Supramolecular Polycaprolactone-Based Polyurethanes with Thermally Activated Shape-Memory Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
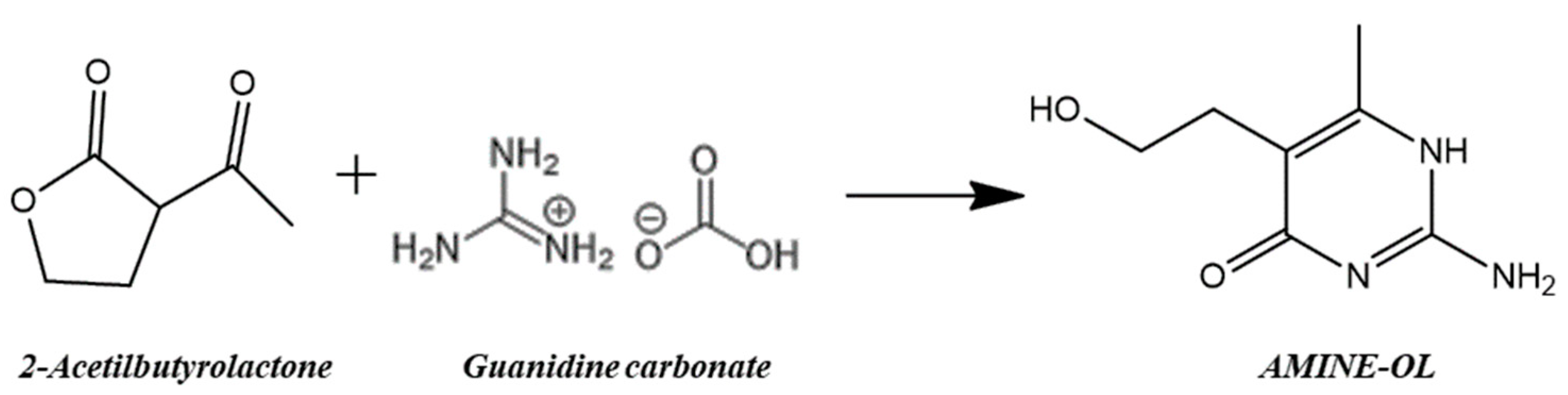
2.2. Preparation of 2-Amino-5-(2-hydroxyethyl)-6-methylpyrimidin-4-ol (AMINE-OL)
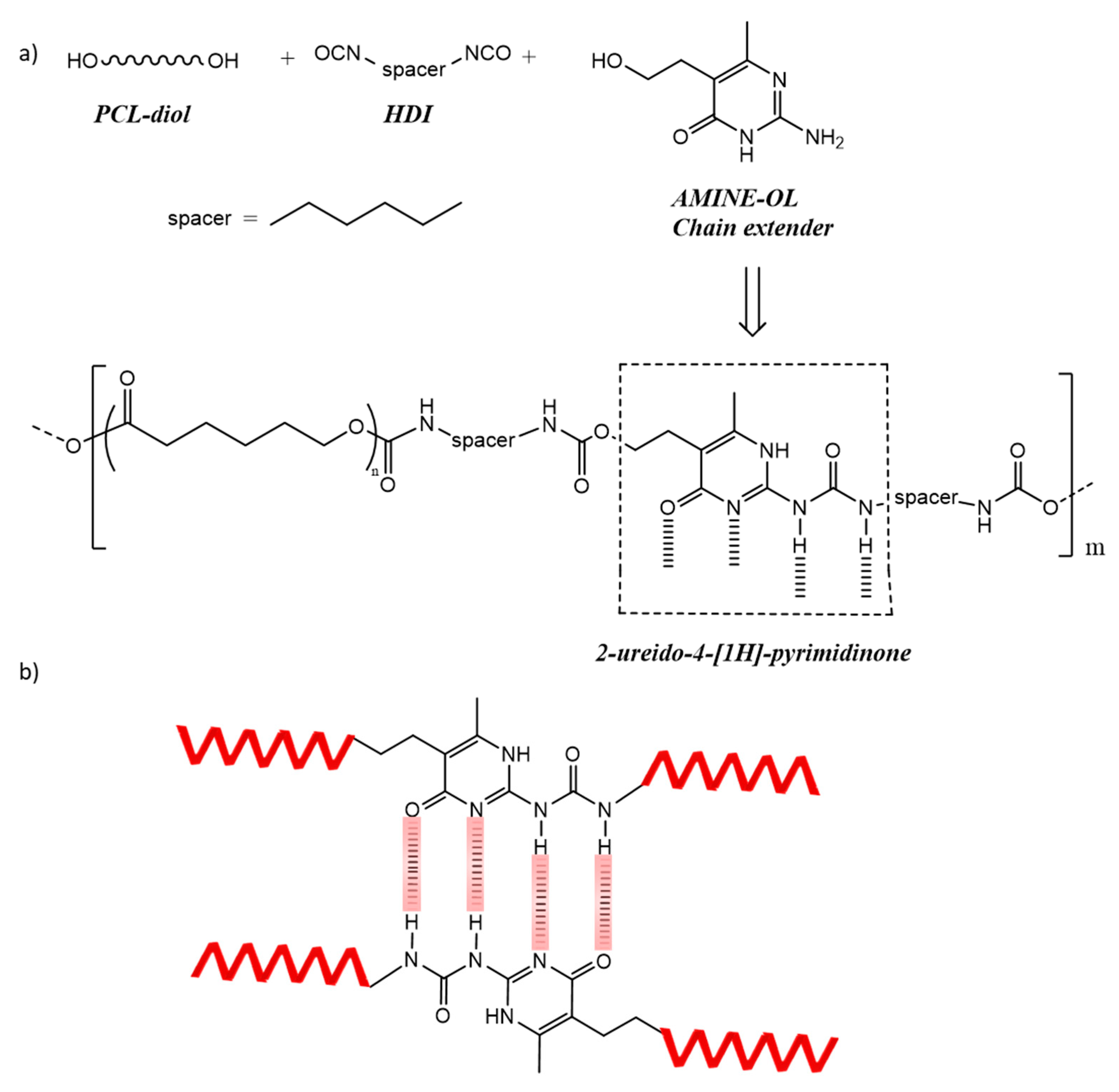
2.3. Preparation of the Polyurethane Based on PCL531 with a 45% Hard Segment (PUPCL531)
2.4. Preparation of the Polyurethane Based on PCL2054 with a 45% Hard Segment (PUPCL2054)
2.5. Characterization Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lendlein, A.; Behl, M.; Hiebl, B.; Wischke, C. Shape-memory polymers as a technology platform for biomedical applications. Expert. Rev. Med. Devic. 2010, 7, 357–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilate, F.; Toncheva, A.; Dubois, P.; Raquez, J.-M. Shape-memory polymers for multiple applications in the materials world. Eur. Polym. J. 2016, 80, 268–294. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Lekka, M.; Marzec, M.; Harhay, K.; Ohar, H.; Ostapiv, D.; Sharan, M.; Yaremchuk, I.; et al. Temperature-responsive grafted polymer brushes obtained from renewable sources with potential application as substrates for tissue engineering. App. Surf. Sci. 2017, 407, 546–554. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Kenny, J.M. Shape memory polymers: Properties, synthesis and applications. In Smart Polymers and Their Applications; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 204–236. [Google Scholar]
