Antimicrobial Efficacy of Quercetin against Vibrio parahaemolyticus Biofilm on Food Surfaces and Downregulation of Virulence Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Culture and Growth Conditions
2.2. Preparation of Samples (Crabs and Shrimp)
2.3. Quercetin Preparation and Determination of Minimum Inhibitory Concentration (MIC)
2.4. Analysis of Motility
2.5. Biofilm Formation and Detachment Process
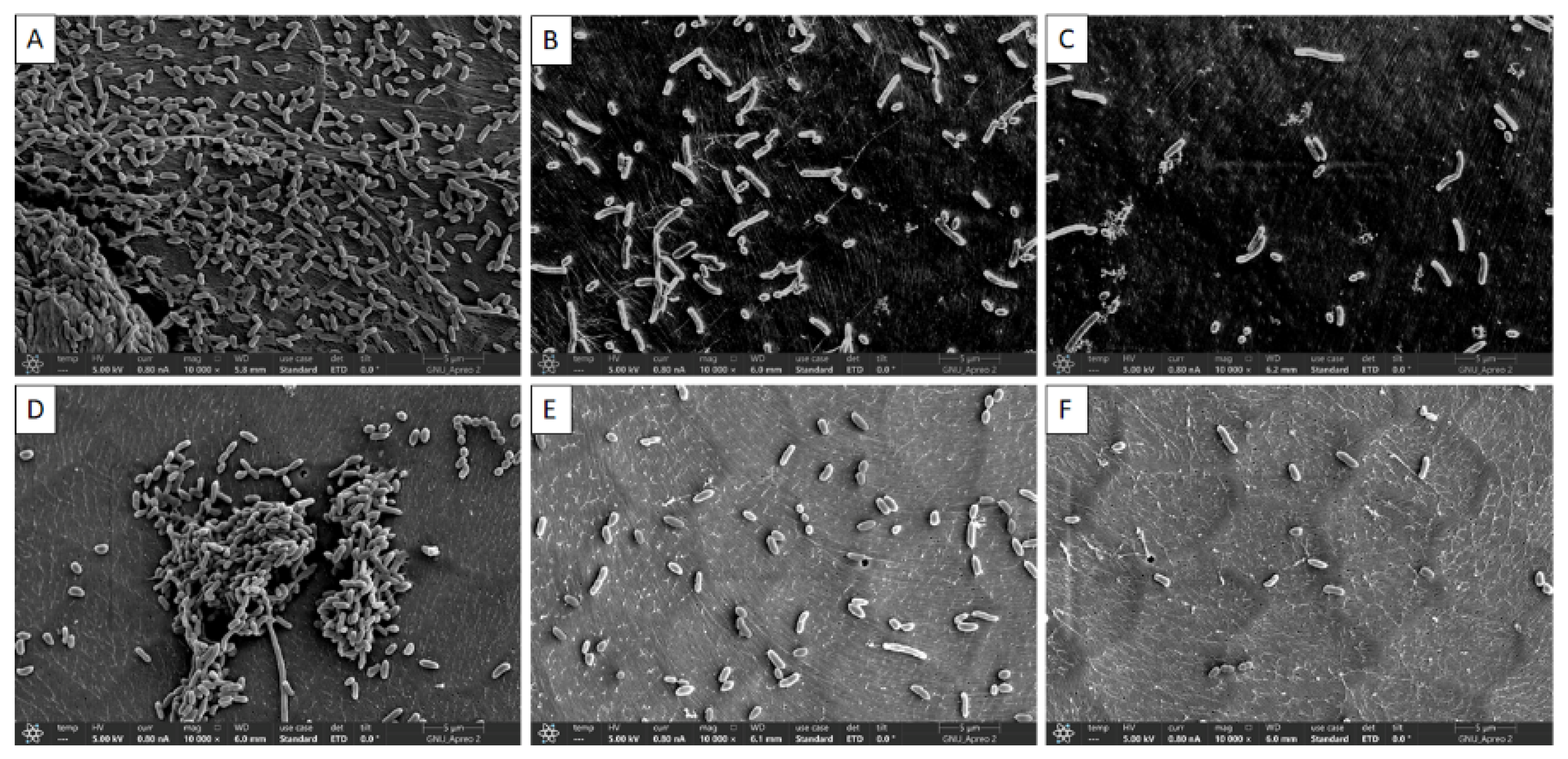
2.6. Confirmation of Biofilms Inhibition by FE-SEM
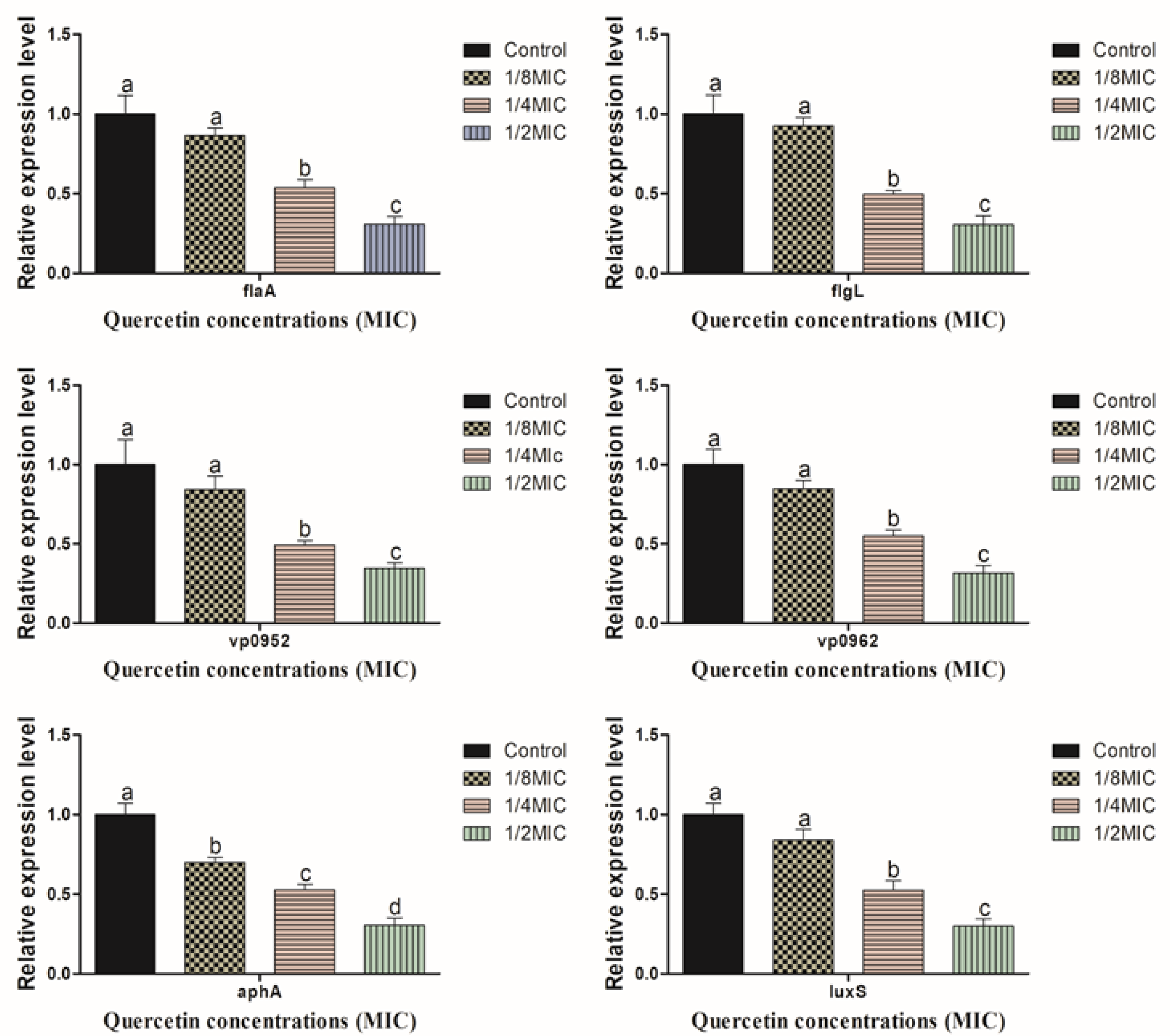
2.7. Relative Gene Expression by Real-Time PCR (RT-PCR)
2.8. Statistical Analysis
3. Results
