Synthesis, Chemical and Biomedical Aspects of the Use of Sulfated Chitosan
Abstract
:1. Introduction
1.1. Methods for the Synthesis of Sulfated Chitosans
1.2. Mechanisms of Formation of Vascular Atherosclerosis and Signs of Cholesterol Extraction
2. Materials and Research Methods
2.1. Simplified Method for the Synthesis of Sulfoethylated Chitosans
2.2. Medical and Biological Aspects of the Study
2.3. Modeling the Early Stage of Atherosclerosis
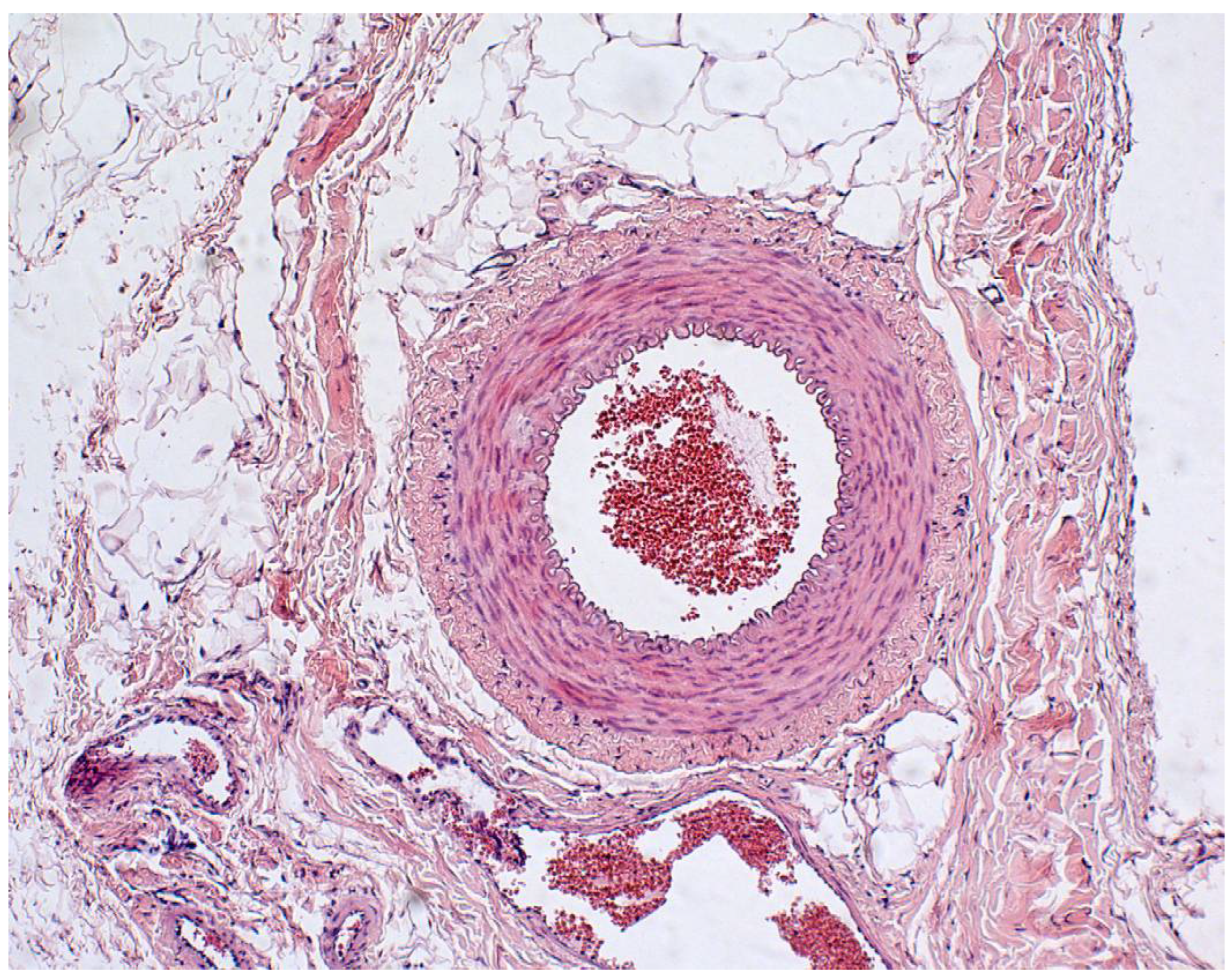
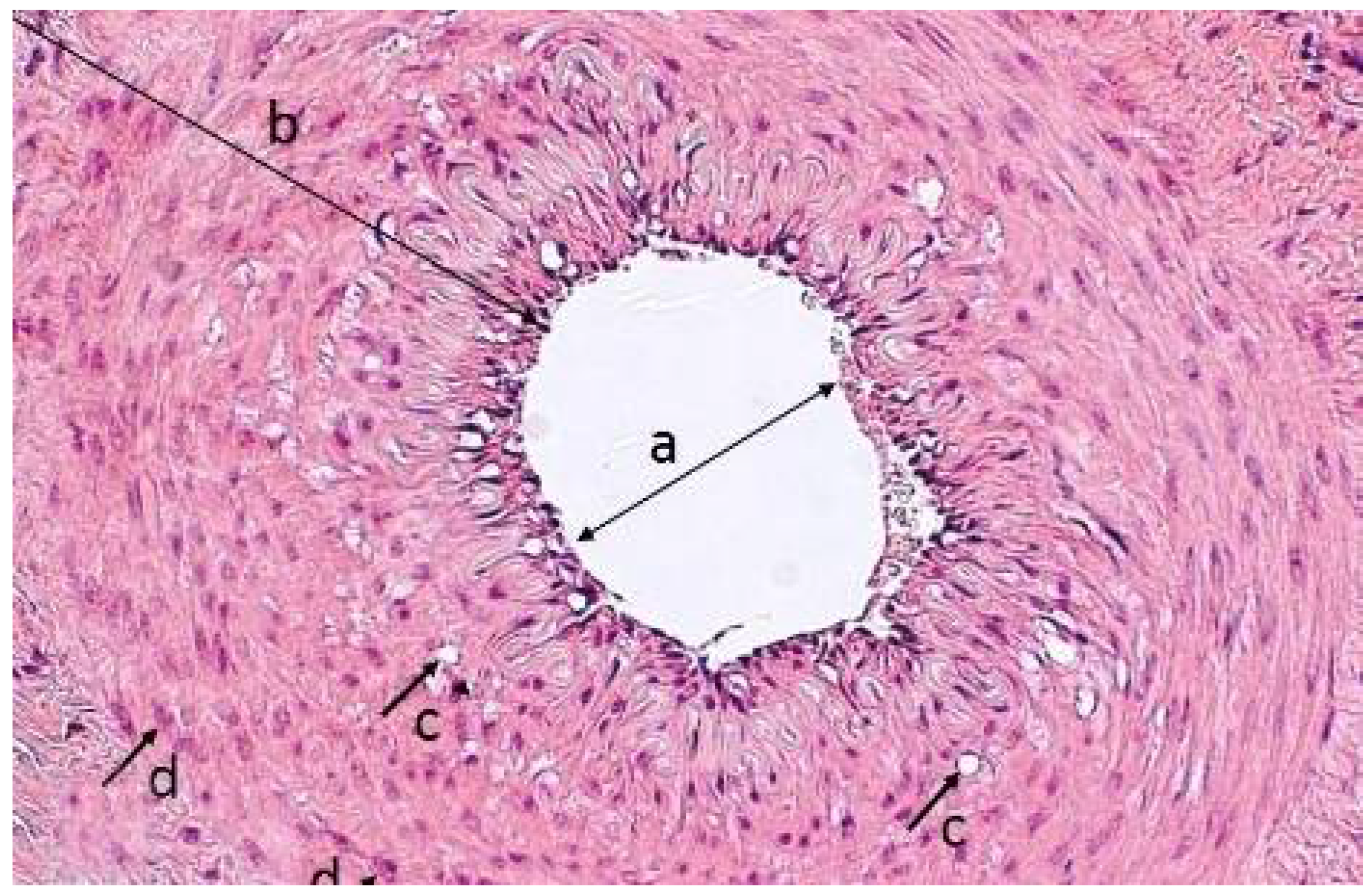
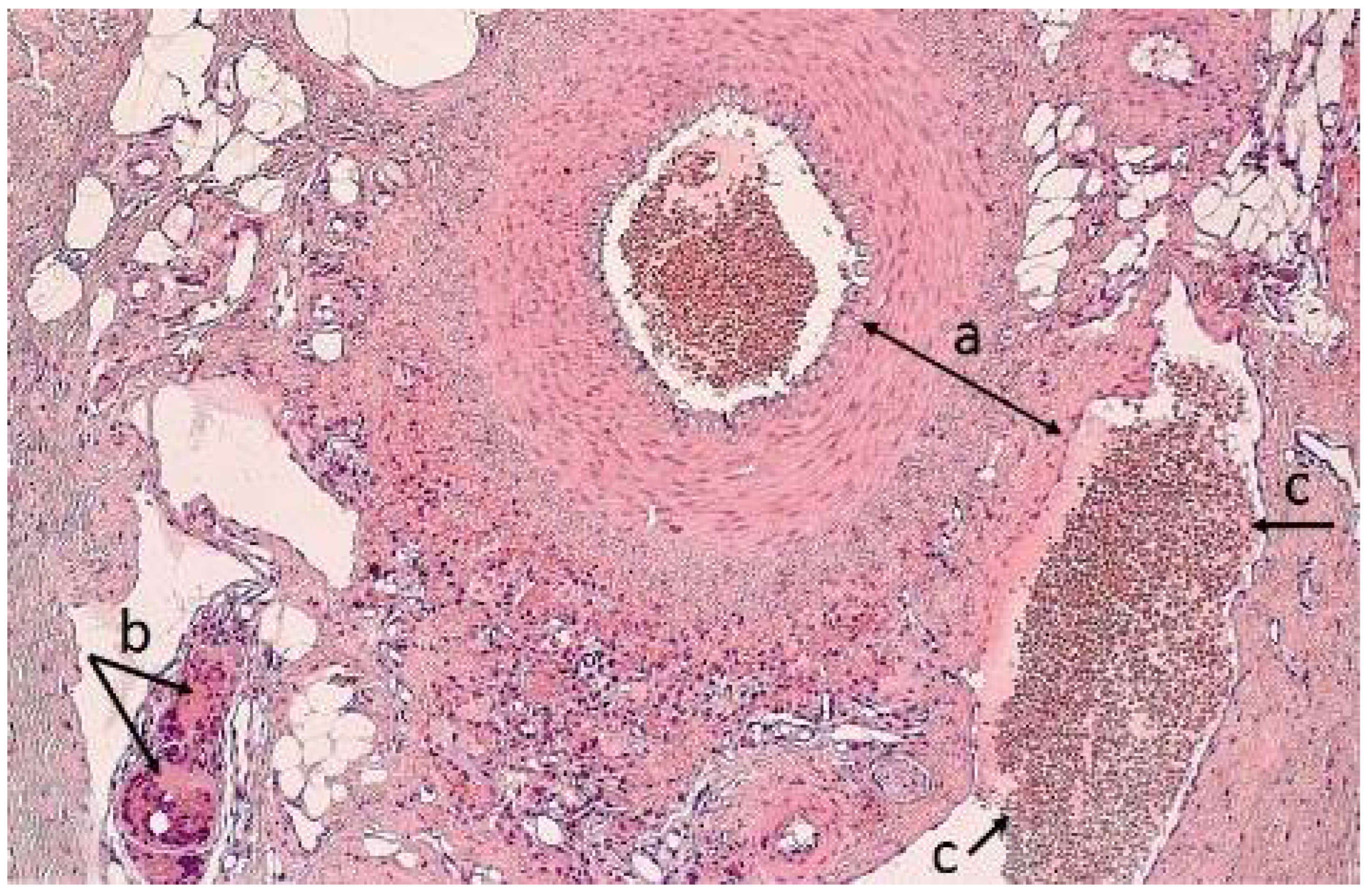
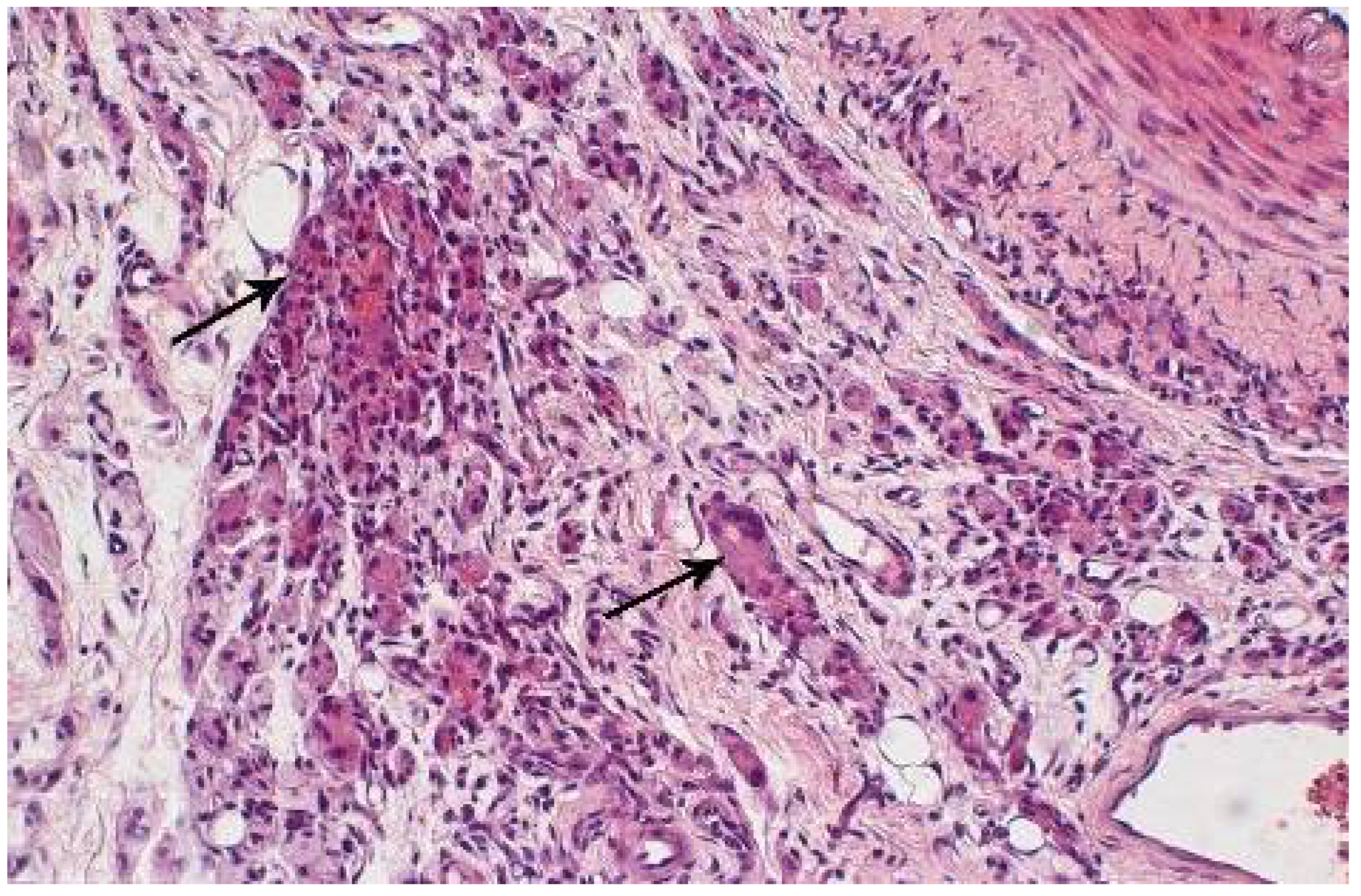
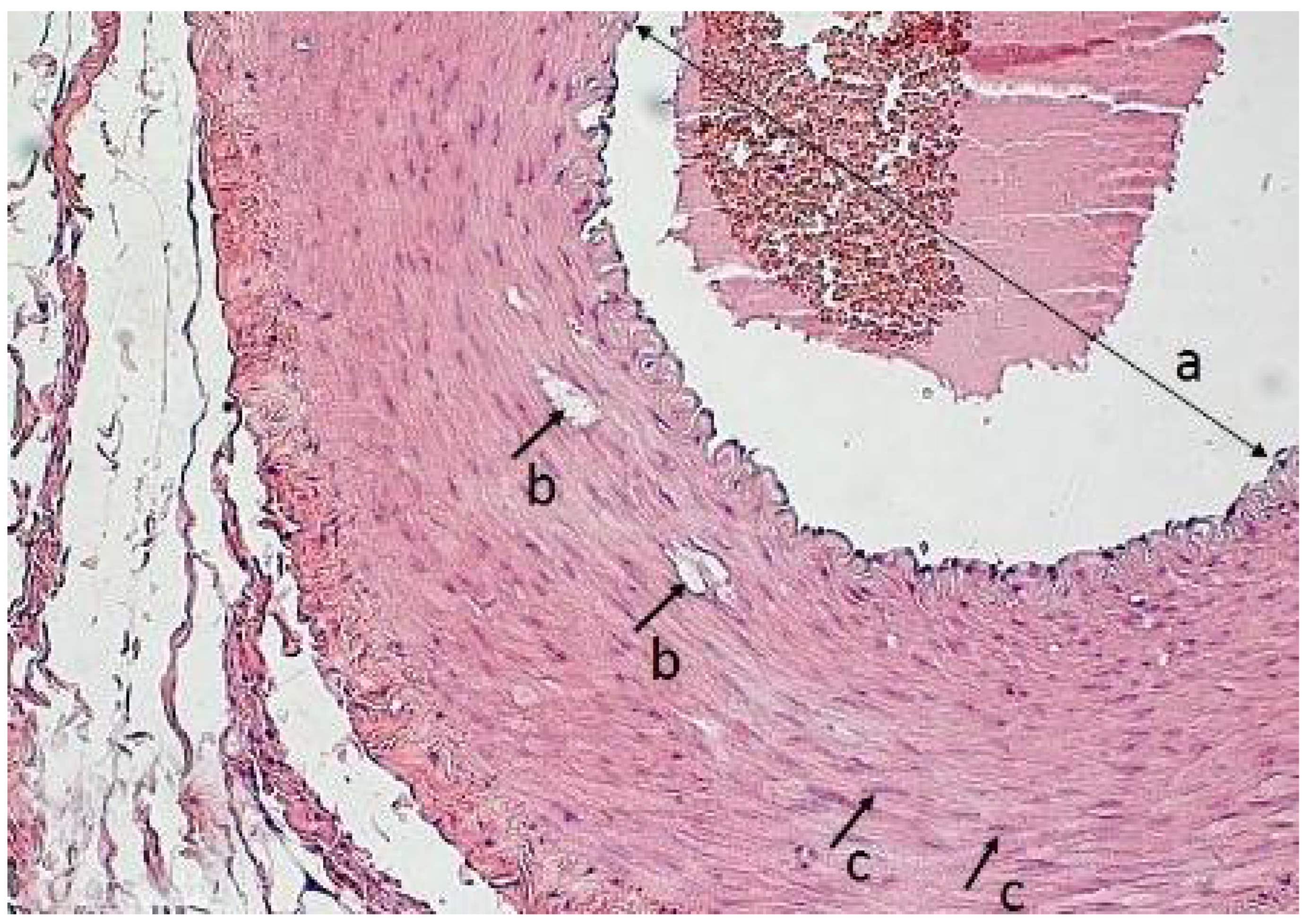
2.4. Morphological Analysis of the Vascular Wall
2.5. Biopolymer Implantation Technique
2.6. Biochemical Research Methods
2.7. Statistics
3. Results
3.1. Synthesis of Sulfoethylated Chitosan
3.2. Lipid Spectrum of Blood Plasma of Rabbits under the Influence of Sulfated Chitosan
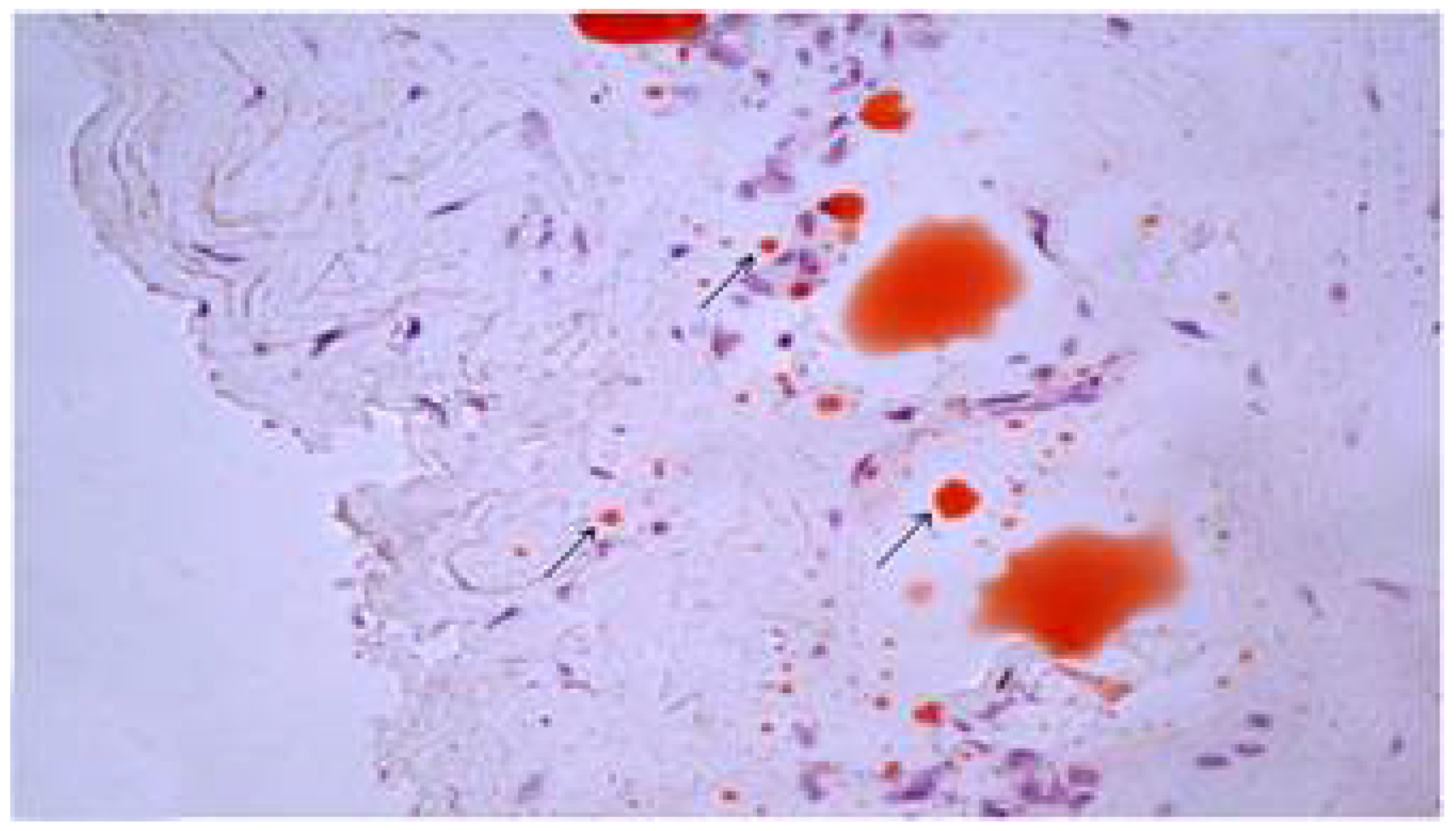
3.3. Signs of Cholesterol Extraction in Experimental Atherosclerosis
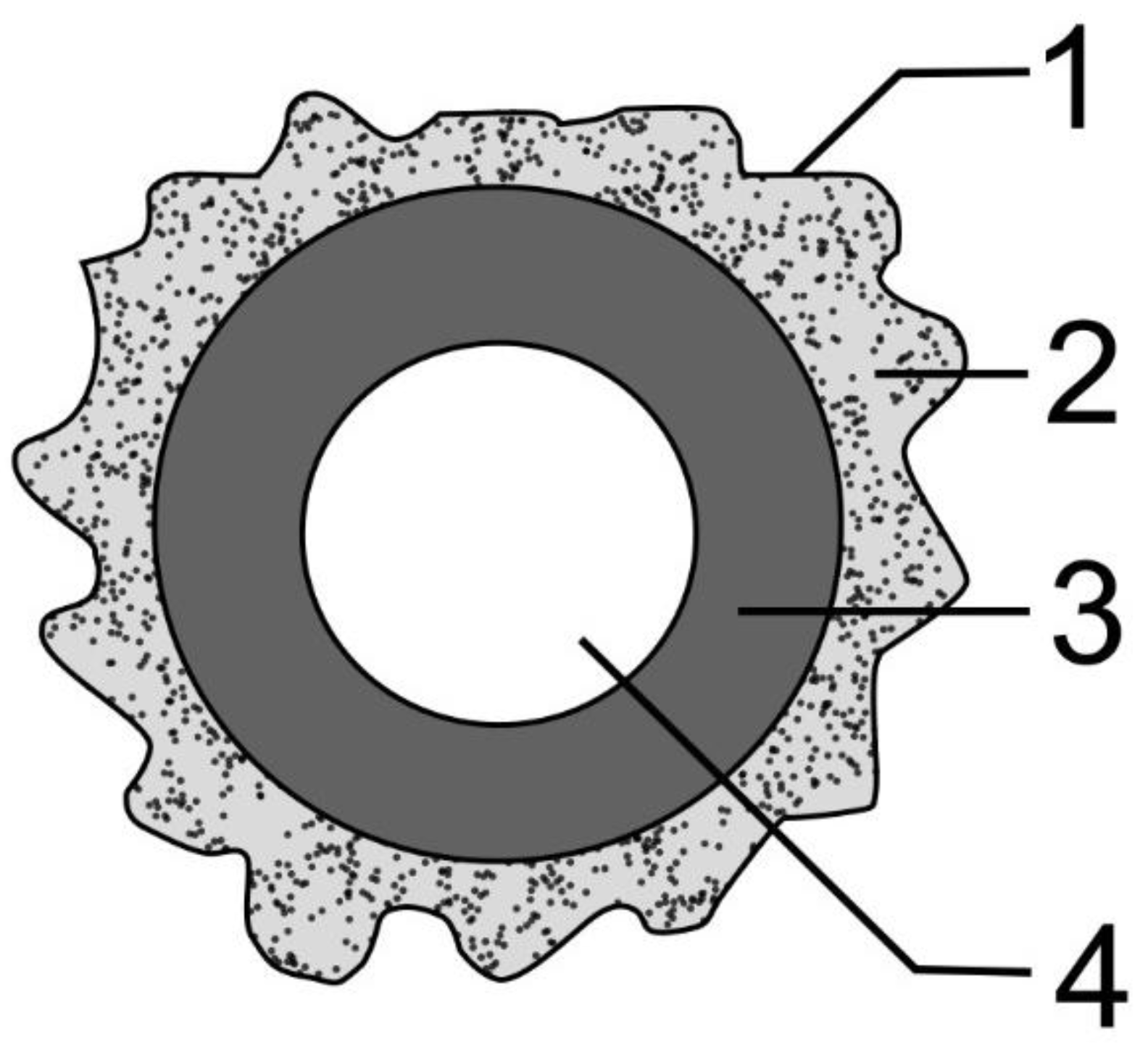
3.4. Morphometric Characteristics of the Wall of Main Arteries of Rabbit Hind Limbs after Injection of Sulfated Chitosan into the Para-Adventitial Area
3.5. Sorption of Human Blood Plasma on Sulfated Chitosan
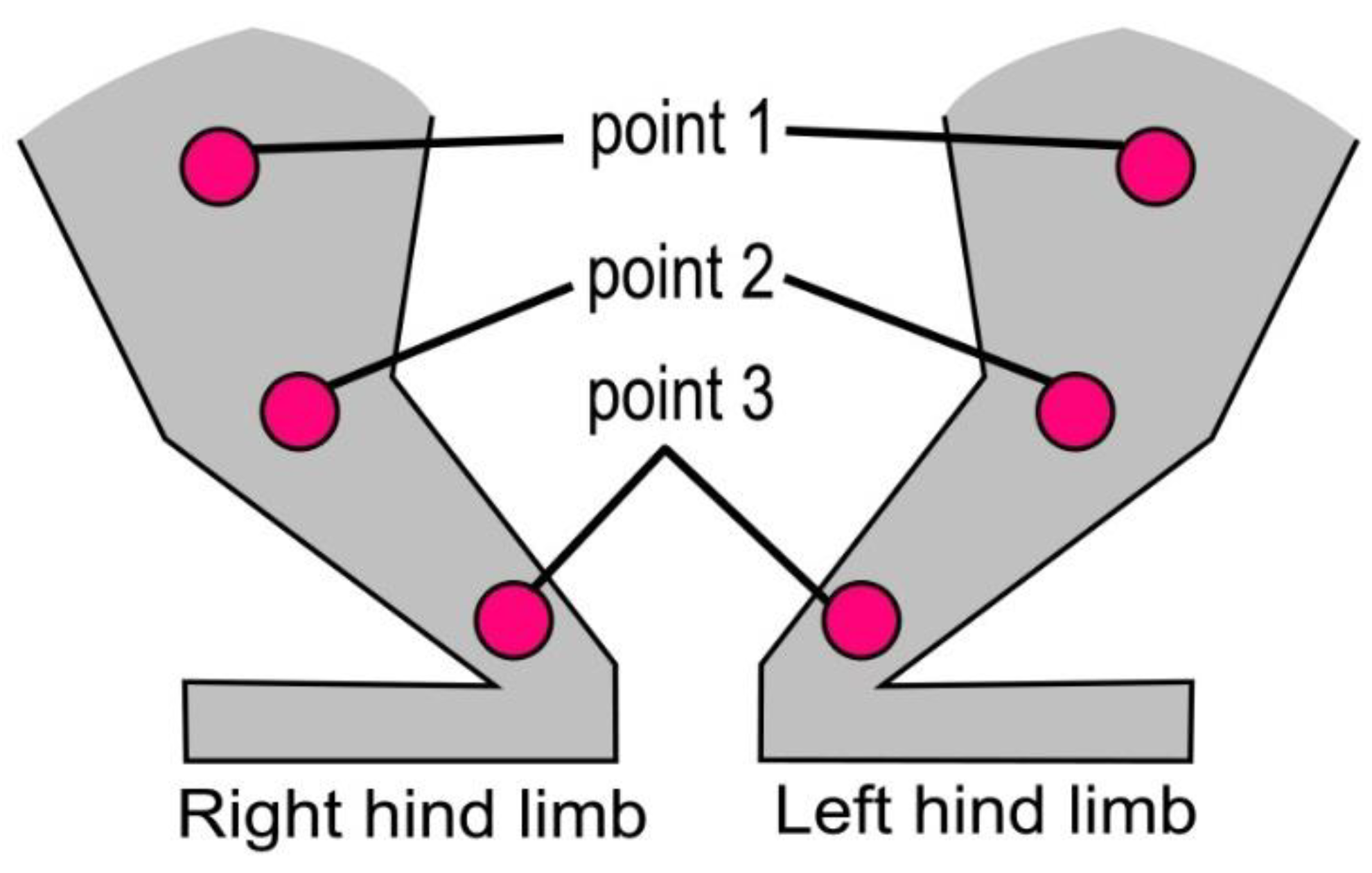
3.6. Microcirculation Index (MI) Evaluated by Laser Doppler Flowmetry of the Hind Limbs of Rabbits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skryabin, K.G.; Vikhoreva, G.A.; Varlamov, V.P. (Eds.) Chitin and chitosan production properties and usage. In Production, Properties and Usage; Nauka: Moscow, Russia, 2002; p. 368. [Google Scholar]
- Wang, Z.; Zheng, L.; Li, C.; Wu, S.; Xiao, Y. Preparation and antimicrobial activity of sulfopropyl chitosan in an ionic liquid aqueous solution. J. Appl. Polym. Sci. 2017, 134, 44989. [Google Scholar] [CrossRef]
- Petrova, Y.S.; Neudachina, L.K.; Mekhaev, A.V.; Pestov, A.V. Simple synthesis and chelation capacity of n-(2-sulfoethyl)chitosan, a taurine derivative. Carbohydr. Polym. 2014, 112, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Pestov, A.V.; Petrova, Y.S.; Bukharova, A.V.; Neudachina, L.K.; Koryakova, O.V.; Matochkina, E.G.; Kodess, M.I.; Yatluk, Y.u.G. Synthesis in a gel and sorption properties of N-2-sulfoethyl chitosan. Russ. J. Appl. Chem. 2013, 86, 269–272. [Google Scholar] [CrossRef]
