Study on Thermal Conductivity of P-Phenylenediamine Modified Graphene/Epoxy Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
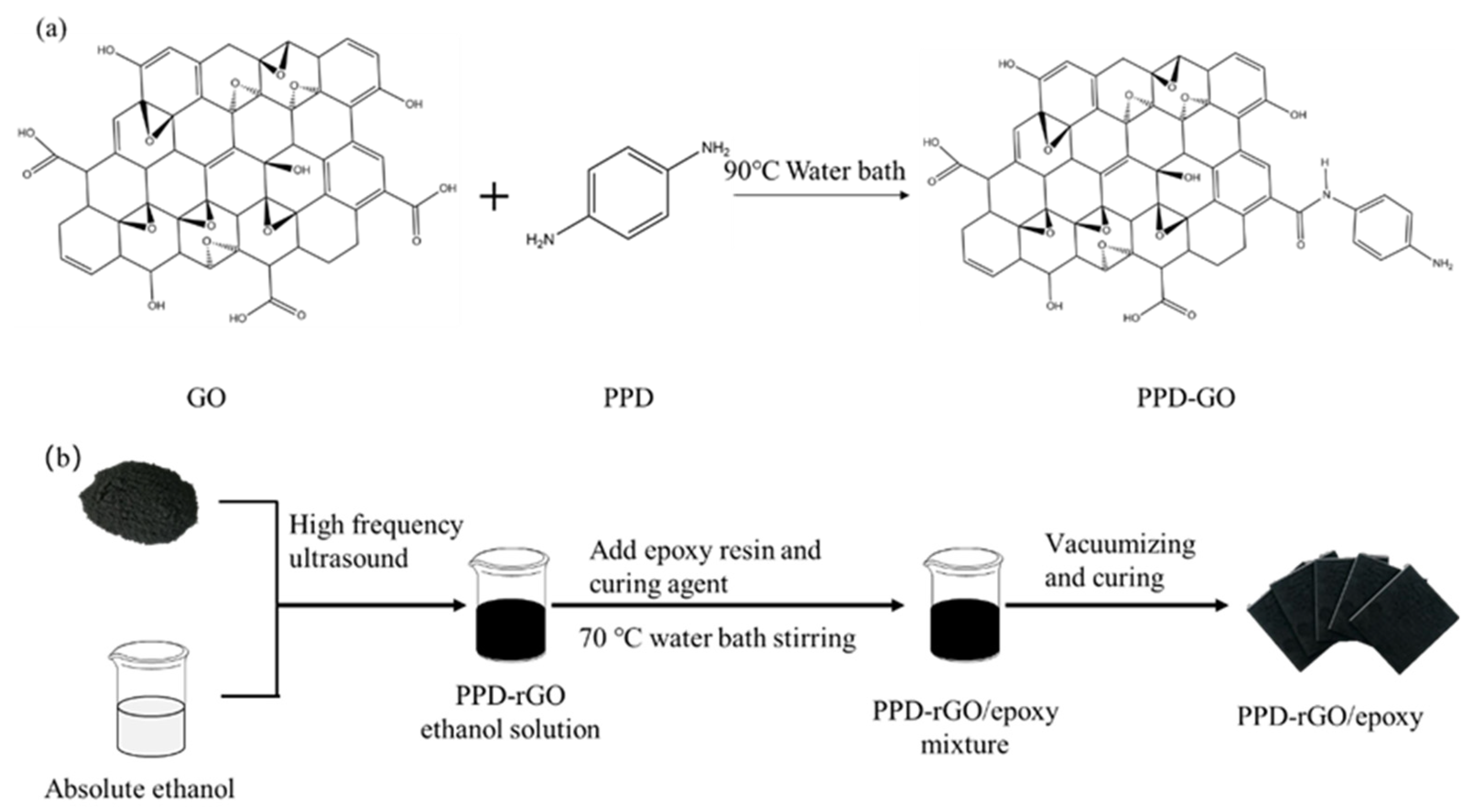
2.2. Preparation of GO Modified by P-Phenylenediamine
2.3. Reduction of PPD-GO by Ascorbic Acid
2.4. Preparation of PPD-RGO Epoxy Composites
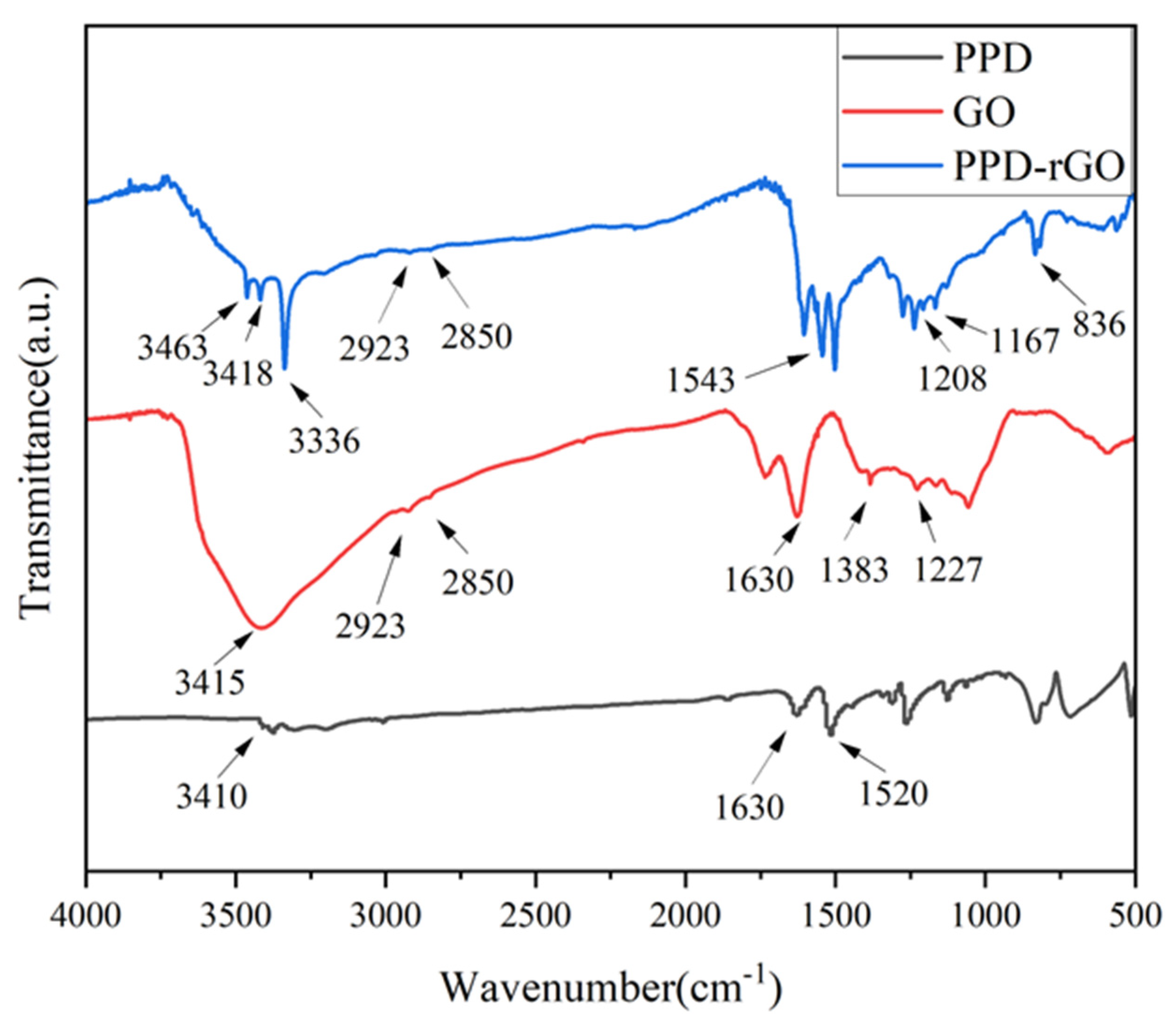
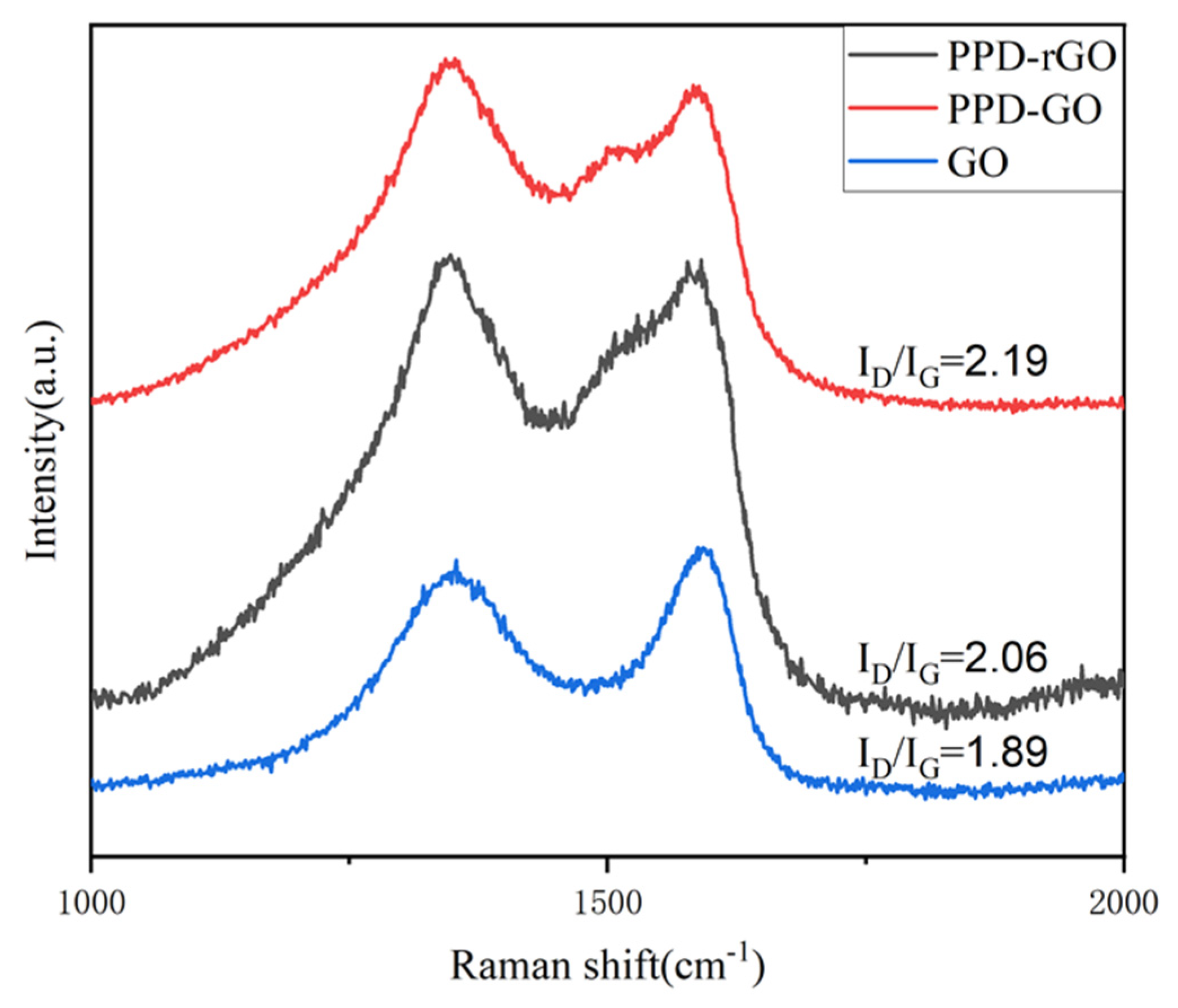
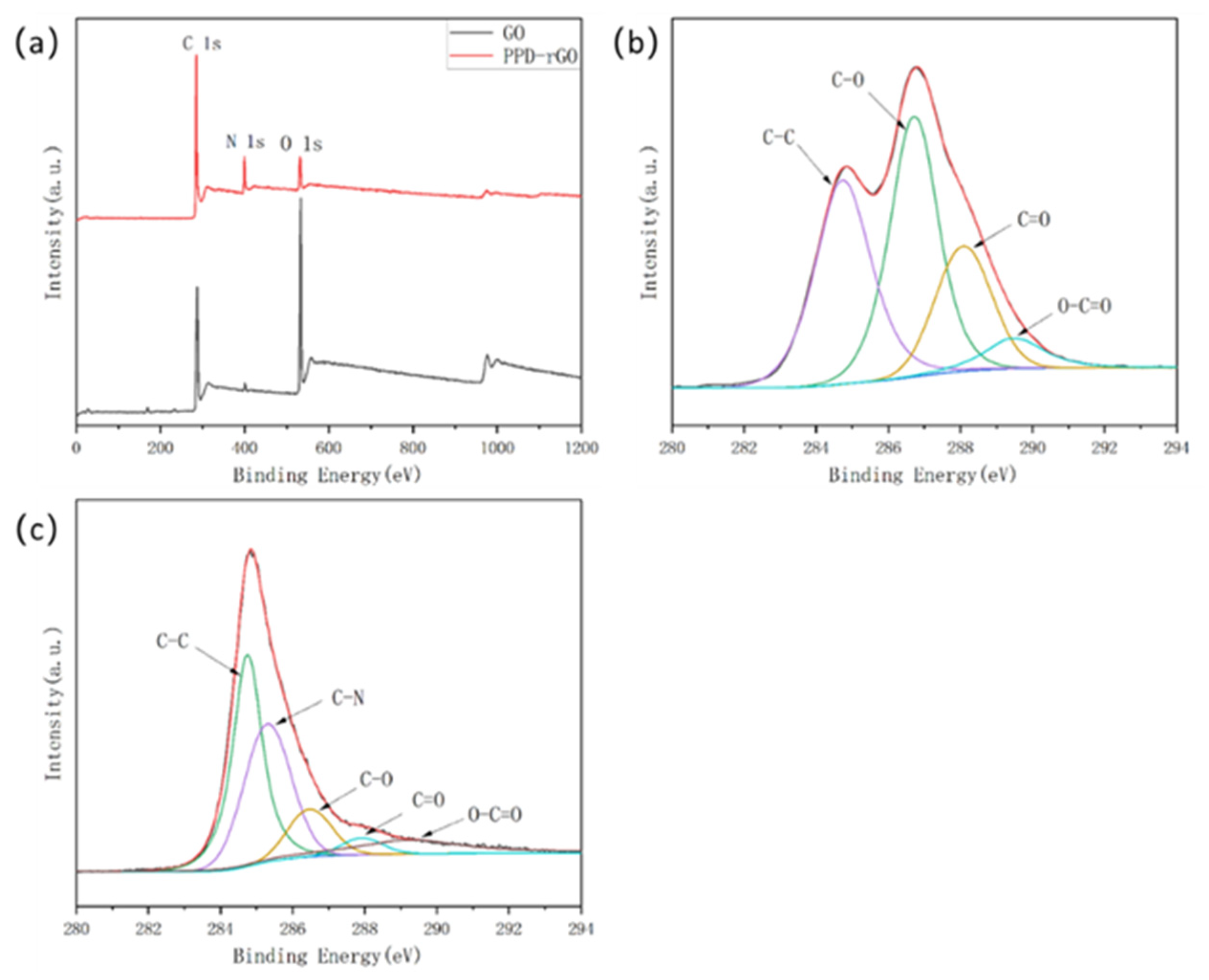
2.5. Characterization
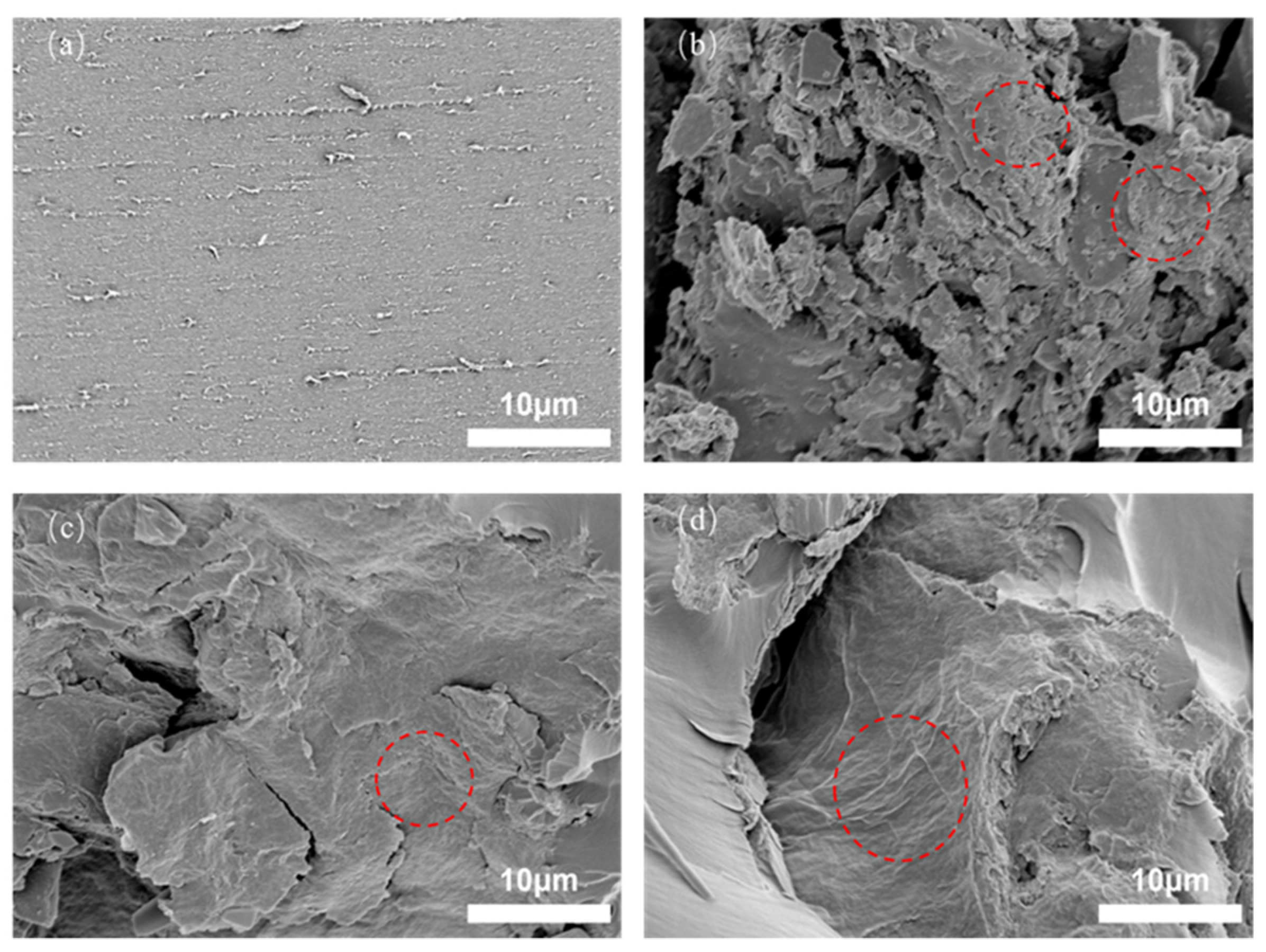
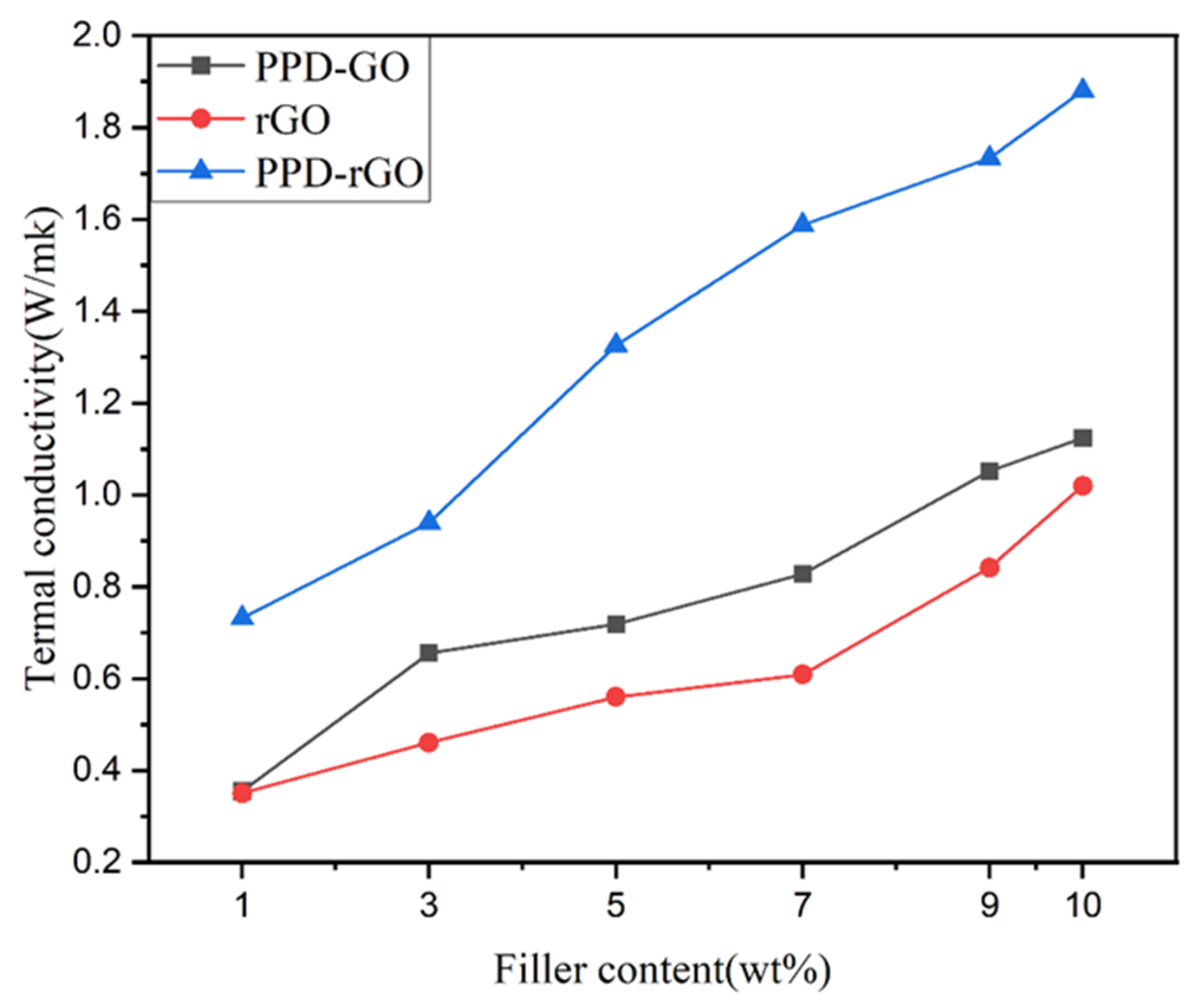
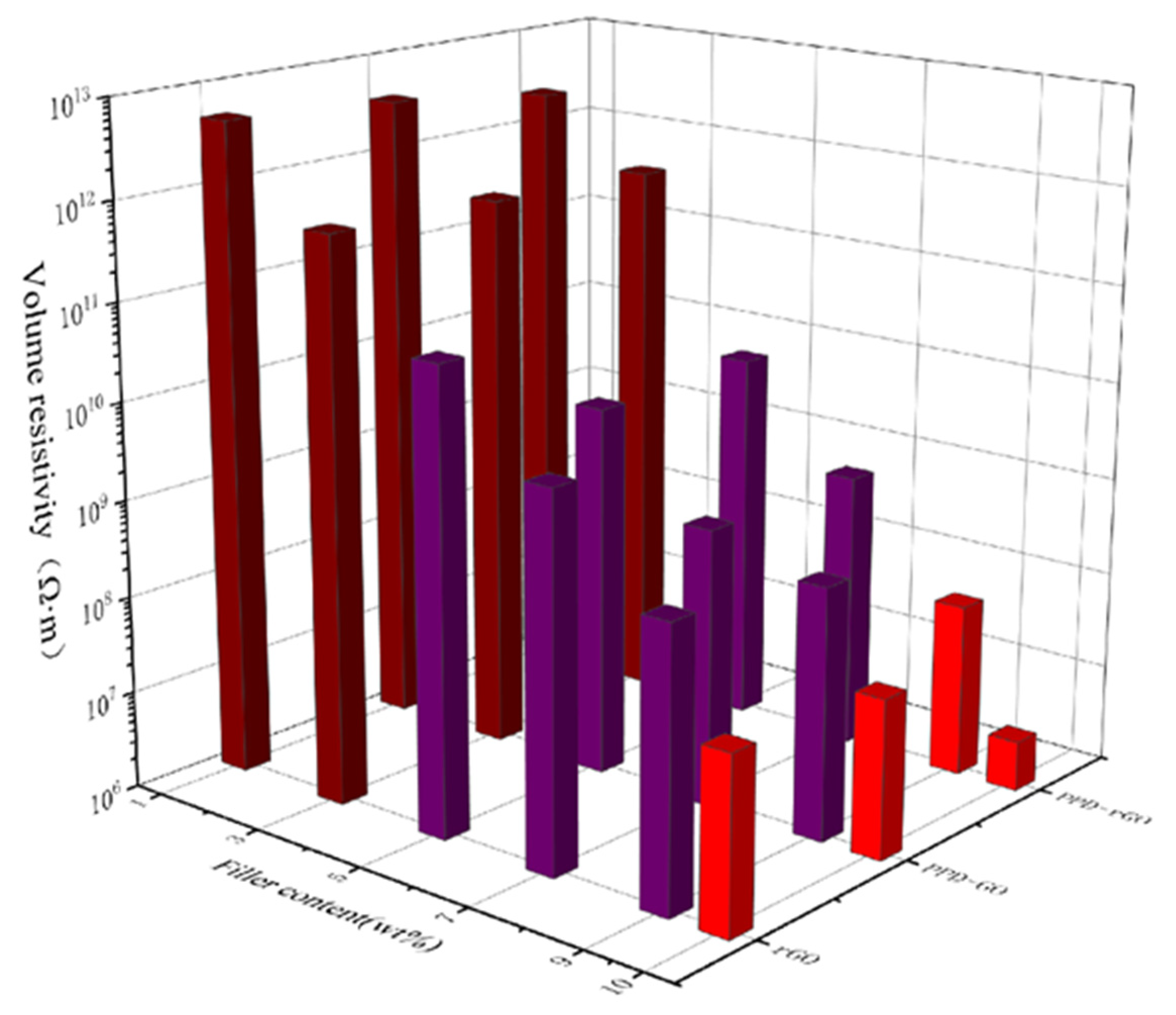
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, C.; Kou, K.C.; Jia, Q.; Zhang, Y.; Wu, G.L.; Ji, T.Z. Improved thermal conductivity and dielectric properties of hBN/PTFE composites via surface treatment by silane coupling agent. Compos. Part B Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Huang, X.Y.; Jiang, P.K.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, F.X.; Zhuang, K.Y.; Chen, D.Q.; Chen, G.H. Composites of epoxy/graphene-modified-diamond filler show enhanced thermal conductivity and high electrical insulation. RSC Adv. 2017, 7, 40761–40766. [Google Scholar] [CrossRef]