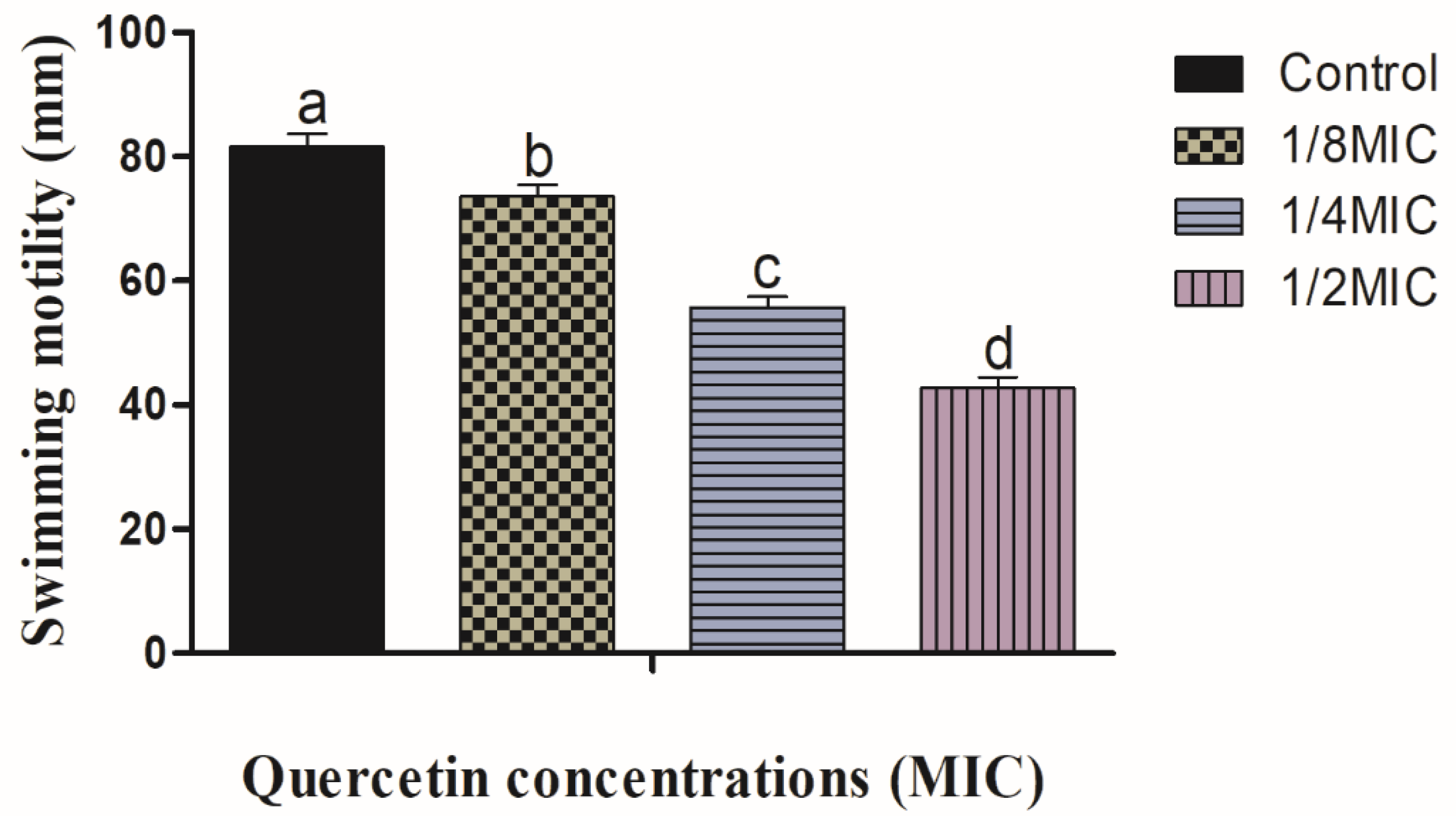
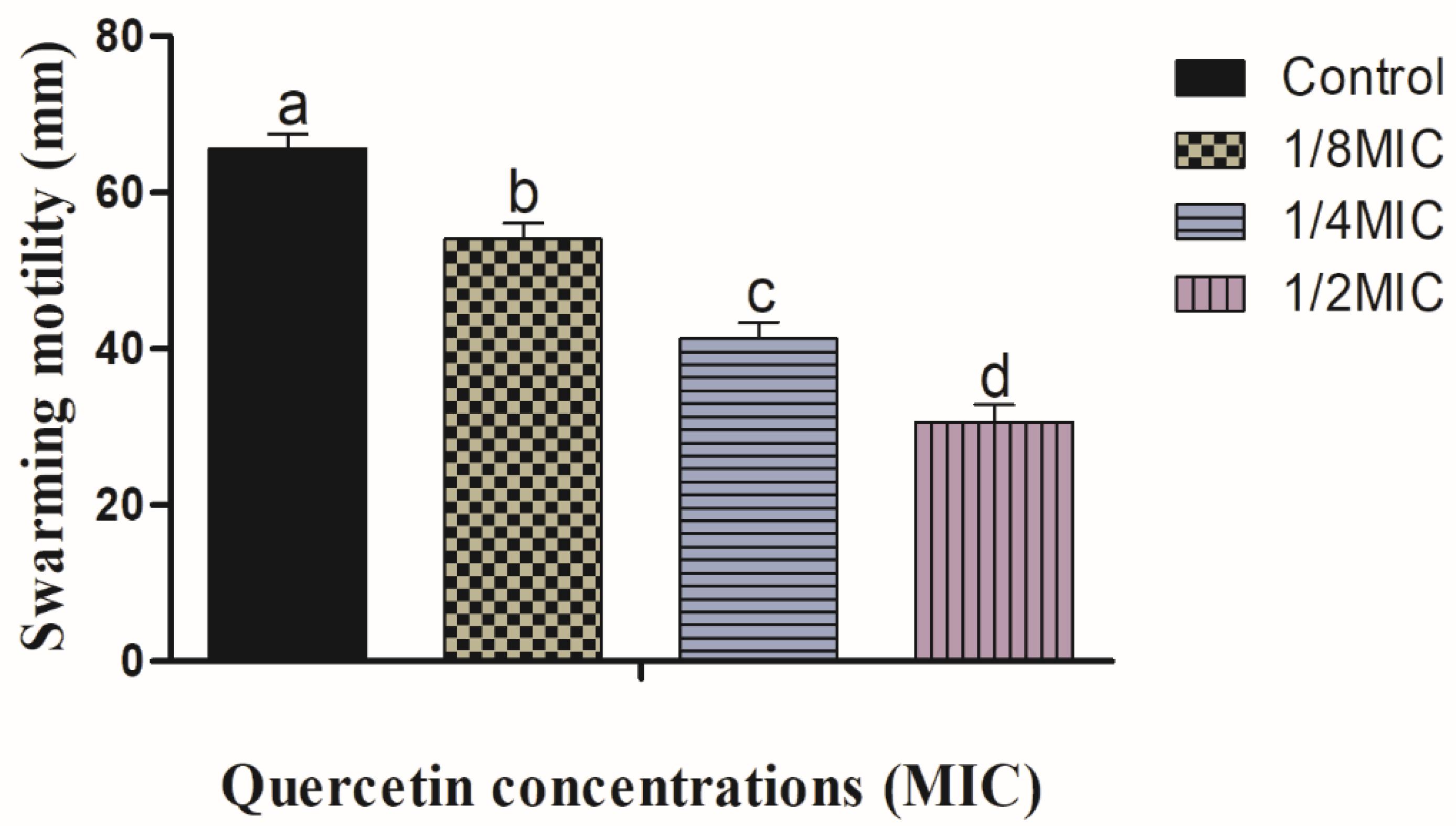
3.1. Swimming and Swarming Motility Assays
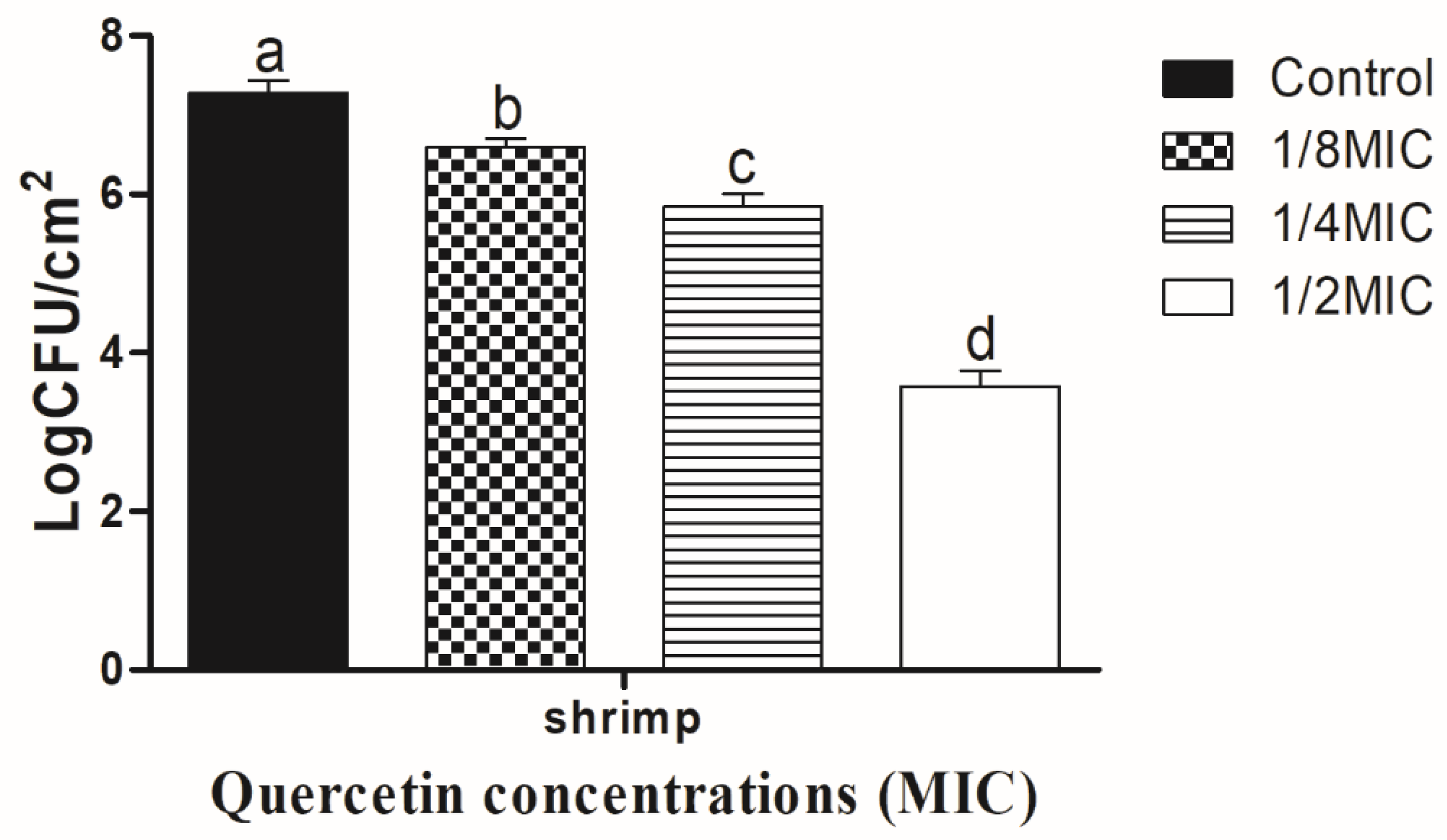
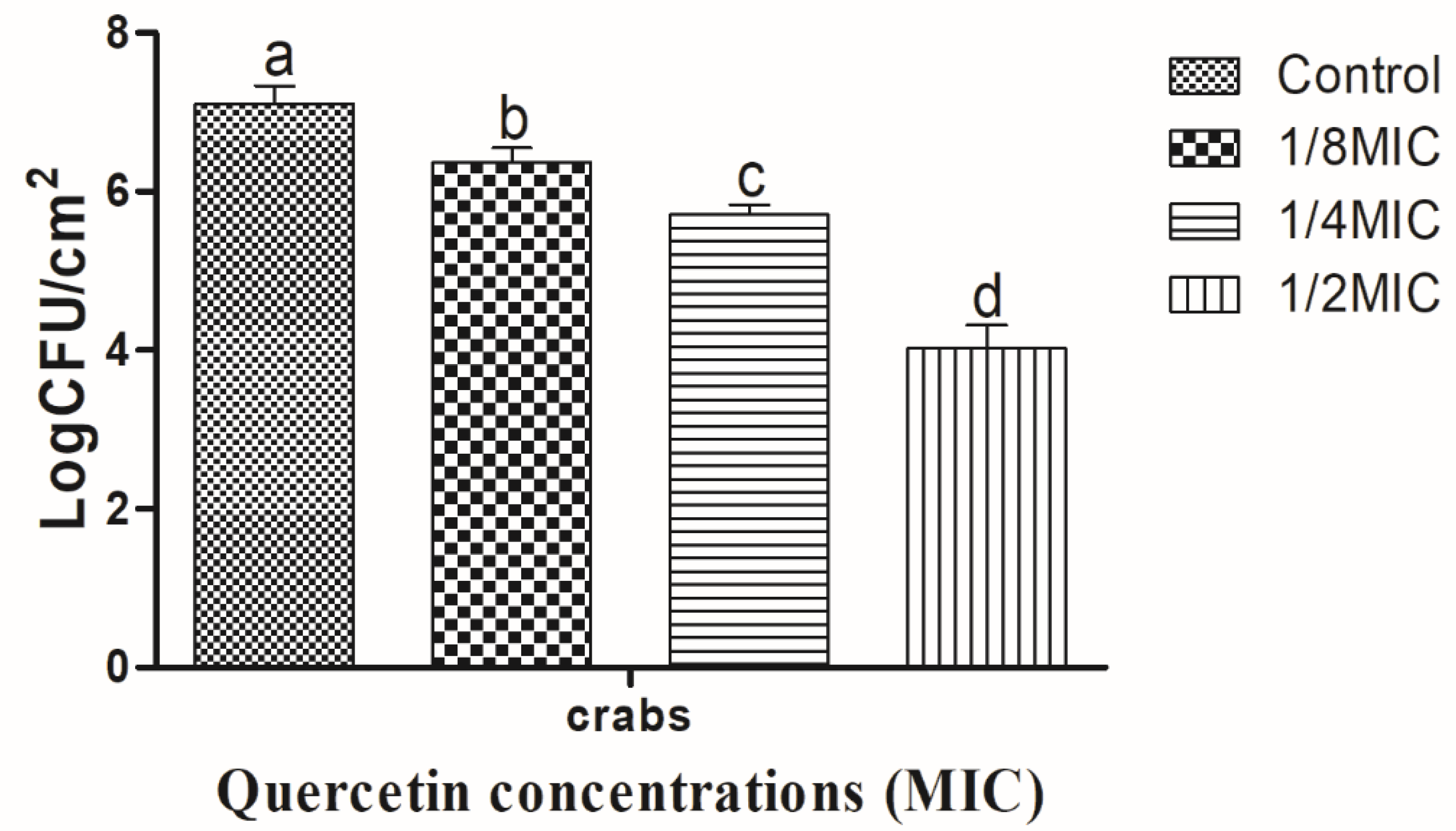
3.2. Eradication Effect of Food Additive Quercetin on Shrimp and Crab Shell Surfaces against V. parahaemolyticus
3.3. Visual Confirmation of Biofilm Reduction by Quercetin under FE-SEM
3.4. Motility, Biofilm Forming, Virulence, and QS Sensing Relative Gene Expression Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selamoglu, M. Importance of the cold chain logistics in the marketing process of aquatic products: An update study. Surv. Fish. Sci. 2021, 8, 25–29. [Google Scholar] [CrossRef]