- Sessini, V.; Raquez, J.-M.; Lo Re, G.; Mincheva, R.; Kenny, J.M.; Dubois, P.; Peponi, L. Multiresponsive shape memory blends and nanocomposites based on starch. ACS Appl. Mater. Inter. 2016, 8, 19197–19201. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A review of shape memory polymers and composites: Mechanisms, materials, and applications. Adv. Mater. 2020, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.-C.; Xiao, Y.-Y.; Kang, Y.; Pan, M.; Li, B.-J.; Zhang, S. Shape memory polymers based on supramolecular interactions. ACS Appl. Mater. Inter. 2017, 9, 20276–20293. [Google Scholar] [CrossRef]
- Woodward, P.J.; Hermida Merino, D.; Greenland, B.W.; Hamley, I.W.; Light, Z.; Slark, A.T.; Hayes, W. Hydrogen bonded supramolecular elastomers: Correlating hydrogen bonding strength with morphology and rheology. Macromolecules 2010, 43, 2512–2517. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Hu, J.; Yuen, C.-W.; Chan, L. Fourier transform infrared study of supramolecular polyurethane networks containing pyridine moieties for shape memory materials. Polym. Int. 2010, 59, 529–538. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977. [Google Scholar] [CrossRef]
- Roy, N.; Tomović, Ž.; Buhler, E.; Lehn, J.M. An easily accessible self-healing transparent film based on a 2D supramolecular network of hydrogen-bonding interactions between polymeric chains. Chem-Eur. J. 2016, 22, 13513–13520. [Google Scholar] [CrossRef] [PubMed]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.; Hirschberg, J.K.; Lange, R.F.; Lowe, J.K.; Meijer, E. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Meijer, E.W. Quadruple hydrogen bonded systems. Chem. Commun. 2003, 1, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Zirbs, R. Supramolecular polymers and networkswith hydrogen bonds in the main-and side-chain. In Hydrogen Bonded Polymers; Binder, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–78. [Google Scholar]
- Feula, A.; Tang, X.; Giannakopoulos, I.; Chippindale, A.M.; Hamley, I.W.; Greco, F.; Buckley, C.P.; Siviour, C.R.; Hayes, W. An adhesive elastomeric supramolecular polyurethane healable at body temperature. Chem. Sci. 2016, 7, 4291–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, C.L.; Dell, E.M. A review of shape memory polymers bearing reversible binding groups. J. Polym. Sci. Pol. Phys. 2016, 54, 1340–1364. [Google Scholar] [CrossRef]
- Li, J.; Viveros, J.A.; Wrue, M.H.; Anthamatten, M. Shape-memory effects in polymer networks containing reversibly associating side-groups. Adv. Mater. 2007, 19, 2851–2855. [Google Scholar] [CrossRef]
- Li, J.; Lewis, C.L.; Chen, D.L.; Anthamatten, M. Dynamic mechanical behavior of photo-cross-linked shape-memory elastomers. Macromolecules 2011, 44, 5336–5343. [Google Scholar] [CrossRef]
