Chemical Structures and Antioxidant Activities of Polysaccharides from Carthamus tinctorius L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental materials
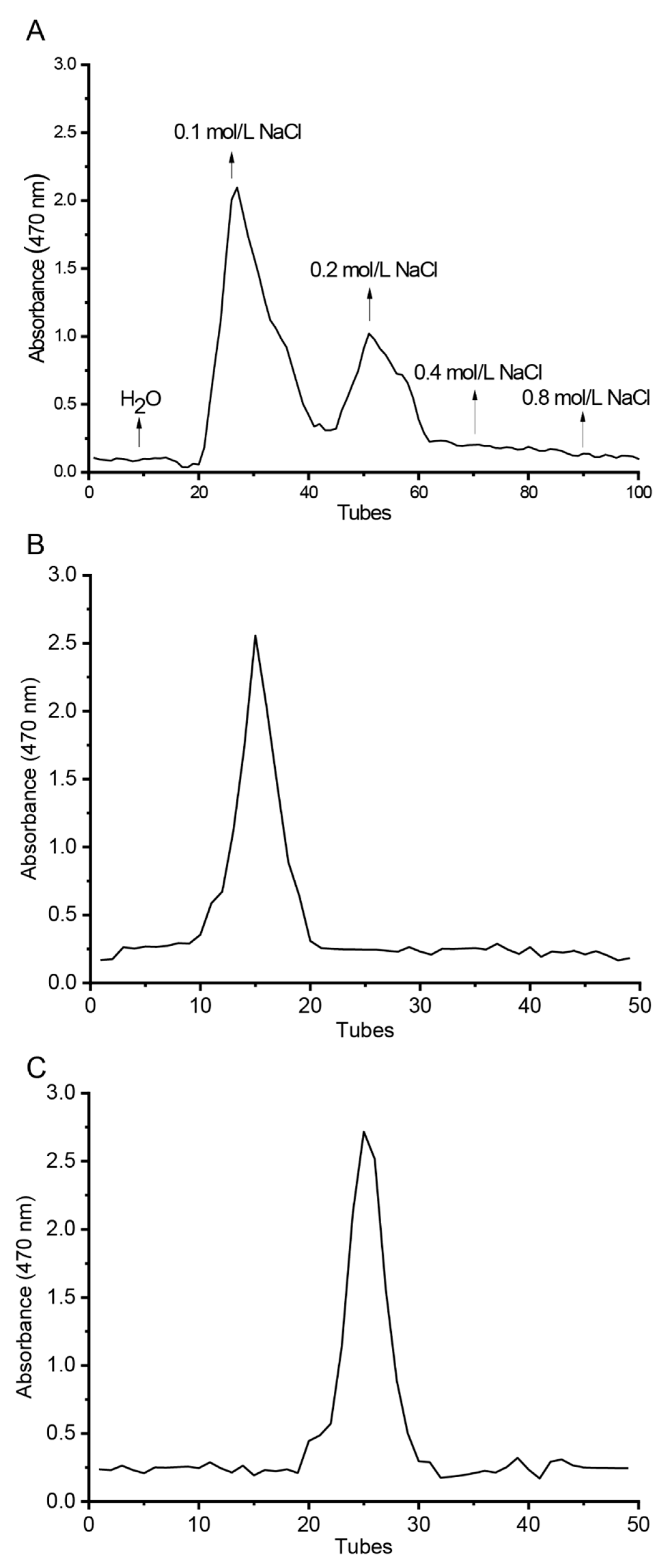
2.2. Purification of CTLP
2.3. Molecular Weight Detection of CTLP
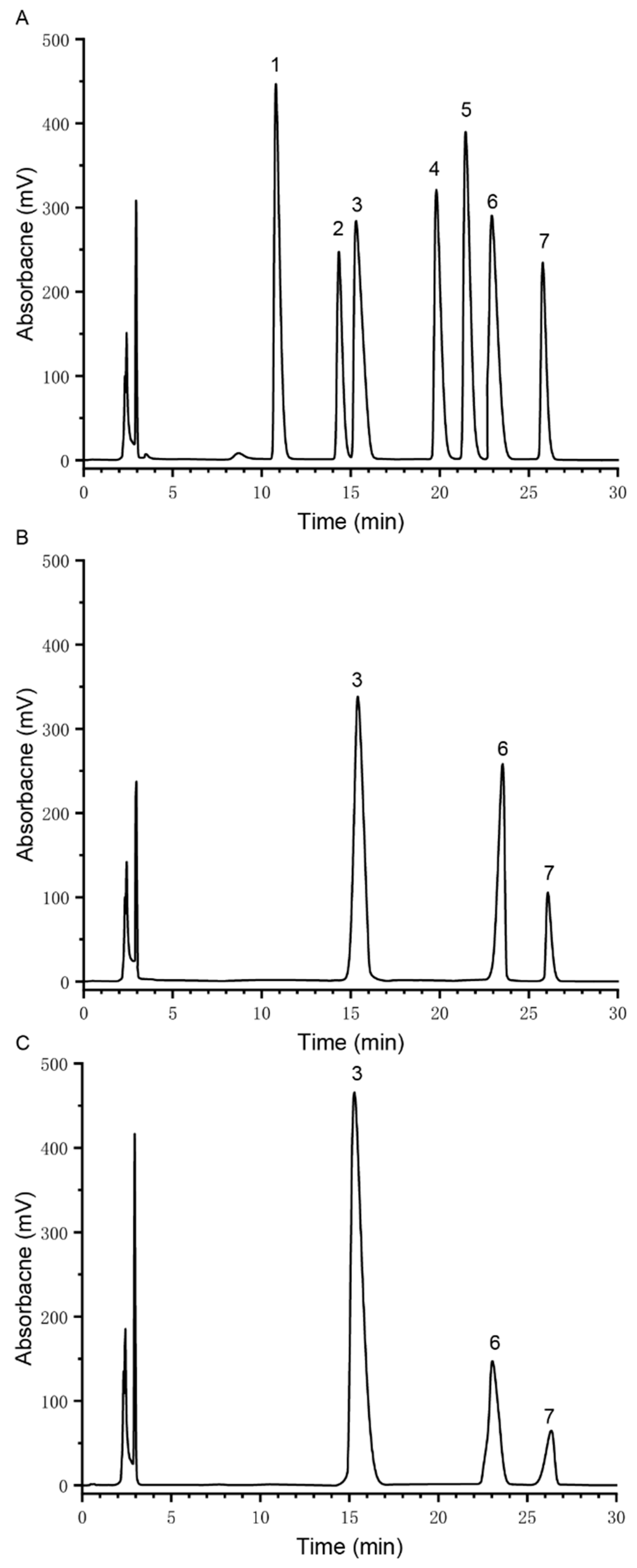
2.4. Monosaccharide Composition Analysis
2.5. Periodate Oxidation
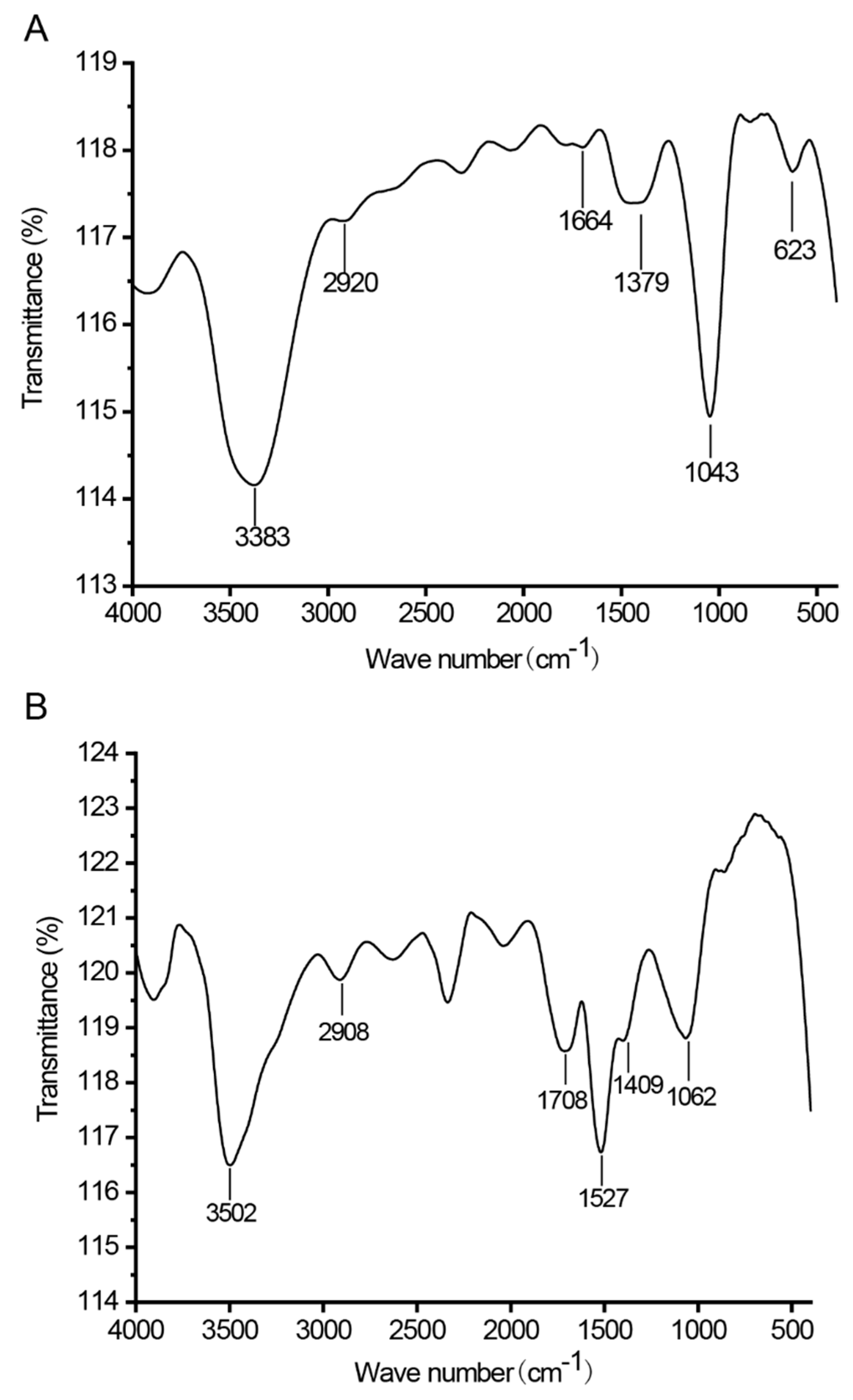
2.6. FT-IR Analysis of CTLP
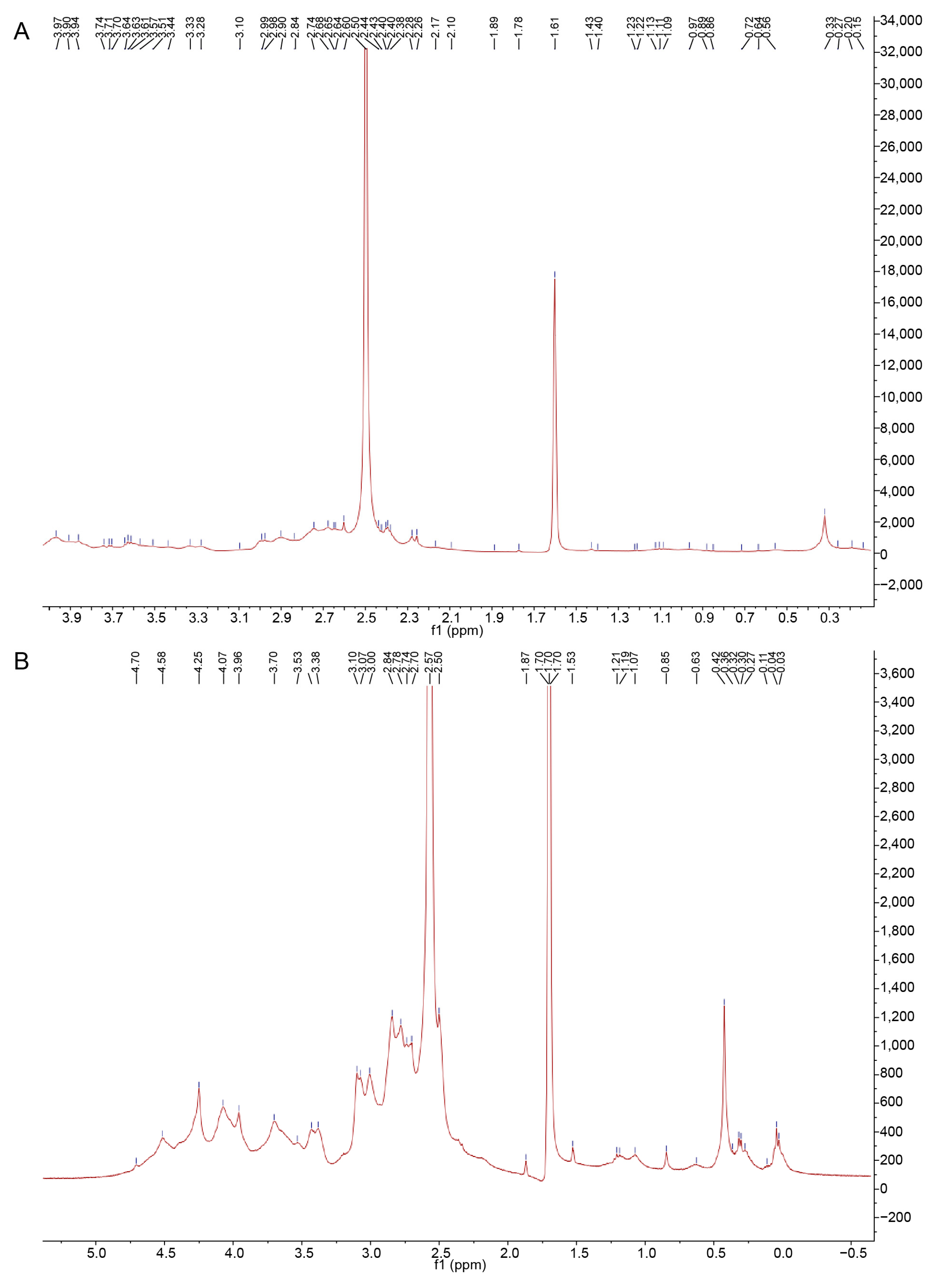
2.7. NMR Spectroscopy
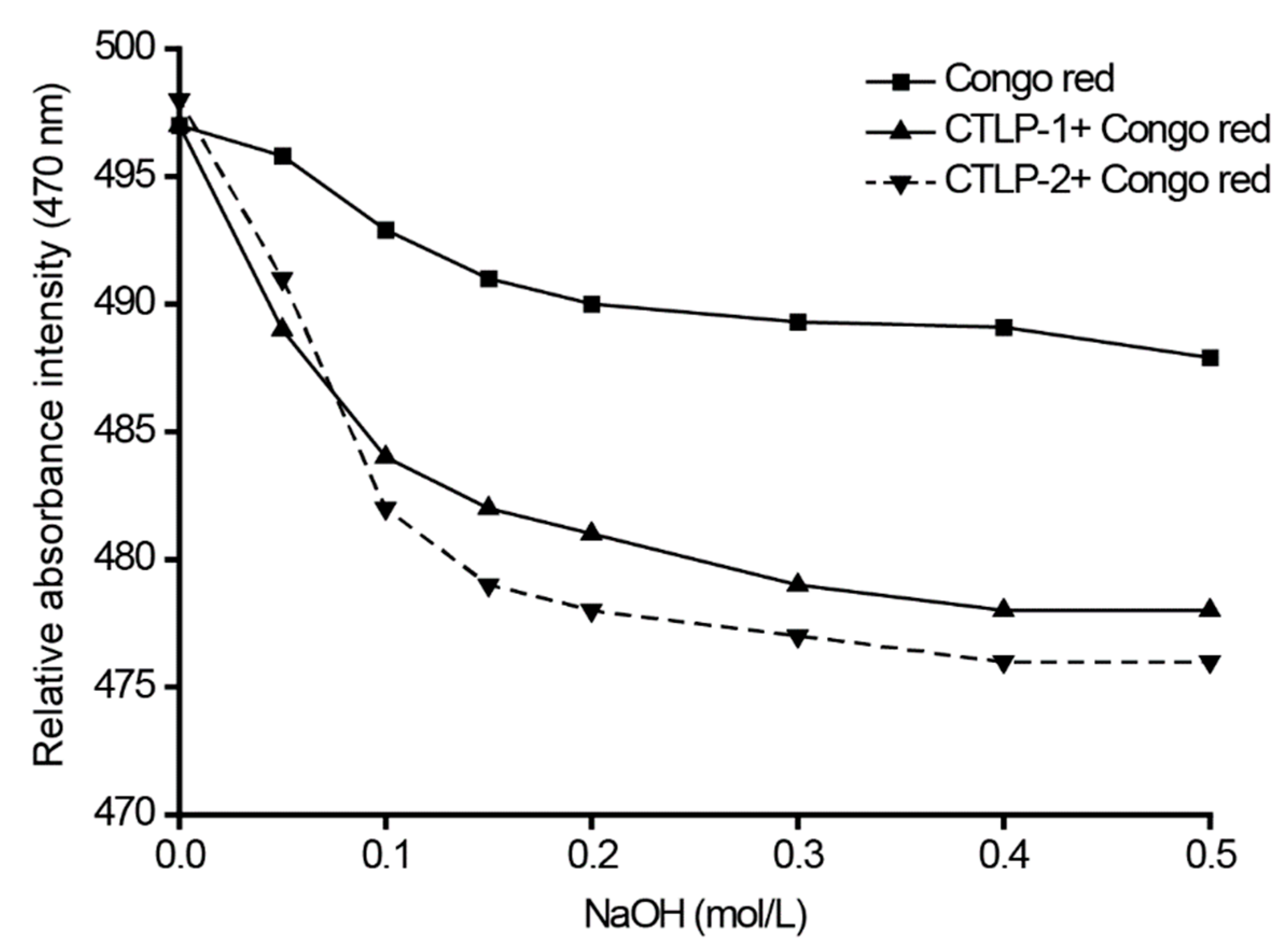
2.8. Congo Red Analysis
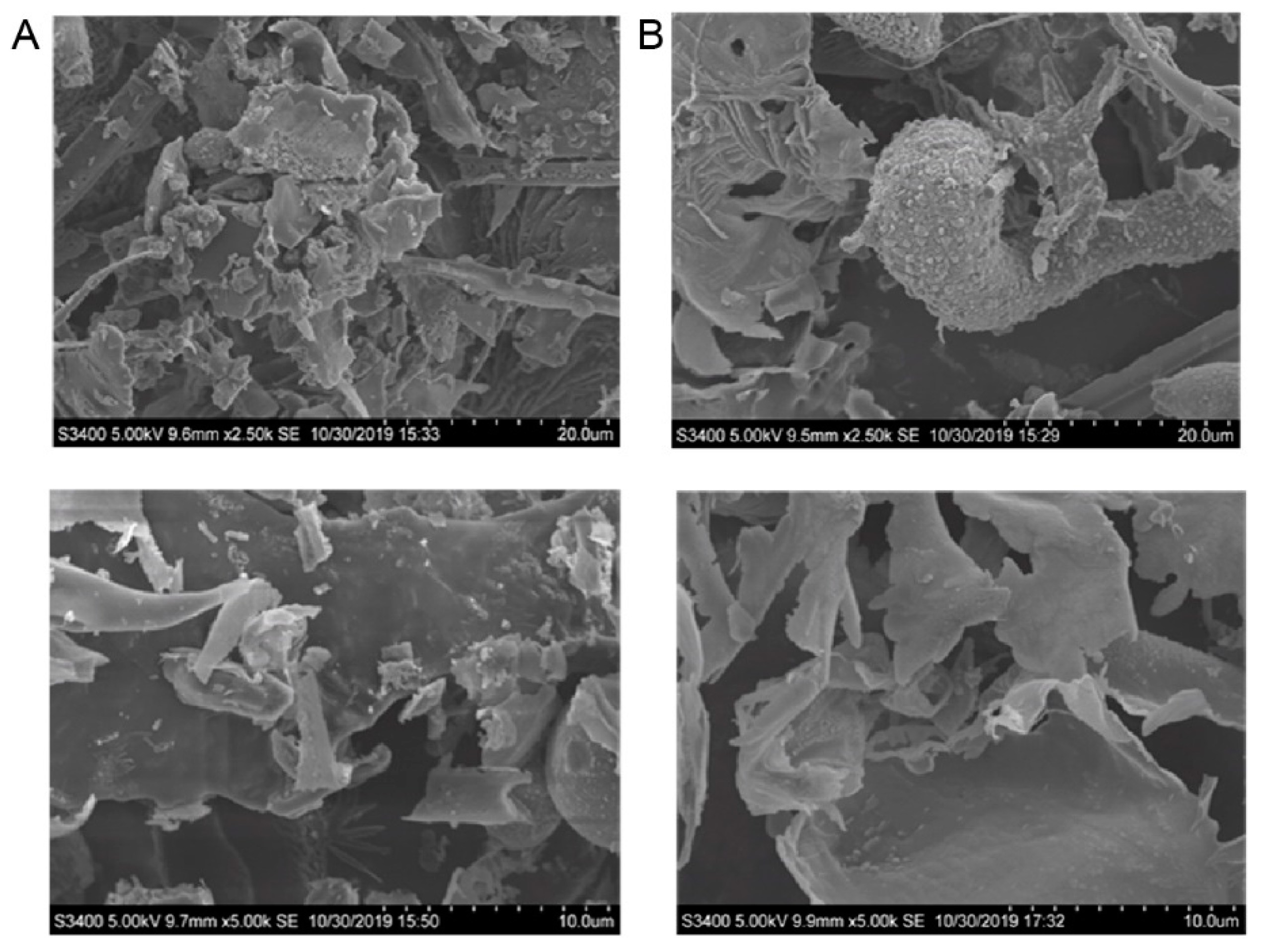
2.9. SEM Anaylsis of CTLP
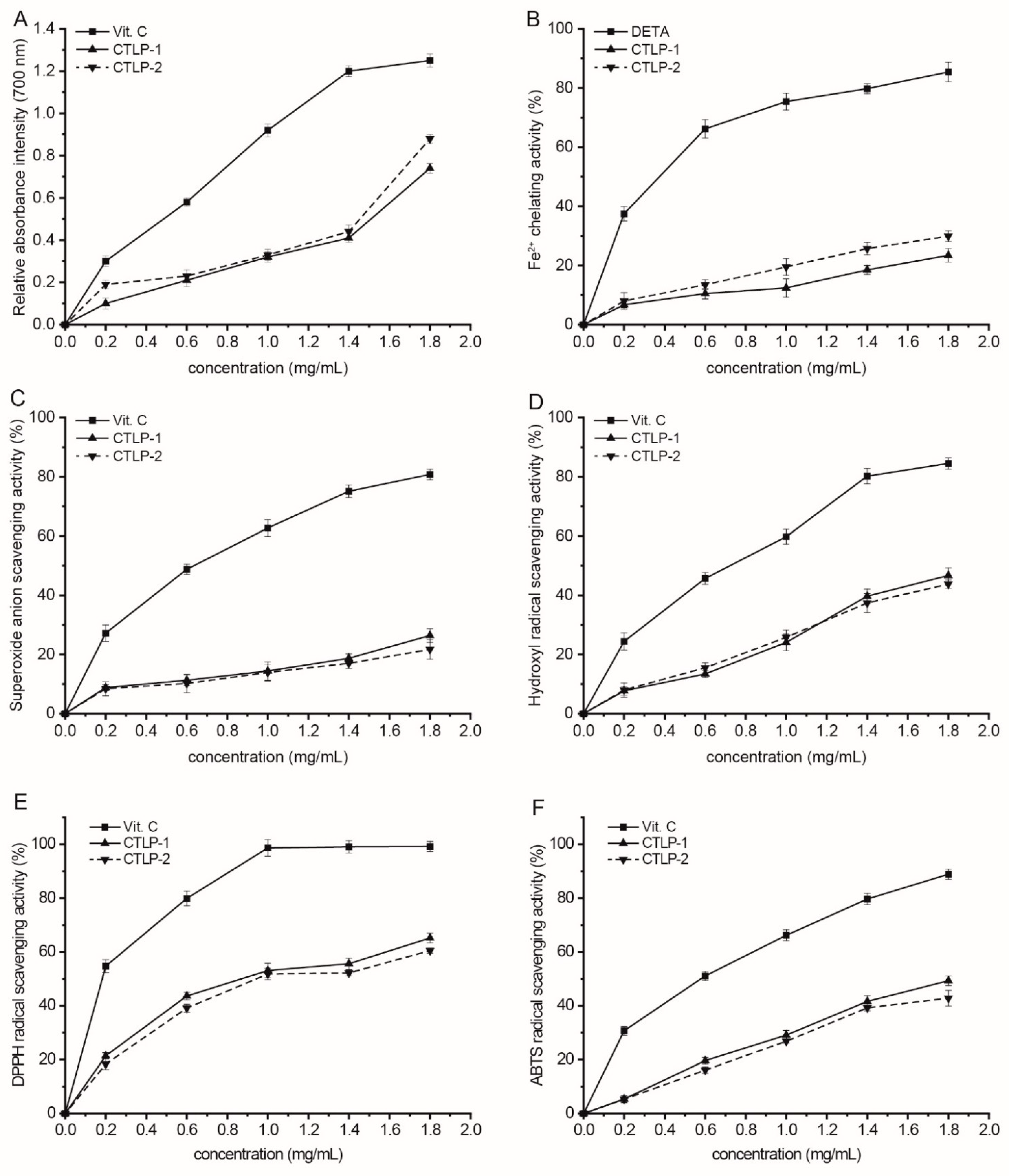
2.10. Antioxidant Activity Assays
3. Results and Discussion
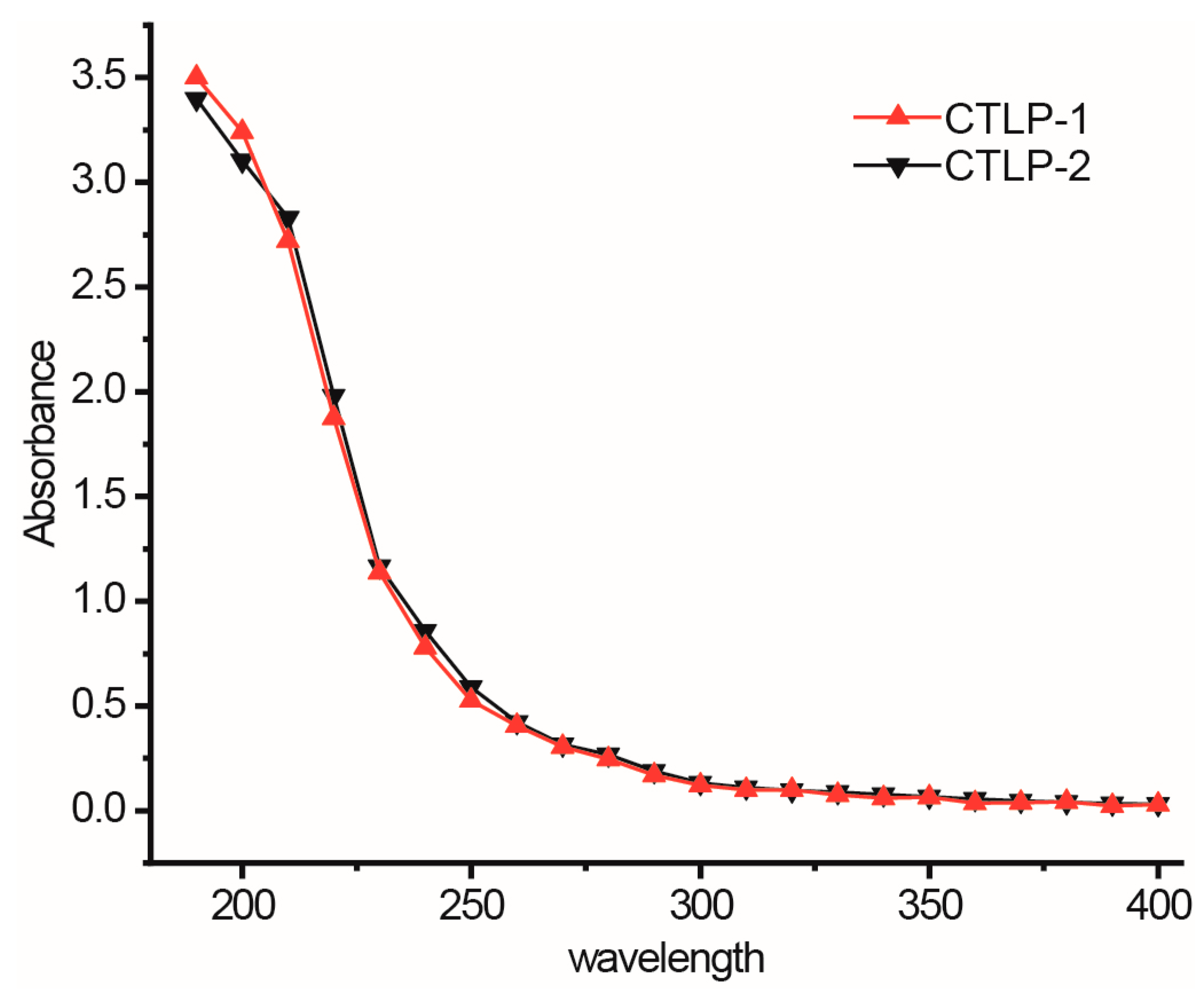
3.1. Structural Analysis
3.2. Antioxidant Activities of CTLP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuley, E.; Yavuzer, M.N.; Yavuzer, E.; Durmuş, M.; Yazgan, H.; Gezginç, Y.; Özogul, F. Inhibitory