- Toushik, S.H.; Kim, K.; Ashrafudoulla, M.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Kim, Y.; Ha, S.D. Korean kimchi-derived lactic acid bacteria inhibit foodborne pathogenic biofilm growth on seafood and food processing surface materials. Food Control 2021, 129, 108276. [Google Scholar] [CrossRef]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Fact. 2016, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Song, X.; Zhang, Z.; Fu, J.; Wang, X.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. Removal of foodborne pathogen biofilms by acidic electrolyzed water. Front. Microbiol. 2017, 8, 988. [Google Scholar] [CrossRef] [PubMed]
- Alabdullatif, M.; Atreya, C.D.; Ramirez-Arcos, S. Antimicrobial peptides: An effective approach to prevent bacterial biofilm formation in platelet concentrates. Transfusion 2018, 58, 2013–2021. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alamo, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhu, W.X.; Cao, Y.; Gao, J.Z.; Jin, T.; Qin, N.B.; Xia, X.D. Punicalagin inhibits biofilm formation and virulence gene expression of Vibrio parahaemolyticus. Food Control 2022, 139, 109045. [Google Scholar] [CrossRef]
- Ralph, A.; Currie, B.J. Vibrio vulnificus and V. parahaemolyticus necrotising fasciitis in fishermen visiting an estuarine tropical northern Australian location. J. Infect. 2007, 54, e111–e114. [Google Scholar] [CrossRef]
- Park, K.S.; Ono, T.; Rokuda, M.; Jang, M.H.; Okada, K.; Iida, T.; Honda, T. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 2004, 72, 6659–6665. [Google Scholar] [CrossRef]
- FAO. Advances in Science and Risk Assessment Tools for Vibrio parahaemolyticus and V. vulnificus Associated with Seafood; Meeting Report; Food & Agriculture Organization: Rome, Italy, 2021; Volume 35. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Listeria Surveillance Annual Summary, 2013; Department of Health and Human Services, CDC: Atlanta, GA, USA, 2015.