- Chen, L.F.; Yu, W.; Xie, H.Q. Enhanced thermal conductivity of nanofluids containing Ag/MWNT. composites Powder Technol. 2012, 231, 18–20. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.F.; Zhi, C.Y.; Zhang, W.J. High thermal conductivity and temperature probing of copper nanowire/upconversion nanoparticles/epoxy composite. Compos. Sci. Technol. 2016, 130, 63–69. [Google Scholar] [CrossRef]
- Mu, Q.W.; Li, Q.M.; Liu, H. Enhancing the thermal conductivities of SiO2/Epoxy composites by orientation. Polym. Compos. 2016, 37, 818–823. [Google Scholar] [CrossRef]
- Long, J.J.; Li, C.X.; Li, Y. Enhancement of mechanical and bond properties of epoxy adhesives modified by SiO2 nanoparticles with active groups. Polymers 2022, 14, 2052. [Google Scholar] [CrossRef]
- Zhang, D.W.; Liu, F.S.; Wang, S.; Yan, M.X.; Hu, X.; Xu, M.Y. D-GQDs modified epoxy resin enhances the thermal conductivity of AlN/Epoxy resin thermally conductive composites. Polymers 2021, 13, 4074. [Google Scholar] [CrossRef]
- Hu, M.C.; Feng, J.Y.; Ng, K.M. Thermally conductive PP/AlN composites with a 3-D segregated structure. Compos. Sci. Technol. 2015, 110, 26–34. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired modification of h-BN for high thermal conductive composite films with aligned structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5708. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.F.; Kong, X.X.; Gao, D.H.; Wang, Y.; Hu, T.T.; Zhang, L.Q. Mussel-inspired modification of boron nitride for natural rubber composites with high thermal conductivity and low dielectric constant. Compos. Sci. Technol. 2019, 177, 18–25. [Google Scholar] [CrossRef]
- Yu, J.; Choi, H.K.; Kim, H.S.; Kim, S.Y. Synergistic effect of hybrid graphene nanoplatelet and multi-walled carbon nanotube fillers on the thermal conductivity of polymer composites and theoretical modeling of the synergistic effect. Compos. Part A Appl. Sci. Manuf. 2016, 88, 79–85. [Google Scholar] [CrossRef]
