Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications
Abstract
:1. Introduction
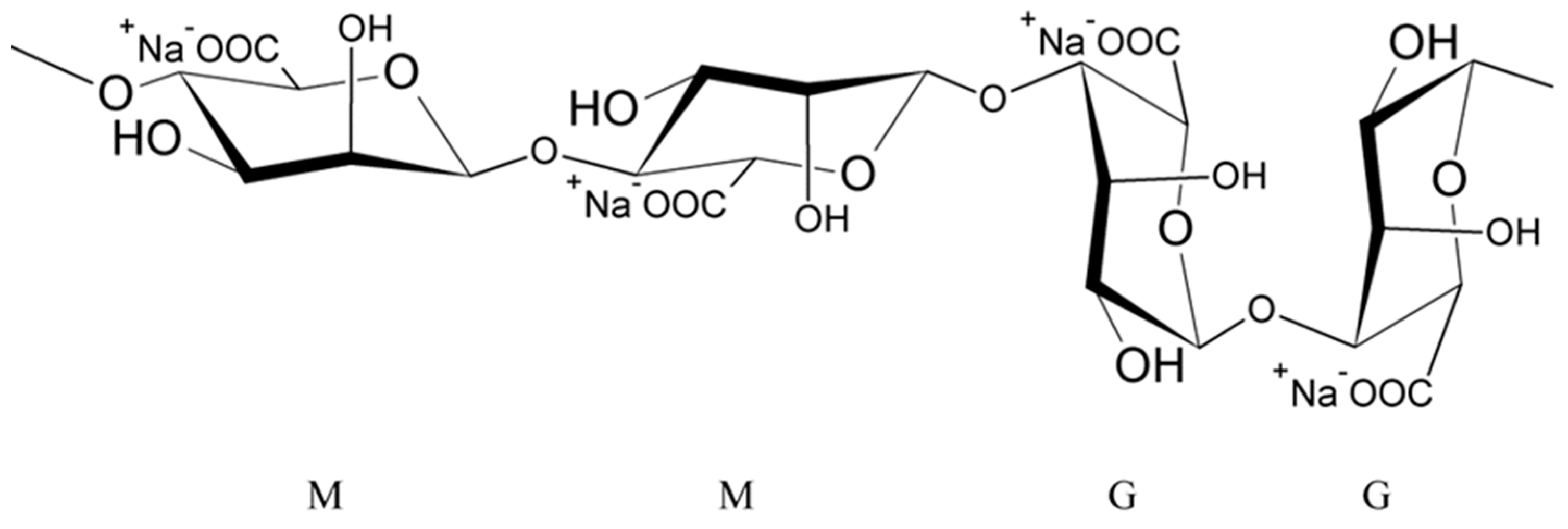
2. Alginate Characteristics
Cross-Linking of Alginates
3. Alginate Encapsulation Techniques
- (a)
- Physical methods such as spray drying, extrusion, lyophilization, supercritical fluid precipitation and solvent evaporation;
- (b)
- Physico-chemical methods including coacervation, liposomes and ionic gelation;
- (c)
- Chemical methods such as interfacial polymerization and molecular inclusion complexation.
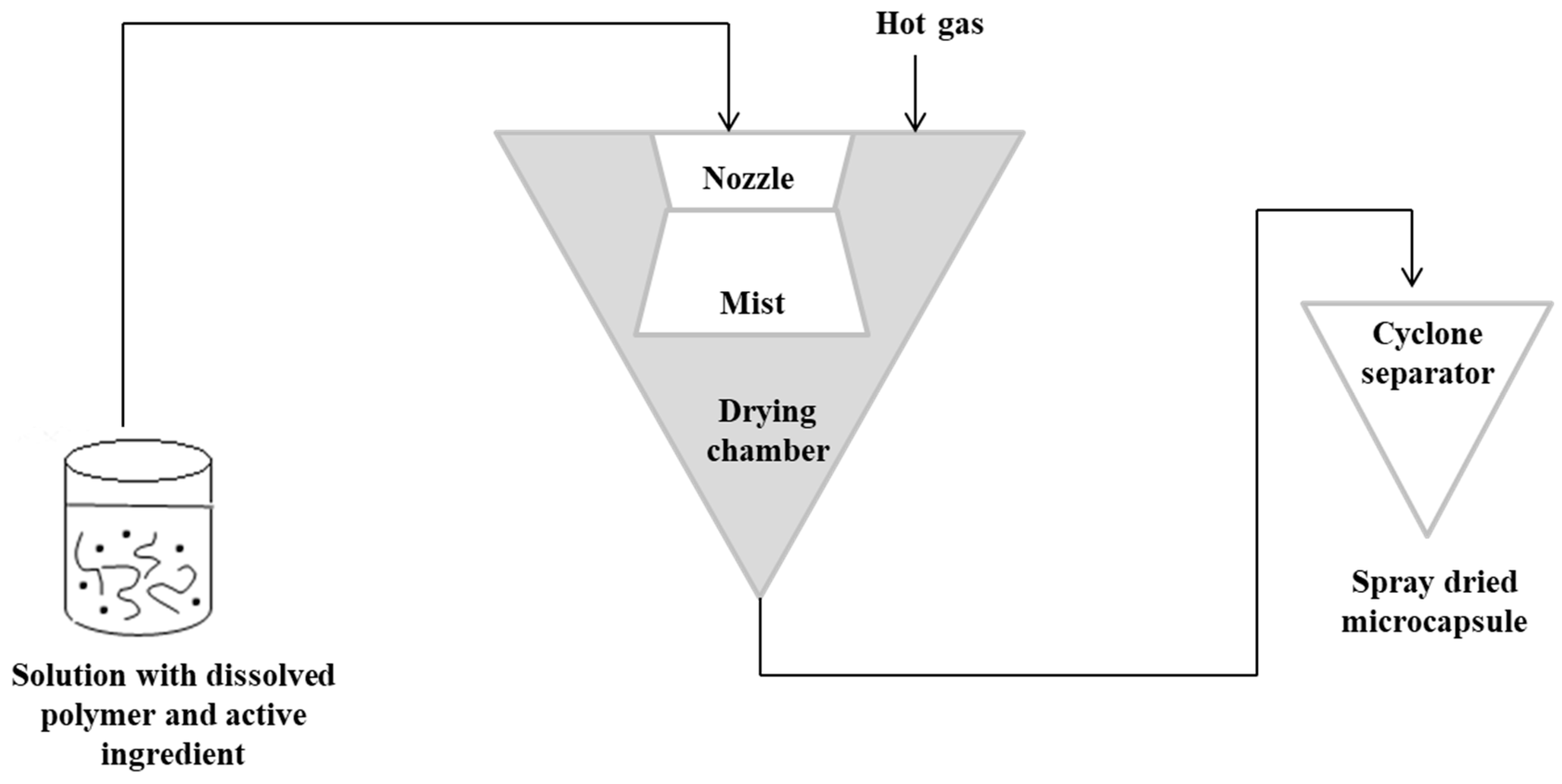
3.1. Spray-Drying Technique
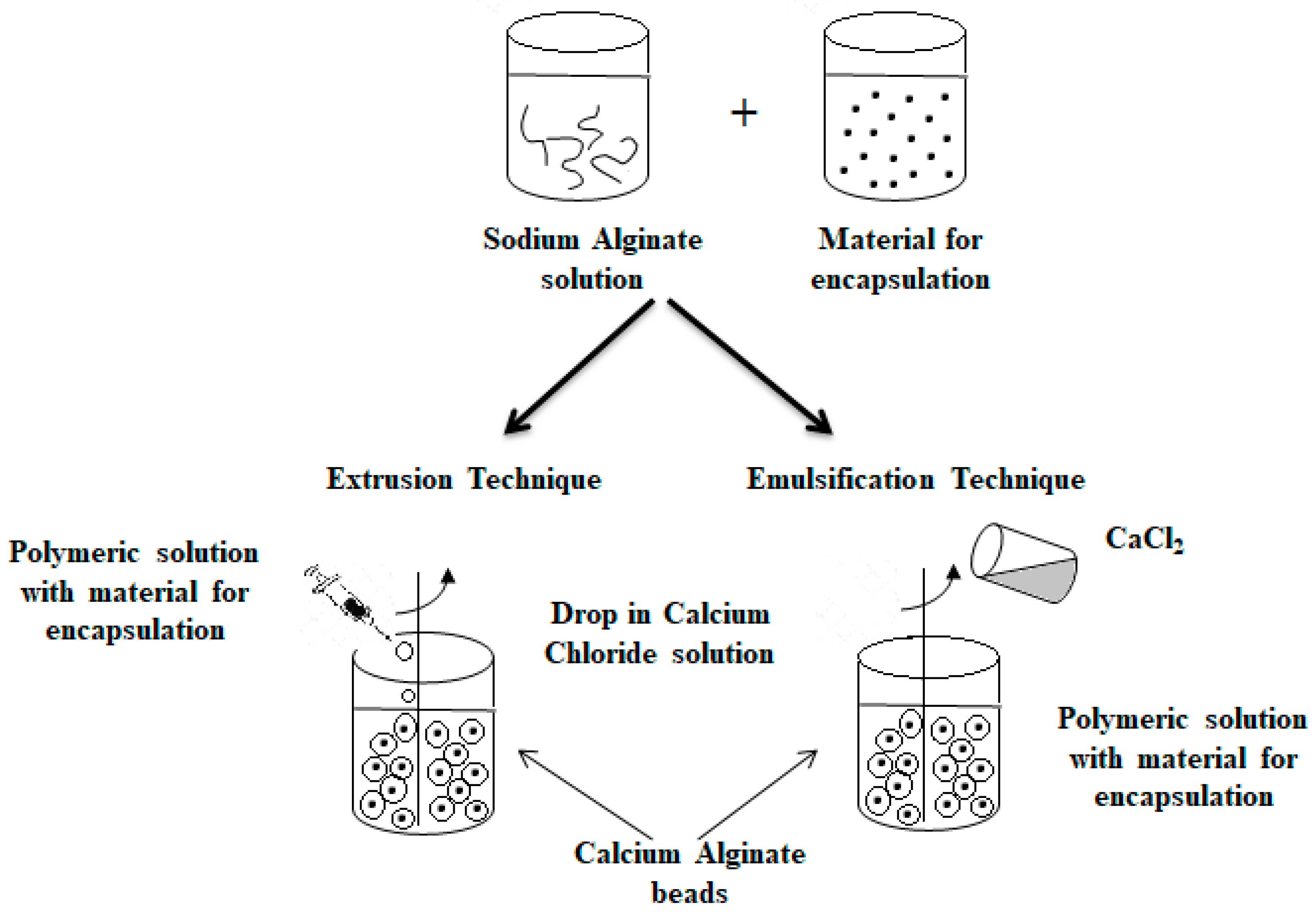
3.2. Extrusion Technique
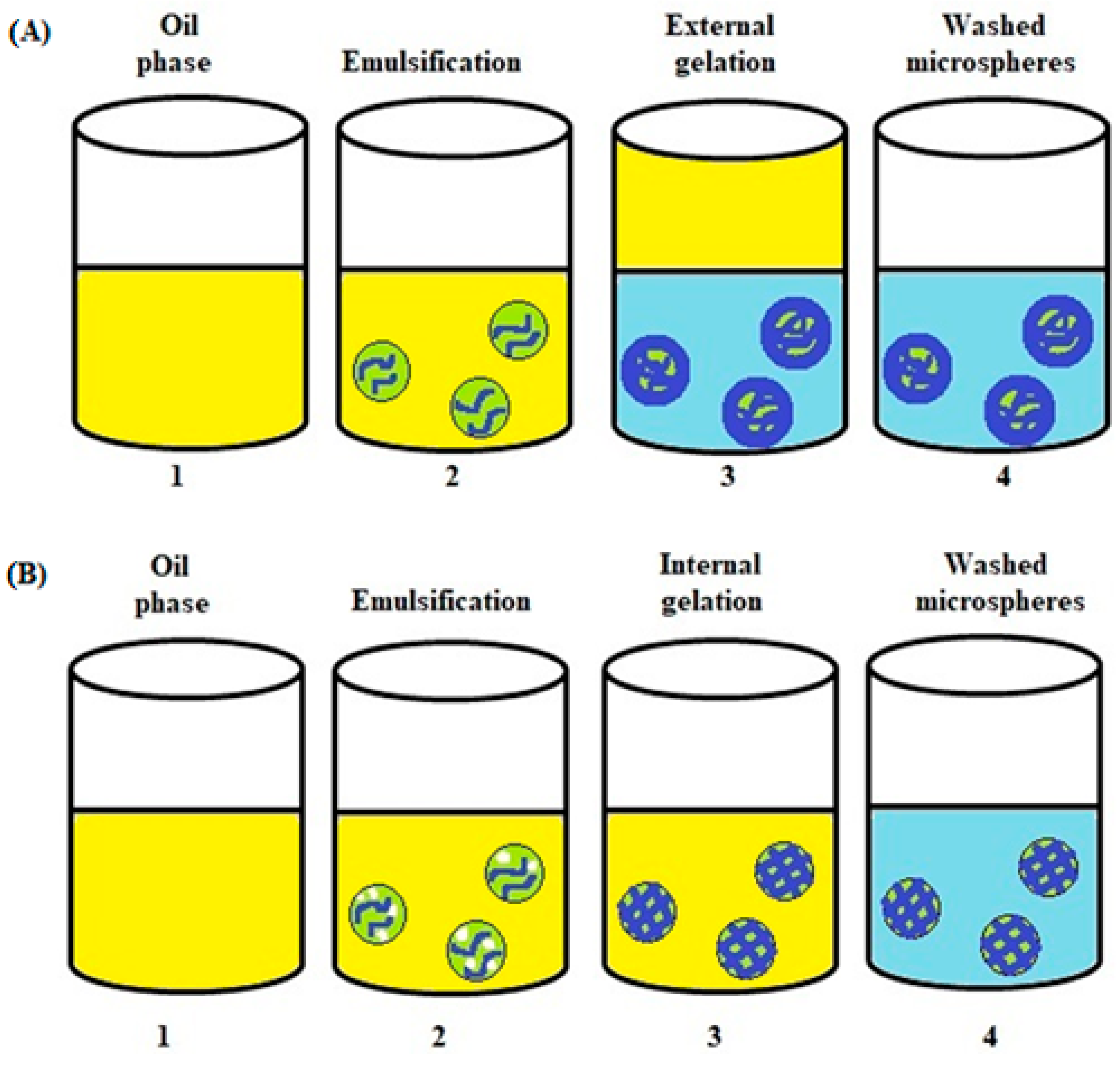
3.3. Emulsification Technique
- The balls have a porous network, while the capsules have a liquid core (water or oil);
- The dimensions of the balls are much larger than in the case of the capsules;
- The balls are uniform in size and shape in contrast to differing in size in capsules.
4. Microencapsulation of Alginates Based on Emulsification Technique
4.1. Alginate Microspheres Formed in a W/O Emulsion—External Gelation
4.2. Alginate Microspheres Formed in a W/O Emulsion—Internal Gelation
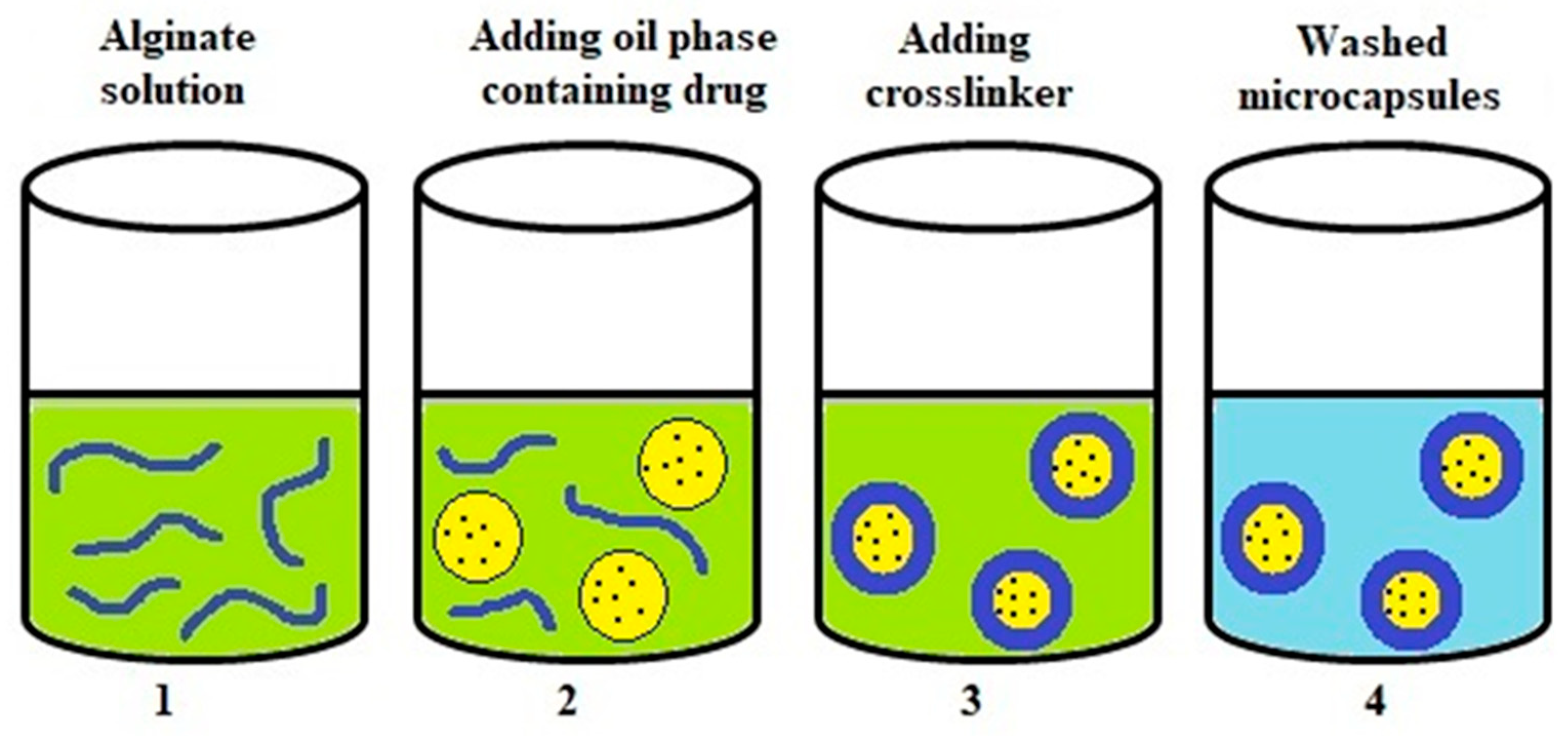
4.3. Gelation at the Interfacial Area of Emulsion (Complexation)
5. Factors Influencing the Alginate Microparticle Formation Process
5.1. Concentration of Sodium Alginate
5.2. Type and Concentration of Cross-Linking Agent
5.3. Type and Concentration of the Emulsifier
5.4. Effect of an Oil Type on Alginate Capsule Properties
5.5. pH Values
5.6. Agitation Speed
5.7. Cross-Linking Time
6. Application of Alginate Microparticles
6.1. The Application of AMP as Active Substance Carriers in the Food Industry
| Capsules Components | Concentration | Active Ingredients | Encapsulation Technique | References | |
|---|---|---|---|---|---|
| [%] | [M] | ||||
| sodium alginate chitosan calcium chloride | 0.05 0.05 - | - - 0.0002 | Nisin | Extrusion | [20] |
| sodium alginate guar gum calcium chloride | 1.5–2.5 0.2–0.6 - | - - 0.1 | Nisin | Extrusion | [163] |
| sodium alginate polyvinyl alcohol whey protein concentrate calcium chloride | 31.2 31.2 18.8 5 | - - - - | Sea buckthorn berries bio-oil and amaranth seeds bio-oil | Extrusion | [164] |
| sodium alginate calcium chloride fructooligosaccharides, isomaltooligosaccharides or peptide coated | 1–3 - - | - 0.1 - | L. acidophilus, L. casei, B. bifidum and B. longum | Extrusion | [165] |
| sodium alginate inulin or Jerusalem artichoke calcium chloride chitosan coated | 3 3 - - | - - 0.1 - | L. acidophilus | Extrusion | [152] |
| sodium alginate starch lecithin calcium chloride | 2 2 0–4 - | - - - 0.1 | L. casei, L. plantarum, L. acidophilus, L. gasperi, L. bulgaricus, B. adolescenti and L. lactis | Extrusion | [166] |
| sodium alginate xanthan gum fructose maltose glycerol calcium chloride | 2 0.15 3 3 5.5 | - - - - - | L. plantarum | Extrusion | [167] |
| sodium alginate sugarbeet calcium chloride | 2 2 - | - - 0.1 | Staphylococcus succinus and Enterococcus fecium | Extrusion | [168] |
| sodium alginate calcium chloride | 2 0.5 | - - | Saccharomyces cerevisiae | Extrusion | [169] |
| sodium alginate guar gum calcium chloride whey protein and chitosan coated | 3 5 - 2 | - - 0.1 - | Yarrowialipolytica, Kluyveromyces lactis, Lipomycesstarkeyi, Saccharomycopsisfibuligera and Brettanomycescustersianus | Extrusion | [170] |
| sodium alginate calcium chloride | 1.875 - | - 1.5 | L. bulgaricus and Streptococcus thermophilus | Extrusion | [171] |
| sodium alginate calcium chloride | 1.875 - | - 1.5 | Streptococcus lactis, Streptococcus lactis subsp. diacetylactis and Streptococcus cremoris | Extrusion | [172] |
| sodium alginate calcium chloride | 3 - | - 0.5 | L. reuteri and B. longum | Extrusion | [173] |
| sodium alginate calcium chloride | 1 - | - 0.1 | Streptococcus lactis and Streptococcus cremoris | Extrusion | [174] |
| sodium alginate calcium chloride | 0.75–2 - | - 0.1; 0.2; 1 | L. acidophilus | Extrusion | [175] |
| sodium alginate locust bean gum xanthan gum calcium chloride chitosan coated | 2 - - - - | - - - 0.1 - | L. rhamnosus | Extrusion | [176] |
| sodium alginate calcium chloride | 3 - | - 0.15 | L. plantarum | Extrusion | [177] |
| sodium alginate pancreatic digested casein fructooligosacharides isomaltooligosaccharides calcium chloride | 1–3 0–1 0–3 0–3 - | - - - - 1 | L. casei, L. acidophilus, B. longum and B. bifidum | Extrusion | [178] |
| sodium alginate calcium chloride | 3 - | - 0.5 | L. reuteri | Extrusion | [179] |
| sodium alginate calcium chloride | 2 0.5; 0.8 | - - | L. plantarum | Extrusion | [180] |
| sodium alginate Hi-maize resistant starch calcium chloride | 2 2 - | - - 0.1 | L. acidophilus | Extrusion | [181] |
| sodium alginate fruktooligosacharides pancreatic digested casein calcium chloride | 1–3 0–3 0–1 - | - - - 0.1 | B. bifidum | Extrusion | [182] |
| sodium alginate calcium chloride | 3 - | - 0.5 | L. reuteri | Extrusion | [183] |
| sodium alginate starch calcium chloride | 2 2 - | - - 0.5 | L. reuteri | Extrusion | [183] |
| sodium alginate glycerol xanthan gum Tween 20 calcium chloride chitosan coated | 2 5 0.26 0.1 - 0.8 | - - - - 0.5 - | L. bulgaricus | Extrusion | [128] |
| sodium alginate calcium chloride | 2 - | - 1 | L. acidophilus and B. bifidum | Extrusion | [184] |
| sodium alginate or palmitoylated alginate calcium chloride | 2 30 | - - | B. longum | Extrusion | [185] |
| sodium alginate calcium chloride | 1 - | - 0.1 | L. lactis subsp. cremoris | Extrusion | [186] |
| sodium alginate glycerol xanthan gum calcium chloride chitosan or gelatin coated | 1–3 5 0.9 - - | - - - 1 - | L. plantarum | Extrusion | [187] |
| sodium alginate calcium chloride | - 2 | 0.1 - | L. acidophilus | Extrusion | [188] |
| whey proteins concentrate sodium alginate calcium chloride | 2.5–4 0.125 - | - - 0.1 | L. acidophilus | Extrusion | [188] |
| sodium alginate whey protein isolate | - - | - - | L. plantarum | Spray-drying | [189] |
| sodium alginate calcium chloride | 2 - | - 0.1 | L. rhamnosus | Spray-drying | [190] |
| sodium alginate chitosan Tween 40 calcium chloride | 1–2 - 0.5–1.5 - | - - - 0.5 | Coriander essential oil | Emulsification (external gelation) | [97] |
| sodium alginate and alginate-resistant starch sunflower oil Span 80 Tween 80 calcium chloride | 1 - 1 1 25 | - - - - - | Nisin | Emulsification (external gelation) | [19] |
| sodium alginate CaCO3 Span 80 polyglycerol polyricinoleate (PGPR) sunflower oil glacial acetic acid Tween 20 calcium chloride | 2 - - 4–15 - - - 0.03–01 | - - - - - - - - | Cocoa extract | Emulsification (internal gelation) | [131] |
| sodium alginate vegetable oil Tween 80 calcium chloride | - - - - | - - - 0.0625 | L. acidophilus and L. rhamnosus | Emulsification (external gelation) | [191] |
| sodium alginate Hi-maize resistant starch Tween 80 calcium chloride | 2 2 0.02 - | - - - 0.1 | L. acidophilus and Bifidobacterium spp. | Emulsification (external gelation) | [83] |
| sodium alginate guar gum xanthan gum locust bean gum carrageenan gum vegetable oil Tween 80 calcium chloride | 3 - - - - - - - | - - - - - - - 0.01 | L. rhamnosus, B. longum, L. salivarius, L. plantarum, B. lactis, L. paracasei, L. acidophilus and B. bifidum | Emulsification (external gelation) | [192] |
| sodium alginate Hi-maize resistant starch corn oil Tween 80 calcium chloride chitosan coated | 3 2 - - - - | - - - - 0.1 - | L. acidophilus and L. casei | Emulsification (external gelation) | [129] |
| sodium alginate faxseed mucilage canola oil Tween 80 calcium chloride | 1–3 0.9 - 0.05 - | - - - - 0.15 | L. casei | Emulsification (external gelation) | [133] |
| sodium alginate vegetable oil Tween 80 calcium chloride | 3.6 - 2 - | - - - 0.05 | L. bulgaricus | Emulsification (external gelation) | [193] |
| sodium alginate Hi-maize resistant starch canola oil lecithin calcium chloride | 2 2 - 0.02 - | - - - - 0.1 | L. casei and B. lactis | Emulsification (external gelation) | [194] |
| sodium alginate corn oil Tween 80 calcium chloride | 3 - 0.02 - | - - - 0.1 | L. reuteri | Emulsification (external gelation) | [174] |