- Elexson, N.; Afsah-Hejri, L.; Rukayadi, Y.; Soopna, P.; Lee, H.Y.; Zainazor, T.C.T.; Ainy, M.N.; Nakaguchi, Y.; Mitsuaki, N.; Son, R. Effect of detergents as antibacterial agents on biofilm of antibiotics-resistant Vibrio parahaemolyticus isolates. Food Control 2014, 35, 378–385. [Google Scholar] [CrossRef]
- Reddy, P. Empiric antibiotic therapy of nosocomial bacterial infections. Am. J. Ther. 2016, 23, E982–E994. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; El Bayomi, R.M.; Hussein, M.A.; Khedr, M.H.E.; Remela, E.M.A.; El-Ashram, A.M.M. Molecular characterization, antibiotic resistance pattern and biofilm formation of Vibrio parahaemolyticus and Vibrio cholerae isolated from crustaceans and humans. Int. J. Food Microbiol. 2018, 274, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Rukayadi, Y.; Hasan, H.; Thung, T.Y.; Lee, E.; Rollon, W.D.; Hara, H.; Kayali, A.Y.; Nishibuchi, M.; Radu, S. Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia. Saudi J. Biol. Sci. 2020, 27, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.F.; Wu, Q.P.; Zhang, J.M.; Xu, X.K.; Cheng, J.H. Comparison of Vibrio parahaemolyticus isolates from aquatic products and clinical by antibiotic susceptibility, virulence, and molecular characterisation. Food Control 2017, 71, 315–321. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcus, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Brannon, J.R.; Hadjifrangiskou, M. The arsenal of pathogens and antivirulence therapeutic strategies for disarming them. Drug Des. Dev. Ther. 2016, 10, 1795–1806. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.; Hernandez-Oñate, M.; Martinez-Tellez, M.; Lopez-Zavala, A.; Gonzalez-Aguilar, G.; Gutierrez-Pacheco, M.; Ayala-Zavala, J. Quercetin repressed the stress response factor (sigB) and virulence genes (prfA, actA, inlA, and inlC), lower the adhesion, and biofilm development of L. monocytogenes. Food Microbiol. 2019, 87, 103377. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.Y.; Ma, L.; Qian, Y.L.; Liu, Z.Y. Combined anti-biofilm enzymes strengthen the eradicate effect of Vibrio parahaemolyticus biofilm: Mechanism on cpsA-J expression and application on different carriers. Foods 2022, 11, 1305. [Google Scholar] [CrossRef]
- Meireles, A.; Borges, A.; Giaouris, E.; Simoes, M. The current knowledge on the application of anti-biofilm enzymes in the food industry. Food Res. Int. 2016, 86, 140–146. [Google Scholar] [CrossRef]
- Malone, M.; Goeres, D.M.; Gosbell, I.; Vickery, K.; Jensen, S.; Stoodley, P. Approaches to biofilm-associated infections: The need for standardized and relevant biofilm methods for clinical applications. Expert Rev. Anti-Infect. Ther. 2017, 15, 147–156. [Google Scholar] [CrossRef]
- Nahar, S.; Mizan, M.F.R.; Ha, A.J.W.; Ha, S.D. Advances and future prospects of enzyme-based biofilm prevention Approaches in the food industry. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1484–1502. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Mizan, M.F.R.; Jahid, I.K.; Ha, S.D. Biofilm formation by Vibrio parahaemolyticus on food and food contact surfaces increases with rise in temperature. Food Control 2016, 70, 161–166. [Google Scholar] [CrossRef]
- Malcolm, T.T.H.; Chang, W.S.; Loo, Y.Y.; Cheah, Y.K.; Radzi, C.W.J.W.M.; Kantilal, H.K.; Nishibuchi, M.; Son, R. Simulation of improper food hygiene practices: A quantitative assessment of Vibrio parahaemolyticus distribution. Int. J. Food Microbiol. 2018, 284, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Bai, L.; Li, W.W.; Han, H.H.; Fu, P.; Ma, X.C.; Bi, Z.W.; Yang, X.R.; Zhang, X.L.; Zhen, S.Q.; et al. Trends of foodborne diseases in China: Lessons from laboratory-based surveillance since 2011. Front. Med. 2018, 12, 48–57. [Google Scholar] [CrossRef]
- Mizan, M.F.R.; Jahid, I.K.; Kim, M.; Lee, K.H.; Kim, T.J.; Ha, S.D. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation. Biofouling 2016, 32, 497–509. [Google Scholar] [CrossRef]
- Elgamoudi, B.A.; Korolik, V. Campylobacter biofilms: Potential of natural compounds to disrupt Campylobacter jejuni transmission. Int. J. Mol. Sci. 2021, 22, 12159. [Google Scholar] [CrossRef]
- Ramić, D.; Ogrizek, J.; Bucar, F.; Jeršek, B.; Jeršek, M.; Možina, S.S. Campylobacter jejuni biofilm control with lavandin essential oils and by-products. Antibiotics 2022, 11, 854. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Ashrafudoulla, M.; Nahar, S.; Joo, H.-J.; Jahid, I.K.; Park, S.H.; Kim, K.-S.; Ha, S.-D. Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBEC™ biofilm device. LWT 2020, 118, 108864. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. Impact of Quercetin against Salmonella typhimurium biofilm formation on food-contact surfaces and molecular mechanism pattern. Foods 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Ashrafudoulla, M.; Mizan, M.F.R.; Ha, A.J.; Park, S.H.; Ha, S.D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef] [PubMed]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2019, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. BioMed Res. Int. 2014, 2014, 761741. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin rich onion extracts on bacterial quorum sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef]