- Ware, T.; Hearon, K.; Lonnecker, A.; Wooley, K.L.; Maitland, D.J.; Voit, W. Triple-shape memory polymers based on self-complementary hydrogen bonding. Macromolecules 2012, 45, 1062–1069. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, Y.; Tao, G.; Wang, L.; Zhou, S. Thermo-and water-induced shape memory poly (vinyl alcohol) supramolecular networks crosslinked by self-complementary quadruple hydrogen bonding. Polym. Chem. 2016, 7, 6637–6644. [Google Scholar] [CrossRef]
- Pilate, F.; Wen, Z.-B.; Khelifa, F.; Hui, Y.; Delpierre, S.; Dan, L.; Mincheva, R.; Dubois, P.; Yang, K.-K.; Raquez, J.-M. Design of melt-recyclable poly (ε-caprolactone)-based supramolecular shape-memory nanocomposites. RSC Adv. 2018, 8, 27119–27130. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Zhan, M.; Yu, D.; Xie, H.; He, M.; Yang, K.; Wang, Y. Novel poly (tetramethylene ether) glycol and poly (ε-caprolactone) based dynamic network via quadruple hydrogen bonding with triple-shape effect and self-healing capacity. ACS Appl. Mater. Inter. 2015, 7, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wei, M.; Zhan, M.; Zhang, J.; Xie, H.; Deng, X.; Yang, K.; Wang, Y. Novel triple-shape PCU/PPDO interpenetrating polymer networks constructed by self-complementary quadruple hydrogen bonding and covalent bonding. Polym. Chem. 2014, 5, 2231–2241. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Y.; Luo, Y.; Wang, Y. Poly(dl-Lactic Acid)-based linear polyurethanes capable of forming physical crosslinking via UPy quadruple hydrogen bonding assembly. Macromol. Mater. Eng. 2020, 305, 2000042. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Yuan, W.; Liu, K.; Zhou, J.; Shan, G.; Bao, Y.; Pan, P. Separate crystallization and melting of polymer blocks and hydrogen bonding units in double-crystalline supramolecular polymers. Polymer 2021, 222, 123670. [Google Scholar] [CrossRef]
- Song, Q.; Chen, H.; Yu, W.; Ni, H.; Hu, P.; Zhao, K. Multi-stimuli responsive polyurethane based on quadruple hydrogen bonding interactions. Mater. Today Proc. 2019, 16, 1405–1409. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Chen, R.; Zhang, H.; Shi, D.; Wang, Y.; Dong, W.; Ma, P.; Zhao, Y. Use of quadruple hydrogen bonding as the switching phase in thermo- and light-responsive shape memory hydrgel. ACS Appl. Polym. Mat. 2021, 3, 2884–2888. [Google Scholar] [CrossRef]
- Briz-López, E.M.; Navarro, R.; Martínez-Hernández, H.; Téllez-Jurado, L.; Marcos-Fernández, Á. Design and synthesis of bio-inspired polyurethane films with high performance. Polymers 2020, 12, 2727. [Google Scholar] [CrossRef]
- Navarro, R.; Seoane-Rivero, R.; Cuevas, J.M.; Marcos-Fernandez, Á. A novel strategy to polyurethanes with improved mechanical properties by photoactivation of amidocoumarin moieties. RSC Adv. 2020, 10, 29935–29944. [Google Scholar] [CrossRef]
- Abraham, G.A.; Marcos-Fernández, A.; Román, J.S. Bioresorbable poly (ester-ether urethane) s from L-lysine diisocyanate and triblock copolymers with different hydrophilic character. J. Biomed. Mater. Res. A 2006, 76, 729–736. [Google Scholar] [CrossRef]