- Du, X.S.; Liu, H.Y.; Mai, Y.W. Ultrafast synthesis of multifunctional N-doped graphene foam in an ethanol flame. ACS Nano 2016, 10, 453–462. [Google Scholar] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Gong, J.R.; Liu, Z.D.; Yu, J.H.; Dai, D.; Dai, W.; Du, S.Y.; Li, C.Y.; Jiang, N.; Zhan, Z.L.; Lin, C.T. Graphene woven fabric-reinforced polyimide films with enhanced and anisotropic thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2016, 87, 290–296. [Google Scholar]
- Lian, G.; Tuan, C.C.; Li, L.Y.; Jiao, S.L.; Wang, Q.L.; Moon, K.S.; Cui, D.L.; Wong, C.P. Vertically aligned and interconnected graphene networks for high thermal conductivity of epoxy composites with ultralow loading. Chem. Mater. 2016, 28, 6096–6104. [Google Scholar] [CrossRef]
- Alam, F.E.; Dai, W.; Yang, M.H.; Du, S.Y.; Li, X.M.; Yu, J.H.; Jiang, N.; Lin, C.T. In situ formation of a cellular graphene framework in thermoplastic composites leading to superior thermal conductivity. J. Mater. Chem. A 2017, 5, 6164–6169. [Google Scholar] [CrossRef]
- Li, R.J.; Yang, Y.; Wu, D.T.; Li, K.L.; Qin, Y.; Tao, Y.X.; Kong, Y. Covalent functionalization of reduced graphene oxide aerogels with polyaniline for high performance supercapacitors. Chem. Commun. 