- Hossain, M.I.; Kim, K.; Mizan, M.F.R.; Toushik, S.H.; Ashrafudoulla, M.; Roy, P.K.; Nahar, S.; Jahid, I.K.; Choi, C.; Park, S.H.; et al. Comprehensive molecular, probiotic, and quorum-sensing characterization of anti-listerial lactic acid bacteria, and application as bioprotective in a food (milk) model. J. Dairy Sci. 2021, 104, 6516–6534. [Google Scholar] [CrossRef]
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Cobo, A.; Ortega-Morente, E.; Galvez, A.; Alejo-Armijo, A.; Salido, S.; Altarejos, J. Antimicrobial and antioxidant activities of flavonoids isolated from wood of sweet cherry tree (Prunus avium L.). J. Wood Chem. Technol. 2021, 41, 104–117. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Zhang, X.; Song, Z.C.; Li, L.; Chang, H.S.; Li, S.L.; Zhou, W. Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.Q.; Zeng, H.; Chen, W. Quercetin inhibits biofilm formation by decreasing the production of EPS and altering the composition of EPS in Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 631058. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Jayachitra, A. Targeting biofilm inhibition using Quercetin—Interaction with bacterial cell membrane and ROS mediated biofilm control. Funct. Foods Health Dis. 2018, 8, 292–306. [Google Scholar]
- Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant activity of quercetin: A mechanistic review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. The inhibitory effect of quercetin on biofilm formation of Listeria monocytogenes mixed culture and repression of virulence. Antioxidants 2022, 11, 1733. [Google Scholar] [CrossRef]
- Kost, B.; Svyntkivska, M.; Brzeziński, M.; Makowski, T.; Piorkowska, E.; Rajkowska, K.; Kunicka-Styczyńska, A.; Biela, T. PLA/β-CD-based fibres loaded with quercetin as potential antibacterial dressing materials. Colloids Surf. B Biointerfaces 2020, 190, 110949. [Google Scholar] [CrossRef]
- Debnath, K.; Jana, N.R.; Jana, N.R. Quercetin encapsulated polymer nanoparticle for inhibiting intracellular polyglutamine aggregation. ACS Appl. Bio Mater. 2019, 2, 5298–5305. [Google Scholar] [CrossRef]
- Fraile, M.; Buratto, R.; Gomez, B.; Martin, A.; Cocero, M.J. Enhanced delivery of quercetin by encapsulation in poloxamers by supercritical antisolvent process. Ind. Eng. Chem. Res. 2014, 53, 4318–4327. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current anti-biofilm strategies and potential of antioxidants in biofilm control. Expert Rev. Anti-Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Yao, J.Y.; Zhou, B.; Yang, J.X.; Chaudry, M.T.; Wang, M.; Xiao, F.L.; Li, Y.; Yin, W.Z. Bacteriostatic effect of Quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Roy, P.K.; Mizan, M.F.R.; Hossain, M.I.; Han, N.; Nahar, S.; Ashrafudoulla, M.; Toushik, S.H.; Shim, W.B.; Kim, Y.M.; Ha, S.D. Elimination of Vibrio parahaemolyticus biofilms on crab and shrimp surfaces using ultraviolet C irradiation coupled with sodium hypochlorite and slightly acidic electrolyzed water. Food Control 2021, 128, 108179. [Google Scholar] [CrossRef]
- Roy, P.K.; Ha, A.J.; Mizan, M.F.R.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Nahar, S.; Kim, Y.K.; Ha, S.D. Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poult. Sci. 2021, 100, 101209. [Google Scholar] [CrossRef]
- Cho, J.; Kim, G.; Qamar, A.Y.; Fang, X.; Roy, P.K.; Tanga, B.M.; Bang, S.; Kim, J.K.; Galli, C.; Perota, A.; et al. Improved efficiencies in the generation of multigene-modified pigs by recloning and using sows as the recipient. Zygote 2022, 30, 103–110. [Google Scholar] [CrossRef]
- Roy, P.K.; Qamar, A.Y.; Tanga, B.M.; Bang, S.; Seong, G.; Fang, X.; Kim, G.; Edirisinghe, S.L.; De Zoysa, M.; Kang, D.H.; et al. Modified Spirulina maxima Pectin nanoparticles improve the developmental competence of in vitro matured porcine oocytes. Animals 2021, 11, 2483. [Google Scholar] [CrossRef]
- Roy, P.K.; Qamar, A.Y.; Tanga, B.M.; Fang, X.; Kim, G.; Bang, S.; Cho, J. Enhancing oocyte competence with Milrinone as a phosphodiesterase 3A inhibitor to improve the development of porcine cloned embryos. Front. Cell Dev. Biol. 2021, 9, 647616. [Google Scholar] [CrossRef]
- Kim, G.; Roy, P.K.; Fang, X.; Hassan, B.M.S.; Cho, J. Improved preimplantation development of porcine somatic cell nuclear transfer embryos by caffeine treatment. J. Veter Sci. 2019, 20, e31. [Google Scholar] [CrossRef]