- Petrova, V.А.; Chernyakov, D.D.; Moskalenko, Y.E.; Gasilova, E.R.; Strelina, I.А.; Okatova, O.V.; Balagina, Y.G.; Vlasova, E.N.; Skorik, Y.A. O,N-(2-sulfoethyl)chitosan: Synthesis and properties of solutions and films. Carbohydr. Polym. 2017, 157, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Nudga, L.A.; Plisko, E.A.; Danilov, S.N. Synthesis and properties of sulfoethylchitosan. Zhurnal Prikl. Khimii 1974, 47, 872–875. [Google Scholar]
- Nud’ga, L.A.; Petrova, V.A.; Ben’kovich, A.D.; Petropavlovskii, G.A. Comparative study of reactivity of cellulose, chitosan, and chitin-glucan complex in sulfoethylation. Russ. J. Appl. Chem. 2001, 74, 145–148. [Google Scholar] [CrossRef]
- Petrova, Y.S.; Pestov, A.V.; Usoltseva, M.K.; Neudachina, L.K. Selective adsorption of silver(I) ions over copper(II) ions on a sulfoethyl derivative of chitosan. J. Hazard. Mater. 2015, 299, 696–701. [Google Scholar] [CrossRef]
- Sokovnin, S.Y.; Balezin, M.E.; Puzyrev, I.S.; Pestov, A.V.; Yatluk, Y.G.; Zabolotskaya, E.V. Sorbents based on n-(2-carboxyethyl)chitosan cross-linked by nanosecond electron beams. Russ. Chem. Bull. 2009, 58, 1172–1179. [Google Scholar] [CrossRef]
- Petrova, Y.S.; Pestov, A.V.; Neudachina, L.K. Removal of metal ions in fixed bed from multicomponent solutions using N-(2-sulfoethyl) chitosan-based sorbents. Sep. Sci. Technol. 2016, 51, 1437–1445. [Google Scholar] [CrossRef]
- Pestov, A.V.; Koryakova, O.V.; Leonidov, I.I.; Yatluk, Y.G. Gel-synthesis, structure, and properties of sulfur-containing chitosan derivatives. Russ. J. Appl. Chem. 2010, 83, 787–794. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Schanzer, A.; Conte, M.S. Critical Limb Ischemia. Curr. Treat Options Cardiovasc. Med. 2010, 12, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, I. Peripheral artery occlusive disease a major contributor to cardiovascular public health burden. Eur. Heart J. 2015, 36, 894–896. [Google Scholar] [CrossRef]
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Lüders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef]