| sodium alginate Hi-maize starch calcium chloride | 2 2 - | - - 0.1 | L. acidophilus and B. lactis | Emulsification (external gelation) | [195] |
| sodium alginate soybean oil Tween 80 calcium chloride | 2–4 - 0.2 - | - - - 0.05 | L. casei | Emulsification (external gelation) | [111] |
| sodium alginate sodium citrate calcium citrate canola oil glacial acetic acid | 2 0.1 1 - 0.667 | - - - - - | Lactococcus lactis subsp. cremoris | Emulsification (internal gelation) | [196] |
| 3% sodium alginate corn oil Tween 80 calcium chloride | 3 - 0.02 - | - - - 0.1 | L. reuteri | Emulsification (external gelation) | [183] |
| sodium alginate starch corn oil Tween 80 calcium chloride | 2 2 - 0.02 - | - - - - 0.1 | L. reuteri | Emulsification (external gelation) | [183] |
| sodium alginate corn starch soybean oil Tween 80 calcium chloride | 4 2 - - - | - - - - 0.1 | L. acidophilus | Emulsification (external gelation) | [197] |
| sodium alginate CaCO3 canola oil Tween 80 glacial acetic acid | 1.5 - - 1.5 - | - - - - - | B. animalis subsp. lactis | Emulsification (internal gelation) | [198] |
6.2. The Application of AMP as Active Substance Carriers in the Pharmaceutical Industry
| Capsules Components | Concentration | Active Ingredients | Encapsulation Technique | References | |
|---|---|---|---|---|---|
| [%] | [M] | ||||
| sodium alginate citric acid dicalcium phosphate succinic acid latex bovine serum albumin | 2–4 0.6 0.2 4 0.05–0.5 0.15 | - - - - - - | Cellulase | Spray-drying | [70] |
| sodium alginate pectin Tween 80 | 3 3 - | - - - | Carvacrol | Spray-drying | [71] |
| sodium alginate | 2 | - | Insulin | Spray-drying | [72] |
| sodium alginate Tween 80 succinic acid calcium phosphate dibasic dihydrate | 4 - 2 0.2 | - - - - | Corn oil | Spray-drying | [69] |
| sodium alginate calcium chloride chitosan coated | 2 2.5 - | - - - | Bovine serum albumin | Spray-drying | [25] |
| sodium alginate sun flower oil oleic acid ester calcium chloride | 3 - 1 4 | - - - - | Catechin | Extrusion | [18] |
| sodium alginate pectin glycerol Tween 80 calcium chloride | 0.5–2 2 1 0.1 5 | - - - - - | α-tocopherol | Extrusion | [199] |
| sodium alginate calcium chloride | 1 2.5 | - - | Invertase | Extrusion | [200] |
| sodium alginate calcium chloride whey proteins coated | 20 - 20 | - 0.1 - | L. plantarum | Extrusion | [201] |
| sodium alginate alginate/psyllium blend alginate/fenugreek blend calcium chloride | 2 1.5/0.5 1.5/0.5 4 | - - - - | L. plantarum | Extrusion | [202] |
| xanthan gum sodium alginate Tween 80 calcium chloride chitosan coated | 0.15 1.8 1 - - | - - - 0.1 - | L. plantarum | Extrusion | [203] |
| sodium alginate calcium chloride chitosan coated | 2 - - | - 0.1 - | L. acidophilus, B. bifidum and L. casei | Extrusion | [204] |
| sodium alginate calcium chloride | 2–4 - | - 0.1 | B. longum | Extrusion | [205] |
| sodium alginate calcium chloride | 1.8 - | - 0.1 | Saccharomyces boulardii | Extrusion | [206] |
| sodium alginate calcium chloride poly-L-lysine and alginate coated | 1.5 - 0.1 and 0.1 | - 0.1 - | L. reuteri | Extrusion | [207] |
| sodium alginate oligosaccharides (GOS, FOS, IMO, XLO) calcium chloride | 2 1.8 2 | - - - | L. fermentum | Extrusion | [208] |
| sodium alginate zein calcium chloride | 1.4 1–9 - | - - 0.1 | B. bifidum | Extrusion | [209] |
| sodium alginate Tween 80 glycerol calcium chloride | 2 - - - | - - - 2 | Testosterone | Emulsification (complexation) | [106] |
| sodium alginate chitosan Tween 80 ethanol calcium chloride | 0.3–0.6 0.3–0.6 1 - 0.67 | - - - - - | Lemongrass oil and turmeric oil | Emulsification (complexation) | [107] |
| sodium alginate CaCO3 soybean oil glacial acetic acid Tween 80 chitosan coated | 1.5 - - - 1 0.4 | - 0.000625 - - - - | B. longum | Emulsification (internal gelation) | [84] |
| sodium alginate corn starch vegetable oil Tween 80 calcium chloride | 1 2 - 0.2 - | - - - - 0.1 | L. casei and B. bifidum | Emulsification (external gelation) | [210] |
| gelatin genipin tempered oil Span 85 calcium chloride sodium alginate coated | 13 - - 0.5 - 1 | - 0.00125 - - 0.05 - | B. adolescentis | Emulsification (external gelation) | [211] |
6.3. The Application of AMP as Active Substance Carriers in the Cosmetic Industry
6.4. Other AMP Applications
6.5. Advantages and Disadvantages of Alginate Microparticles
- There are mild microencapsulation conditions and no high temperatures (emulsification and extrusion), which is especially important in the case of the encapsulation of bacteria [200], because they are very sensitive to high temperatures;
- Alginates improve the stability and increase the efficiency of delivery to the body of microencapsulated active compounds [72], including probiotic microorganisms;
- Alginates protect bacterial cells against the external environment [152], reduce susceptibility to contamination and protect against damage [205], such as the presence of gastric juices, acidity and low pH [239,240], and in the case of cosmetics, protect probiotics against the effects of preservatives;
- The low efficiency of capsules loaded with bacteria, difficulties in application on an industrial scale due to the high cost of bacteria and the microencapsulation techniques [72];
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing Polymeric Microparticles for Biomedical and Industrial Applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Vilos, C.; Velasquez, L.A. Therapeutic Strategies Based on Polymeric Microparticles. J. Biomed. Biotechnol. 2012, 2012, 672760. [Google Scholar] [CrossRef] [PubMed]
- Solanki, H.K.; Pawar, D.D.; Shah, D.A.; Prajapati, V.D.; Jani, G.K.; Mulla, A.M.; Thakar, P.M. Development of Microencapsulation Delivery System for Long-Term Preservation of Probiotics as Biotherapeutics Agent. BioMed Res. Int. 2013, 2013, 620719. [Google Scholar] [CrossRef] [PubMed]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation Methods of Alginate Nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalized Treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Alhaique, F. Recent Advances and Perspectives on Coated Alginate Microspheres for Modified Drug Delivery. Expert Opin. Drug Deliv. 2008, 5, 417–425. [Google Scholar] [CrossRef]
- Teoh, P.L.; Mirhosseini, H.; Mustafa, S.; Hussin, A.S.M.; Manap, M.Y.A. Recent Approaches in the Development of Encapsulated Delivery Systems for Probiotics. Food Biotechnol. 2011, 25, 77–101. [Google Scholar] [CrossRef]
- Wang, B.; Hu, L.; Siahaan, T.J. Drug Delivery: Principles and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Park, J.H.; Ye, M.; Park, K. Biodegradable Polymers for Microencapsulation of Drugs. Molecules 2005, 10, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural Polymers for the Microencapsulation of Cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Curcio, M.; Spizzirri, U.G.; Iemma, F.; Puoci, F.; Cirillo, G.; Parisi, O.I.; Picci, N. Grafted Thermo-Responsive Gelatin Microspheres as Delivery Systems in Triggered Drug Release. Eur. J. Pharm. Biopharm. 2010, 76, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Ghorab, M.; Kamel, R.; Salama, A.H. A Trial for the Design and Optimization of PH-Sensitive Microparticles for Intestinal Delivery of Cinnarizine. Drug Deliv. Transl. Res. 2016, 6, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Montemagno, C.; Choi, H.J. Smart Microparticles with a PH-Responsive Macropore for Targeted Oral Drug Delivery. Sci. Rep. 2017, 7, 3059. [Google Scholar] [CrossRef] [PubMed]
- Tyle, P. Effect of Size, Shape and Hardness of Particles in Suspension on Oral Texture and Palatability. Acta Psychol. 1993, 84, 111–118. [Google Scholar] [CrossRef]
- Leon, A.M.; Medina, W.T.; Park, D.J.; Aguilera, J.M. Mechanical Properties of Whey Protein/Na Alginate Gel Microparticles. J. Food Eng. 2016, 188, 1–7. [Google Scholar] [CrossRef]
