, E.; et al. Biological validation of physical coastal waters classification along the NE Atlantic region based on rocky macroalgae distribution. Estuar. Coast. Shelf Sci. 2014, 147, 103–112. [Google Scholar] [CrossRef]
- Rinne, H.; Salovius-Laurén, S. The status of brown macroalgae Fucus spp. and its relation to environmental variation in the Finnish marine area, northern Baltic Sea. Ambio 2020, 49, 118–129. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity, antioxidant properties, phenolic content and amino acid profiles of Fucus spiralis L. Protein hydrolysate fractions. Mar. Drugs 2017, 15, 311. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Thomas, O.P.; Pedrosa, R. Antioxidant and cytoprotective activities of Fucus spiralis seaweed on a human cell in vitro model. Int. J. Mol. Sci. 2017, 18, 292. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Barba, F.; Lorenzo, J. Antioxidant Potential of Extracts Obtained from Macro-(Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Lopes, G.; Ferreres, F.; Andrade, P.B.; Pereira, D.M.; Gil-Izquierdo, Á.; Valentão, P. Phlorotannin extracts from Fucales: Marine polyphenols as bioregulators engaged in inflammation-related mediators and enzymes. Algal Res. 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drugs 2016, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Anastyuk, S.D.; Shevchenko, N.M.; Ermakova, S.P.; Vishchuk, O.S.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Anticancer activity in vitro of a fucoidan from the brown alga Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012, 87, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-assisted extraction of polysaccharides from brown alga Fucus evanescens. Structure and biological activity of the new fucoidan fractions. J. Appl. Phycol. 2018, 30, 2039–2046. [Google Scholar] [CrossRef]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2015, 27, 545–553. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Gunnarsson, I.B.; Fotidis, I.A.; Vasilakou, E.; Lyberatos, G.; Angelidaki, I. Laminaria digitata as a potential carbon source for succinic acid and bioenergy production in a biorefinery perspective. Algal Res. 2015, 9, 126–132. [Google Scholar] [CrossRef]
- Kostas, E.T.; White, D.A.; Cook, D.J. Development of a bio-refinery process for the production of speciality chemical, biofuel and bioactive compounds from Laminaria digitata. Algal Res. 2017, 28, 211–219. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential hydrothermal-assisted extraction followed by ultrasound and thermal technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef]
- Kim, G.Y.; Seo, Y.H.; Kim, I.; Han, J.I. Co-production of biodiesel and alginate from Laminaria japonica. Sci. Total Environ. 2019, 673, 750–755. [Google Scholar] [CrossRef]
- Balboa, E.M.; Moure, A.; Domínguez, H. Valorization of Sargassum muticum biomass according to the biorefinery concept. Mar. Drugs 2015, 13, 3745–3760. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, Y.; Jazini, M.; Karimi, K. Sargassum angustifolium brown macroalga as a high potential substrate for alginate and ethanol production with minimal nutrient requirement. Algal Res. 2018, 36, 29–36. [Google Scholar] [CrossRef]
