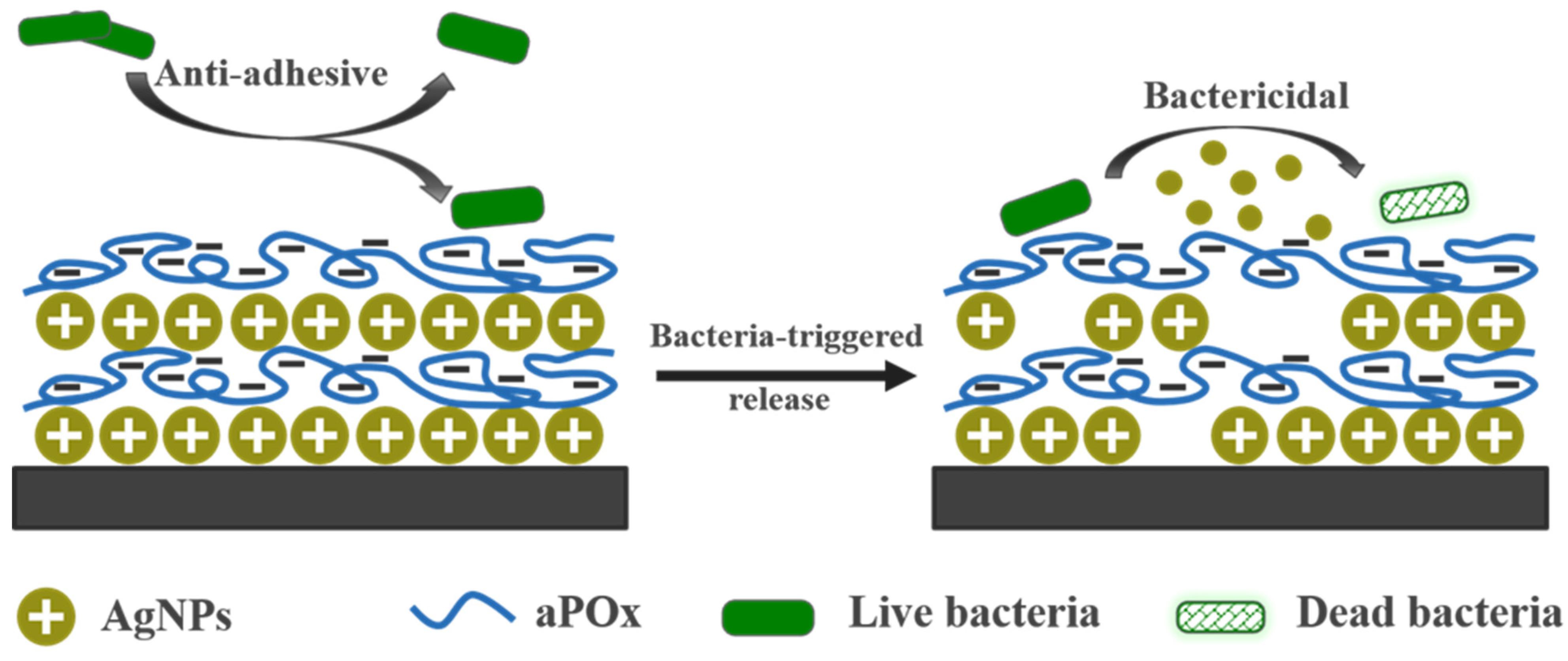
Bactericidal Anti-Adhesion Potential Integrated Polyoxazoline/Silver Nanoparticle Composite Multilayer Film with pH Responsiveness
Abstract
:1. Introduction
2. Experiments
2.1. Materials
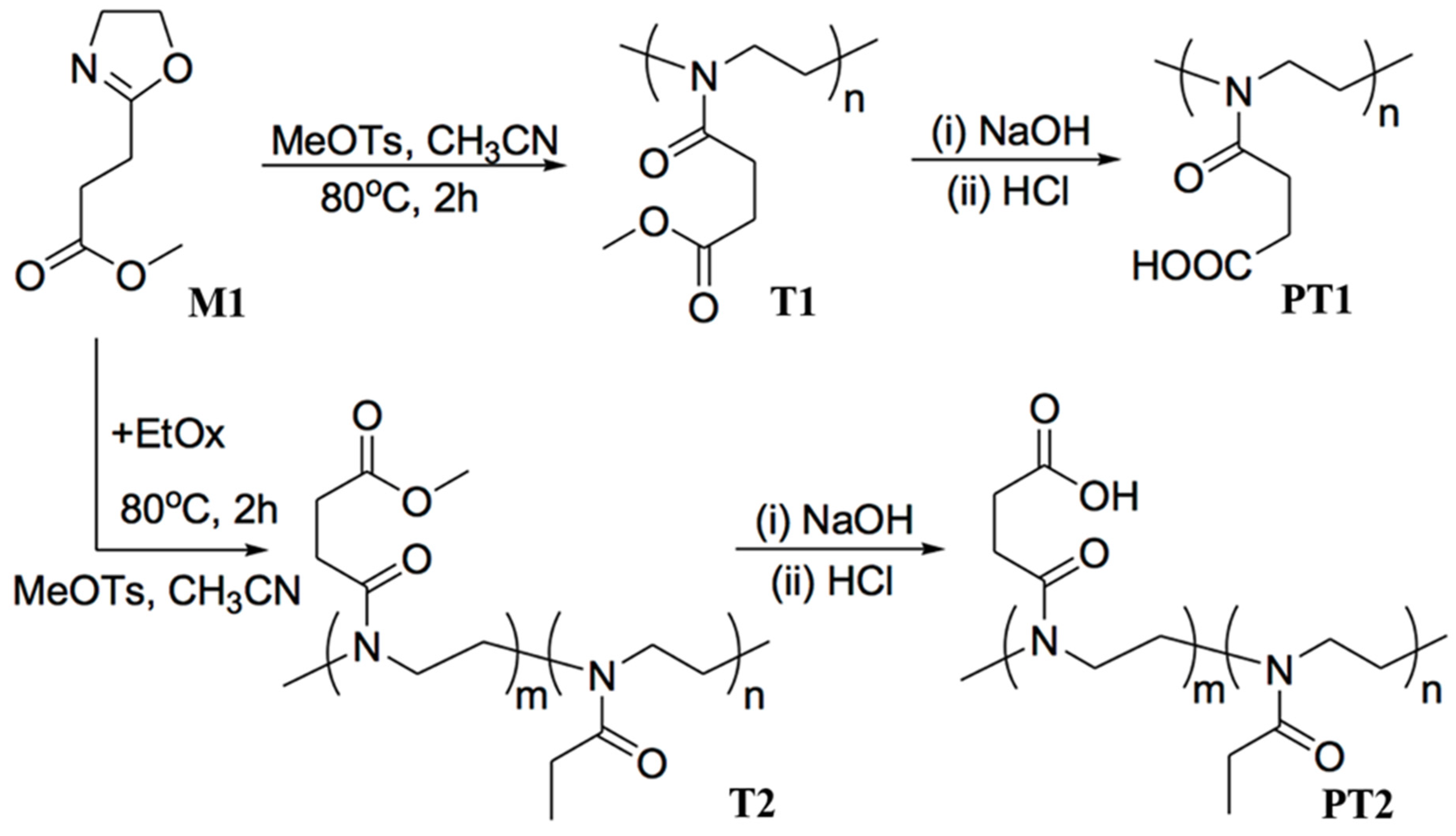
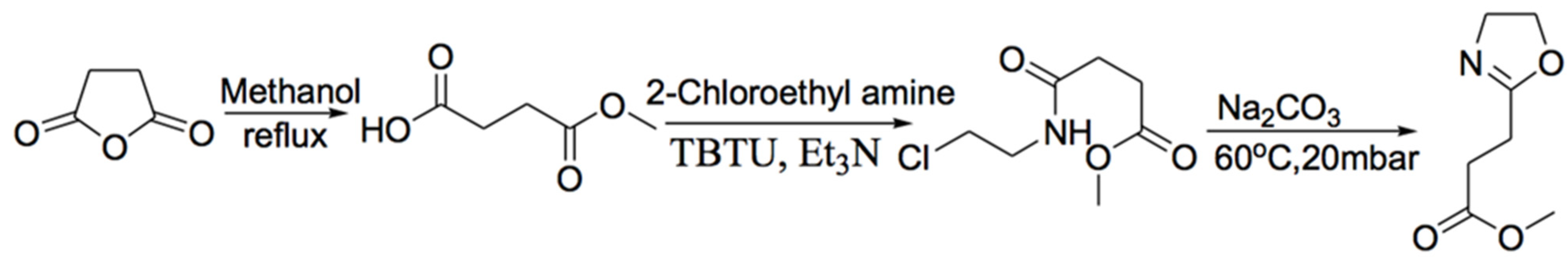
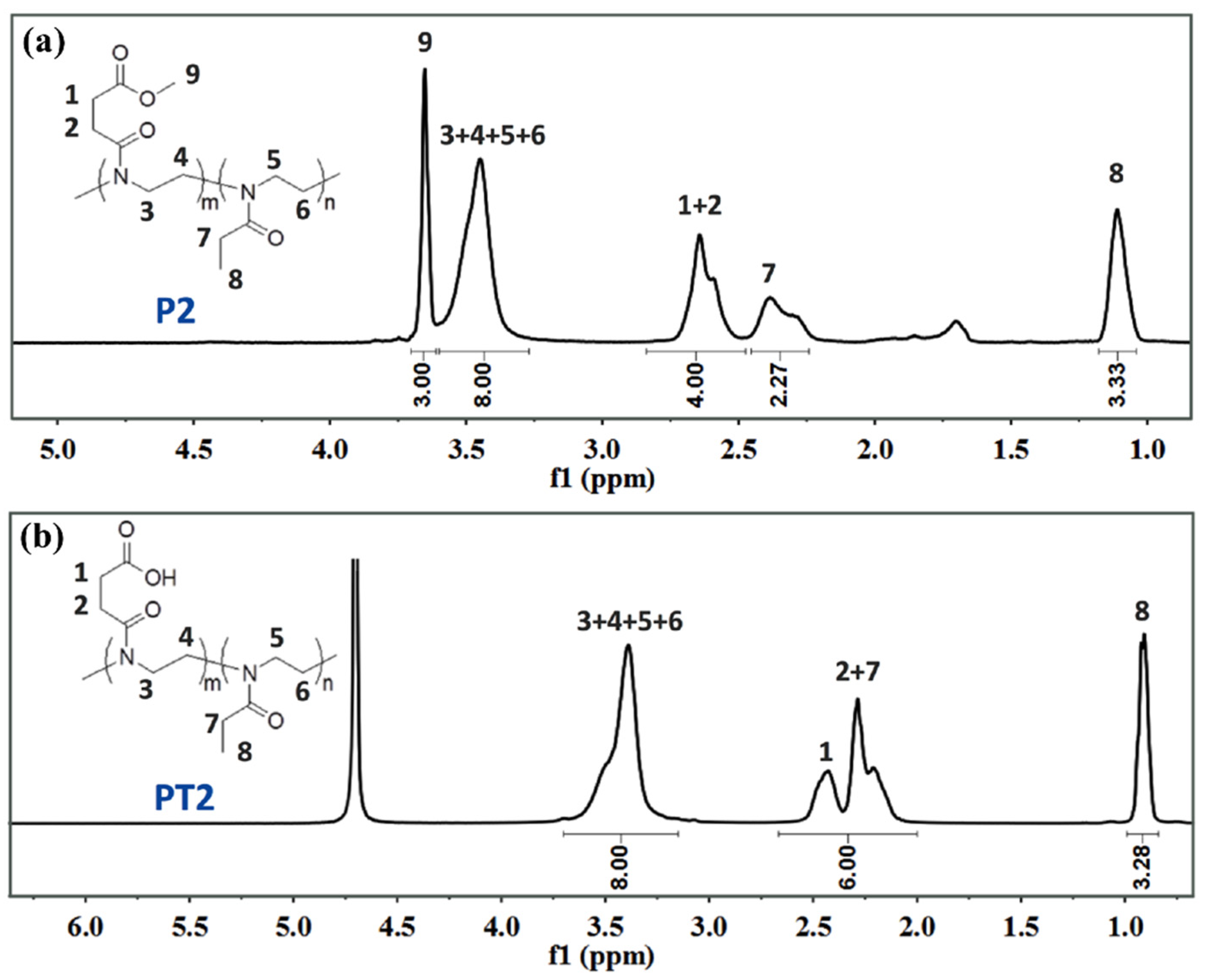
2.2. Synthesis and Characterization of aPOx (PT1, PT2 and PT3)
2.3. Preparation and Characterization of AgNPs
2.4. Preparation of aPOx-AgNP Multilayer Film
2.5. Layer-by-Layer Self-Assembly Behavior Tracking of aPOx-AgNP Multilayer Film
2.6. Characterization of AgNPs in aPOx-AgNP Multilayer Film
2.7. Characterization of aPOx-AgNP Multilayer Film
2.8. In Vitro Silver Release Test of aPOx-AgNP Multilayer Film
3. Results and Discussion
3.1. Structures of aPOx (PT1, PT2 and PT3) and M1
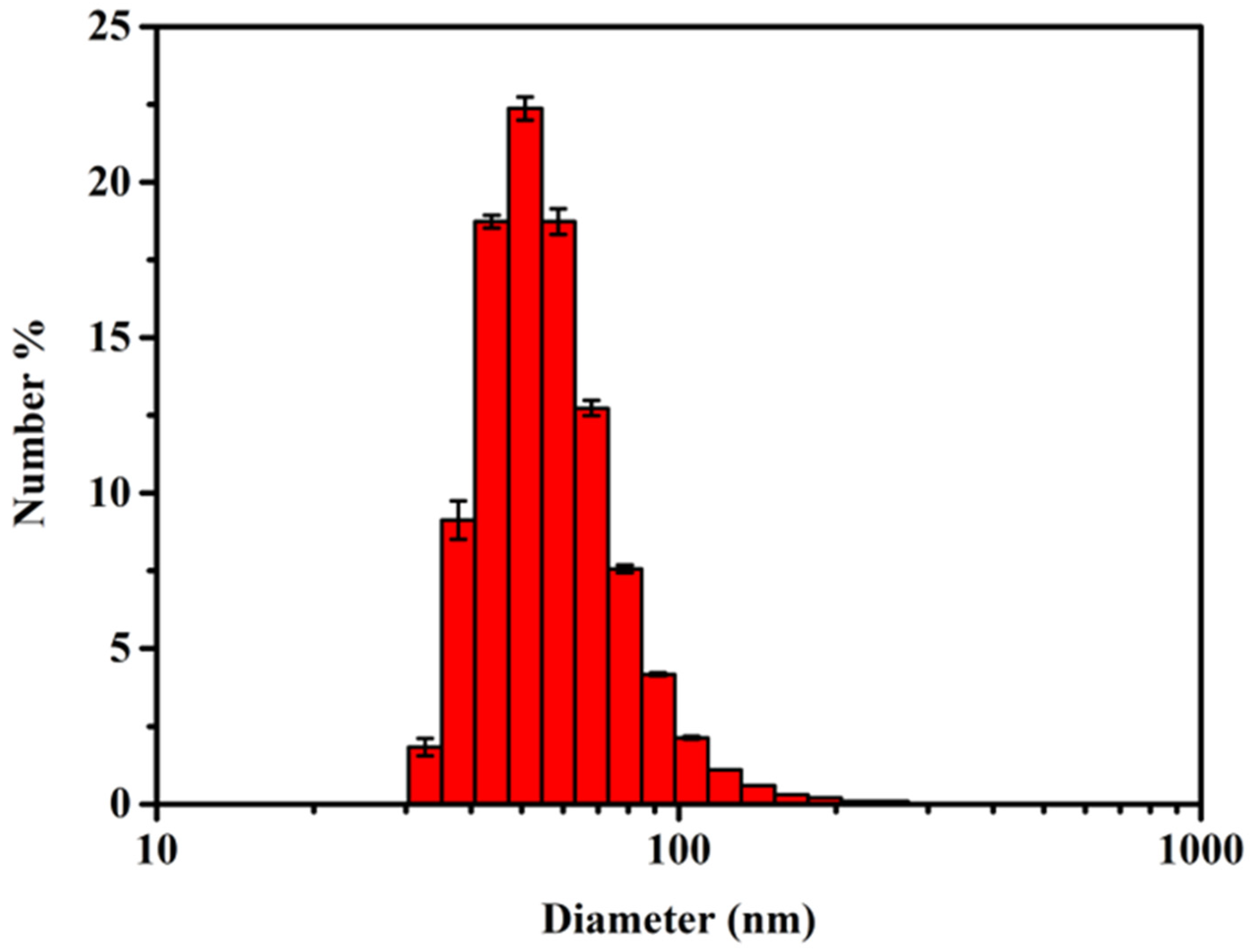
3.2. Size and Distribution of AgNPs
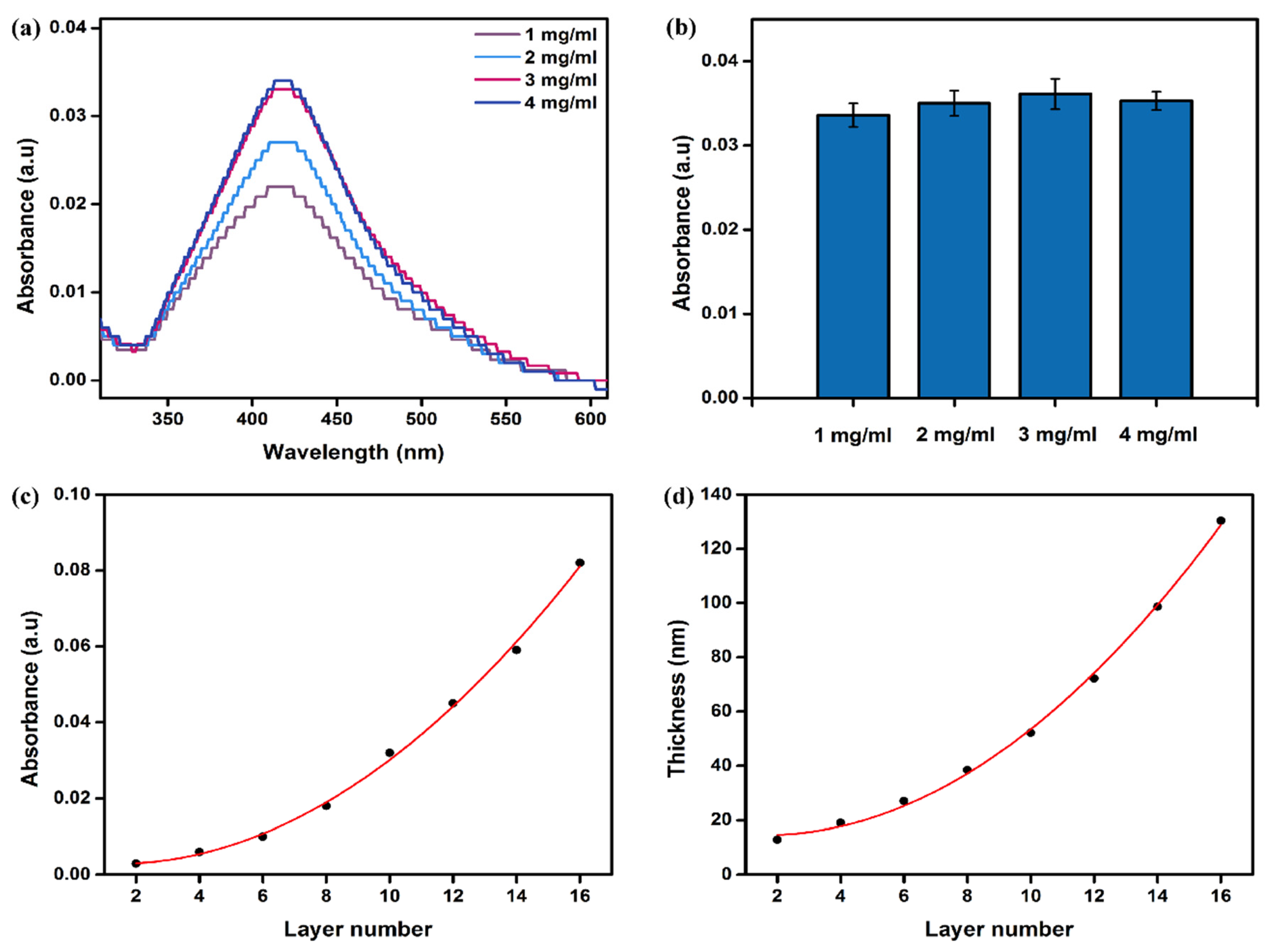
3.3. Layer-by-Layer Self-Assembly Behavior and Parameter Adjustment of aPOx-AgNP Multilayer Film
- (1)
- Effect of polyelectrolyte charge density on self-assembly
- (2)
- Effect of polyelectrolyte concentration on self-assembly
3.4. Properties of aPOx-AgNP Multilayer Film
3.5. pH-Responsive Silver Release of aPOx-AgNP Multilayer Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beilvert, A.; Chaubet, F.; Chaunier, L.; Guilois, S.; Lourdin, D. Shape-memory starch for resorbable biomedical devices. Carbohydr. Polym. 2014, 99, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, M.; Cometa, S.; Ferretti, C.; Latta, R.; Trapani, A.; Ceci, E.; Falconi, M.; Giglio, E. Characterization and cytocompatibility of an antibiotic/chitosan/cyclodextrins nanocoating on titanium implants. Carbohydr. Polym. 2014, 110, 173–182. [Google Scholar] [CrossRef]
