Ionic Push–Pull Polythiophenes: A Further Step towards Eco-Friendly BHJ Organic Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Characterization
2.2. Cyclic Voltammetry (CV)
2.3. Atomic Force Microscopy (AFM) and Field-Emission Scanning Electron Microscopy (FE-SEM)
2.4. X-Ray Diffraction (XRD)
2.5. Organic Solar Cells
3. Results and Discussion
3.1. Synthesis
3.2. NMR and FT-IR Characterization
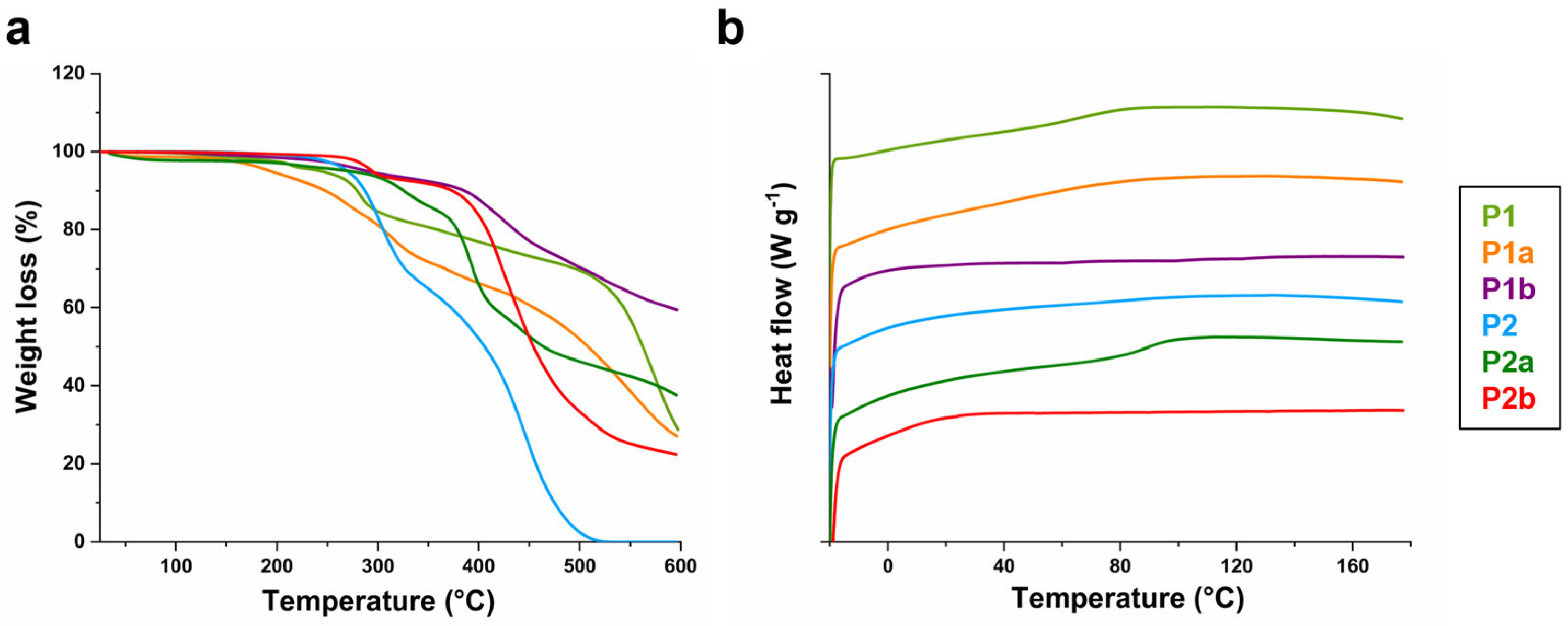
3.3. Thermal Characterization
3.4. Electrochemical Properties
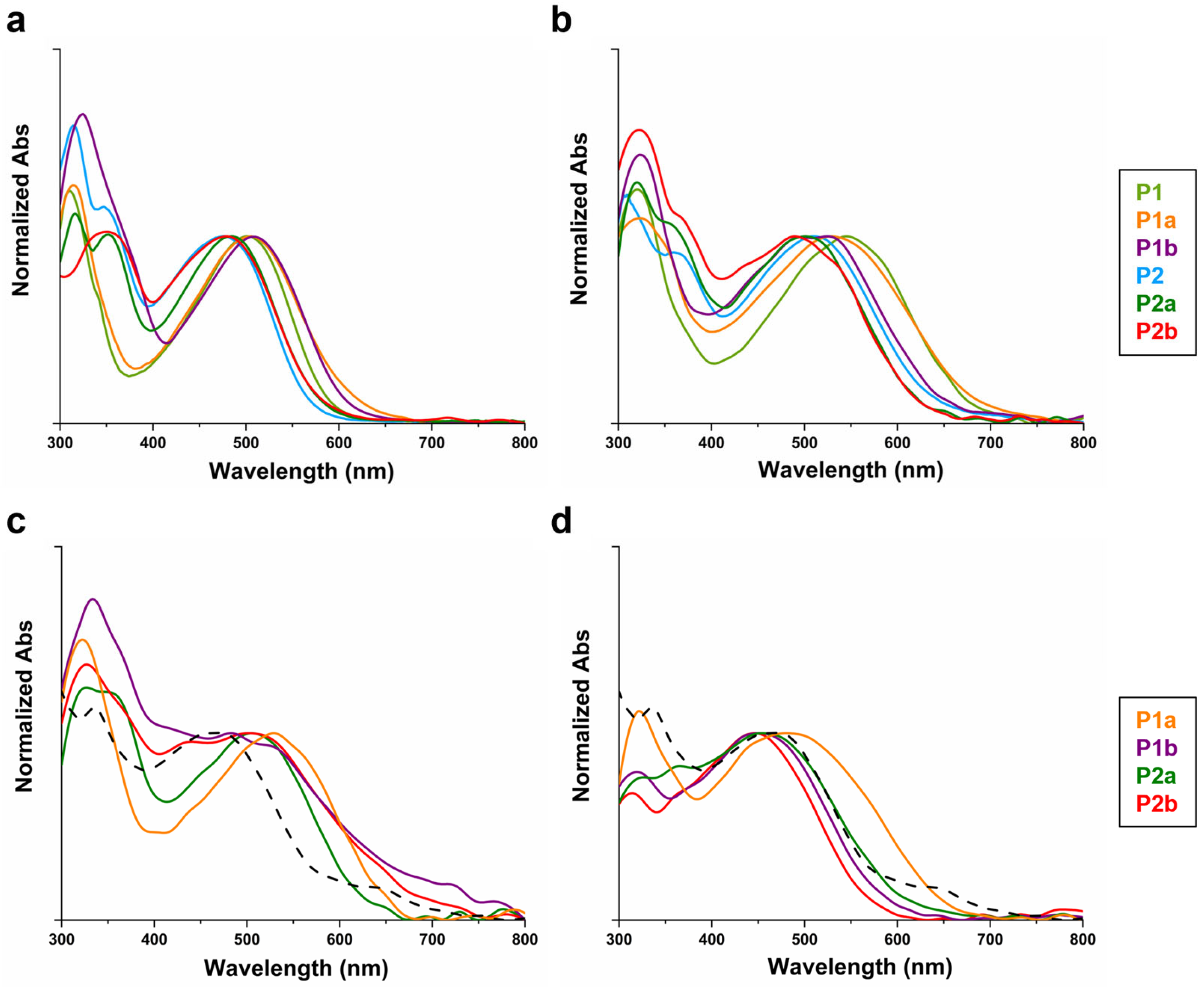
3.5. UV–Vis Characterization
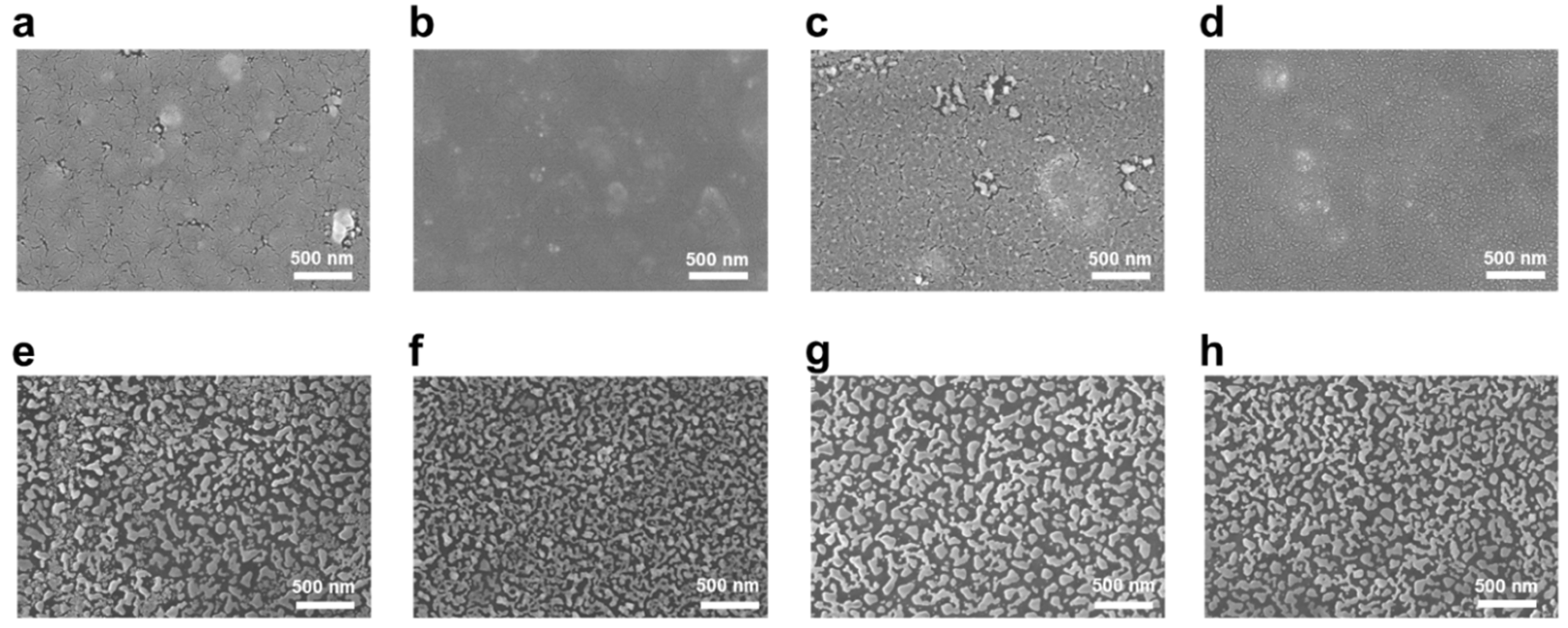
3.6. Morphology
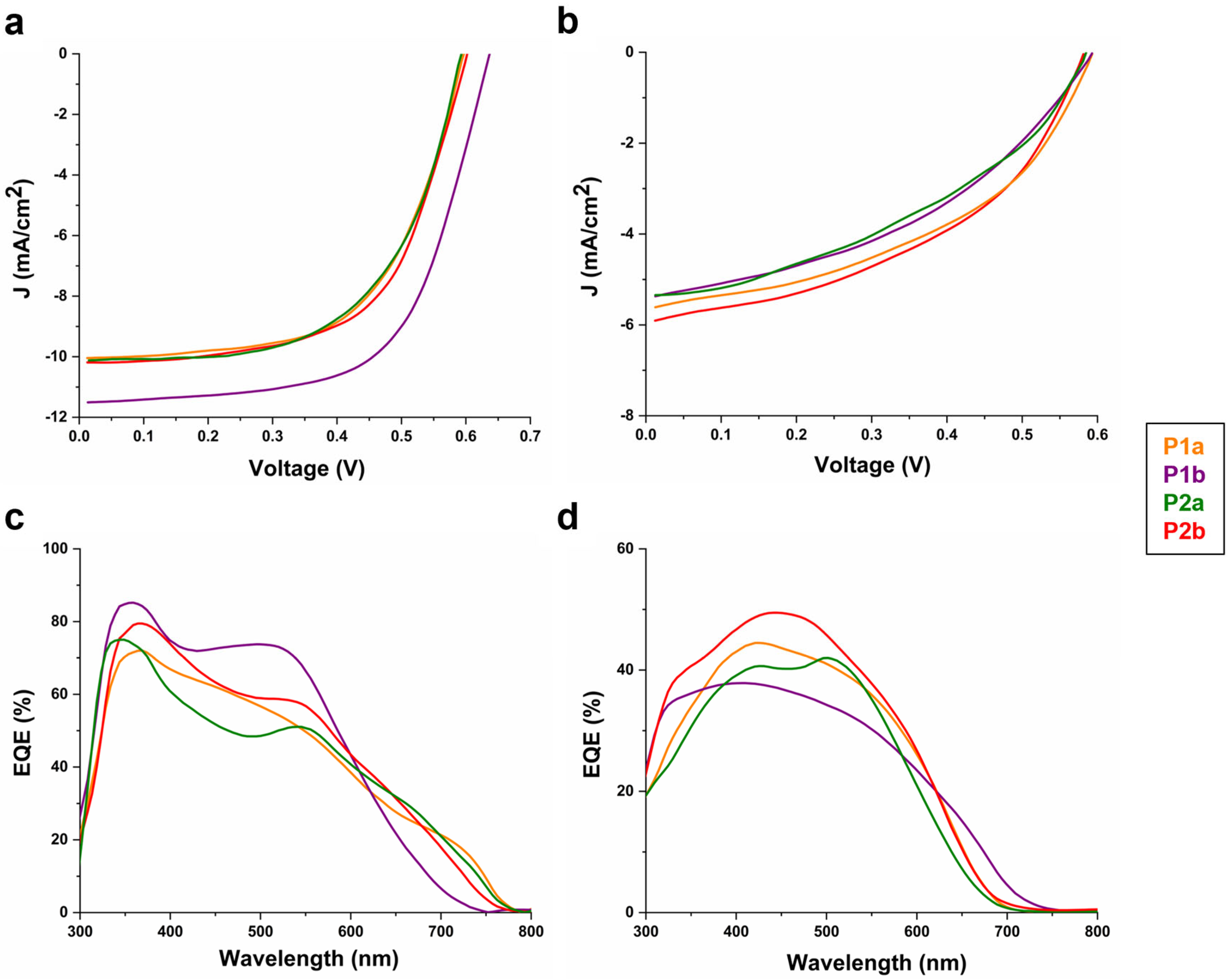
3.7. Organic Solar Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amna, B.; Siddiqi, H.M.; Hassan, A.; Ozturk, T. Recent Development in the Synthesis of Regioregular Thiophene-Based Conjugated Polymers for Electronic and Optoelectronic Application Using Nickel and Palladium-Based Catalytic Systems. RSC Adv. 2020, 10, 4322–4396. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, S.; Kim, G.U.; Lee, W.; Kim, B.J. Recent Advances, Design Guidelines, and Prospects of All-Polymers Solar Cells. Chem. Rev. 2019, 119, 8028–8086. [Google Scholar] [CrossRef] [PubMed]
- Pathiranage, T.M.S.K.; Dissanayake, D.S.; Niermann, C.N.; Ren, Y.; Biewer, M.C.; Stefan, M.C. Role of Polythiophenes as Electroactive Materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3327–3346. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Welsh, T.A.; Draper, E.R. Water-Soluble Organic Electrochromic Materials. RSC Adv. 2021, 11, 5245–5264. [Google Scholar] [CrossRef]
- Lanzi, M.; Salatelli, E.; Giorgini, L.; Mucci, A.; Pierini, F.; Di Nicola, F.P. Water-Soluble Polythiophenes as Efficient Charge-Transport Layers for the Improvement of Photovoltaic Performance in Bulk Heterojunction Polymeric Solar Cells. Eur. Polym. J. 2017, 97, 378–388. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, K.; Zhong, C.; Huang, F.; Cao, Y. Recent Advances in Water/Alcohol-Soluble π-Conjugated Materials: New Materials and Growing Applications in Solar Cells. Chem. Soc. Rev. 2013, 42, 9071–9104. [Google Scholar] [CrossRef]