- Lorbeer, A.J.; Charoensiddhi, S.; Lahnstein, J.; Lars, C.; Franco, C.M.M.; Bulone, V.; Zhang, W. Sequential extraction and characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 2017, 29, 1515–1526. [Google Scholar] [CrossRef]
- Leyton, A.; Lienqueo, M.E.; Shene, C. Macrocystis pyrifera: Substrate for the production of bioactive compounds. J. Appl. Phycol. 2020, 32, 2335–2341. [Google Scholar] [CrossRef]
- Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Nguyen, T.; Zhang, W. Multiple-response optimization of the acidic treatment of the brown alga Ecklonia radiata for the sequential extraction of fucoidan and alginate. Bioresour. Technol. 2015, 197, 302–309. [Google Scholar] [CrossRef]
- Lazar, L.; Talmaciu, A.I.; Volf, I.; Popa, V.I. Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochem. 2016, 32, 191–197. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave assisted step-by-step process for the production of fucoidan, alginate sodium, sugars and biochar from Ascophyllum nodosum through a biorefinery concept. Bioresour. Technol. 2015, 198, 819–827. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Int. Union Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Pregl, F. Die Quantitative Organische Mikroanalyse; Spinger: Berlin/Heidelberg, Germany, 1930. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Schöniger, W. Eine mikroanalytisehe Sehnellbestimmung von Halogen in organischen Substanzen. Mikrochim. Acta 1955, 43, 123–129. [Google Scholar] [CrossRef]
- Schöniger, W. Die mikroanalytische Schnellbestimmung von Halogenen und Schwefel in organischen Verbindungen. Mikrochim. Acta 1956, 44, 869–876. [Google Scholar] [CrossRef]
- Oleksi, A.; Blanco, A.G.; Boer, R.; Usón, I.; Aymamí, J.; Rodger, A.; Hannon, M.J.; Coll, M. Molecular recognition of a three-way DNA junction by a metallosupramolecular helicate. Angew. Chem.-Int. Ed. 2006, 45, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Zaheer, M.; Qureshi, R.; Akhter, Z.; Faizan Nazar, M. Voltammetric and spectroscopic investigations of 4-nitrophenylferrocene interacting with DNA. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2010, 75, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicryl-hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Manns, D.; Deutschle, A.L.; Saake, B.; Meyer, A.S. Methodology for quantitative determination of the carbohydrate composition of brown seaweeds (Laminariaceae). RSC Adv. 2014, 4, 25736–25746. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Hansen, J.H.; Bjerre, A.B. Integrated bioethanol and protein production from brown seaweed Laminaria digitata. Bioresour. Technol. 2015, 197, 310–317. [Google Scholar] [CrossRef]
- Bruton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Review of the Potential of Marine Algae as a Source of Biofuel in Ireland. Sustain. Energy. 2009, pp. 1–88. Available online: https://www.researchgate.net/publication/309185965_A_review_of_the_potential_of_Marine_Algae_as_a_Source_of_Biofuel_in_Ireland_Sustainable_Energy (accessed on 10 July 2022).
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Jothisaraswathi, S.; Babu, B.; Rengasamy, R. Seasonal studies on alginate and its composition II: Turbinaria conoides (J. Ag.) Kütz. (Fucales, Phaeophyceae). J. Appl. Phycol. 2006, 18, 161–166. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, D.; Nah, J.W.; Jeon, Y.J. Advances in functionalizing fucoidans and alginates (bio)polymers by structural modifications: A review. Chem. Eng. J. 2019, 355, 33–48. [Google Scholar] [CrossRef]