- Marcos-Fernández, A.; Abraham, G.; Valentín, J.; San Román, J. Synthesis and characterization of biodegradable non-toxic poly (ester-urethane-urea) s based on poly (ε-caprolactone) and amino acid derivatives. Polymer 2006, 47, 785–798. [Google Scholar] [CrossRef]
- Bosman, A.W.; Janssen, H.M.; Van Gemert, G.M.L.; Versteegen, R.M.; Meijer, E.W.; Sijbesma, R.P. Siloxane Polymers with Quadruple Hydrogen Bonding Units. U.S. Patent No. 7,622,131, 24 November 2009. [Google Scholar]
- Seefried, C.; Koleske, J.; Critchfield, F. Thermoplastic urethane elastomers. I. Effects of soft-segment variations. J. Appl. Polym. Sci. 1975, 19, 2493–2502. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Patrício, T.; Bártolo, P. Thermal stability of PCL/PLA blends produced by physical blending process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zavargo, Z.; Flyn, J.H.; Macknight, W.J. Thermal degradation of segmented polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Fernandez, A.; Lozano, A.; Gonzalez, L.; Rodriguez, A. Hydrogen bonding in copoly (ether−urea) s and its relationship with the physical properties. Macromolecules 1997, 30, 3584–3592. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hu, J.; Liu, Y. Shape memory effect of thermoplastic segmented polyurethanes with self-complementary quadruple hydrogen bonding in soft segments. Eur. Phys. J. E 2009, 28, 3–10. [Google Scholar] [CrossRef]