- Leon, A.M.; Medina, W.T.; Park, D.J.; Aguilera, J.M. Properties of Microparticles from a Whey Protein Isolate/Alginate Emulsion Gel. Food Sci. Technol. Int. 2018, 24, 414–423. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, J.S.; Lee, H.G. Calcium-Alginate Microparticles for Sustained Release of Catechin Prepared via an Emulsion Gelation Technique. Food Sci. Biotechnol. 2016, 25, 1337–1343. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; German, J.B.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Khaksar, R. Preparation and Characterization of Alginate and Alginate-Resistant Starch Microparticles Containing Nisin. Carbohydr. Polym. 2014, 103, 573–580. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Coupland, J.N.; Anantheswaran, R.C. Characterization of Nisin Containing Chitosan-Alginate Microparticles. Food Hydrocoll. 2017, 69, 301–307. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Yuen, M.C.W.; Kan, C.W.; Cheuk, K.K.L.; Chui, C.H.; Lam, K.H. Cosmetic Textiles with Biological Benefits: Gelatin Microcapsules Containing Vitamin C. Int. J. Mol. Med. 2009, 24, 411–419. [Google Scholar] [CrossRef]
- Chelaru, C.; Ignat, M.; Albu, M.; Meghea, A. Polymeric Microcapsules for Cosmetic Applications, Based on Lemon Essential Oil (Citrus limon). UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2015, 77, 101–112. [Google Scholar]
- Yingngam, B.; Kacha, W.; Rungseevijitprapa, W.; Sudta, P.; Prasitpuriprecha, C.; Brantner, A. Response Surface Optimization of Spray-Dried Citronella Oil Microcapsules with Reduced Volatility and Irritation for Cosmetic Textile Uses. Powder Technol. 2019, 355, 372–385. [Google Scholar] [CrossRef]
- Tran, V.T.; Benoît, J.P.; Venier-Julienne, M.C. Why and How to Prepare Biodegradable, Monodispersed, Polymeric Microparticles in the Field of Pharmacy? Int. J. Pharm. 2011, 407, 1–11. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V.; Leo, E.; Bernabei, M.T.; Cameroni, R. Chitosan-Alginate Microparticles as a Protein Carrier. Drug Dev. Ind. Pharm. 2001, 27, 393–400. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V.; Leo, E.; Bernabei, M.T.; Cameroni, R. Protein Immobilization in Crosslinked Alginate Microparticles. J. Microencapsul. 2002, 19, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate Microparticles as Oral Colon Drug Delivery Device: A Review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V. Technology and Potential Applications of Probiotic Encapsulation in Fermented Milk Products. J. Food Sci. Technol. 2015, 52, 4679–4696. [Google Scholar] [CrossRef]
- Yang, M.; Liang, Z.; Wang, L.; Qi, M.; Luo, Z.; Li, L. Microencapsulation Delivery System in Food Industry-Challenge and the Way Forward. Adv. Polym. Technol. 2020, 2020, 7531810. [Google Scholar] [CrossRef]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of Alginate Microspheres in Therapeutics Delivery and Cell Culture: Past, Present and Future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-Based Composites for Environmental Applications: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Gomathi, T.; Susi, S.; Abirami, D.; Sudha, P.N. Size Optimization And Thermal Studies on Calcium Alginate Nanoparticles. IOSR J. Pharm. 2017, 48, 1–7. [Google Scholar]
- Van Vlierberghe, S.; Graulus, G.J.; Samal, S.K.; Van Nieuwenhove, I.; Dubruel, P. Porous Hydrogel Biomedical Foam Scaffolds for Tissue Repair. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing Limited: Thorston, UK, 2014; pp. 335–390. [Google Scholar] [CrossRef]
- Wyrębska, Ł.; Szuster, L.; Stawska, H. Synteza i Aplikacja Nowych Pochodnych Wybranych Polisacharydów Część I: Przegląd Literatury. Technol. i Jakość Wyr. 2014, 59, 3–16. [Google Scholar]
- Asgari, S.; Pourjavadi, A.; Licht, T.R.; Boisen, A.; Ajalloueian, F. Polymeric Carriers for Enhanced Delivery of Probiotics. Adv. Drug Deliv. Rev. 2020, 161–162, 1–21. [Google Scholar] [CrossRef]
- El Din, K.S. Deposition and Structural Formation of 3D Alginate Tissue Scaffolds. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2005. [Google Scholar]
- Walczak, J.; Marchewka, J.; Laska, J. Hydrożele Alginianowe Sieciowane Jonowo i Kowalencyjnie. Eng. Biomater. 2015, 18, 17–23. [Google Scholar]
- Paques, J.P. Formation of Alginate Nanospheres. Ph.D. Thesis, Wageningen University, Wageningen, NL, USA, 2014. [Google Scholar]
- Kim, S.; Jeong, C.; Cho, S.; Kim, S.B. Effects of Thermal Treatment on the Physical Properties of Edible Calcium Alginate Gel Beads: Response Surface Methodological Approach. Foods 2019, 8, 578. [Google Scholar] [CrossRef]
- Jarmoluk, A.; Wrzeszcz, K.; Zimoch, A.; Marycz, K. Sieciowanie Alginianu Sodu Do Hydrożeli Dla Inżynierii Tkankowej. Przem. Chem. 2013, 92, 1018–1022. [Google Scholar]
- Gombotz, W.R.; Wee, S.F. Protein Release from Alginate Matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotics for Gastrointestinal Delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Emmerichs, N.; Wingender, J.; Flemming, H.C.; Mayer, C. Interaction between Alginates and Manganese Cations: Identification of Preferred Cation Binding Sites. Int. J. Biol. Macromol. 2004, 34, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Bolla, S.; Barr, J.; Miedema, J.; Li, X.; Jasti, B. Alginate Microparticles Prepared by Spray-Coagulation Method: Preparation, Drug Loading and Release Characterization. Int. J. Pharm. 2005, 303, 171–181. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T. Probiotic Encapsulation Technology: From Microencapsulation to Release into the Gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef]
- Keyan, K.; Ramachandran, T.; Shumugasundaram, O.L.; Balasubramaniam, M.; Ragavendra, T. Microencapsulation of PCMs in Textiles: A Review. J. Text. Apparel Technol. Manag. 2012, 7, 1–10. [Google Scholar]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of Active Ingredients in Polysaccharide–Protein Complex Coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]
- Avula, B.; Parveen, I.; Zhao, J.; Wang, M.; Techen, N.; Wang, Y.H.; Riaz, M.; Bae, J.Y.; Shami, A.A.; Chittiboyina, A.G.; et al. A Comprehensive Workflow for the Analysis of Bio-Macromolecular Supplements: Case Study of 20 Whey Protein Products. J. Diet. Suppl. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Drobník, J. Biodegradable Soluble Macromolecules as Drug Carriers. Adv. Drug Deliv. Rev. 1989, 3, 229–245. [Google Scholar] [CrossRef]
- Kiyohara, H.; Nonaka, K.; Sekiya, M.; Matsumoto, T.; Nagai, T.; Tabuchi, Y.; Yamada, H. Polysaccharide-Containing Macromolecules in a Kampo (Traditional Japanese Herbal) Medicine, Hochuekkito: Dual Active Ingredients for Modulation of Immune Functions on Intestinal Peyer’s Patches and Epithelial Cells. Evid.-Based Complement. Altern. Med. 2011, 2011, 492691. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, A.; Bhatnagar, A.; Nishad, D.; Karwasra, R.; Khanna, K.; Sharma, D.; Kumar, N.; Jain, G.K. Novel Gum Acacia Based Macroparticles for Colon Delivery of Mesalazine: Development and Gammascintigraphy Study. J. Drug Deliv. Sci. Technol. 2019, 54, 101224. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Callahan, D.J.; Chilkoti, A. Stimulus-Responsive Macromolecules and Nanoparticles for Cancer Drug Delivery. Nanomedicine 2010, 5, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Kaurav, H.; Hari Kumar, S.L.; Kaur, A. Mucoadhesive Microspheres as Carriers in Drug Delivery: A Review. Int. J. Drug Dev. Res. 2012, 4, 21–34. [Google Scholar]
- Gurung, B.D.; Kakar, S. An Overview on Microspheres. Int. J. Health Clin. Res. 2020, 3, 11–24. [Google Scholar]
- Popović, D.A.; Milinčić, D.D.; Pešić, M.B.; Kalušević, A.M.; Tešić, Ž.L.; Nedović, V.A. Encapsulation Technologies for Polyphenol-Loaded Microparticles in Food Industry. In Green Food Processing Techniques; Academic Press: Cambridge, MA, USA, 2019; pp. 335–367. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. The Chemotherapeutic Potential of Gold Nanoparticles Against Human Carcinomas: A Review. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of Phase Change Materials Based Microencapsulated Technology for Buildings: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Ockun, M.A.; Uner, B.; Tas, C.; Ivanauskas, L. Effect of the Amount of Polysorbate 80 and Oregano Essential Oil on the Emulsion Stability and Characterization Properties of Sodium Alginate Microcapsules. Molecules 2021, 26, 6304. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Percy, S.R. Improvement in Drying and Concentrating Liquid Substances by Atomizing. US Patent No. 125406, 1872. [Google Scholar]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Keshani, S.; Daud, W.R.W.; Nourouzi, M.M.; Namvar, F.; Ghasemi, M. Spray Drying: An Overview on Wall Deposition, Process and Modeling. J. Food Eng. 2015, 146, 152–162. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of Bacteria: A Review of Different Technologies and Their Impact on the Probiotic Effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Kailasapathy, K. Microencapsulation of Probiotic Bacteria: Technology and Potential Applications. Curr. Issues Intest. Microbiol. 2002, 3, 39–48. [Google Scholar] [PubMed]