effects of safflower and bitter melon extracts on biogenic amine formation by fish spoilage bacteria and food borne pathogens. Food Biosci. 2019, 32, 100478. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Işik, M.Z.; Aydin, H. Investigation on the effects of gasoline reactivity controlled compression ignition application in a diesel generator in high loads using safflower biodiesel blends. Renew. Energy 2019, 133, 177–189. [Google Scholar] [CrossRef]
- Fu, F.; Su, C.; Liu, K. Effect of safflower yellow a on the blood pressure in dog and man. Am. J. Hypertens. 2005, 18, A59. [Google Scholar] [CrossRef]
- Yang, J.; Wang, R.; Feng, Q.; Wang, Y.X.; Zhang, Y.Y.; Wu, W.H.; Ge, P.L.; Qi, J.P. Safflower polysaccharide induces cervical cancer cell apoptosis via inhibition of the PI3K/Akt pathway. S. Afr. J. Bot. 2018, 118, 209–215. [Google Scholar] [CrossRef]
- Yu, G.; Luo, Z.; Zhou, Y.; Zhang, L.; Wu, Y.; Ding, L.; Shi, Y. Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach. Biomed. Pharmacother. 2019, 117, 109094. [Google Scholar] [CrossRef]
- Lu, J.-X.; Zhang, C.-X.; Hu, Y.; Zhang, M.-H.; Wang, Y.-N.; Qian, Y.-X.; Yang, J.; Yang, W.-Z.; Jiang, M.-M.; Guo, D.-A. Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites. J. Phymed. 2019, 58, 152826. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.A.-O.; Xie, M.A.-O. Antidiabetic Mechanism of Dietary Polysaccharides Based on Their Gastrointestinal Functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef]
- Yang, X.; Ji, H.; Feng, Y.; Yu, J.; Liu, A.A.-O. Structural Characterization and Antitumor Activity of Polysaccharides from Kaempferia galanga L. Oxidative Med. Cell Longev. 2018, 2018, 9579262. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.-D.; Feng, Y.-Y.; Liu, Y.-N.; Ji, H.-Y.; Yu, S.-S.; Liu, A.; Yu, J. A novel polysaccharide from Castanea mollissima Blume: Preparation, characteristics and antitumor activities in vitro and in vivo. Carbohydr. Polym. 