- Schmatz, B.; Yuan, Z.; Lang, A.W.; Hernandez, J.L.; Reichmanis, E.; Reynolds, J.R. Aqueous Processing for Printed Organic Electronics: Conjugated Polymers with Multistage Cleavable Side Chains. ACS Cent. Sci. 2017, 3, 961–967. [Google Scholar] [CrossRef]
- Ghoos, T.; Brassinne, J.; Fustin, C.; Gohy, J.; Defour, M.; Van den Brande, N.; Van Mele, B.; Lutsen, L.; Vanderzande, D.J.; Maes, W. Imidazolium-Substituted Ionic (Co) Polythiophenes: Compositional Influence on Solution Behavior and Thermal Properties. Polymer 2013, 54, 6293–6304. [Google Scholar] [CrossRef]
- Govaerts, S.; Kesters, J.; Defour, M.; Van Mele, B.; Penxten, H.; Neupane, S.; Renner, F.U.; Lutsen, L.; Vanderzande, D.; Maes, W. Conjugated Ionic (Co) Polythiophene-Based Cathode Interlayers for Bulk Heterojunction Organic Solar Cells. Eur. Polym. J. 2017, 97, 49–56. [Google Scholar] [CrossRef]
- Schmode, P.; Savva, A.; Kahl, R.; Ohayon, D.; Meichsner, F.; Dolynchuk, O.; Thurn-Albrecht, T.; Inal, S.; Thelakkat, M. The Key Role of Side Chain Linkage in Structure Formation and Mixed Conduction of Ethylene Glycol Substituted Polythiophenes. ACS Appl. Mater. Interfaces 2020, 12, 13029–13039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, B.; Sekine, J.; Luo, S.C.; Yu, H.H. Oligoethylene-Glycol Functionalized Polyoxythiophenes for Cell Engineering: Synthesis, Characterization, and Cell Compatibilities. ACS Appl. Mater. Interfaces 2012, 4, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Carreon, A.C.; Santos, W.L.; Matson, J.B.; So, R.C. Cationic Polythiophenes as Responsive DNA-Binding Polymers. Polym. Chem. 2014, 5, 314–317. [Google Scholar] [CrossRef]
- Fossépré, M.; Trévisan, M.E.; Cyriaque, V.; Wattiez, R.; Beljonne, D.; Richeter, S.; Clément, S.; Surin, M. Detection of the Enzymatic Cleavage of DNA Through Supramolecular Chiral Induction to a Cationic Polythiophene. ACS Appl. Bio Mater. 2019, 2, 2125–2136. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Wang, S. Water-Soluble Conjugated Organic Molecules as Optical Electrochemical Materials for Interdisciplinary Biological Applications. Acc. Chem. Res. 2019, 52, 3211–3222. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, D.P.; Ghosh, R.; Nandi, A.K. Water-Soluble Polythiophenes: Preparation and Applications. RSC Adv. 2015, 5, 20160–20177. [Google Scholar] [CrossRef]