- Knoben, W. Chain Stoppers in Reversible Supramolecular Polymer Solutions. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2007. [Google Scholar]
- Zhu, B.; Feng, Z.; Zheng, Z.; Wang, X. Thermoreversible supramolecular polyurethanes with self-complementary quadruple hydrogen-bonded end groups. J. Appl. Polym. Sci. 2012, 123, 1755–1763. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Sonseca, A.; Gimenez, E.; Marcos-Fernandez, A.; Kenny, J.M. Synthesis and characterization of PCL–PLLA polyurethane with shape memory behavior. Eur. Polym. J. 2013, 49, 893–903. [Google Scholar] [CrossRef] [Green Version]

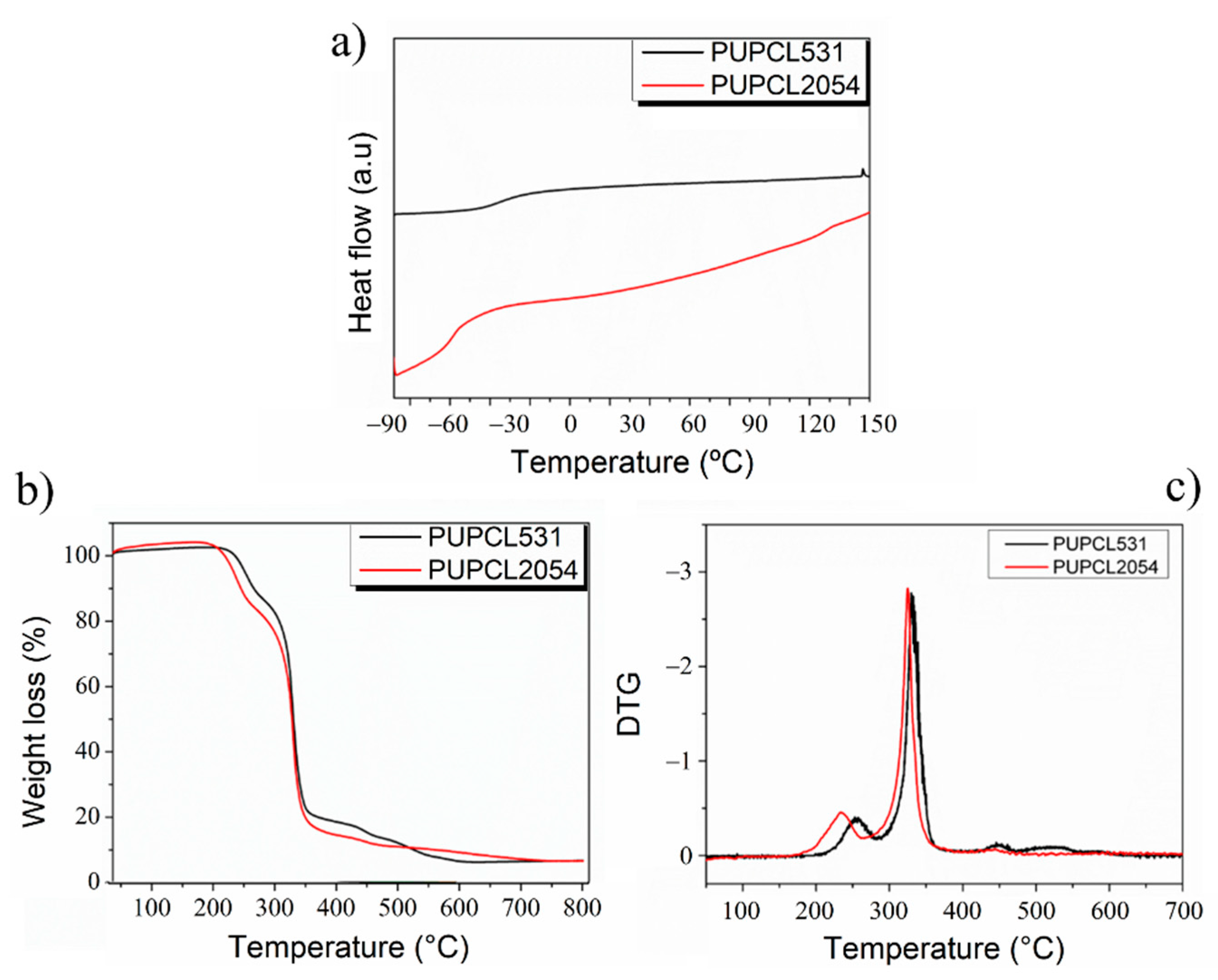
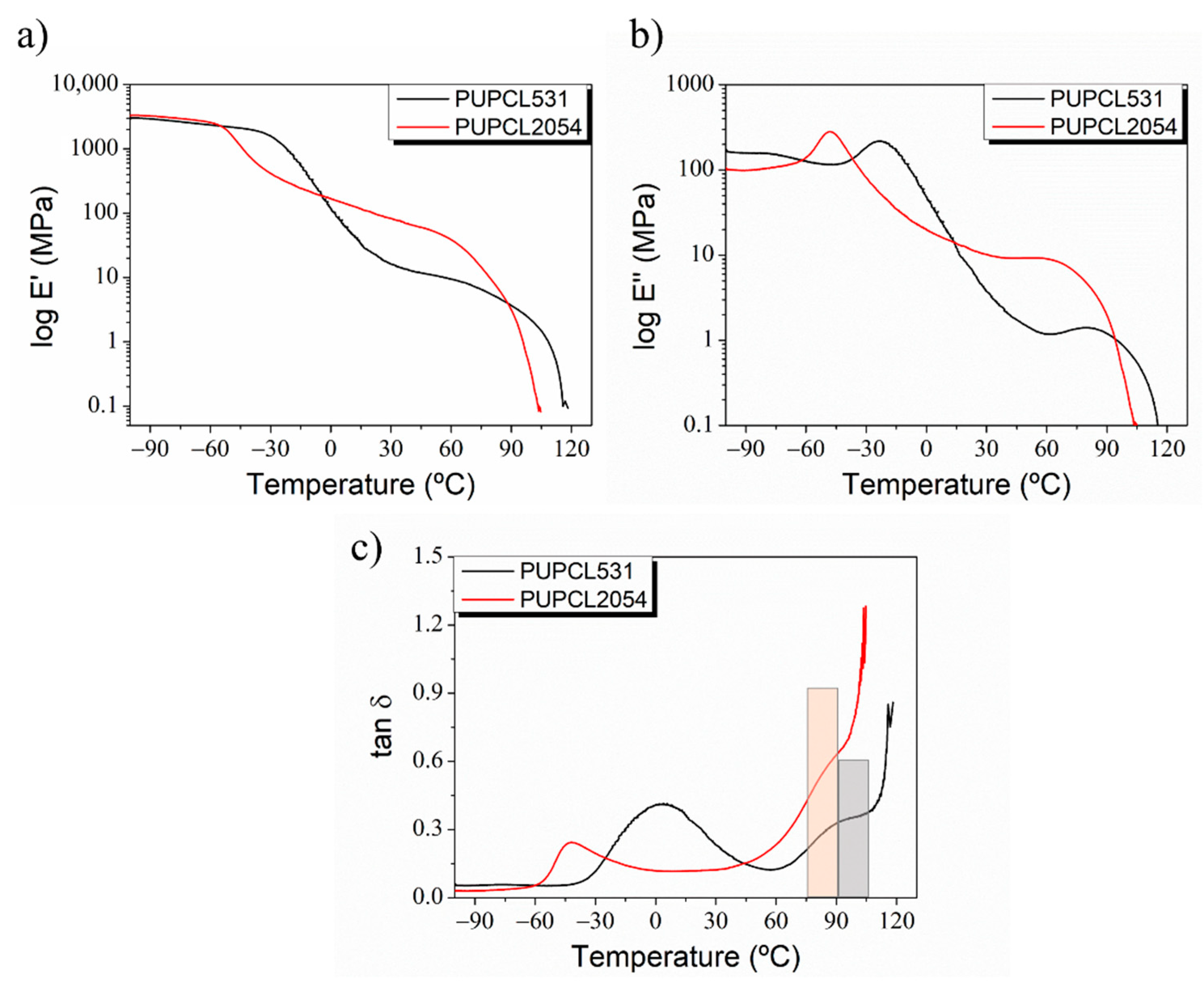
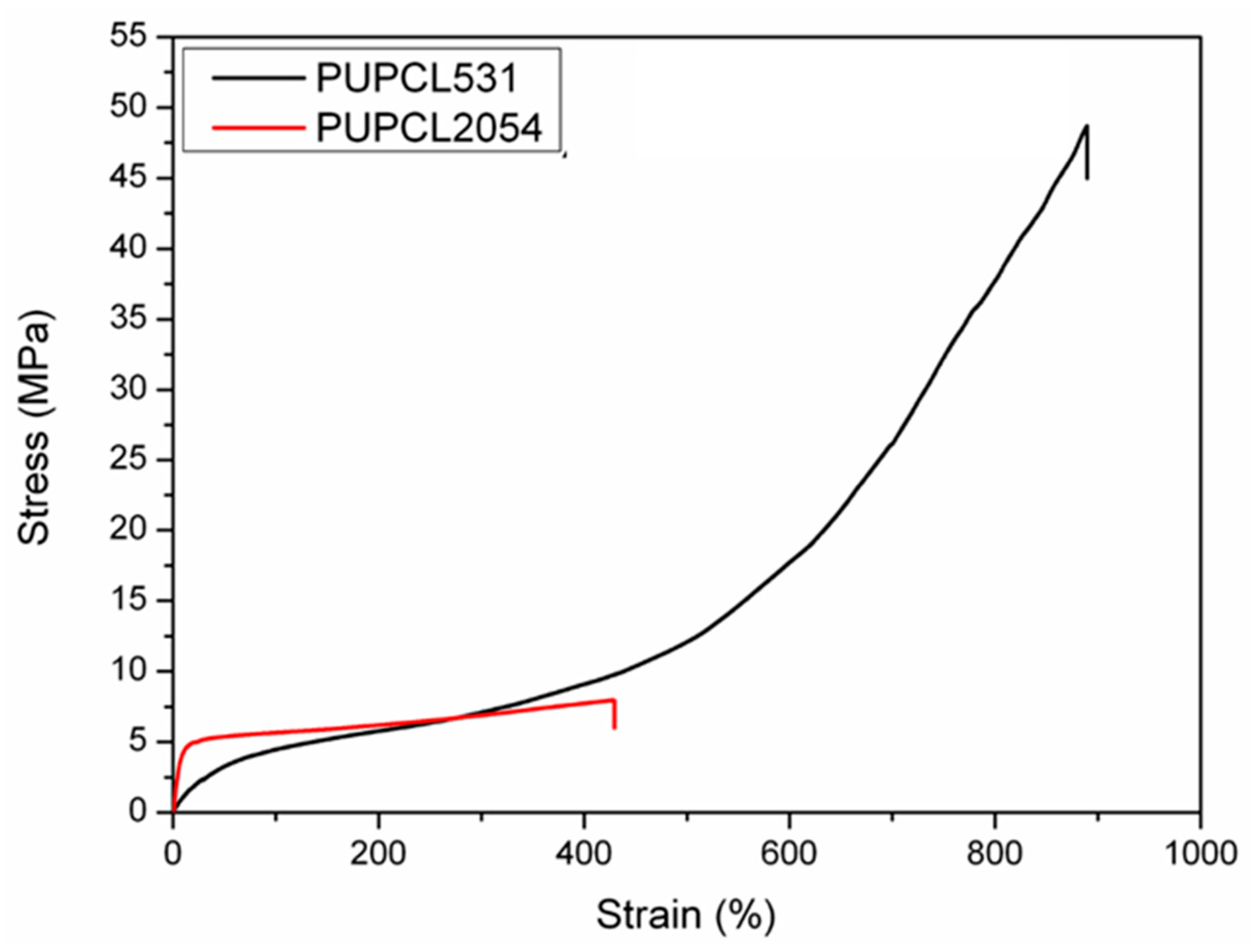
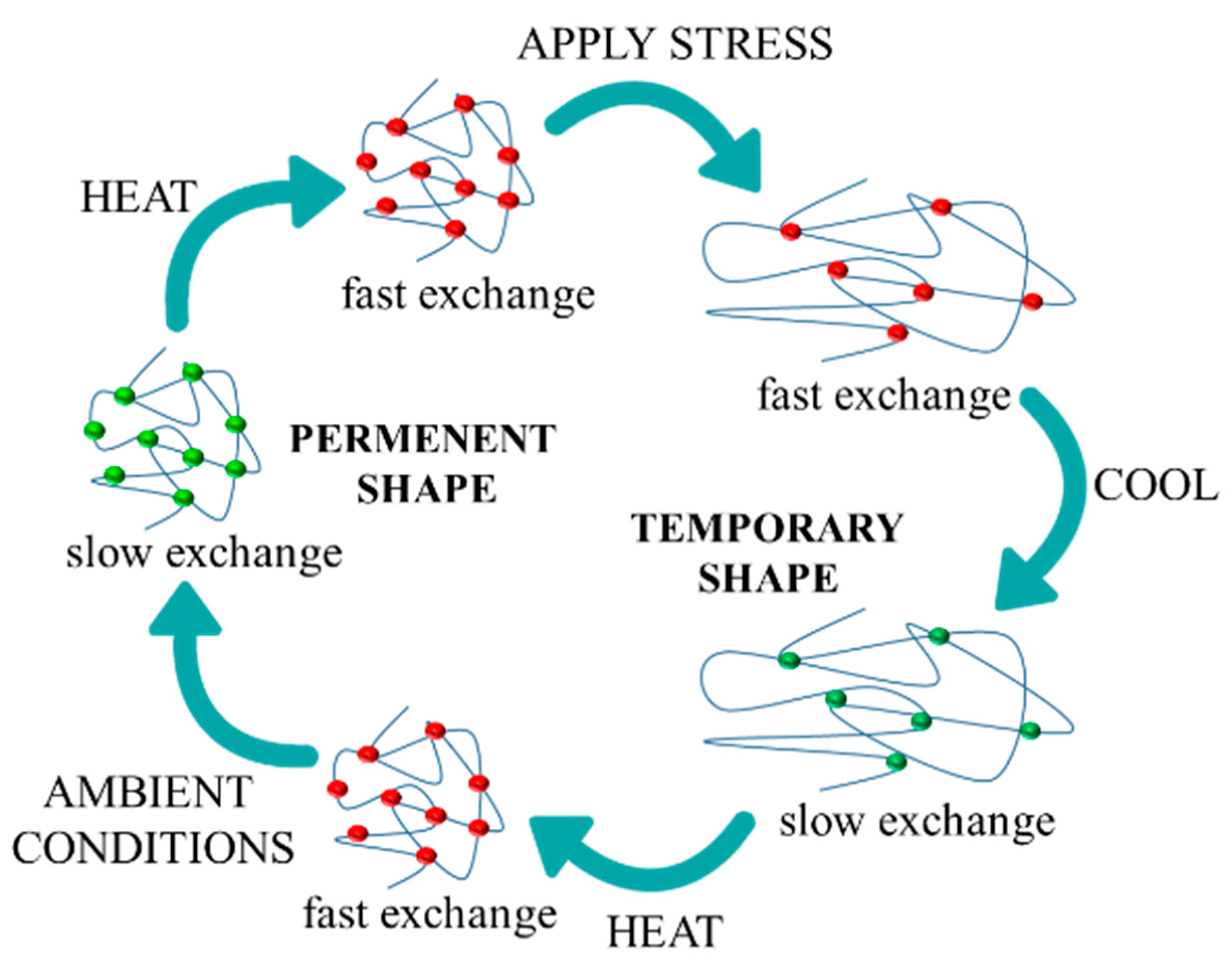
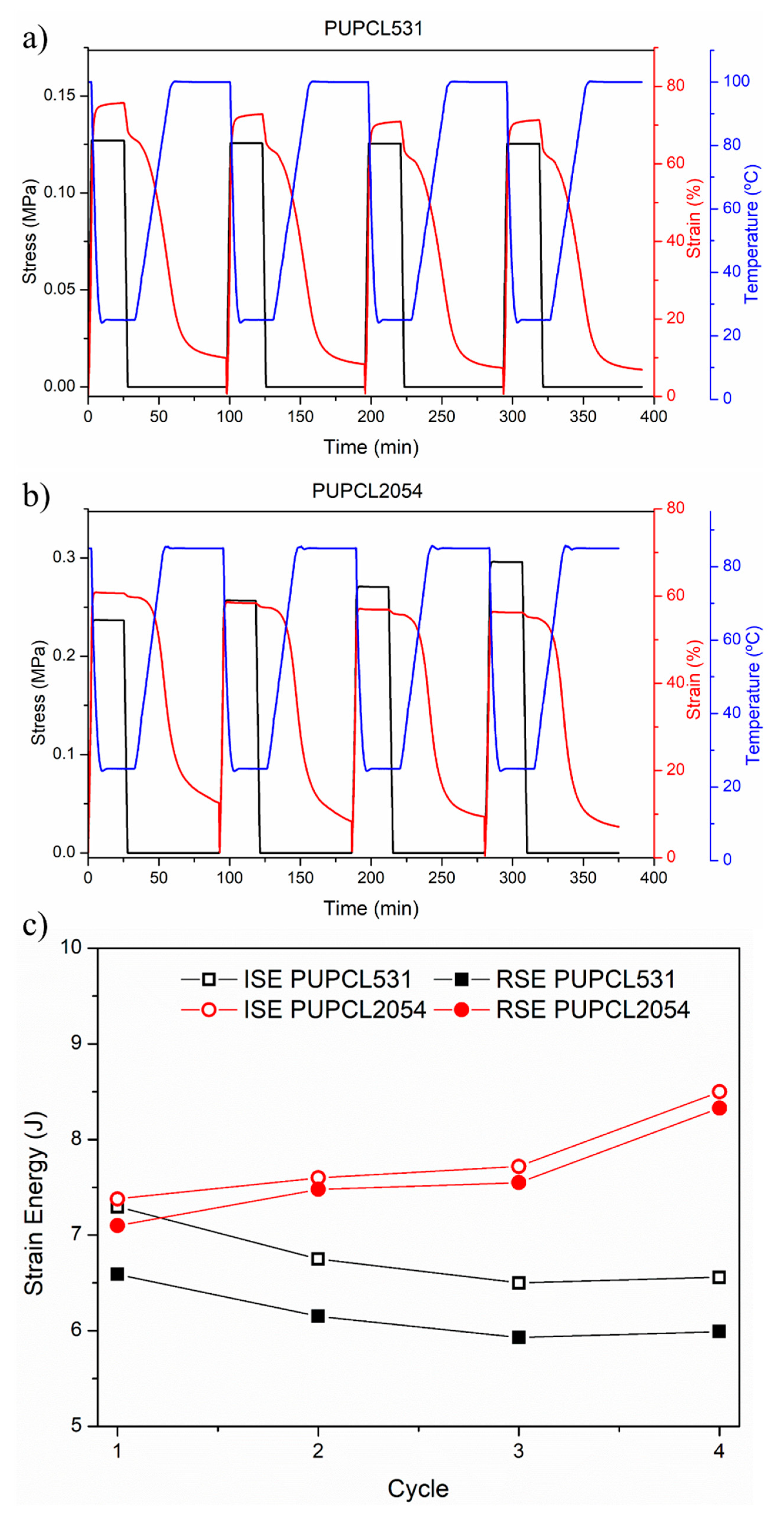
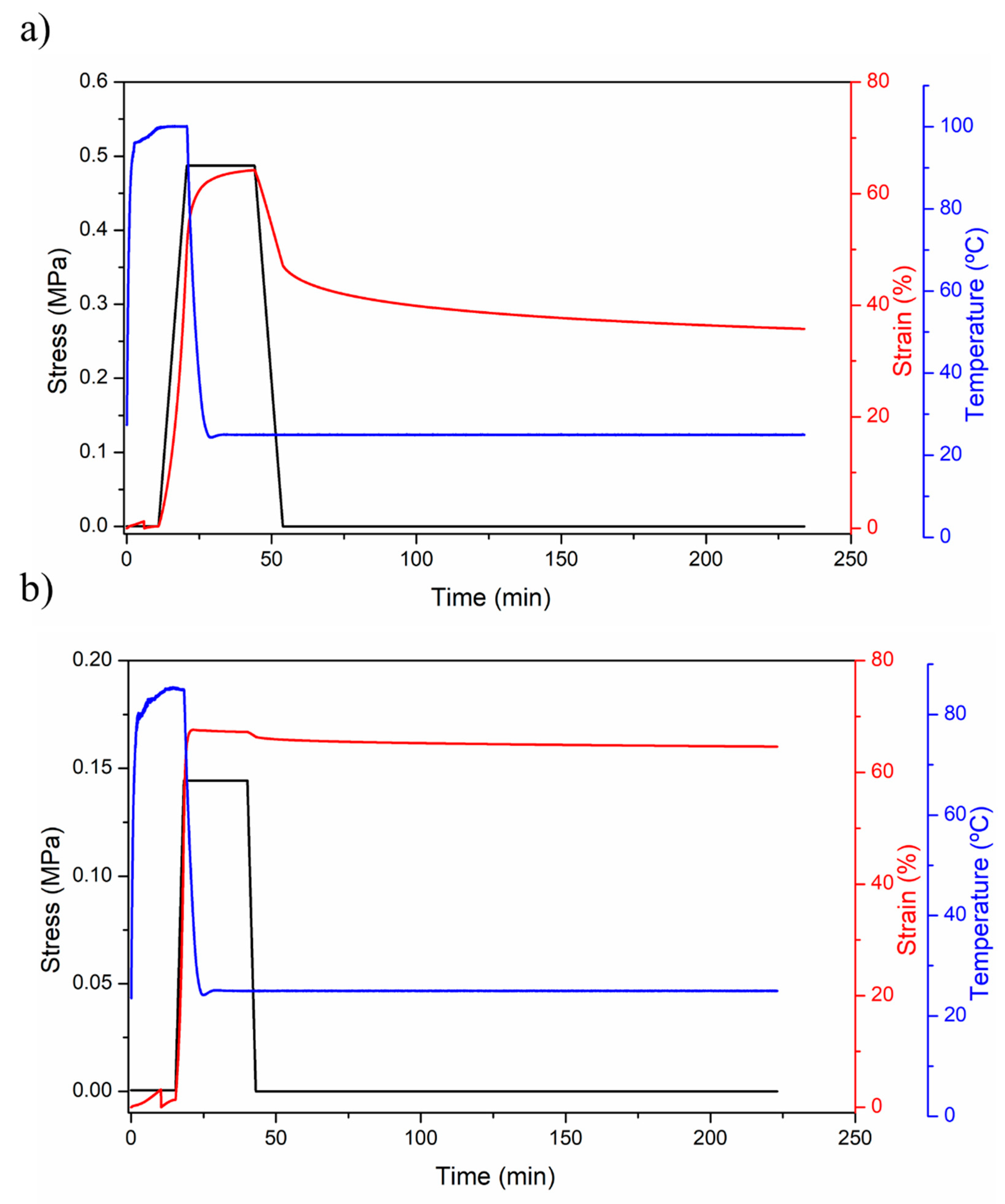
| Sample | Reaction Path | Reaction T (°C) | PCL-Diol (g·Mol−1) | Hard Segment (%) | Tg (°C) | T5% (°C) | Tmax1 (°C) | Tmax2 (°C) |
|---|---|---|---|---|---|---|---|---|
| PUPCL531 | One step | 80 | 531 | 45 | −38 | 200 | 230 | 330 |
| PUPCL2054 | Two steps | 80/92 | 2054 | 45 | −68 | 180 | 215 | 310 |
| Sample | Elastic Modulus E (MPa) | Tensile Strength σT (MPa) | Elongation at Break ε (%) |
|---|---|---|---|
| PUPCL531 | 13 ± 1 | 46 ± 5 | 880 ± 18 |
| PUPCL2054 | 72 ± 3 | 6 ± 1 | 274 ± 4 |