- Nehler, M.R.; Duval, S.; Diao, L.; Annex, B.H.; Hiatt, W.R.; Rogers, K.; Zakharyan, A.; Hirsch, A.T. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J. Vasc. Surg. 2014, 60, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Garcia, L.; Acharji, S. Epidemiology of chronic critical limb ischemia. In Critical Limb Ischemia; Dieter, R., Dieter, R.A., Jr., Dieter, R.A., III, Nanjundappa, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 9–14. [Google Scholar] [CrossRef]
- Karpov, I.A.; Sorokin, E.V. Intensive medical treatment of patients with atherosclerosis. Kardiologiia 2005, 45, 4–7. [Google Scholar] [PubMed]
- Tabas, I.; Williams, K.J.; Boren, J. Subendothe lial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.B.; Gronholdt, M.L.; Schroeder, T.V.; Stender, S.; Nordestgaard, B.G. In vivo transfer of lipoprotein(a) into human atherosclerotic carotid arterial intima. Arter. Thromb. Vasc. Biol. 1997, 17, 905–911. [Google Scholar] [CrossRef]
- Bartels, E.D.; Christoffersen, C.; Lindholm, M.W.; Nielsen, L.B. Altered Metabolism of LDL in the Arterial Wall Precedes Atherosclerosis Regression. Circ. Res. 2015, 117, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Groyer, E.; Caligiuri, G.; Laschet-Khallou, J.; Nicoletti, A. Immunological aspects of atherosclerosis. Presse Med. 2006, 35, 475–486. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Grufman, H.; Asciutto, G.; Nitulescu, M.; Persson, A.; Nilsson, M.; Nilsson, J.; Goncalves, I. Circulating cytokines reflect the expression of pro-inflammatory cytokines in atherosclerotic plaques. Atherosclerosis 2015, 241, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Deev, R.V.; Grigoryan, A.S.; Potapov, I.V.; Kiselev, S.L.; Isaev, A.A. Worldwide experience and recent trends in gene therapy of ischemic diseases. Angiol. Vasc. Surg. 2011, 17, 145–154. [Google Scholar]
- Kalinin, R.E.; Suchkov, I.A.; Pshennikov, A.S.; Mzhavanadze, N.D.; Krylov, A.A.; Plaksa, I.L.; Deev, R.V. Efficacy of medication for therapeutic angiogenesis in combined treatment of patients with diabetes mellitus and critical limb ischemia. Kazan Med. J. 2016, 97, 674–680. [Google Scholar] [CrossRef]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Polignano, R.; Baggiore, C.; Falciani, F.; Restelli, U.; Troisi, N.; Michelagnoli, S.; Panigada, G.; Tatini, S.; Farina, A.; Landini, G. Efficacy, safety and feasibility of intravenous iloprost in the domiciliary treatment of patients with ischemic disease of the lower limbs. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3720–3726. [Google Scholar]