- Kesters, J.; Govaerts, S.; Pirotte, G.; Drijkoningen, J.; Chevrier, M.; Van den Brande, N.; Liu, X.; Fahlman, M.; Van Mele, B.; Lutsen, L.; et al. High-Permittivity Conjugated Polyelectrolyte Interlayers for High-Performance Bulk Heterojunction Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 6309–6314. [Google Scholar] [CrossRef]
- Kesters, J.; Ghoos, T.; Penxten, H.; Drijkoningen, J.; Vangerven, T.; Lyons, D.M.; Verreet, B.; Aernouts, T.; Lutsen, L.; Vanderzande, D.; et al. Imidazolium-Substituted Polythiophene as Efficient Electron Transport Materials Improving Photovoltaic Performance. Adv. Energy Mater. 2013, 3, 1180–1185. [Google Scholar] [CrossRef]
- Lassi, E.; Squeo, B.M.; Sorrentino, R.; Scavia, G.; Mrakic-Sposta, S.; Gussoni, M.; Vercelli, B.; Galeotti, F.; Pasini, M.; Luzzati, S. Sulfonate-Conjugated Polyelectrolytes as Anode Interfacial Layers in Inverted Organic Solar Cells. Molecules 2021, 26, 763. [Google Scholar] [CrossRef]
- Campana, F.; Kim, C.; Marrocchi, A.; Vaccaro, L. Green Solvent-Processed Organic Electronic Devices. J. Mater. Chem. C 2020, 8, 15027–15047. [Google Scholar] [CrossRef]
- Dong, B.X.; Liu, Z.; Onorato, J.W.; Ma, T.; Strzalka, J.; Bennington, P.; Luscombe, C.K.; Ober, C.K.; Nealey, P.F.; Patel, S.N. Ionic Dopant-Induced Ordering Enhances the Thermoelectric Properties of a Polythiophene-Based Block Copolymer. Adv. Funct. Mater. 2021, 31, 2106991. [Google Scholar] [CrossRef]
- Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, A.W. Ab Initio Molecular Dynamics Assessment on the Mixed Ionic−Electronic Transport for Crystalline Poly(3-Hexylthiophene) Using Full Explicit Lithium-Based Dopants and Additives. Macromolecules 2022, 55, 113–124. [Google Scholar] [CrossRef]