- Liu, Y.; Busscher, H.; Zhao, B.; Li, Y.; Zhang, Z.; Mei, H.; Ren, Y.; Shi, L. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in Staphylococcal biofilms. ACS Nano 2016, 10, 4779–4789. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Tang, B.; Yuan, L.; Wang, K.; Liu, X.; Zhu, X.; Li, J.; Ge, Z.; Chen, S. New insights into synergistic antimicrobial and antifouling cotton fabrics via dually finished with quaternary ammonium salt and zwitterionic sulfobetaine. Chem. Eng. J. 2018, 336, 123–132. [Google Scholar] [CrossRef]
- Golberg, K.; Emuna, N.; Vinod, T.P.; Moppes, D.; Marks, R.; Arad, S.; Kushmaro, A. Novel anti-adhesive biomaterial patches: Preventing biofilm with metal complex films (MCF) derived from a microalgal polysaccharide. Adv. Mater. Interfaces 2016, 3, 1500486. [Google Scholar] [CrossRef]
- Costerton, J.; Stewart, P.; Greenberg, E. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Lushnikova, T.; Golla, R.; Wang, X.; Wang, G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017, 49, 316–328. [Google Scholar] [CrossRef]
- Tan, Y.; Matthias, L.; Leonhard, M.; Moser, D.; Stickler, B. Antibiofilm activity of carboxymethyl chitosan on the biofilms of non-Candida albicans Candida species. Carbohydr. Polym. 2016, 14, 77–82. [Google Scholar] [CrossRef]
- Junter, G.; Thebault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef]