2020, 240, 116323. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Young, B. A new thermodynamic approach for struvite product quality prediction. Environ. Sci. Pollut. Res. 2019, 26, 3954–3964. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-J.; Hu, Y.; Xiong, S.-L.; Wu, H.-C.; Jia, H.-F.; Lin, D.; Liu, Y.; Xiao, H. Ultrasonic-Assisted Extraction Optimization, Structural Elucidation and Antioxidant Activity of Polysaccharides from Carthamus tinctorius L. Basic Clin. Pharmacol. Toxicol. 2020, 127, 253. [Google Scholar]
- Saqib, A.A.N.; Whitney, P.J. Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono- and di-saccharide sugars. Biomass. Bioenergy 2011, 35, 4748–4750. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Molecular weight distribution of polysaccharides from edible seaweeds by high-performance size-exclusion chromatography (HPSEC). Talanta 2012, 93, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Vold, I.M.N.; Christensen, B.E. Periodate oxidation of chitosans with different chemical compositions. Carbohydr. Res. 2005, 340, 679–684. [Google Scholar] [CrossRef]
- An, Q.; Ye, X.; Han, Y.; Zhao, M.; Chen, S.; Liu, X.; Li, X.; Zhao, Z.; Zhang, Y.; Ouyang, K.; et al. Structure analysis of polysaccharides purified from Cyclocarya paliurus with DEAE-Cellulose and its antioxidant activity in RAW264.7 cells. Int. J. Biol. Macromol. 2020, 157, 604–615. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Xing, X.; Cheng, T.-J.R.; Liang, P.-H.; Bulone, V.; Park, J.H.; Hsieh, Y.S.Y. Structural analysis and biological activity of cell wall polysaccharides extracted from Panax ginseng marc. Int. J. Biol. Macromol. 2019, 135, 29–37. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, C.-Q.; Li, Y.-B.; Lu, S.-L.; Zhang, J.; Mao, X.-Y.; Zhu, X.-R.; Guo, X.-B.; Xiao, H.; Xu, C.-J. Structural elucidation and antioxidant activities of polysaccharides from Pleurotus ferulae. Intl. J. Agric. Biol. 2019, 22, 1258–1264. [Google Scholar] [CrossRef]