- Danielsen, S.P.O.; Thompson, B.J.; Fredrickson, G.H.; Nguyen, T.-Q.; Bazan, G.C.; Segalman, R.A. Ionic Tunability of Conjugated Polyelectrolyte Solutions. Macromolecules 2022, 55, 3437–3448. [Google Scholar] [CrossRef]
- Marinelli, M.; Lanzi, M.; Liscio, A.; Zanelli, A.; Zangoli, M.; Di Maria, F.; Salatelli, E. Single-Material Organic Solar Cells with Fully Conjugated Electron-Donor Alkoxy-Substituted Bithiophene Units and Electron-Acceptor Benzothiadiazole Moieties Alternating in the Main Chain. J. Mater. Chem. C 2020, 8, 4124–4132. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Sui, X.; Wu, H.; Wang, C.; Feng, G.; Wu, Y.; Liu, F.; Liu, X.; Tang, Z.; et al. Crystalline Cooperativity of Donor and Acceptor Segments in Double-Cable Conjugated Polymers Toward Efficient Single-Component Organic Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 15532–15540. [Google Scholar] [CrossRef]
- Roncali, J.; Grosu, I. The Dawn of Single Material Organic Solar Cells. Adv. Sci. 2019, 6, 1801026. [Google Scholar] [CrossRef]
- Hou, J.; Inganas, O.; Friend, R.H.; Gao, F. Organic Solar Cells Based on Non-Fullerene Acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Karki, A.; Gillett, A.J.; Friend, R.H.; Nguyen, T.-Q. The Path to 20% Power Conversion Efficiencies in Nonfullerene Acceptor Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2003441. [Google Scholar] [CrossRef]
- Kumada, M.; Tamao, K. Aliphatic Organopolysilanes. Adv. Organomet. Chem. 1968, 6, 19–117. [Google Scholar]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R.F.; Bassler, H.; Porsch, M.; Daub, J. Efficient Two Layer Leds on a Polymer Blend Basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- Andrade, B.W.D.; Datta, S.; Forrest, S.R.; Djurovich, P.; Polikarpov, E.; Thompson, M.E. Relationship Between the Ionization and Oxidation Potentials of Molecular Organic Semiconductors. Org. Electron. 2005, 6, 11–20. [Google Scholar] [CrossRef]