- Strobel, S.A.; Scher, H.B.; Nitin, N.; Jeoh, T. In Situ Cross-Linking of Alginate during Spray-Drying to Microencapsulate Lipids in Powder. Food Hydrocoll. 2016, 58, 141–149. [Google Scholar] [CrossRef]
- Santa-Maria, M.; Scher, H.; Jeoh, T. Microencapsulation of Bioactives in Cross-Linked Alginate Matrices by Spray Drying. J. Microencapsul. 2012, 29, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cameron, R.G.; Bai, J. Effect of Spray-Drying Temperature on Physicochemical, Antioxidant and Antimicrobial Properties of Pectin/Sodium Alginate Microencapsulated Carvacrol. Food Hydrocoll. 2020, 100, 105420. [Google Scholar] [CrossRef]
- Bowey, K.; Swift, B.E.; Flynn, L.E.; Neufeld, R.J. Characterization of Biologically Active Insulin-Loaded Alginate Microparticles Prepared by Spray Drying. Drug Dev. Ind. Pharm. 2013, 39, 457–465. [Google Scholar] [CrossRef] [PubMed]
- King, A.H. Encapsulation of Food Ingredients. A Review of Available Technology, Focusing on Hydrocolloids. ACS Symp. Ser. 1995, 590, 26–39. [Google Scholar]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of Probiotic Cells for Food Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial Appraisal of Existing Technologies and Trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Kailasapathy, K. Encapsulation Technologies for Functional Foods and Nutraceutical Product Development. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2009, 4, 1–19. [Google Scholar] [CrossRef]
- de Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for Preservation of Functionality and Targeted Delivery of Bioactive Food Components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of Encapsulation Techniques of Probiotics for Yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and Methods of Microencapsulation of Probiotic Microorganisms. Iran. J. Biotechnol. 2007, 5, 1–18. [Google Scholar]
- Groboillot, A.F.; Champagne, C.P.; Darling, G.D.; Poncelet, D.; Neufeld, R.J. Membrane Formation by Interfacial Cross-Linking of Chitosan for Microencapsulation of Lactococcus Iactis. Biotechnol. Bioeng. 1993, 42, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Shima, M.; Morita, Y.; Yamashita, M.; Adachi, S. Protection of Lactobacillus Acidophilus from the Low PH of a Model Gastric Juice by Incorporation in a W/O/W Emulsion. Food Hydrocoll. 2006, 20, 1164–1169. [Google Scholar] [CrossRef]
- Mortazavian, A.M.; Ehsani, M.R.; Azizi, A.; Razavi, S.H.; Mousavi, S.M.; Sohrabvandi, S.; Reinheimer, J.A. Viability of Calcium-Alginate-Microencapsulated Probiotic Bacteria in Iranian Yogurt Drink (Doogh) during Refrigerated Storage and under Simulated Gastrointestinal Conditions. Aust. J. Dairy Technol. 2008, 63, 25–30. [Google Scholar]
- Sultana, K.; Godward, G.; Reynolds, N.; Arumugaswamy, R.; Peiris, P.; Kailasapathy, K. Encapsulation of Probiotic Bacteria with Alginate-Starch and Evaluation of Survival in Simulated Gastrointestinal Conditions and in Yoghurt. Int. J. Food Microbiol. 2000, 62, 47–55. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending Viability of Bifidobacterium Longumin Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Kearney, L.; Upton, M.; McLoughlin, A. Enhancing the Viability of Lactobacillus Plantarum Inoculum by Immobilizing the Cells in Calcium-Alginate Beads Incorporating Cryoprotectants. Appl. Environ. Microbiol. 1990, 56, 3112–3116. [Google Scholar] [CrossRef]
- Gentile, F.T.; Doherty, E.J.; Rein, D.H.; Shoichet, M.S.; Winn, S.R. Reactive Polymers Polymer Science for Macroencapsulation of Cells for Central Nervous System Transplantation. React. Polym. 1995, 25, 207–227. [Google Scholar] [CrossRef]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Muangsin, N.; Shah, A.A.; Prapasarakul, N. The Efficacy of Three Double-Microencapsulation Methods for Preservation of Probiotic Bacteria. Sci. Rep. 2021, 11, 13753. [Google Scholar] [CrossRef]
- Przybysławska, M.; Winnicka, K. Technologie Otrzymywania Mikrokapsułek. Farm. Pol. 2012, 68, 283–289. Available online: https://www.ptfarm.pl/en/wydawnictwa/czasopisma/farmacja-polska/103/-/14209 (accessed on 22 August 2022).
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour Encapsulation and Controlled Release-A Review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E.; Tang, Z.X. Alginate-Based and Protein-Based Materials for Probiotics Encapsulation: A Review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Chan, L.W.; Lee, H.Y.; Heng, P.W.S. Mechanisms of External and Internal Gelation and Their Impact on the Functions of Alginate as a Coat and Delivery System. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Liu, X.; Xue, W.; Liu, Q.; Yu, W.; Fu, Y.; Xiong, X.; Ma, X.; Yuan, Q. Swelling Behaviour of Alginate-Chitosan Microcapsules Prepared by External Gelation or Internal Gelation Technology. Carbohydr. Polym. 2004, 56, 459–464. [Google Scholar] [CrossRef]
- Quong, D.; Neufeld, R.J.; Skjåk-Bræk, G.; Poncelet, D. External versus Internal Source of Calcium during the Gelation of Alginate Beads for DNA Encapsulation. Biotechnol. Bioeng. 1998, 57, 438–446. [Google Scholar] [CrossRef]
- Liu, X.D.; Yu, W.Y.; Zhang, Y.; Xue, W.M.; Yu, W.T.; Xiong, Y.; Ma, X.J.; Chen, Y.; Yuan, Q. Characterization of Structure and Diffusion Behaviour of Ca-Alginate Beads Prepared with External or Internal Calcium Sources. J. Microencapsul. 2002, 19, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Kaklamani, G.; Cheneler, D.; Grover, L.M.; Adams, M.J.; Bowen, J. Mechanical Properties of Alginate Hydrogels Manufactured Using External Gelation. J. Mech. Behav. Biomed. Mater. 2014, 36, 135–142. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Diryak, R.; Kontogiorgos, V.; Morris, G.A.; Smith, A.M. In Situ Rheological Measurements of the External Gelation of Alginate. Food Hydrocoll. 2016, 55, 77–80. [Google Scholar] [CrossRef]
- Dima, C.; Gitin, L.; Alexe, P.; Dima, S. Encapsulation of Coriander Essential Oil in Alginate and Alginate/Chitosan Microspheres by Emulsification External Gelation Method. Insid. Food Symp. 2013, 9, 1–6. [Google Scholar]
- Paques, J.P. Alginate Nanospheres Prepared by Internal or External Gelation with Nanoparticles. In Microencapsulation and Microspheres for Food Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 39–55. [Google Scholar] [CrossRef]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.X.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Vilela, S.; Ribeiro, A.J.; Veiga, F. Review and Current Status of Emulsion/Dispersion Technology Using an Internal Gelation Process for the Design of Alginate Particles. J. Microencapsul. 2006, 23, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Joo, S.H.; Toborek, M. Treatment of Antibiotic-Resistant Bacteria by Encapsulation of ZnO Nanoparticles in an Alginate Biopolymer: Insights into Treatment Mechanisms. J. Hazard. Mater. 2019, 373, 122–130. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madrakian, T.; Ghavami, S. Preparation and Characterization of Simvastatin Nanocapsules: Encapsulation of Hydrophobic Drugs in Calcium Alginate. Methods in Molecular Biology; Humana: New York, NY, USA, 2018; pp. 47–56. [Google Scholar] [CrossRef]
- Šafaříkova, M.; Roy, I.; Gupta, M.N.; Šafařík, I. Magnetic Alginate Microparticles for Purification of α-Amylases. J. Biotechnol. 2003, 105, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lertsutthiwong, P.; Noomun, K.; Jongaroonngamsang, N.; Rojsitthisak, P.; Nimmannit, U. Preparation of Alginate Nanocapsules Containing Turmeric Oil. Carbohydr. Polym. 2008, 74, 209–214. [Google Scholar] [CrossRef]