- Vian, M.; Breil, C.; Vernes, L.; Chaabani, E.; Chemat, F. Green solvents for sample preparation in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2017, 5, 44–48. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Pressurized GRAS solvents for the green extraction of phenolic compounds from Hibiscus sabdariffa calyces. Food Res. Int. 2020, 137, 109466. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Freitas, O.M.M.; Martins, R.J.E.; Delerue-Matos, C.M.; Boaventura, R.A.R. Removal of Cd(II), Zn(II) and Pb(II) from aqueous solutions by brown marine macro algae: Kinetic modelling. J. Hazard. Mater. 2008, 153, 493–501. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Filote, C.; Volf, I.; Santos, S.C.R.; Botelho, C.M.S. Bioadsorptive removal of Pb(II) from aqueous solution by the biorefinery waste of Fucus spiralis. Sci. Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Filote, C.; Ungureanu, G.; Boaventura, R.; Santos, S.; Volf, I.; Botelho, C. Green macroalgae from the Romanian coast of Black Sea: Physico-chemical characterization and future perspectives on their use as metal anions biosorbents. Process Saf. Environ. Prot. 2017, 108, 34–43. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Biosorption of heavy metals by Fucus spiralis. Bioresour. Technol. 2008, 99, 4684–4693. [Google Scholar] [CrossRef]
- Mazur, L.P.; Cechinel, M.A.P.; de Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Brown marine macroalgae as natural cation exchangers for toxic metal removal from industrial wastewaters: A review. J. Environ. Manag. 2018, 223, 215–253. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Equilibrium and kinetic modelling of Cd(II) biosorption by algae Gelidium and agar extraction algal waste. Water Res. 2006, 40, 291–302. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Kinetics and equilibrium modelling of lead uptake by algae Gelidium and algal waste from agar extraction industry. J. Hazard. Mater. 2007, 143, 396–408. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Copper removal by algae Gelidium, agar extraction algal waste and granulated algal waste: Kinetics and equilibrium. Bioresour. Technol. 2008, 99, 750–762. [Google Scholar] [CrossRef]
- Romero-Gonzalez, M.E.; Williams, C.J.; Gardiner, P.H.E. Study of the mechanisms of cadmium biosorption by dealginated seaweed waste. Environ. Sci. Technol. 2001, 35, 3025–3030. [Google Scholar] [CrossRef]
- Romero-González, M.E.; Williams, C.J.; Gardiner, P.H.E.; Gurman, S.J.; Habesh, S. Spectroscopic studies of the biosorption of gold(III) by dealginated seaweed waste. Environ. Sci. Technol. 2003, 37, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Masri, M.A.; Jurkowski, W.; Shaigani, P.; Haack, M.; Mehlmer, N.; Brück, T. A waste-free, microbial oil centered cyclic bio-refinery approach based on flexible macroalgae biomass. Appl. Energy 2018, 224, 1–12. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Bigham, A.; Rahimkhoei, V.; Sharifi, S.; Jabbari, E. Antiviral Polymers: A Review. Polymers 2022, 14, 1634. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.; Hong, Y.; Tsai, Y.; Lee, Y.; Patel, A.K.; Guo, H.; Kuo, C.; Huang, C. Extraction, Biochemical Characterization, and Health Effects of Native and Degraded Fucoidans from Sargassum crispifolium. Polymers 2022, 14, 1812. [Google Scholar] [CrossRef]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Ross, A.; Jones, J.; Kubacki, M.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Yu, L.J.; Wang, S.; Jiang, X.M.; Wang, N.; Zhang, C.Q. Thermal analysis studies on combustion characteristics of seaweed. J. Anal. Calorim. 2008, 93, 611–617. [Google Scholar] [CrossRef]