- Coluccio, A.; Neiman, A.M. Interspore bridges: A new feature of the Saccharomyces cerevisiae spore wall. Microbiology 2004, 150, 3189–3196. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-Y.; Liao, A.-M.; Huang, J.-H.; Zhang, J.-G.; Thakur, K.; Wei, Z.-J. Evaluation of structural, functional, and anti-oxidant potential of differentially extracted polysaccharides from potatoes peels. Int. J. Biol. Macromol. 2019, 129, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Arora, S. Superoxide anion radical scavenging activity of Cassiasiamea and Cassia javanica. Med. Chem. Res. 2011, 20, 9–15. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.; Miao, F.; Xue, Q.; Qin, S. The hydroxyl radical scavenging activity of chitosan, hyaluronan, starch and their O-carboxymethylated derivatives. Carbohydr. Polym. 2010, 82, 1043–1045. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Chen, W.; Zhang, W.-M.; Zhang, H. Adulteration identification of some fungal polysaccharides with SEM, XRD, IR and optical rotation: A primary approach. Carbohydr. Polym. 2009, 78, 620–625. [Google Scholar] [CrossRef]
- Devanthéry, T.; Nakamura, K.; Hashiguchi, S.; Hashimoto, M. Structure of a heptose-containing polysaccharide derived from Komagataeibacter europaeus NBRC 3261. Carbohydr. Res. 2020, 492, 107989. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2−1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Bellich, B.; Distefano, M.; Syrgiannis, Z.; Bosi, S.; Guida, F.; Rizzo, R.; Brady, J.W.; Cescutti, P. The polysaccharide extracted from the biofilm of Burkholderia multivorans strain C1576 binds hydrophobic species and exhibits a compact 3D-structure. Int. J. Biol. Macromol. 2019, 136, 944–950. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Xu, C.-J.; Liu, Y.; Zhou, Y.; Xiong, S.-L.; Wu, H.-C.; Deng, J.; Yi, Y.-W.; Qiao, M.-F.; Xiao, H.; et al. Chemical Structures and Antioxidant Activities of Polysaccharides from Carthamus tinctorius L. Polymers 2022, 14, 3510. https://doi.org/10.3390/polym14173510
Lin D, Xu C-J, Liu Y, Zhou Y, Xiong S-L, Wu H-C, Deng J, Yi Y-W, Qiao M-F, Xiao H, et al. Chemical Structures and Antioxidant Activities of Polysaccharides from Carthamus tinctorius L. Polymers. 2022; 14(17):3510. https://doi.org/10.3390/polym14173510
Chicago/Turabian StyleLin, Dan, Cheng-Jian Xu, Yang Liu, Yu Zhou, Shuang-Li Xiong, Hua-Chang Wu, Jing Deng, Yu-Wen Yi, Ming-Feng Qiao, Hang Xiao, and et al. 2022. "Chemical Structures and Antioxidant Activities of Polysaccharides from Carthamus tinctorius L." Polymers 14, no. 17: 3510. https://doi.org/10.3390/polym14173510
APA StyleLin, D., Xu, C.-J., Liu, Y., Zhou, Y., Xiong, S.-L., Wu, H.-C., Deng, J., Yi, Y.-W., Qiao, M.-F., Xiao, H., Chan, S.-W., & Lu, Y. (2022). Chemical Structures and Antioxidant Activities of Polysaccharides from Carthamus tinctorius L. Polymers, 14(17), 3510. https://doi.org/10.3390/polym14173510