- Bhowmik, B.B.; Sa, B.; Mukherjee, A. Preparation and in Vitro Characterization of Slow Release Testosterone Nanocapsules in Alginates. Acta Pharm. 2006, 56, 417–429. [Google Scholar]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of Essential Oil-Loaded Chitosan-Alginate Nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Dubashynskaya, N.V.; Petrova, V.A.; Romanov, D.P.; Skorik, Y.A. PH-Sensitive Drug Delivery System Based on Chitin Nanowhiskers–Sodium Alginate Polyelectrolyte Complex. Materials 2022, 15, 5860. [Google Scholar] [CrossRef]
- Chan, E.S.; Lim, T.K.; Voo, W.P.; Pogaku, R.; Tey, B.T.; Zhang, Z. Effect of Formulation of Alginate Beads on Their Mechanical Behavior and Stiffness. Particuology 2011, 9, 228–234. [Google Scholar] [CrossRef]
- Liu, X.D.; Bao, D.C.; Xue, W.M.; Xiong, Y.; Yu, W.T.; Yu, X.J.; Ma, X.J.; Yuan, Q. Preparation of Uniform Calcium Alginate Gel Beads by Membrane Emulsification Coupled with Internal Gelation. J. Appl. Polym. Sci. 2002, 87, 848–852. [Google Scholar] [CrossRef]
- Mandal, S.; Puniya, A.K.; Singh, K. Effect of Alginate Concentrations on Survival of Microencapsulated Lactobacillus Casei NCDC-298. Int. Dairy J. 2006, 16, 1190–1195. [Google Scholar] [CrossRef]
- Lotfipour, F.; Mirzaeei, S.; Maghsoodi, M. Evaluation of the Effect of CaCl2 and Alginate Concentrations and Hardening Time on the Characteristics of Lactobacillus Acidophilus Loaded Alginate Beads Using Response Surface Analysis. Adv. Pharm. Bull. 2012, 2, 71–78. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the Egg-Box Model in Calcium-Alginate Gels with X-ray Diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Harper, B.A.; Barbut, S.; Lim, L.T.; Marcone, M.F. Effect of Various Gelling Cations on the Physical Properties of “Wet” Alginate Films. J. Food Sci. 2014, 79, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Lu, C.; Liu, P. Disintegration-Controllable Stimuli-Responsive Polyelectrolyte Multilayer Microcapsules via Covalent Layer-by-Layer Assembly. Colloids Surf. B Biointerfaces 2011, 82, 385–390. [Google Scholar] [CrossRef]
- Merakchi, A.; Bettayeb, S.; Drouiche, N.; Adour, L.; Lounici, H. Cross-Linking and Modification of Sodium Alginate Biopolymer for Dye Removal in Aqueous Solution. Polym. Bull. 2019, 76, 3535–3554. [Google Scholar] [CrossRef]
- Kesselman, L.R.B.; Shinwary, S.; Selvaganapathy, P.R.; Hoare, T. Synthesis of Monodisperse, Covalently Cross-Linked, Degradable “Smart” Microgels Using Microfluidics. Small 2012, 8, 1092–1098. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Guo, N. Sodium Alginate/Na+-Rectorite Composite Microspheres: Preparation, Characterization, and Dye Adsorption. Carbohydr. Polym. 2012, 90, 853–858. [Google Scholar] [CrossRef]
- Won, K.; Kim, S.; Kim, K.J.; Park, H.W.; Moon, S.J. Optimization of Lipase Entrapment in Ca-Alginate Gel Beads. Process. Biochem. 2005, 40, 2149–2154. [Google Scholar] [CrossRef]
- Esquisabel, A.; Hernandez, R.M.; Igartua, M.; Gascon, A.R.; Calvo, B.; Pedraz, J.L. Effect of Lecithins on BCG-Alginate-PLL Microcapsule Particle Size and Stability upon Storage. J. Microencapsul. 2000, 17, 363–372. [Google Scholar] [CrossRef]
- Alnaief, M.; Alzaitoun, M.A.; García-González, C.A.; Smirnova, I. Preparation of Biodegradable Nanoporous Microspherical Aerogel Based on Alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Gedam, S.; Jadhav, P.; Talele, S.; Jadhav, A. Effect of Crosslinking Agent on Development of Gastroretentive Mucoadhesive Microspheres of Risedronate Sodium. Int. J. Appl. Pharm. 2018, 10, 133–140. [Google Scholar] [CrossRef]
- Mali, K.K.; Dias, R.J.; Ghorpade, V.S.; Havaldar, V.D. Sodium Alginate Microspheres Containing Multicomponent Inclusion Complex of Domperidone. Lat. Am. J. Pharm. 2010, 29, 1199–1207. [Google Scholar]
- Lemoine, D.; Wauters, F.; Bouchend’homme, S.; Préat, V. Preparation and Characterization of Alginate Microspheres Containing a Model Antigen. Int. J. Pharm. 1998, 176, 9–19. [Google Scholar] [CrossRef]
- Chan, L.W.; Lee, H.Y.; Heng, P.W.S. Production of Alginate Microspheres by Internal Gelation Using an Emulsification Method. Int. J. Pharm. 2002, 242, 259–262. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Chen, R.; Chen, L.; Ho, H. Improving the Stability of Astaxanthin by Microencapsulation in Calcium Alginate Beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, D.S.; Park, H.J. Survival of Freeze-Dried Lactobacillus Bulgaricus KFRI 673 in Chitosan-Coated Calcium Alginate Microparticles. J. Agric. Food Chem. 2004, 52, 7300–7305. [Google Scholar] [CrossRef]
- Seyedain-Ardabili, M.; Sharifan, A.; Tarzi, B.G. The Production of Synbiotic Bread by Microencapsulation. Food Technol. Biotechnol. 2016, 54, 52–59. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Neufeld, R.J.; Arnaud, P.; Chaumeil, J.C. Microencapsulation of Lipophilic Drugs in Chitosan-Coated Alginate Microspheres. Int. J. Pharm. 1999, 187, 115–123. [Google Scholar] [CrossRef]
- Lupo, B.; Maestro, A.; Porras, M.; Gutiérrez, J.M.; González, C. Preparation of Alginate Microspheres by Emulsification/Internal Gelation to Encapsulate Cocoa Polyphenols. Food Hydrocoll. 2014, 38, 56–65. [Google Scholar] [CrossRef]
- Price, R.; Monshipouri, M. Internal Gelation Methodmfor Forming Multilayer Microspheres and Product Thereof. U.S. Patent No. 5,744,337, 28 April 1998. [Google Scholar]
- Shafizadeh, A.; Golestan, L.; Ahmadi, M.; Darjani, P.; Ghorbani, A. Encapsulation of Lactobacillus Casei in Alginate Microcapsules: Improvement of the Bacterial Viability under Simulated Gastrointestinal Conditions Using Flaxseed Mucilage. J. Food Meas. Charact. 2020, 14, 1901–1908. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ma, G.H.; Su, Z.G. Preparation of Uniform Sized Chitosan Microspheres by Membrane Emulsification Technique and Application as a Carrier of Protein Drug. J. Control. Release 2005, 106, 62–75. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, B.; Kang, T.W.; Noh, J.H.; Kim, M.J.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. Electrostatically Interactive Injectable Hydrogels for Drug Delivery. Tissue Eng. Regen. Med. 2018, 15, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Gierszewska, M.; Ostrowska-Czubenko, J.; Chrzanowska, E. PH-Responsive Chitosan/Alginate Polyelectrolyte Complex Membranes Reinforced by Tripolyphosphate. Eur. Polym. J. 2018, 101, 282–290. [Google Scholar] [CrossRef]
- Jardim, K.V.; Palomec-Garfias, A.F.; Andrade, B.Y.G.; Chaker, J.A.; Báo, S.N.; Márquez-Beltrán, C.; Moya, S.E.; Parize, A.L.; Sousa, M.H. Novel Magneto-Responsive Nanoplatforms Based on MnFe2O4 Nanoparticles Layer-by-Layer Functionalized with Chitosan and Sodium Alginate for Magnetic Controlled Release of Curcumin. Mater. Sci. Eng. C 2018, 92, 184–195. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Alginate Nanoparticles Protect Ferrous from Oxidation: Potential Iron Delivery System. Int. J. Pharm. 2016, 513, 404–409. [Google Scholar] [CrossRef]
- Poncelet, D. Production of Alginate Beads by Emulsification/Internal Gelation. Ann. N. Y. Acad. Sci. 2001, 944, 74–82. [Google Scholar] [CrossRef]
- Zhai, P.; Chen, X.B.; Schreyer, D.J. Preparation and Characterization of Alginate Microspheres for Sustained Protein Delivery within Tissue Scaffolds. Biofabrication 2013, 5, 015009. [Google Scholar] [CrossRef]
- Ahmed, M.M.; El-Rasoul, S.A.; Auda, S.H.; Ibrahim, M.A. Emulsification/Internal Gelation as a Method for Preparation of Diclofenac Sodium-Sodium Alginate Microparticles. Saudi Pharm. J. 2013, 21, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Perumal, D. Microencapsulation of Ibuprofen and Eudragit® RS 100 by the Emulsion Solvent Evaporation Technique. Int. J. Pharm. 2001, 218, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Hirsch, D.; Figueiredo, H.C.P.; Logato, P.V.R.; Moraes, Â.M. Production and Characterisation of Alginate Microparticles Incorporating Aeromonas Hydrophila Designed for Fish Oral Vaccination. Process. Biochem. 2006, 41, 638–643. [Google Scholar] [CrossRef]
- Shukla, S.; Jain, D.; Verma, K.; Verma, S. Formulation and in Vitro Characterization of Alginate Microspheres Loaded with Diloxanide Furoate for Colon- Specific Drug Delivery. Asian J. Pharm. 2010, 4, 199–204. [Google Scholar] [CrossRef]
- Esposito, E.; Cortesi, R.; Luca, G.; Nastruzzi, C. Pectin-Based Microspheres: A Preformulatory Study. Ann. N. Y. Acad. Sci. 2001, 944, 160–179. [Google Scholar] [CrossRef]