- Herrmann, J.; Lerman, L.O.; Rodriguez-Porcel, M.; Holmes Jr, D.R.; Richardson, D.M.; Ritman, E.L.; Lerman, A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc. Res. 2001, 51, 762–766. [Google Scholar] [CrossRef]
- Shahzadi, L.; Yar, M.; Jamal, A.; Siddiqi, S.A.; Chaudhry, A.A.; Zahid, S.; Tariq, M.; Rehman, I.U.; MacNeil, S. Triethyl orthoformate covalently cross-linked chitosan-(poly vinyl) alcohol based biodegradable scaffolds with heparin-binding ability for promoting neovascularization. J. Biomater. Appl. 2016, 31, 582–593. [Google Scholar] [CrossRef]
- Yar, M.; Gigliobianco, G.; Shahzadi, L.; Dew, L.; Siddiqi, S.A.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; MacNeil, S. Production of chitosan PVA PCL hydrogels to bind heparin and induce angiogenesis. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 466–476. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, Y.T.J. Effect of basic fibroblast growth factor released from chitosan-fucoidan nanoparticles on neurite extension. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef]
- Beisiegel, U. Lipoprotein metabolism. Eur. Heart J. 1998, 19 (Suppl. A), A20–A23. [Google Scholar]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethyleneoxide nanoparticles as protein carriers. J. Appl. Pol. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Taravel, M.N.; Domard, A. Relation between the physicochemical characteristics of collagen and its interactions with chitosan. I. Biomater. 1993, 14, 930–938. [Google Scholar] [CrossRef]
- Du, W.L.; Xu, Z.R.; Han, X.Y.; Xu, Y.L.; Miao, Z.G. Preparation, characterization and adsorption properties of chitosan nanoparticles for eosinY as a model an ionic dye. J. Hazard. Mater. 2008, 153, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Deuchi, K.; Imasato, Y.; Shizukuishi, M.; Kobayashi, E. Mechanism for the inhibition of fat digestion by chitosan and for the synergistic effect of ascorbate. Biosci. Biotechnol. Biochem. 1995, 59, 786–790. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z. In vivo antitumor activity of chitosan nanoparticles. Bioorg. Med. Chem. Lett. 2006, 16, 4243–4245. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical activity of chitin/chitosan based materials—Influence of physicochemical properties apart from molecular weight and degree of N-acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Hou, J.; Xing, W.; Liu, C. Vascularization and bone regeneration in a critical sized defect using 2-N, 6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials 2014, 35, 684–698. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Ways, M.T.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte complexes of natural polymers and their biomedical applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Bolshakov, I.N.; Levenets, A.A.; Furtsev, T.V.; Kotikov, A.R.; Patlataya, N.N.; Ryaboshapko, E.I.; Dmitrienko, A.E.; Nikolaenko, M.M.; Matveeva, N.D.; Ibragimov, I.G. Experimental reconstruction of critical size defects of bone tissue in the maxillofacial region when using modified chitosan. Biomed. Translat. Sci. 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Xu, J.; Liu, Y.; Cui, Z.; He, Z.; Zheng, Z.; Huang, X.; Zhang, Y. Self-assembled angelica sinensis polysaccharide nanoparticles with an instinctive liver-targeting ability as a drug carrier for acute alcoholic liver damage protection. Int. J. Pharm. 2020, 577, 118996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Pa, J.-H.; Yu, T.L. Light scattering study of chitosan in acetic acid aqueous solutions in acetic acid aqueous solutions. Macromol Chem. Phys. 2001, 202, 985–991. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J. Preparation and characterization of the fluorescent chitosan nanoparticle probe. Chin. J. Anal. Chem. 2006, 34, 1555–1559. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. (Ed.) Chitosan Per Os: From Dietary Supplement to Drug Carrier; Atec: Grottamare, Italy, 2000. [Google Scholar]
- Tao, X.; Tao, T.; Wen, Y.; Yi, J.; He, L.; Huang, Z.; Nie, Y.; Yao, X.; Wang, Y.; He, C.; et al. Novel delivery of mitoxantrone with hydrophobically modified pullulan nanoparticles to inhibit bladder cancer cell and the effect of nano-drug size on inhibition efficiency. Nanoscale Res. Lett. 2018, 13, 345. [Google Scholar] [CrossRef]