- Morra, M.; Cassineli, C. Non-fouling properties of polysaccharide-coated surfaces. J. Biomater. Sci. Polym. Ed. 1999, 10, 1107–1124. [Google Scholar] [CrossRef]
- Peng, Z.; Tu, B.; Shen, Y.; Du, L.; Wang, L.; Guo, S.; Tang, T. Quaternized Chitosan Inhibits icaA Transcription and Biofilm Formation by Staphylococcus on a Titanium Surface. Antimicrob. Agents Chemother. 2011, 55, 860–866. [Google Scholar] [CrossRef]
- Kuo, J.; Tan, S.; Hsiao, Y.; Mutalik, C.; Chen, H.; Yougbare, S.; Kuo, T. Unveiling the Antibacterial Mechanism of Gold Nanoclusters via In Situ Transmission Electron Microscopy. ACS Sustain. Chem. Eng. 2022, 10, 464–471. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, L.; Huang, L.; Xiao, S.; Yang, Y.; Zhong, M.; Yang, J. Salt-induced regenerative surface for bacteria killing and release. Langmuir 2017, 33, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Liu, J.; Yang, Y.; Lai, Q.; Wu, X.; Yang, J.; Zeng, Q.; Zhang, G.; Zhao, S. Reinforcing hydration layer on membrane surface via nano-capturing and hydrothermal crosslinking for fouling reduction. J. Membr. Sci. 2022, 644, 120076. [Google Scholar] [CrossRef]
- Ranawat, Y.; Jaques, Y.J.; Foster, A. Predicting hydration layers on surfaces using deep learning. Nanoscale Adv. 2021, 3, 3447–3453. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Liu, Y.; Ren, Y.; Wang, Z.; Zhao, B.; Huang, S.; Xu, J.; Li, Z. Highly improved aqueous lubrication of polymer surface by noncovalently bonding hyaluronic acid-based hydration layer for endotracheal intubation. Biomaterials 2020, 262, 120336. [Google Scholar] [CrossRef]