- Rahman, Z.; Kohli, K.; Khar, R.K.; Ali, M.; Charoo, N.A.; Shamsher, A.A.A. Characterization of 5-Fluorouracil Microspheres for Colonic Delivery. AAPS PharmSciTech 2006, 7, 113–121. [Google Scholar] [CrossRef]
- Rastogi, R.; Sultana, Y.; Aqil, M.; Ali, A.; Kumar, S.; Chuttani, K.; Mishra, A.K. Alginate Microspheres of Isoniazid for Oral Sustained Drug Delivery. Int. J. Pharm. 2007, 334, 71–77. [Google Scholar] [CrossRef]
- Patil, S.B.; Sawant, K.K. Development, Optimization and in Vitro Evaluation of Alginate Mucoadhesive Microspheres of Carvedilol for Nasal Delivery. J. Microencapsul. 2009, 26, 432–443. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-Alginate Biocomposite Containing Fucoidan for Bone Tissue Engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef]
- Schroeder, G. Nanotechnologia, Kosmetyki, Chemia Supramolekularna; Cursiva: Krakow, Poland, 2010. [Google Scholar]
- Jantarathin, S.; Borompichaichartkul, C.; Sanguandeekul, R. Microencapsulation of Probiotic and Prebiotic in Alginate-Chitosan Capsules and Its Effect on Viability under Heat Process in Shrimp Feeding. Mater. Today Proc. 2017, 4, 6166–6172. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of Probiotic Saccharomyces Cerevisiae Var: Boulardii with Different Wall Materials by Spray Drying. LWT-Food Sci. Technol. 2015, 63, 685–690. [Google Scholar] [CrossRef]
- Maleki, O.; Khaledabad, M.A.; Amiri, S.; Asl, A.K.; Makouie, S. Microencapsulation of Lactobacillus Rhamnosus ATCC 7469 in Whey Protein Isolate-Crystalline Nanocellulose-Inulin Composite Enhanced Gastrointestinal Survivability. LWT-Food Sci. Technol. 2020, 126, 109224. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Trząskowska, M. Probiotyki w Produktach Pochodzenia Roślinnego. Zywn. Nauk. Technol. Jakosc 2013, 4, 5–20. [Google Scholar]
- Fuller, R. Probiotics in Man and Animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar]
- Toczek, K.; Glibowski, P. Bakterie Probiotyczne w Żywności Nowe Kierunki Stosowania. Przem. Spożywczy 2015, 69, 42–45. [Google Scholar]
- Sánchez-Portilla, Z.; Melgoza-Contreras, L.M.; Reynoso-Camacho, R.; Pérez-Carreón, J.I.; Gutiérrez-Nava, A. Incorporation of Bifidobacterium Sp. into Powder Products through a Fluidized Bed Process for Enteric Targeted Release. J. Dairy Sci. 2020, 103, 11129–11137. [Google Scholar] [CrossRef]
- Muszyński, J.; Hudemowicz, P.; Gawlik, M. Mikroenkapsulacja–Innowacyjna Technologia Zwiększająca Biodostępność Probiotyków Dojelitowych. Gaz. Farm. 2019, 3, 1–3. [Google Scholar]
- Kołodziej, M.; Łukasik, J.; Szajewska, H. Mikroenkapsulacja Probiotyków. Pediatr. Pol. 2017, 92, 594–601. [Google Scholar] [CrossRef]
- Rokka, S.; Rantamäki, P. Protecting Probiotic Bacteria by Microencapsulation: Challenges for Industrial Applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Wilson, R.A.; Mandge, H.M.; Manikantan, M.R. Optimizing Microencapsulation of Nisin with Sodium Alginate and Guar Gum. J. Food Sci. Technol. 2014, 51, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Dajnowiec, F.; Biegaj, M.; Banaszczyk, P.; Kubiak, A.; Zander, L. Otrzymywanie Mikrokapsułek Alginianowych Za Pomocą Ultradźwięków. Inżynieria Apar. Chem. 2015, 54, 29–30. [Google Scholar]
- Chen, K.N.; Chen, M.J.; Liu, J.E.R.; Lin, C.W.; Chiu, H.Y.I. Optimization of Incorporated Prebiotics as Coating Materials for Probiotic Microencapsulation. J. Food Sci. 2005, 70, M260–M266. [Google Scholar] [CrossRef]
- Donthidi, A.R.; Tester, R.F.; Aidoo, K.E. Effect of Lecithin and Starch on Alginate-Encapsulated Probiotic Bacteria. J. Microencapsul. 2010, 27, 67–77. [Google Scholar] [CrossRef]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in Alginate and Alginate Coated-Chitosan Improved the Survival of Newly Probiotic in Oxgall and Gastric Juice. Int. J. Biol. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef]
- Sathyabama, S.; Kumar, M.R.; Devi, P.B.; Vijayabharathi, R.; Priyadharisini, V.B. Co-Encapsulation of Probiotics with Prebiotics on Alginate Matrix and Its Effect on Viability in Simulated Gastric Environment. LWT-Food Sci. Technol. 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Microencapsulation of Saccharomyces Cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods 2020, 9, 1051. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Das, N. Process Optimization for Microencapsulation of Probiotic Yeasts. Front. Biol. 2018, 13, 197–207. [Google Scholar] [CrossRef]
- Prevost, H.; Divies, C.; Rousseau, E. Continuous Yoghurt Production with Lactobacillus Bulgaricus and Streptococcus Thermophilus Entrapped in Ca-Alginate. Biotechnol. Lett. 1985, 7, 247–252. [Google Scholar] [CrossRef]
- Prevost, H.; Divies, C. Fresh Fermented Cheese Production with Continuous Pre-Fermented Milk by a Mixed Culture of Mesophilic Lactic Streptococci Entrapped in Ca-Alginate. Biotechnol. Lett. 1987, 9, 789–794. [Google Scholar] [CrossRef]
- Muthukumarasamy, P.; Holley, R.A. Survival of Escherichia Coli O157:H7 in Dry Fermented Sausages Containing Micro-Encapsulated Probiotic Lactic Acid Bacteria. Food Microbiol. 2007, 24, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Steenson, L.R.; Klaenhammer*, T.R.; Swaisgood, H.E. Calcium Alginate-Immobilized Cultures of Lactic Streptococci Are Protected from Bacteriophages. J. Dairy Sci. 1987, 70, 1121–1127. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M. An Improved Method of Microencapsulation and Its Evaluation to Protect Lactobacillus Spp. in Simulated Gastric Conditions. J. Microbiol. Methods 2004, 56, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Controlled Release of Lactobacillus Rhamnosus Biofilm Probiotics from Alginate-Locust Bean Gum Microcapsules. Carbohydr. Polym. 2014, 103, 587–595. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Lenton, D.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan Coated Alginate Beads for the Survival of Microencapsulated Lactobacillus Plantarum in Pomegranate Juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef]
- Chen, K.N.; Chen, M.J.; Lin, C.W. Optimal Combination of the Encapsulating Materials for Probiotic Microcapsules and Its Experimental Verification (R1). J. Food Eng. 2006, 76, 313–320. [Google Scholar] [CrossRef]
- Muthukumarasamy, P.; Holley, R.A. Microbiological and Sensory Quality of Dry Fermented Sausages Containing Alginate-Microencapsulated Lactobacillus Reuteri. Int. J. Food Microbiol. 2006, 111, 164–169. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Speranza, B.; Di Maggio, B.; Gallo, M.; Sinigaglia, M. Use of Alginate Beads as Carriers for Lactic Acid Bacteria in a Structured System and Preliminary Validation in a Meat Product. Meat Sci. 2016, 111, 198–203. [Google Scholar] [CrossRef]
- Mirzaei, H.; Pourjafar, H.; Homayouni, A. Effect of Calcium Alginate and Resistant Starch Microencapsulation on the Survival Rate of Lactobacillus Acidophilus La5 and Sensory Properties in Iranian White Brined Cheese. Food Chem. 2012, 132, 1966–1970. [Google Scholar] [CrossRef]
- Chen, M.J.; Chen, K.N.; Kuo, Y.T. Optimal Thermotolerance of Bifidobacterium Bifidum in Gellan-Alginate Microparticles. Biotechnol. Bioeng. 2007, 98, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, P.; Wojtas, P.; A Holley, R. Stability of Lactobacillus Reuteri in Different Types of Microcapsules. J. Food Sci. 2006, 71, 20–24. [Google Scholar] [CrossRef]
- Özer, B.; Kirmaci, H.A.; Şenel, E.; Atamer, M.; Hayaloǧlu, A. Improving the Viability of Bifidobacterium Bifidum BB-12 and Lactobacillus Acidophilus LA-5 in White-Brined Cheese by Microencapsulation. Int. Dairy J. 2009, 19, 22–29. [Google Scholar] [CrossRef]
- Amine, K.M.; Champagne, C.P.; Raymond, Y.; St-Gelais, D.; Britten, M.; Fustier, P.; Salmieri, S.; Lacroix, M. Survival of Microencapsulated Bifidobacterium Longum in Cheddar Cheese during Production and Storage. Food Control 2014, 37, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Yeung, T.W.; Arroyo-Maya, I.J.; McClements, D.J.; Sela, D.A. Microencapsulation of Probiotics in Hydrogel Particles: Enhancing Lactococcus Lactis Subsp. Cremoris LM0230 Viability Using Calcium Alginate Beads. Food Funct. 2016, 7, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, I.; Ayadi, D.; Bejar, W.; Bejar, S.; Chouayekh, H.; Ben Salah, R. Effects of Lactobacillus Plantarum Immobilization in Alginate Coated with Chitosan and Gelatin on Antibacterial Activity. Int. J. Biol. Macromol. 2014, 64, 84–89. [Google Scholar] [CrossRef]