- Roy, P.K.; Qamar, A.Y.; Fang, X.; Kim, G.; Bang, S.; De Zoysa, M.; Shin, S.T.; Cho, J. Chitosan nanoparticles enhance developmental competence of in vitro-matured porcine oocytes. Reprod. Domest. Anim. 2020, 56, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Qamar, A.Y.; Fang, X.; Hassan, B.M.S.; Cho, J. Effects of cobalamin on meiotic resumption and developmental competence of growing porcine oocytes. Theriogenology 2020, 154, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef]
- Damte, D.; Gebru, E.; Lee, S.; Suh, J.; Park, S. Evaluation of anti-quorum sensing activity of 97 indigenous plant extracts from Korea through bioreporter bacterial strains Chromobacterium violaceum and Pseudomonas aeruginosa. J. Microb. Biochem. Technol. 2013, 5, 42–46. [Google Scholar] [CrossRef]
- Niu, C.; Afre, S.; Gilbert, E.S. Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett. Appl. Microbiol. 2006, 43, 489–494. [Google Scholar] [CrossRef]
- Thenmozhi, R.; Nithyanand, P.; Rathna, J.; Pandian, S.K. Antibiofilm activity of coral-associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol. Med. Microbiol. 2009, 57, 284–294. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Tapia-Rodriguez, M.R.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Martinez-Tellez, M.A.; Hernandez-Onate, M.A.; Ayala-Zavala, J.F. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control 2018, 90, 266–273. [Google Scholar] [CrossRef]
- Stepanovic, S.; Cirkovic, I.; Ranin, L.; Svabic-Vlahovic, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.Y.; Roy, P.K.; Mizan, M.F.R.; Hossain, M.I.; Park, S.H.; Ha, S.D. Viability of Salmonella typhimurium biofilms on major food-contact surfaces and eggshell treated during 35 days with and without water storage at room temperature. Poult. Sci. 2020, 99, 4558–4565. [Google Scholar] [CrossRef]
- Sinde, E.; Carballo, J. Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: The influence of free energy and the effect of commercial sanitizers. Food Microbiol. 2000, 17, 439–447. [Google Scholar] [CrossRef]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef] [PubMed]
- McCarter, L.L. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001, 65, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yang, Z.Y.; Zheng, X.Y.; Kang, S.M.; Yang, Z.K.; Xu, Y.F.; Shi, C.; Tian, H.Y.; Xia, X.D. Thymoquinone inhibits biofilm formation and attachment-invasion in host cells of Vibrio parahaemolyticus. Foodborne Pathog. Dis. 2019, 16, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.F.; Cohen, A.L.V.; Naughton, L.M.; Ussery, D.W.; Binnewies, T.T.; Stine, O.C.; Parent, M.A. Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus. BMC Microbiol. 2008, 8, 110. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, D.; Hua, Z.; Sun, H.H.; Zheng, Z.W.; Xia, X.D.; Shi, C. Attenuation of multiple Vibrio parahaemolyticus virulence factors by citral. Front. Microbiol. 2019, 10, 894. [Google Scholar] [CrossRef]
- Rutherford, S.T.; van Kessel, J.C.; Shao, Y.; Bassler, B.L. AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 2011, 25, 397–408. [Google Scholar] [CrossRef]
- Guo, M.H.; Fang, Z.J.; Sun, L.J.; Sun, D.F.; Wang, Y.L.; Li, C.; Wang, R.D.; Liu, Y.; Hu, H.Q.; Liu, Y.; et al. Regulation of thermostable direct hemolysin and biofilm formation of Vibrio parahaemolyticus by quorum-sensing genes luxM and luxS. Curr. Microbiol. 2018, 75, 1190–1197. [Google Scholar] [CrossRef]
| Target Gene | Sequence of Primers (5′-3′) | Product Size (bp) | NCBI Accessions No. |
|---|---|---|---|
| flaA | F: CGGACTAAACCGTATCGCTGAAA R: GGCTGCCCATAGAAAGCATTACA | 128 | GQ433373.1 |
| flgL | F: CGTCAGCGTCCACCACTT R: GCGGCTCTGACTTACTGCTA | 141 | CP066246.1 |
| luxS | F: GGATTTTGTTCTGGCTTTCCACTT R: GGGATGTCGCACTGGTTTTTAC | 119 | CP066246.1 |
| aphA | F: ACACCCAACCGTTCGTGATG R: GTTGAAGGCGTTGCGTAGTAAG | 162 | CP066246.1 |
| vp0952 | F: TATGATGGTGTTTGGTGC R: TGTTTTTCTGAGCGTTTC | 276 | CP064041.1 |
| vp0962 | F: GACCAAGACCCAGTGAGA R: GGTAAAGCCAGCAAAGTT | 358 | CP064041.1 |
| 16S rRNA | F: TATCCTTGTTTGCCAGCGAG R: CTACGACGCACTTTTTGGGA | 186 | CP085308.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.K.; Park, S.-H.; Song, M.G.; Park, S.Y. Antimicrobial Efficacy of Quercetin against Vibrio parahaemolyticus Biofilm on Food Surfaces and Downregulation of Virulence Genes. Polymers 2022, 14, 3847. https://doi.org/10.3390/polym14183847
Roy PK, Park S-H, Song MG, Park SY. Antimicrobial Efficacy of Quercetin against Vibrio parahaemolyticus Biofilm on Food Surfaces and Downregulation of Virulence Genes. Polymers. 2022; 14(18):3847. https://doi.org/10.3390/polym14183847
Chicago/Turabian StyleRoy, Pantu Kumar, Sung-Hee Park, Min Gyu Song, and Shin Young Park. 2022. "Antimicrobial Efficacy of Quercetin against Vibrio parahaemolyticus Biofilm on Food Surfaces and Downregulation of Virulence Genes" Polymers 14, no. 18: 3847. https://doi.org/10.3390/polym14183847
APA StyleRoy, P. K., Park, S.-H., Song, M. G., & Park, S. Y. (2022). Antimicrobial Efficacy of Quercetin against Vibrio parahaemolyticus Biofilm on Food Surfaces and Downregulation of Virulence Genes. Polymers, 14(18), 3847. https://doi.org/10.3390/polym14183847