- Rajesh, S.; Leiske, M.N.; Leitch, V.; Zhai, J.; Drummond, C.J.; Kempe, K.; Tran, N. Lipidic poly(2-oxazoline)s as PEG replacement steric stabilisers for cubosomes. J. Colloid Interface Sci. 2022, 623, 1142–1150. [Google Scholar] [CrossRef]
- Makhayeva, D.; Filippov, S.; Yestemes, S.; Irmukhametova, G.; Khutoryanskiy, Y. Polymeric iodophors with poly(2-ethyl-2-oxazoline) and poly(N-vinylpyrrolidone): Optical, hydrodynamic, thermodynamic, and antimicrobial properties. Eur. Polym. J. 2022, 165, 111005. [Google Scholar] [CrossRef]
- Tryba, A.; Borkowicz, M.; Kula, M.; Piergies, N.; Marzec, M.; Wegener, E.; Fraczyk, J.; Jordan, R.; Kolesinska, B.; Scharnweber, D.; et al. Surface Functionalization of Poly(l-lactide-co-glycolide) Membranes with RGD-Grafted Poly(2-oxazoline) for Periodontal Tissue Engineering. J. Funct. Biomater. 2022, 13, 4. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, M.; Cong, Z.; Xie, J.; Zhang, W.; Chen, S.; Zou, J.; Ji, Z.; Shao, N.; Chen, X.; et al. Short Guanidinium-Functionalized Poly(2-oxazoline)s Displaying Potent Therapeutic Efficacy on Drug-Resistant Fungal Infections. Angew. Chem. Int. Ed. 2022, 61, e202200778. [Google Scholar] [CrossRef]
- Shubha, A.; Manohara, S.; Siddlingeshwar, B.; Daima, H.; Singh, M.; Revaprasadu, N. Ternary poly(2-ethyl-2-oxazoline)-polyvinylpyrrolidone-graphene nanocomposites: Thermal, electrical, dielectric, mechanical, and antibacterial profiling. Diam. Relat. Mater. 2022, 125, 109001. [Google Scholar] [CrossRef]