2019, 55, 1738–1741. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Y.F.; Lin, Y.; Yan, H.X.; Zhang, W.B.; Bao, Y.; Ma, J.Z. Enhanced mechanical and tribological properties of graphene/bismaleimide composites by using reduced graphene oxide with non-covalent functionalization. Compos. Part B Eng. 2019, 165, 491–499. [Google Scholar] [CrossRef]
- Wang, X.D.; Liu, X.H.; Yuan, H.Y.; Liu, H.; Liu, C.T.; Li, T.X.; Yan, C.; Yan, X.R.; Shen, C.Y.; Guo, Z.H. Non-covalently functionalized graphene strengthened poly (vinyl alcohol). Mater. Des. 2018, 139, 372–379. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lu, M.P.; Wu, K.; Jiao, E.X.; Liang, L.Y.; Shi, J.; Lu, M.E. Enhanced thermal conduction of functionalized graphene nanoflake/polydimethylsiloxane composites via thermoluminescence strategy. Compos. Sci. Technol. 2021, 213, 108940. [Google Scholar] [CrossRef]
- Jr, W.S.H.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Liu, S.; Yu, B.; Zhang, T. Preparation of crumpled reduced graphene oxide–poly(p-phenylenediamine) hybrids for the detection of dopamine. J. Mater. Chem. A 2013, 1, 13314–13320. [Google Scholar] [CrossRef]