| Sample | Rr (%) | Rf (%) | Energy Efficiency (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle Number | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| PUPCL531 | 85 | 87 | 88 | 89 | 87 | 87 | 87 | 86 | 90 | 91 | 91 | 91 |
| PUPCL2054 | 79 | 85 | 83 | 87 | 98 | 98 | 98 | 98 | 96 | 98 | 98 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscas, F.; Sessini, V.; Peponi, L.; López, A.J.; Ureña, A.; Navarro, R.; Marcos-Fernández, Á. Supramolecular Polycaprolactone-Based Polyurethanes with Thermally Activated Shape-Memory Behavior. Polymers 2022, 14, 3447. https://doi.org/10.3390/polym14173447
Muscas F, Sessini V, Peponi L, López AJ, Ureña A, Navarro R, Marcos-Fernández Á. Supramolecular Polycaprolactone-Based Polyurethanes with Thermally Activated Shape-Memory Behavior. Polymers. 2022; 14(17):3447. https://doi.org/10.3390/polym14173447
Chicago/Turabian StyleMuscas, Fabio, Valentina Sessini, Laura Peponi, Antonio Julio López, Alejandro Ureña, Rodrigo Navarro, and Ángel Marcos-Fernández. 2022. "Supramolecular Polycaprolactone-Based Polyurethanes with Thermally Activated Shape-Memory Behavior" Polymers 14, no. 17: 3447. https://doi.org/10.3390/polym14173447
APA StyleMuscas, F., Sessini, V., Peponi, L., López, A. J., Ureña, A., Navarro, R., & Marcos-Fernández, Á. (2022). Supramolecular Polycaprolactone-Based Polyurethanes with Thermally Activated Shape-Memory Behavior. Polymers, 14(17), 3447. https://doi.org/10.3390/polym14173447