- Calce, E.; Mercurio, F.A.; Leone, M.; Saviano, M.; De Luca, S. Eco-friendly microwave-assisted protocol to prepare hyaluronan-fatty acid conjugates and to induce their self-assembly process. Carbohydr. Polym. 2016, 143, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Alhaique, F.; Matricardi, P.; Meo, C.; Coviello, T.; Montanari, E. Polysaccharide-based self-assembling nanohydrogels: An overview on 25-years research on pullulan. J. Drug Deliv. Sci. Technol. 2015, 30, 300–309. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Hirano, S.; Takahashi, H.; Loethen, S.; Thompson, D.H.; Akiyoshi, K. Self-assembled ph-sensitive cholesteryl pullulan nanogel as a protein delivery vehicle. Biomacromolecules 2013, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, P. Versatile injectable supramolecular hydrogels containing drug loaded micelles for delivery of various drugs. Polym. Chem. 2014, 5, 1072–1081. [Google Scholar] [CrossRef]
- Al-Remawi, M.M. Properties of chitosan nanoparticles formed using sulfate anions as crosslinking bridges. Amer. J. Appl. Sci. 2012, 9, 1091–1100. [Google Scholar]
- Martien, R.; Loretz, B.; Sandbichler, A.M.; Schnürch, A.B. Thiolated chitosan nanoparticles: Transfection study in the caco-2 differentiated cell culture. Nanotechnology 2008, 19, 045101. [Google Scholar] [CrossRef]
- Cohen, Y.; Rutenberg, R.; Cohen, G.; Veltman, B.; Gvirtz, R.; Fallik, E.; Danino, D.; Eltzov, E.; Poverenov, E. Aminated polysaccharide-based nanoassemblies as stable biocompatible vehicles enabling crossing of biological barriers: An effective transdermal delivery of diclofenac medicine. ACS Appl. Bio. Mater. 2020, 3, 2209–2217. [Google Scholar] [CrossRef]
- Wang, Y.; Karmakar, T.; Ghosh, N.; Basak, S.; Sahoo, N.G. Targeting mangiferin loaded N-succinyl chitosan-alginate grafted nanoparticles against atherosclerosis–A case study against diabetes mediated hyperlipidemia in rat. Food Chem. 2022, 370, 131376. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.-C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of microRNAs by chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Kirichenko, А.К.; Patlataya, N.N.; Sharkova, А.F.; Pevnev, А.А.; Kontorev, К.V.; Bolshakov, I.N. Pathomorphism of limb major vessels in experimental atherogenic inflammation. The role of adventitial intimal relations (review). Mod. Technol. Med. 2017, 9, 162–173. [Google Scholar] [CrossRef]