- Namazian, M.; Lin, C.Y.; Coote, M.L. Benchmark Calculations of Absolute Reduction Potential of Ferrocinium/Ferrocene Couple in Nonaqueous Solutions. J. Chem. Theory Comput. 2010, 6, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, M.; Quadretti, D.; Marinelli, M.; Ziai, Y.; Salatelli, E.; Pierini, F. Influence of the Active Layer Structure on the Photovoltaic Performance of Water-Soluble Polythiophene-Based Solar Cells. Polymers 2021, 13, 1640. [Google Scholar] [CrossRef] [PubMed]
- Di Maria, F.; Biasiucci, M.; Di Nicola, F.P.; Fabiano, E.; Zanelli, A.; Gazzano, M.; Salatelli, E.; Lanzi, M.; Della Sala, F.; Gigli, G.; et al. Nanoscale Characterization and Unexpected Photovoltaic Behavior of Low Band Gap Sulfur-Overrich-Thiophene/Benzothiadiazole Decamers and Polymers. J. Phys. Chem. C 2015, 119, 27200–27211. [Google Scholar] [CrossRef]
- Hladysh, S.; Murmiliuk, A.; Vohlídal, J.; Havlíček, D.; Sedlařík, V.; Štěpánek, M.; Zedník, J. Combination of Phosphonium and Ammonium Pendant Groups in Cationic Conjugated Polyelectrolytes Based on Regioregular Poly(3-hexylthiophene) Polymer Chains. Eur. Polym. J. 2018, 100, 200–208. [Google Scholar] [CrossRef]
- Lanzi, M.; Salatelli, E.; Marinelli, M.; Pierini, F. Effect of Photocrosslinking of D-A Thiophene Copolymers on the Performance of Single-Material Solar Cells. Macromol. Chem. Phys. 2020, 221, 1900433. [Google Scholar] [CrossRef]
- Iraqi, A.; Crayston, J.A.; Walton, J.C. Synthesis, Spectroelectrochemistry and Thermochromism of Regioregular Head-to-tail Oligo- and Poly-[3-aryloxyhexylthiophenes]. J. Mater. Chem. 1995, 5, 1831–1836. [Google Scholar] [CrossRef]
- Della Casa, C.; Costa Bizzari, P.; Lanzi, M.; Bertinelli, F. Clear Evidence of the Sensitivity of the Solvatochromic Effect to Side Chain Functionalization in 3-Hexyl-substituted Polythiophenes. Acta Polym. 1997, 48, 251–255. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Lai, Y.; Williams, R.V. N-Bromosuccinimide-Dimethylformamide: A Mild, Selective Nuclear Monobromination Reagent for Reactive Aromatic Compounds. J. Org. Chem. 1979, 44, 4733–4735. [Google Scholar] [CrossRef]
- Wang, J.; Ye, H.; Li, H.; Mei, C.; Ling, J.; Li, W.; Shen, Z. Donor-Acceptor Oligomers and Polymers Composed of Benzothiadiazole and 3-Hexylthiophene: Effect of Chain Length and Regioregularity. Chin. J. Chem. 2013, 31, 1367–1379. [Google Scholar] [CrossRef]