- Bae, Y.J.; Ryu, C.; Jeon, J.-K.; Park, J.; Suh, D.J.; Suh, Y.-W.; Chang, D.; Park, Y.-K. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Balboa, E.M.; Rivas, S.; Moure, A.; Domínguez, H.; Parajó, J.C. Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar. Drugs 2013, 11, 4612–4627. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Khajouei, R.A.; Keramat, J.; Hamdami, N.; Ursu, A.V.; Delattre, C.; Laroche, C.; Gardarin, C.; Lecerf, D.; Desbrières, J.; Djelveh, G.; et al. Extraction and characterization of an alginate from the Iranian brown seaweed Nizimuddinia zanardini. Int. J. Biol. Macromol. 2018, 118, 1073–1081. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; McHugh, D.J.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E. Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1. Effect of pre-extraction treatments on yield and quality of alginate. J. Appl. Phycol. 1999, 10, 507–513. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Abraham, R.E.; Su, P.; Puri, M.; Raston, C.L.; Zhang, W. Optimisation of biorefinery production of alginate, fucoidan and laminarin from brown seaweed Durvillaea potatorum. Algal Res. 2019, 38, 101389. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Dyer, P.W.; Greenwell, H.C.; Selby, D.; Theodorou, M.K. Copper(II)-mediated thermolysis of alginates: A model kinetic study on the influence of metal ions in the thermochemical processing of macroalgae. Interface Focus 2013, 3, 20120046. [Google Scholar] [CrossRef]
- Cong, Q.; Xiao, F.; Liao, W.; Dong, Q.; Ding, K. Structure and biological activities of an alginate from Sargassum fusiforme, and its sulfated derivative. Int. J. Biol. Macromol. 2014, 69, 252–259. [Google Scholar] [CrossRef]
- Elbayomi, S.M.; Wang, H.; Tamer, T.M.; You, Y. Enhancement of antioxidant and hydrophobic properties of alginate via aromatic derivatization: Preparation, characterization, and evaluation. Polymers 2021, 13, 2575. [Google Scholar] [CrossRef]
- Kok, J.M.L.; Wong, C.L. Physicochemical properties of edible alginate film from Malaysian Sargassum polycystum C. Agardh. Sustain. Chem. Pharm. 2018, 9, 87–94. [Google Scholar] [CrossRef]
- Kinh, C.; Kinh, C.D.; Thien, T.V.; Hoa, T.T.; Khieu, D.Q. Interpretation of 1H-NMR spectrum of alginate by 1H-1H TOCSY and COSY spectrum. Vietnam J. Chem. 2007, 45, 772–775. [Google Scholar]
- Jamil, M.; Ali, A.; Badshah, A.; Ahmad, I.; Zubair, M.; Kemal, S.; Irshad, M. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Naked Eye DNA detection: Synthesis, characterization and DNA binding studies of a novel azo-guanidine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 165–170. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, J.; Zhang, J.; Chen, H. Voltammetric studies of the interaction of methylene blue with DNA by means of β-cyclodextrin. Anal. Chim. Acta 1999, 394, 337–344. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry; Macmillan: New York, NY, USA, 2006; Volume 78, ISBN 9780716787594. [Google Scholar]