- Mazarov, D.; Ezhov, I.; Yudintceva, N.; Shevtsov, M.; Rudakova, A.; Kalganov, V.; Tolmachev, V.; Zharova, Y.; Lutakov, O.; Kraeva, L.; et al. Antibacterial and osteogenic properties of Ag nanoparticles and Ag/TiO2 nanostructures prepared by atomic layer deposition. J. Funct. Biomater. 2022, 13, 62. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Li, P.; Li, Z. Lignin as a multi-functional agent for the synthesis of Ag nanoparticles and its application in antibacterial coatings. J. Mater. Res. Technol.-JMRT 2022, 17, 3211–3220. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Jia, Q.; He, L.; Duan, S.; Fu, Y.; Zhang, Z.; Du, M. Copper-based polymer-metal-organic framework embedded with Ag nanoparticles: Long-acting and intelligent antibacterial activity and accelerated wound healing. Chem. Eng. J. 2017, 9, 24440–24445. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties applications and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.; Rhim, J.; Adhikari, B. Switchable dual-functional and bioresponsive materials to control bacterial infections. Appl. Mater. Interfaces 2019, 11, 22897–22914. [Google Scholar] [CrossRef]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.X.; Xiao, S.W.; Yang, Y.; Chen, F.; Fan, P.; Zhao, Z.; Zhong, M.; Yang, J. Bacteria killing and release of salt-responsive, regenerative, double-layered polyzwitterionic brushes. Chem. Eng. J. 2018, 333, 1–10. [Google Scholar] [CrossRef]
- Huang, X.; Bao, X.; Liu, Y.; Wang, Z.; Hu, Q. Catechol-functional chitosan/silver nanoparticles composite as a highly effective antibacterial agent with species-specific mechanisms. Sci. Rep. 2017, 7, 1860. [Google Scholar] [CrossRef]
- Huang, X.; Pang, Y.; Liu, Y.; Zhou, Y.; Wang, Z.; Hu, Q. Green synthesis of silver nanoparticles with high antimicrobial activity and low cytotoxicity using catechol-conjugated chitosan. RSC Adv. 2016, 6, 64357–64363. [Google Scholar] [CrossRef]