- Lanzi, M.; Salatelli, E.; Giorgini, L.; Marinelli, M.; Pierini, F. Effect of the Incorporation of an Ag Nanoparticle Interlayer on the Photovoltaic Performance of Green Bulk Heterojunction Water-soluble Polythiophene Solar Cells. Polymer 2018, 149, 273–285. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A Survey of Solvent Selection Guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Brendel, J.C.; Schmidt, M.M.; Hagen, G.; Moos, R.; Thelakkat, M. Controlled Synthesis of Water-Soluble Conjugated Polyelectrolytes Leading to Excellent Hole Transport Mobility. Chem. Mater. 2014, 26, 1992–1998. [Google Scholar] [CrossRef]
- Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, A.W. Unraveling the Lithium Bis(trifluoromethanesulfonyl)imide (LiTFSI) Doping Mechanism of Regioregular Poly(3-hexylthiophene): Experimental and Theoretical Study. J. Phys. Chem. C 2020, 124, 7061–7070. [Google Scholar] [CrossRef]
- Trasatti, S. The Absolute Electrode Potential: An Explanatory Note. Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Gritzr, G.; Kuta, J. Recommendations on Reporting Electrode Potentials in Nonaqueous Solutions. Pure Appl. Chem. 1984, 56, 461–466. [Google Scholar] [CrossRef]
- Deng, L.L.; Xia, S.L.; Yuan, C.; Lu, R.F.; Feng, J.; Shun, L.C.; Lu, X.; Xie, S.Y.; Huang, R.B.; Zheng, L.S. High LUMO Energy Level C60(OCH3)4 Derivatives: Electronic Acceptors for Photovoltaic Cells with Higher Open-circuit Voltage. Sol. Energy Mater. Sol. Cells 2013, 111, 193–199. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Wang, M. Synthesis of Conjugated Polymers Via an Exclusive Direct-Arylation Coupling Reaction: A Facile and Straightforward Way to Synthesize Thiophene-Flanked Benzothiadiazole Derivatives and Their Copolymers. Polym. Chem. 2015, 6, 1846–1855. [Google Scholar] [CrossRef]
- El-Shehawy, A.A.; Abdo, N.I.; El-Barbary, A.A.; Choi, J.W.; El-Sheshtawy, H.S.; Lee, J. Thiophene, Benzothiadiazole Copolymers: Synthesis, Optoelectronic Properties and Electrical Characterization for Photovoltaic Application. J. Mater. Sci. Nanomater. 2018, 2, 103. [Google Scholar]