- Ding, P.; Zhang, J.; Song, N.; Tang, S.F.; Liu, Y.M.; Shi, L.Y. Anisotropic thermal conductive properties of hot-pressed polystyrene/graphene composites in the through-plane and in-plane directions. Compos. Sci. Technol. 2015, 109, 25–31. [Google Scholar] [CrossRef]
- Jing, L.C.; Wang, T.; Rhen, D.S.; Yuan, X.T.; Tian, Y.; Xie, Q.X.; Geng, H.Z. Wrinkled p-phenylenediamine grafted graphene oxide as reinforcement for polyvinyl butyral anti-corrosive coating. J. Mater. Sci. 2021, 56, 12686–12699. [Google Scholar] [CrossRef]
- Wazalwar, R.; Raichur, A.M. Model-free cure kinetics of tetra-functional epoxy reinforced with GO and p-Phenylenediamine modified GO. Thermochim. Acta 2021, 697, 178857. [Google Scholar] [CrossRef]
- Han, L.; Li, K.; Fu, Y.; Yin, X.; Jiao, Y.; Song, Q. Multifunctional electromagnetic interference shielding 3D reduced graphene oxide/vertical edge-rich graphene/epoxy nanocomposites with remarkable thermal management performance. Compos. Sci. Technol. 2022, 222, 109407. [Google Scholar] [CrossRef]