- Huang, X.; Bao, X.; Wang, Z.; Hu, Q. A novel silver-loaded chitosan composite sponge with sustained silver release as a long-lasting antimicrobial dressing. RSC Adv. 2017, 7, 34655–34663. [Google Scholar] [CrossRef]
- Ballester, N.; Sciortino, F.; Mir, S.; Rydzek, G. Weak Polyelectrolytes as Nanoarchitectonic Design Tools for Functional Materials: A Review of Recent Achievements. Molecules 2022, 27, 3263. [Google Scholar] [CrossRef]
- Ni, J.; Li, J.; Jian, J.; He, J.; Chen, H.; Leng, X.; Liu, X. Recent Studies on the Fabrication of Multilayer Films by Magnetron Sputtering and Their Irradiation Behaviors. Coatings 2021, 11, 1468. [Google Scholar] [CrossRef]
- Javaid, S.; Mahnood, A.; Nasir, H.; Iqbal, M.; Ahmed, N.; Ahmed, N. Layer-By-Layer Self-Assembled Dip Coating for Antifouling Functionalized Finishing of Cotton Textile. Polymers 2022, 14, 2540. [Google Scholar] [CrossRef]
- Bodon, J.; Olmo, J.; Alonso, J.; Benitez, I.; Vilela, J.; Alvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2022, 14, 165. [Google Scholar] [CrossRef]
- Wei, S.; Peng, R.; Bian, S.; Han, W.; Xiao, B.; Peng, X. Facile and Scalable Synthesis and Self-Assembly of Chitosan Tartaric Sodium. Polymers 2022, 14, 69. [Google Scholar] [CrossRef]
- Yu, D.; Lin, W.; Yang, M. Surface Modification of Poly (l-lactic acid) Membrane via Layer-by-Layer Assembly of Silver Nanoparticle-Embedded Polyelectrolyte Multilayer. Bioconjug. Chem. 2007, 18, 1521–1529. [Google Scholar] [CrossRef]