- Aslanoǧlu, M.; Öge, N. Voltammetric, UV absorption and viscometric studies of the interaction of norepinephrine with DNA. Turk. J. Chem. 2005, 29, 477–485. [Google Scholar]
- Aslanoglu, M.; Ayne, G. Voltammetric studies of the interaction of quinacrine with DNA. Anal. Bioanal. Chem. 2004, 380, 658–663. [Google Scholar] [CrossRef]
- Husain, M.A.; Sarwar, T.; Rehman, S.U.; Ishqi, H.M.; Tabish, M. Ibuprofen causes photocleavage through ROS generation and intercalates with DNA: A combined biophysical and molecular docking approach. Phys. Chem. Chem. Phys. 2015, 17, 13837–13850. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Kashanian, S.; Mahdavi, M.; Sourinejad, N. DNA interaction and DNA cleavage studies of a new platinum(II) complex containing aliphatic and aromatic dinitrogen ligands. Bioinorg. Chem. Appl. 2011, 2011, 525794. [Google Scholar] [CrossRef] [PubMed]
- Houlton, A.; Isaac, C.J.; Gibson, A.E.; Horrocks, B.R.; Clegg, W.; Elsegood, M.R.J. Synthesis, structure and redox properties of ferrocenylmethyl-nucleobases. J. Chem. Soc. Dalton Trans. 1999, 18, 3229–3234. [Google Scholar] [CrossRef]
- Benabdesselam, S.; Lanez, T. Evaluation of total antioxidant capacity and free radicals scavenging of 2 and 4-Nitrophenylferrocene by electrochemical and chemical assays. J. Chem. Pharm. Res. 2015, 7, 825–831. [Google Scholar]
- Ahmed, S.; Shakeel, F. Voltammetric determination of antioxidant character in Berberis lycium Royel, Zanthoxylum armatum and Morus nigra Linn plants. Pak. J. Pharm. Sci. 2012, 25, 501–507. [Google Scholar]
- Ahmed, S.; Shakeel, F. Antioxidant activity coefficient, mechanism, and kinetics of different derivatives of flavones and flavanones towards superoxide radical. Czech J. Food Sci. 2012, 30, 153–163. [Google Scholar] [CrossRef]
- Lanez, T.; Hemmami, H. Antioxidant Activities of N-ferrocenylmethyl-2- and -3-nitroaniline and Determination of their Binding Parameters with Superoxide Anion Radicals. Curr. Pharm. Anal. 2017, 13, 110–116. [Google Scholar] [CrossRef]
- Ozsoz, M.; Erdem, A.; Kara, P.; Kerman, K.; Ozkan, D. Electrochemical Biosensor for the Detection of Interaction Between Arsenic Trioxide and DNA Base on Guanine Signal. Electroanalysis 2003, 15, 613–619. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, K.; Zhang, M.; Liu, H.; Kang, J. Voltammetric studies of the interaction of transition-metal complexes with DNA. J. Biochem. Biophys. Methods 2002, 52, 189–200. [Google Scholar] [CrossRef]
- Paleček, E. Past, present and future of nucleic acids electrochemistry. Talanta 2002, 56, 809–819. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry. Principles, Methods and Applications. Electrochim. Acta 1994, 39, 853–854. [Google Scholar]



| Property Value | Unit | Value |
|---|---|---|
| Proximate analysis | ||
| Moisture content | wt% (dry) | 11.5 ± 0.7 |
| Ash content | wt% (dry) | 22.5 ± 0.9 |
| Ultimate analysis | ||
| Carbon | wt% (dry) | 36.6 ± 0.6 |
| Hydrogen | wt% (dry) | 3.9 ± 0.1 |
| Nitrogen | wt% (dry) | 1.1 ± 0.1 |
| Sulphur | wt% (dry) | 0.5 ± 0.1 |
| Carbohydrate analysis | ||
| Reducing sugars | wt% (dry) | 16.3 ± 0.7 |
| Protein characterization | ||
| Protein content | wt% (dry) | 5.7 ± 0.5 |
| Nitrogen-to-protein conversion factor | - | 6.25 |