- Marinelli, M.; Candini, A.; Monti, F.; Boschi, A.; Zangoli, M.; Salatelli, E.; Pierini, F.; Lanzi, M.; Zanelli, A.; Gazzano, M.; et al. Push-Pull Thiophene-Based Small Molecules with Donor and Acceptor Units of Varying Strength for Photovoltaic Application: Beyond P3HT and PCBM. J. Mater. Chem. C 2021, 9, 11216–11228. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, M.; Fan, Z.; Deng, L.; Lu, Y. Direct (Hetero) Arylation Polymerization, Electrochemical and Optical Properties of Regioregular 3-Substituted Polythiophenes with Alkylsulphanyl and Alkylsulfonyl Groups. Eur. Polym. J. 2022, 166, 111032. [Google Scholar] [CrossRef]
- Dkhissi, A. Exciton in Organic Semiconductors. Synth. Met. 2011, 161, 1441–1443. [Google Scholar] [CrossRef]
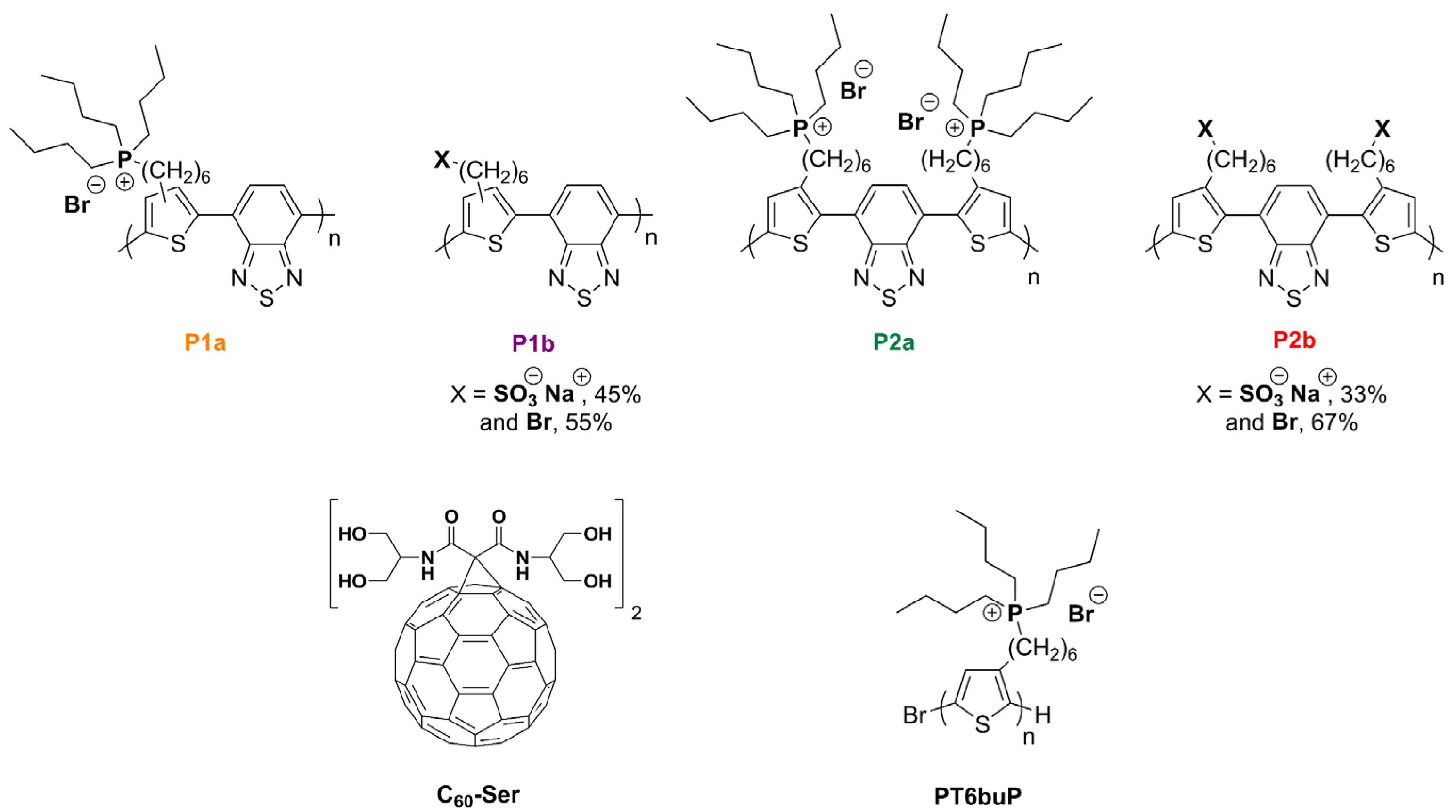
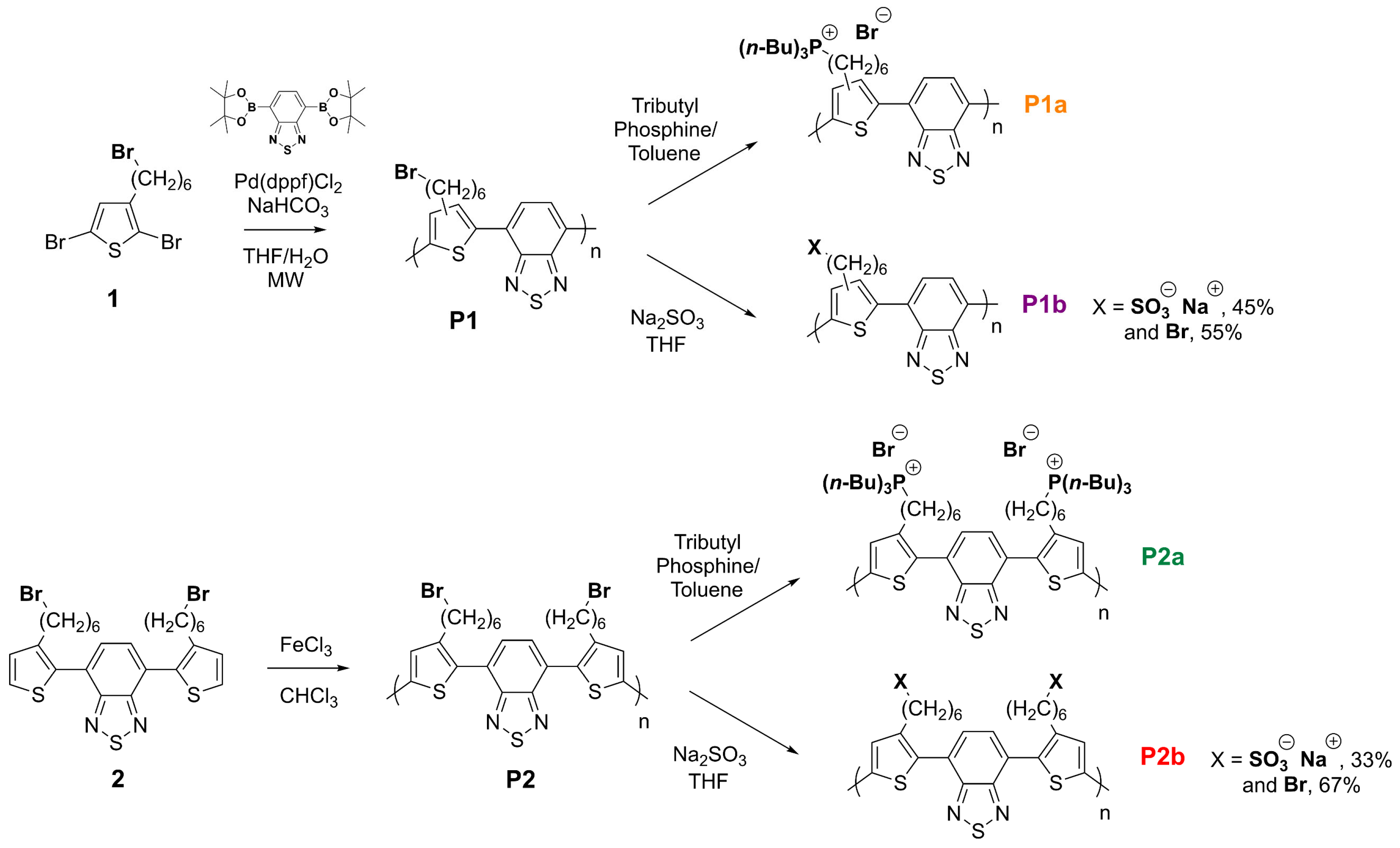
| Reaction Yields (%) | Ionic Group Content (% mol) [a] | Mn (Da) | Xn | Ð | Td (°C) [d] | Tg (°C) [e] | |
|---|---|---|---|---|---|---|---|
| P1 | 77 | 0 | 2500 [b] | 6.6 [b] | 1.7 [b] | 206 | 68 |
| P1a | 86 | 100 | 3800 [c] | 6.6 [b] | 1.7 [b] | 248 | 49 |
| P1b | 79 | 45 | 2600 [c] | 6.6 [b] | 1.7 [b] | 218 | 63 |
| P2 | 82 | 0 | 15,600 [b] | 25.0 [b] | 1.3 [b] | 273 | - |
| P2a | 89 | 100 | 23,700 [c] | 25.0 [b] | 1.3 [b] | 305 | 89 |
| P2b | 81 | 33 | 15,900 [c] | 25.0 [b] | 1.3 [b] | 272 | - |
| Eox (V vs. SCE) | Ered (V vs. SCE) | HOMO (eV) | LUMO (eV) | (eV) | |
|---|---|---|---|---|---|
| P1a [a] | 1.04 | −1.01 | −5.28 | −3.23 | 2.05 |
| P1b [b] | 1.08 | −1.11 | −5.50 | −3.31 | 2.19 |
| P2a [a] | 1.02 | −1.10 | −5.26 | −3.14 | 2.12 |
| P2b [b] | 0.99 | −1.22 | −5.41 | −3.20 | 2.21 |
| C60-Ser [a] | - | −0.37 | - | −3.87 | - |
| PT6buP [a] | 0.54 | - | −4.78 | - | - |
| Solution | Film | |||
|---|---|---|---|---|
| Solvent | λmax (nm) [a] | λmax(nm) [a] | (eV) [b] | |
| P1 | CHCl3 | 311, 501 | 320, 545 | 1.84 |
| P1a | EtOH | 314, 505 | 323, 529 | 1.81 |
| P1b | Anisole | 320, 508 | 323, 525 | 1.84 |
| P2 | CHCl3 | 315, 347, 477 | 310, 360, 509 | 1.95 |
| P2a | EtOH | 316, 352, 485 | 320, 354, 501 | 1.98 |