- De Araújo Etchepare, M.; Nunes, G.L.; Nicoloso, B.R.; Barin, J.S.; Moraes Flores, E.M.; de Oliveira Mello, R.; Ragagnin de Menezes, C. Improvement of the Viability of Encapsulated Probiotics Using Whey Proteins. LWT-Food Sci. Technol. 2020, 117, 108601. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of Whey Protein-Alginate Wall Systems on Survival of Microencapsulated Lactobacillus Plantarum in Simulated Gastrointestinal Conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. The Viability of Probiotic Lactobacillus Rhamnosus (Non-Encapsulated and Encapsulated) in Functional Reduced-Fat Cream Cheese and Its Textural Properties during Storage. Food Control 2019, 100, 8–16. [Google Scholar] [CrossRef]
- Mokarram, R.R.; Mortazavi, S.A.; Najafi, M.B.H.; Shahidi, F. The Influence of Multi Stage Alginate Coating on Survivability of Potential Probiotic Bacteria in Simulated Gastric and Intestinal Juice. Food Res. Int. 2009, 42, 1040–1045. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Effect of Various Encapsulating Materials on the Stability of Probiotic Bacteria. J. Food Sci. 2009, 74, M100–M107. [Google Scholar] [CrossRef] [PubMed]
- Sheu, T.Y.; Marshall, R.T.; Heymann, H. Improving Survival of Culture Bacteria in Frozen Desserts by Microentrapment. J. Dairy Sci. 1993, 76, 1902–1907. [Google Scholar] [CrossRef]
- Homayouni, A.; Azizi, A.; Ehsani, M.R.; Yarmand, M.S.; Razavi, S.H. Effect of Microencapsulation and Resistant Starch on the Probiotic Survival and Sensory Properties of Synbiotic Ice Cream. Food Chem. 2008, 111, 50–55. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Masondole, L. Survival of Free and Microencapsulated Lactobacillus Acidophilus and Bifidobacterium Lactis and Their Effect on Texture of Feta Cheese. Aust. J. Dairy Technol. 2005, 60, 252–258. [Google Scholar]
- Larisch, B.C.; Poncelet, D.; Champagne, C.P.; Neufeld, R.J. Microencapsulation of Lactococcus Lactis Subsp. Cremoris. J. Microencapsul. 1994, 11, 189–195. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of Microencapsulated Lactobacillus Acidophilus LA1 to Processing Treatments and Simulated Gut Conditions. Food Bioprocess. Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
- Holkem, A.T.; Raddatz, G.C.; Barin, J.S.; Moraes Flores, É.M.; Muller, E.I.; Codevilla, C.F.; Jacob-Lopes, E.; Ferreira Grosso, C.R.; de Menezes, C.R. Production of Microcapsules Containing Bifidobacterium BB-12 by Emulsification/Internal Gelation. LWT-Food Sci. Technol. 2017, 76, 216–221. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, K.; Kumar, P. Optimizing Microencapsulation of α-Tocopherol with Pectin and Sodium Alginate. J. Food Sci. Technol. 2018, 55, 3625–3631. [Google Scholar] [CrossRef]
- Santagapita, P.R.; Mazzobre, M.F.; Buera, M.P. Formulation and Drying of Alginate Beads for Controlled Release and Stabilization of Invertase. Biomacromolecules 2011, 12, 3147–3155. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum spp in an Alginate Matrix Coated with Whey Proteins. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef]
- Haghshenas, B.; Abdullah, N.; Nami, Y.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Microencapsulation of Probiotic Bacteria Lactobacillus Plantarum 15HN Using Alginate-Psyllium-Fenugreek Polymeric Blends. J. Appl. Microbiol. 2015, 118, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan Coated Alginate-Xanthan Gum Bead Enhanced PH and Thermotolerance of Lactobacillus Plantarum LAB12. Int. J. Biol. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The Influence of Coating Materials on Some Properties of Alginate Beads and Survivability of Microencapsulated Probiotic Bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Lee, K.; Heo, T.-R. Survival of Bifidobacterium Longum Immobilized in Calcium Alginate Beads in Simulated Gastric Juices and Bile Salt Solution. Appl. Environ. Microbiol. 2000, 66, 869–873. [Google Scholar] [CrossRef]
- Graff, S.; Chaumeil, J.C.; Boy, P.; Lai-Kuen, R.; Charrueau, C. Formulations for Protecting the Probiotic Saccharomyces Boulardii from Degradation in Acidic Condition. Biol. Pharm. Bull. 2008, 31, 266–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martoni, C.; Bhathena, J.; Urbanska, A.M.; Prakash, S. Microencapsulated Bile Salt Hydrolase Producing Lactobacillus Reuteri for Oral Targeted Delivery in the Gastrointestinal Tract. Appl. Microbiol. Biotechnol. 2008, 81, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Luo, B.; Gao, J.; Li, X.; Zhao, Z.; Zhang, Y.; Ni, Y.; Tian, F. Oligosaccharides as Co-Encapsulating Agents: Effect on Oral Lactobacillus Fermentum Survival in a Simulated Gastrointestinal Tract. Biotechnol. Lett. 2019, 41, 263–272. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Saeed, M.; Yasmin, I.; Hassanin, H.A.M.; Mahmood, S.; Rehman, A. In Vitro Survival of Bifidobacterium Bifidum Microencapsulated in Zein-Coated Alginate Hydrogel Microbeads. J. Microencapsul. 2019, 36, 192–203. [Google Scholar] [CrossRef]
- Zanjani, M.A.K.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N. Microencapsulation of Probiotics by Calcium Alginate-Gelatinized Starch with Chitosan Coating and Evaluation of Survival in Simulated Human Gastro-Intestinal Condition. Iran. J. Pharm. Res. IJPR 2014, 13, 843–852. [Google Scholar]
- Annan, N.T.; Borza, A.D.; Hansen, L.T. Encapsulation in Alginate-Coated Gelatin Microspheres Improves Survival of the Probiotic Bifidobacterium Adolescentis 15703T during Exposure to Simulated Gastro-Intestinal Conditions. Food Res. Int. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- Sikora, E.; Miastkowska, M.; Lasoń, E. Selected Skin Delivery Systems; Wydawnictwo PK: Kraków, Poland, 2020. [Google Scholar]
- Patravale, V.B.; Mandawgade, S.D. Novel Cosmetic Delivery Systems: An Application Update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Rac, V.; Manojlovi, V.; Raki, V.; Bugarski, B.; Flock, T.; Krzyczmonik, K.E.; Nedovi, V. Limonene Encapsulation in Alginate/Poly (Vinyl Alcohol). Procedia Food Sci. 2011, 1, 1816–1820. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil Encapsulation Techniques Using Alginate as Encapsulating Agent: Applications and Drawbacks. J. Microencapsul. 2017, 38, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Oerlemans, E.; Claes, I.; Henkens, T. Topical Cream with Live Lactobacilli Modulates the Skin Microbiome and Reduce Acne Symptoms. bioRxiv 2018, 463307. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P. Chitosan-Alginate Nanocapsules for Encapsulation of Turmeric Oil. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 911–915. [Google Scholar] [CrossRef]
- Shalaka, D.; Naik, S.R.; Amruta, A.; Parimal, K. Vitamin E Loaded Pectin Alginate Microspheres for Cosmetic Application. J. Pharm. Res. 2009, 2, 1098–1102. [Google Scholar]
- Riseh, R.S.; Skorik, Y.A.; Thakur, V.K.; Pour, M.M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Riseh, R.S.; Ebrahimi-Zarandi, M.; Vazvani, M.G.; Skorik, Y.A. Reducing Drought Stress in Plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides. Int. J. Mol. Sci. 2021, 22, 12979. [Google Scholar] [CrossRef]
- Fathi, F.; Saberi-Riseh, R.; Khodaygan, P. Survivability and Controlled Release of Alginate-Microencapsulated Pseudomonas Fluorescens VUPF506 and Their Effects on Biocontrol of Rhizoctonia Solani on Potato. Int. J. Biol. Macromol. 2021, 183, 627–634. [Google Scholar] [CrossRef]
- Meng, L.; Ivanov, A.S.; Kim, S.; Zhao, X.; Kumar, N.; Young-gonzales, A.; Saito, T.; Bras, W.; Gluesenkamp, K.; Bocharova, V. Alginate—Sodium Sulfate Decahydrate Phase Change Composite with Extended Stability. ACS Appl. Polym. Mater. 2022, 4, 6563–6571. [Google Scholar] [CrossRef]
- Hia, I.L.; Lam, W.H.; Chai, S.P.; Chan, E.S.; Pasbakhsh, P. Surface Modified Alginate Multicore Microcapsules and Their Application in Self-Healing Epoxy Coatings for Metallic Protection. Mater. Chem. Phys. 2018, 215, 69–80. [Google Scholar] [CrossRef]