- Kirichenko, A.K.; Shulmin, A.V.; Sharkova, A.F.; Patlataya, N.N.; Bolshakov, I.N. Morphological reconstruction of major arteries by perivascular implantation of sulfated chitosan in experimental atherosclerosis. Mod. Technol. Med. 2017, 9, 115–122. [Google Scholar] [CrossRef]
- RU. Method for Reconstructing Main Arterial Vessels in Case of Their Atherosclerotic Lesion. Patent RU. No. 2311139, 27 November 2007. [Google Scholar]
- Bolshakov, I.N.; Shestakova, L.A.; Kotikov, A.R.; Kaptyuk, G.I. Experimental atherosclerosis in rats. Morphological reconstruction of the main artery wall with the polysaccharide biopolymers. Fundam. Res. 2013, 10, 557–563. [Google Scholar]








| Group Number | Experimental Series | Number of Animals | ChD (Days) | Time of Polymer Implantation (Days) | Lipid Blood Fractions | Laser Doppler Blood Flow Measurement (Days) | Morphometric Analysis of Great Vessels |
|---|---|---|---|---|---|---|---|
| 1 | Intact animals, standard vivarium diet | 6 | - | - | + | 1, 80, 100, 110 | + |
| 2 | ChD, without intervention | 6 | 110 | - | + | 1, 80, 100, 110 | + |
| 3 | ChD, 1% gel of SEC | 6 | 110 | from 80 to 110 | + | 1, 80, 100, 110 | + |
| 4 | ChDf, 1% gel of SEC | 6 | - | 30 | + | 1, 80, 100, 110 | + |
| 5 | ChD, implantation of 1% ChAsc | 6 | 110 | from 80 to 110 | + | 1, 80, 100, 110 | + |
| 6 | ChDf, implantation of 1% ChAsc | 6 | 110 | from 80 to 110 | + | 1, 80, 100, 110 | + |
| In total | 36 | 110 | 30 | + | 1, 80, 100, 110 | + |
| Animal Group (n = 18) | TG mmol/L | Ch mmol/L | LHL mmol/L | LDL mmol/L | vLDL mmol/L | AC |
|---|---|---|---|---|---|---|
| Intact animals | 1.68 ± 0.35 | 0.77 ± 0.13 | 0.27 ± 0.05 | 0.33 ± 0.18 | 0.76 ± 0.16 | 1.18 ± 0.72 |
| ChD-treated animals | 4.16 ± 0.91 ** | 22.95 ± 2.13 * | 2.93 ± 0.76 * | 18.14 ± 1.55 * | 1.89 ± 0.41 * | 10.95 ± 1.81 * |
| №. | Animal Groups | Total Lipid Fraction (g/L) | TG (mmol/L) | Non-Esterified Fatty Acids (mmol/L) | |||
|---|---|---|---|---|---|---|---|
| Control (Right) | Experience (Left) | Control (Right) | Experience (Left) | Control (Right) | Experience (Left) | ||
| 1. | Intact animals | 2.68 ± 0.01 (n = 12) | 0.95 ± 0.03 (n = 12) | 7.80 ± 2.66 (n = 12) | |||
| 2. | Animals on a cholesterol diet (ChD) | 8.15 ± 0.06 + (n = 6) | 2.43 ± 0.84 (n = 6) | 8.66 ± 1.96 (n = 6) | |||
| 3. | ChD, 1% gel implantation SEC | 4.98 ± 0.70 (n = 30) | 2.60 ± 0.24 ** (n = 6) | 2.14 ± 0.25 (n = 30) | 1.08 ± 0.01 * (n = 6) | 8.11 ± 1.0 (n = 30) | 9.67 ± 1.57 (n = 6) |