- Lopez-Barajas, F.; Ramos-deValle, L.F.; Sanchez-Valdes, S.; Ramirez-Vargas, E.; Martinez-Colunga, J.G.; Espinoza-Martinez, A.B.; da Silva, L.; Hernandez-Gamez, J.F.; Rodriguez-Fernandez, O.S.; Beltran-Ramirez, F.I. Epoxy/hybrid graphene-copper nanocomposite materials with enhanced thermal conductivity. J. Appl. Polym. Sci. 2022, 139, 52419. [Google Scholar] [CrossRef]
- Sun, Z.; Wong, R.; Yu, M.; Li, J.; Zhang, M.; Mele, L.; Hah, J.; Kathaperumal, M.; Wong, C.P. Nanocomposites for future electronics device Packaging: A fundamental study of interfacial connecting mechanisms and optimal conditions of silane coupling agents for Polydopamine-Graphene fillers in epoxy polymers. Chem. Eng. J. 2022, 439, 135621. [Google Scholar] [CrossRef]
| The Name of the Material | The Epoxy Value | Epoxy Equivalent | Viscosity | The Amine Value |
|---|---|---|---|---|
| Epoxy resin (E6002) | 0.46–0.51 (eq/100 g) | 185–210 (g/eq) | 1100–1600 (mPas) | / |
| DETDA | / | / | 280–300 (mPas) | 620–640 (mg KOH/g) |
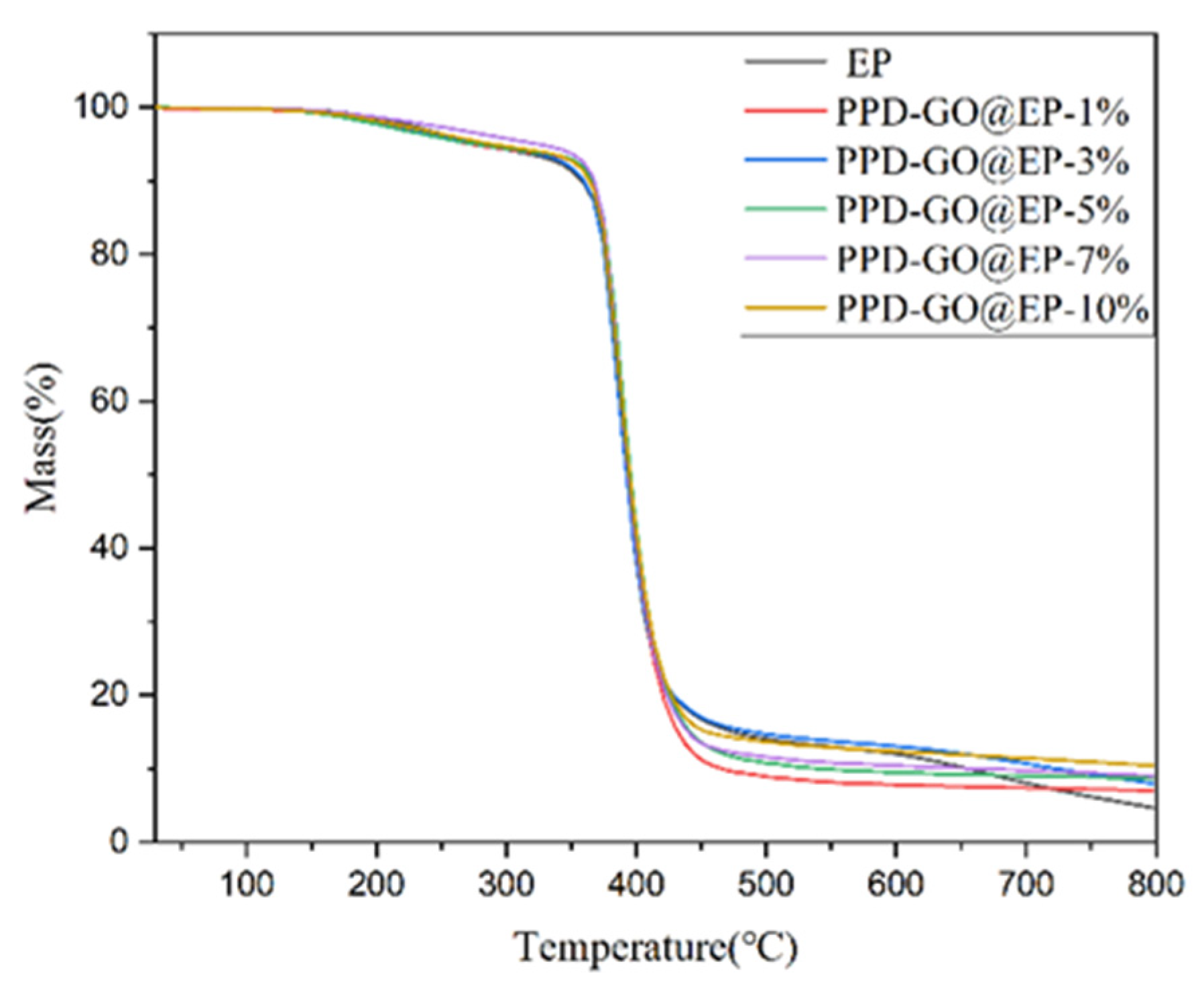
| Samples | T10% (°C) | Residue (%) |
|---|---|---|
| EP | 358 | 4.65 |