- Tohoku, T.; Section, A.; Agency, E. Selective Separation and Recovery of Mo(VI) by Hybrid Microcapsules Containing Organic Extractants-10179. In Proceedings of the WM2010 Conference, Phoenix, AZ, USA, 7–11 March 2010; pp. 3–13. [Google Scholar]
- Wang, L.; Li, H.; Yu, D.; Wang, Y.; Wang, W.; Wu, M. Hyperbranched Polyamide–Functionalized Sodium Alginate Microsphere as a Novel Adsorbent for the Removal of Antimony(III) in Wastewater. Environ. Sci. Pollut. Res. 2019, 26, 27372–27384. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mimura, H.; Niibori, Y. Selective Uptake of Plutonium (IV) on Calcium Alginate Gel Polymer and TBP Microcapsule. J. Radioanal. Nucl. Chem. 2009, 281, 513–520. [Google Scholar] [CrossRef]
- Mimura, H.; Ohta, H.; Akiba, K.; Wakui, Y.; Onodera, Y. Uptake and Recovery of Platinum Group Metals Ions By Alginate Microcapsules Immobilizing Cyanex 302 Emulsions. J. Nucl. Sci. Technol. 2002, 39, 1008–1012. [Google Scholar] [CrossRef]
- Zhao, F.; Qin, X.; Feng, S. Preparation of Microgel/Sodium Alginate Composite Granular Hydrogels and Their Cu2+ Adsorption Properties. RSC Adv. 2016, 6, 100511–100518. [Google Scholar] [CrossRef]
- Hao, D.; Huang, Q.; Wei, W.; Bai, X.; Ni, B.J. A Reusable, Separation-Free and Biodegradable Calcium Alginate/g-C3N4 Microsphere for Sustainable Photocatalytic Wastewater Treatment. J. Clean. Prod. 2021, 314, 128033. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a Useful Natural Polymer for Microencapsulation and Therapeutic Applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Pourjafar, H.; Noori, N.; Gandomi, H.; Akhondzadeh, A. Viability of Microencapsulated and Non-Microencapsulated Lactobacilli in a Commercial Beverage. Biotechnol. Rep. 2020, 25, e00432. [Google Scholar] [CrossRef]
- Haghshenas, B.; Nami, Y.; Haghshenas, M. Effect of Addition of Inulin and Fenugreek on the Survival of Microencapsulated Enterococcus Durans 39C in Alginate-Psyllium Polymeric Blends in Simulated Digestive System and Yogurt. Asian J. Pharm. Sci. 2015, 10, 350–361. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-capillas, C.; Herrero, A.M.; Jiménez-colmenero, F.; Pintado, T.; Ragagnin, C.; Menezes, D.; Lucy, L.; Fries, M. Effect of Different Strategies of Lactobacillus Plantarum Incorporation in Chorizo Sausages. J. Sci. Food Agric. 2019, 99, 6706–6712. [Google Scholar] [CrossRef]
- Karabulut, I.; Candogan, K. Effects of Encapsulated Starter Cultures on Microbial and Physicochemical Properties of Traditionally Produced and Heat Treated Sausages (Sucuks). LWT-Food Sci. Technol. 2017, 75, 425–433. [Google Scholar] [CrossRef]
- Angiolillo, L.; Conte, A.; Nobile, M.A. Del. Microencapsulated Lactobacillus Reuteri Combined with Modified Atmosphere as a Way to Improve Tuna Burger Shelf Life. Int. J. Food Sci. Technol. 2017, 52, 1576–1584. [Google Scholar] [CrossRef]
- Kavas, N.; Kavas, G.; Kinik, Ö.; Ates, M.; Kaplan, M.; Şatir, G. Symbiotic Microencapsulation to Enhance Bifidobacterium Longum and Lactobacillus Paracasei Survival in Goat Cheese. Food Sci. Technol. 2022, 42, 1–7. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of Chitosan-Alginate Encapsulation with Inulin on Survival of Lactobacillus Rhamnosus GG during Apple Juice Storage and under Simulated Gastrointestinal Conditions. LWT-Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Zanjani, K.A.M.; Reza, M.E.; Tarzi, B.G.; Sharifan, A. Promoting Lactobacillus Casei and Bifidobacterium Adolescentis Survival by Microencapsulation with Different Starches and Chitosan and Poly L-Lysine Coatings in Ice Cream. J. Process. Preserv. 2017, 42, e13318. [Google Scholar] [CrossRef]
- Barajas-álvarez, P.; González-ávila, M.; Espinosa-andrews, H.; Barajas-álvarez, P. Recent Advances in Probiotic Encapsulation to Improve Viability under Storage and Gastrointestinal Conditions and Their Impact on Functional Food Formulation Recent Advances in Probiotic Encapsulation to Improve Viability under Storage and Gastrointestina. Food Rev. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Polina, C.C.; Zamprogna, D.F.; Paliga, M.; Wisniewski, M.S.W.; Junges, A.; Steffens, J.; Cansian, R.L.; Backes, G.T. Encapsulation Methods for Probiotic Immobilization with Food Application Métodos de Encapsulação Para Imobilização de Probióticos Com Aplicação Em Alimentos. Brazilian J. Dev. 2021, 7, 22908–22929. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Biol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Prescott, S.L.; Larcombe, D.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Van Etten, E.; Horwitz, P.; Kozyrskyj, A.; et al. The Skin Microbiome: Impact of Modern Environments on Skin Ecology, Barrier Integrity, and Systemic Immune Programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of Probiotic Living Cells: From Laboratory Scale to Industrial Applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Aguirre Calvo, T.; Santagapita, P. Physicochemical Characterization of Alginate Beads Containing Sugars and Biopolymers. J. Qual. Reliab. Eng. 2016, 2016, 9184039. [Google Scholar] [CrossRef]
- Lopes, M.; Abrahim, B.; Veiga, F.; Seiça, R.; Cabral, L.M.; Arnaud, P.; Andrade, J.C.; Ribeiro, A.J. Preparation Methods and Applications behind Alginate-Based Particles. Expert Opin. Drug Deliv. 2017, 14, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Azadi, G.; Ardekani, A.M. Microfluidic Fabrication of Shape-Tunable Alginate Microgels: Effect of Size and Impact Velocity. Carbohydr. Polym. 2015, 120, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Q.; Wang, J.; Zhu, J.; Wang, H.; Yang, Y. Shape Controllable Microgel Particles Prepared by Microfluidic Combining External Ionic Crosslinking. Biomicrofluidics 2012, 6, 026502–265029. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Method | Advantages | Disadvantages |
|---|---|---|
| Extrusion |
|
|
| Emulsification |
|
|
| Spray-drying |
|
|
| Trade Name | Concentration of Emulsifier [%] | Encapsulation Techniques | References |
|---|---|---|---|
| Lecithin | 0.1; 0.5; 1.0; 2.0 | Emulsification/internal gelation | [121] |
| Span 80 | 0.5–2.0 | Emulsification/internal gelation | [110] |
| Span 80 | 0–3.0 | Emulsification/internal gelation | [122] |
| Span 80 | 2.0 | Emulsification/external gelation | [123] |
| Span 80 | 2.0 | Emulsification/external gelation | [124] |
| Span 85 | 2.0 | Emulsification/external gelation | [125] |
| Span 85 | - | Emulsification/internal gelation | [126] |
| Tween 20 | 0.5; 2.0 | Extrusion | [127] |
| Tween 20 | 0.1 | Extrusion | [128] |
| Tween 80 | 0.02 | Emulsification/external gelation | [83] |
| Tween 80 | - | Spray-drying | [69] |
| Tween 80 | - | Spray-drying | [71] |
| Tween 80 | - | Emulsification/complexation | [106] |
| Tween 80 | 0.2 | Emulsification/external gelation | [129] |
| Mixture: Span 80 and Tween 80 | 1.0 Span 80 and 1.0 Tween 80 | Emulsification/external gelation | [19] |
| Mixture: Span 80 and Tween 80 | 1.0 (mixture 60/40) | Emulsification/internal gelation | [130] |
| polyglycerol polyricinoleate (PGPR) | 4.0–15.0 | Emulsification/internal gelation | [131] |
| Capsules Components | Concentration | Actie Ingredients | Encapsulation Technique | References | |
|---|---|---|---|---|---|
| [%] | [M] | ||||
| sodium alginate Tween 20 calcium chloride | 1–3 0.5; 2 2; 10 | - - - | Astaxanthin | Extrusion | [127] |
| sodium alginate calcium chloride | - - | - - | Lactic acid bacteria (Lactobacillus) | Extrusion | [216] |
| sodium alginate solution ethanol Tween 80 calcium chloride | - - - - | - - - - | Tumeric oil | Emulsification | [217] |
| sodium alginate pectin Tween 80 calcium chloride | 0.5–2.5 5 0.1 5 | - - - - | Vitamin E | Emulsification | [218] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. https://doi.org/10.3390/polym14183834
Łętocha A, Miastkowska M, Sikora E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers. 2022; 14(18):3834. https://doi.org/10.3390/polym14183834
Chicago/Turabian StyleŁętocha, Anna, Małgorzata Miastkowska, and Elżbieta Sikora. 2022. "Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications" Polymers 14, no. 18: 3834. https://doi.org/10.3390/polym14183834
APA StyleŁętocha, A., Miastkowska, M., & Sikora, E. (2022). Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers, 14(18), 3834. https://doi.org/10.3390/polym14183834