| P2b | Anisole | 349, 479 | 323, 366, 490 | 1.97 |
| Solvent | Jsc (mA/cm2) [a] | Jsc (mA/cm2) [b] | Voc (V) [c] | FF [d] | PCE (%) [e] | |
|---|---|---|---|---|---|---|
| P1a: C60-Ser | EtOH/MeOH | 10.10 ± 0.97 | 9.39 | 0.59 ± 0.01 | 0.58 ± 0.02 | 3.45 ± 0.31 |
| P1b: C60-Ser | Anisole/MeOH | 11.60 ± 1.02 | 12.10 | 0.64 ± 0.01 | 0.64 ± 0.03 | 4.76 ± 0.42 |
| P2a: C60-Ser | EtOH/MeOH | 10.20 ± 0.95 | 9.69 | 0.60 ± 0.01 | 0.58 ± 0.02 | 3.51 ± 0.35 |
| P2b: C60-Ser] | Anisole/MeOH | 10.40 ± 0.88 | 9.61 | 0.60 ± 0.01 | 0.60 ± 0.01 | 3.74 ± 0.32 |
| P1a: PT6buP | EtOH | 5.68 ± 0.62 | 5.01 | 0.59 ± 0.01 | 0.57 ± 0.03 | 1.91 ± 0.22 |
| P1b: PT6buP | Anisole/EtOH | 5.40 ± 0.61 | 4.89 | 0.59 ± 0.01 | 0.55 ± 0.05 | 1.75 ± 0.21 |
| P2a: PT6buP | EtOH | 5.32 ± 0.58 | 4.87 | 0.58 ± 0.01 | 0.55 ± 0.03 | 1.71 ± 0.28 |
| P2b: PT6buP | Anisole/EtOH | 5.95 ± 0.64 | 5.53 | 0.58 ± 0.01 | 0.56 ± 0.04 | 1.93 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinelli, M.; Lanzi, M.; Pierini, F.; Ziai, Y.; Zanelli, A.; Quadretti, D.; Di Maria, F.; Salatelli, E. Ionic Push–Pull Polythiophenes: A Further Step towards Eco-Friendly BHJ Organic Solar Cells. Polymers 2022, 14, 3965. https://doi.org/10.3390/polym14193965
Marinelli M, Lanzi M, Pierini F, Ziai Y, Zanelli A, Quadretti D, Di Maria F, Salatelli E. Ionic Push–Pull Polythiophenes: A Further Step towards Eco-Friendly BHJ Organic Solar Cells. Polymers. 2022; 14(19):3965. https://doi.org/10.3390/polym14193965
Chicago/Turabian StyleMarinelli, Martina, Massimiliano Lanzi, Filippo Pierini, Yasamin Ziai, Alberto Zanelli, Debora Quadretti, Francesca Di Maria, and Elisabetta Salatelli. 2022. "Ionic Push–Pull Polythiophenes: A Further Step towards Eco-Friendly BHJ Organic Solar Cells" Polymers 14, no. 19: 3965. https://doi.org/10.3390/polym14193965
APA StyleMarinelli, M., Lanzi, M., Pierini, F., Ziai, Y., Zanelli, A., Quadretti, D., Di Maria, F., & Salatelli, E. (2022). Ionic Push–Pull Polythiophenes: A Further Step towards Eco-Friendly BHJ Organic Solar Cells. Polymers, 14(19), 3965. https://doi.org/10.3390/polym14193965