| Element | Fucoidan | Alginate |
|---|---|---|
| C % | 32.1 ± 0.4 | 31.4 ± 0.6 |
| H % | 4.0 ± 0.1 | 5.2 ± 0.3 |
| S % | 0.4 ± 0.0 | 0.3 ± 0.0 |
| N % | ND | 3.2 ± 0.2 |
| Sample | Equation | R2 | K (M−1) | −ΔG (KJ·mol−1) |
|---|---|---|---|---|
| E2 | y = 1.0445x + 4.3039 | 0.972 | 2.01 × 104 | 24.57 |
| E3 | y = 0.9162x + 4.6782 | 0.989 | 4.77 × 104 | 26.71 |
| Adduct | Equation | R2 | s |
|---|---|---|---|
| DNA-E2 | y = 0.02193x − 8.287 × 10−5 | 0.828 | 0.02 |
| DNA-E3 | y = 0.0486x − 0.027 | 0.987 | 0.12 |
| Compound | Equation | R2 | D (cm2/s) |
|---|---|---|---|
| E2 | y = −1.29665x − 4.16404 | 0.938 | 1.161 × 10−7 |
| DNA-E2 | y = −1.31838 x + 0.02649 | 0.968 | 0.721 × 10−7 |
| E3 | y = −2.9437x + 1.3783 | 0.972 | 7.086 × 10−9 |
| DNA-E3 | y = −2.6593 x + 8.1874 | 0.992 | 5.783 × 10−9 |
| Sample | Equation | R2 | K (M−1) | −ΔG (KJ·mol−1) |
|---|---|---|---|---|
| E2 | y = −16.76582x – 0.24616 | 0.986 | 1.47 × 104 | 23.79 |
| E3 | y = −87.1888x – 3.2914 | 0.998 | 3.78 × 104 | 26.13 |
| Sample | Equation | R2 | IC50 |
|---|---|---|---|
| E2 | y = 3.21057x + 0.08397 | 0.918 | 0.13 ± 0.04 |
| E3 | y = 2.3336x + 0.0658 | 0.984 | 0.19 ± 0.06 |
| α-tocopherol | y = 15.99x + 1.37 | 0.950 | 3.04 ± 0.30 |
| Compound | Equation | R2 | K (L−1) | −ΔG (KJ·mol−1) |
|---|---|---|---|---|
| E2-O2.- | y = 0.96108x + 3.02683 | 0.978 | 1.06 × 103 | 17.28 |
| E3-O2.- | y = 1.28666x + 2.55632 | 0.891 | 0.36 × 103 | 14.59 |
| Compound | Epa (V) | Epc (V) | E0 (V) | Kred/Kox | |
|---|---|---|---|---|---|
| E2 | −0.6764 | −0.8411 | −0.75875 | 0.06765 | 14.01 |
| E2-O2.- | −0.6018 | −0.7804 | −0.6911 | ||
| E3 | −0.6575 | −0.8411 | −0.7493 | 0.05095 | 7.03 |
| E3-O2.- | −0.6018 | −0.7949 | −0.69835 |
| Sample | Equation | R2 | IC50 |
|---|---|---|---|
| E2 | y = 0.114x − 0.0123 | 0.997 | 4.49 ± 0.21 |
| E3 | y = 0.1051x − 0.0081 | 0.998 | 4.83 ± 0.34 |
| α-tocopherol | y =1.5646x + 29.093 | 0.996 | 4.81 ± 0.32 |
| Adduct | Equation | R2 | K (M−1) | −ΔG (KJ·mol−1) |
|---|---|---|---|---|
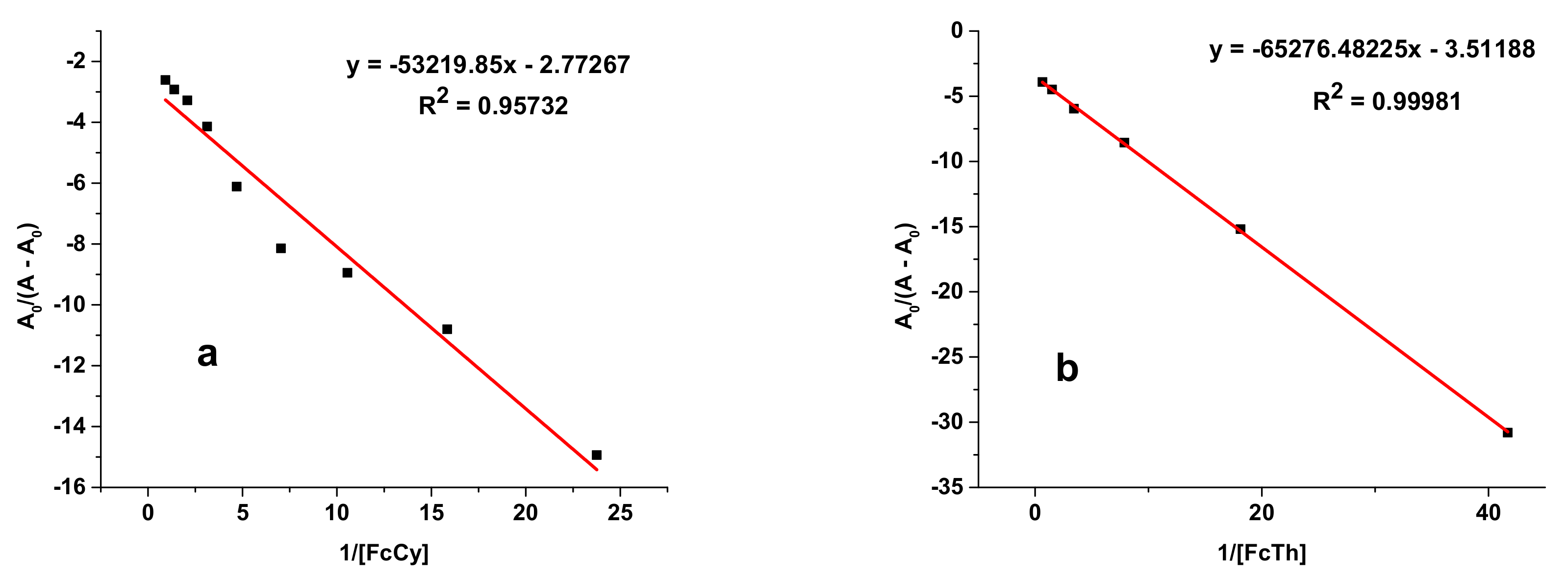
| E2-DPPH | y = −53,219.85x − 2.77267 | 0.957 | 52.1 | 9.80 |
| E3-DPPH | y = −65,276.482x − 3.5119 | 0.999 | 53.8 | 9.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filote, C.; Lanez, E.; Popa, V.I.; Lanez, T.; Volf, I. Characterization and Bioactivity of Polysaccharides Separated through a (Sequential) Biorefinery Process from Fucus spiralis Brown Macroalgae. Polymers 2022, 14, 4106. https://doi.org/10.3390/polym14194106
Filote C, Lanez E, Popa VI, Lanez T, Volf I. Characterization and Bioactivity of Polysaccharides Separated through a (Sequential) Biorefinery Process from Fucus spiralis Brown Macroalgae. Polymers. 2022; 14(19):4106. https://doi.org/10.3390/polym14194106
Chicago/Turabian StyleFilote, Cătălina, Elhafnaoui Lanez, Valentin I. Popa, Touhami Lanez, and Irina Volf. 2022. "Characterization and Bioactivity of Polysaccharides Separated through a (Sequential) Biorefinery Process from Fucus spiralis Brown Macroalgae" Polymers 14, no. 19: 4106. https://doi.org/10.3390/polym14194106
APA StyleFilote, C., Lanez, E., Popa, V. I., Lanez, T., & Volf, I. (2022). Characterization and Bioactivity of Polysaccharides Separated through a (Sequential) Biorefinery Process from Fucus spiralis Brown Macroalgae. Polymers, 14(19), 4106. https://doi.org/10.3390/polym14194106