- Arjan, W.; Khan, M.; Almutairi, H.; Alharbi, S.; Razak, S. pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef]
- Henriquez, G.; Umanzor, F.; Gomez, M.; Inostroza, C.; Campos, E.; Lopez, R.; Vallejos, M.; Herrera, C.; Hernandez, J. Wrinkling on Stimuli-Responsive Functional Polymer Surfaces as a Promising Strategy for the Preparation of Effective Antibacterial/Antibiofouling Surfaces. Polymers 2021, 13, 4262. [Google Scholar] [CrossRef]
- Manouras, T.; Platania, V.; Georgopoulou, A.; Chatzinikolaidou, M.; Vamvakaki, M. Responsive Quaternized PDMAEMA Copolymers with Antimicrobial Action. Polymers 2021, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Boutan, P.; Lava, K.; Hest, J.; Hoogenboom, R. Thermal Properties of Methyl Ester-Containing Poly(2-oxazoline)s. Polymers 2015, 7, 1998–2008. [Google Scholar] [CrossRef]
- Boerman, M.; Laan, H.; Bender, J.; Hoogenboom, R.; Jansen, J.; Leeuwenburgh, S.; Hest, J. Synthesis of pH- and thermoresponsive poly(2-n-propyl-2-oxazoline) based copolymers. J. Polym. Sci. Part A-Polym. Chem. 2016, 54, 1573–1582. [Google Scholar] [CrossRef]
- Kostruba, A.; Stetsyshyn, Y.; Vlokh, R. Method for determination of the parameters of transparent ultrathin films deposited on transparent substrates under conditions of low optical contrast. Appl. Opt. 2015, 54, 6208–6216. [Google Scholar] [CrossRef]
- Schoeler, B.; Kumaraswamy, G.; Caruso, F. Investigation of the Influence of Polyelectrolyte Charge Density on the Growth of Multilayer Thin Films Prepared by the Layer-by-Layer Technique. Macromolecules 2002, 35, 889–897. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Ye, Z.; Nan, K.; Lin, S.; Chen, H.; Wang, B. Antimicrobial efficiency of PAA/(PVP/CHI) erodible polysaccharide multilayer through loading and controlled release of antibiotics. Carbohydr. Polym. 2017, 161, 53–62. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Q.; He, W. Amphipathicity and self-assembly behavior of amphiphilic alginate esters. Carbohydr. Polym. 2013, 92, 223–227. [Google Scholar] [CrossRef]




| aPOx | [M1]:[EtOx] | Mn | Mw | PDI |
|---|---|---|---|---|
| P1 | 100:0 | 12,805 | 15,325 | 1.197 |
| P2 | 50:0 | 14,305 | 18,137 | 1.267 |
| P3 | 10:90 | 10,992 | 12,641 | 1.151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Huang, X.; Jin, X.; Hu, Q. Bactericidal Anti-Adhesion Potential Integrated Polyoxazoline/Silver Nanoparticle Composite Multilayer Film with pH Responsiveness. Polymers 2022, 14, 3685. https://doi.org/10.3390/polym14173685
Bao X, Huang X, Jin X, Hu Q. Bactericidal Anti-Adhesion Potential Integrated Polyoxazoline/Silver Nanoparticle Composite Multilayer Film with pH Responsiveness. Polymers. 2022; 14(17):3685. https://doi.org/10.3390/polym14173685
Chicago/Turabian StyleBao, Xiaojiong, Xiaofei Huang, Xiaoqiang Jin, and Qiaoling Hu. 2022. "Bactericidal Anti-Adhesion Potential Integrated Polyoxazoline/Silver Nanoparticle Composite Multilayer Film with pH Responsiveness" Polymers 14, no. 17: 3685. https://doi.org/10.3390/polym14173685
APA StyleBao, X., Huang, X., Jin, X., & Hu, Q. (2022). Bactericidal Anti-Adhesion Potential Integrated Polyoxazoline/Silver Nanoparticle Composite Multilayer Film with pH Responsiveness. Polymers, 14(17), 3685. https://doi.org/10.3390/polym14173685