| PPD-rGO@EP-1% | 365 | 7.03 |
| PPD-rGO@EP-3% | 360 | 7.93 |
| PPD-rGO@EP-5% | 366 | 8.70 |
| PPD-rGO@EP-7% | 368 | 9.05 |
| PPD-rGO@EP-9% | 370 | 10.22 |
| PPD-rGO@EP-10% | 364 | 10.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Zhou, J.; Guo, M.; Chen, D.; Chen, G. Study on Thermal Conductivity of P-Phenylenediamine Modified Graphene/Epoxy Composites. Polymers 2022, 14, 3660. https://doi.org/10.3390/polym14173660
Lin J, Zhou J, Guo M, Chen D, Chen G. Study on Thermal Conductivity of P-Phenylenediamine Modified Graphene/Epoxy Composites. Polymers. 2022; 14(17):3660. https://doi.org/10.3390/polym14173660
Chicago/Turabian StyleLin, Jun, Jiancheng Zhou, Mengyao Guo, Danqing Chen, and Guohua Chen. 2022. "Study on Thermal Conductivity of P-Phenylenediamine Modified Graphene/Epoxy Composites" Polymers 14, no. 17: 3660. https://doi.org/10.3390/polym14173660
APA StyleLin, J., Zhou, J., Guo, M., Chen, D., & Chen, G. (2022). Study on Thermal Conductivity of P-Phenylenediamine Modified Graphene/Epoxy Composites. Polymers, 14(17), 3660. https://doi.org/10.3390/polym14173660