| 4. | ChD, 1% gel implantation ChAsc | 1.96 ± 0.07 * (n = 6) | 0.57 ± 0.27 * (n = 6) | 5.63 ± 1.26 (n = 6) | |||
| 5. | ChD, 1% gel implantation chitosan hydrochloride, Mw 100 kDa, DD 87% | 2.21 ± 0.70 ** (n = 6) | 1.00 ± 0.46 ** (n = 6) | 10.23 ± 1.86 (n = 6) | |||
| 6. | ChD, 1% gel implantation carrageenan, BCh RAS (Pacific Institute of Bioorganic Chemistry, Vladivostok) | 4.21 ± 0.33 (n = 6) | 1.86 ± 0.10 (n = 6) | 9.95 ± 1.44 (n = 6) | |||
| 7. | ChD, 1% gel implantation polyethylene glycol | 4.52 ± 0.28 (n = 6) | 1.56 ± 0.36 (n = 6) | 10.55 ± 1.64 (n = 6) | |||
| № | Animal Groups | Total Lipid Fraction (g/L) | TG (g/L) | Non-Esterified Fatty Acids (mmol/L) | |||
|---|---|---|---|---|---|---|---|
| Control (Right) | Experience (Left) | Control (Right) | Experience (Left) | Control (Right) | Experience (Left) | ||
| 1. | Intact animals (right and left limbs) | 1.82 ± 0.59 (n = 12) | 1.71 ± 1.34 (n = 12) | 7.33 ± 0.93 (n = 12) | |||
| 2. | Animals on a cholesterol diet (ChD) | 8.15 ± 0.06 + (n = 6) | 2.25 ± 0.64 (n = 6) | 8.58 ± 2.45 (n = 6) | |||
| 3. | ChD, 1% gel implantation SEC | 4.06 ± 0.43 (n = 30) | 4.56 ± 0.28 (n = 6) | 1.35 ± 0.42 (n = 30) | 2.06 ± 0.03 (n = 6) | 10.36 ± 1.14 (n = 30) | 9.57 ± 1.01 (n = 6) |
| 4. | ChD, 1% gel implantation ChAsc | 3.96 ± 0.15 (n = 6) | 2.55 ± 0.85 (n = 6) | 13.35 ± 1.77 (n = 6) | |||
| 5. | ChD, 1% gel implantation chitosan hydrochloride, Mw 100 kDa, DD 87% | 2.37 ± 0.11 * (n = 6) | 4.12 ± 0.10 * (n = 6) | 9.02 ± 0.27 (n = 6) | |||
| 6. | ChD, 1% gel implantation carrageenan, BCh RAS (Pacific Institute of Bioorganic Chemistry, Vladivostok) | 3.9 ± 0.17 (n = 6) | 1.46 ± 0.18 (n = 6) | 13.25 ± 1.76 (n = 6) | |||
| 7. | ChD, 1% gel implantation, polyethylene glycol | 3.80 ± 0.22 (n = 6) | 1.11 ± 0.26 (n = 6) | 12.75 ± 2.05 (n = 6) | |||
| №. | Animal Groups | n | The Levels of Lipid Fractions in the Blood Plasma of Rabbits (mmol/L, M ± m) | |||||
|---|---|---|---|---|---|---|---|---|
| TG mmol/L | Ch mmol/L | LHL mmol/L | LDL mmol/L | vLDL mmol/L | AC | |||
| 1. | ChD, 80 days | 18 | 4.16 ± 0.91 | 22.95 ± 2.13 | 2.93 ± 0.76 | 18.14 ± 1.55 | 1.89 ± 0.41 | 10.95 ± 1.81 |
| 2. | ChDf, 80 days | 18 | 1.68 ± 0.35 | 0.77 ± 0.13 | 0.27 ± 0.05 | 0.33 ± 0.18 | 0.76 ± 0.16 | 1.18 ± 0.72 |
| 3. | ChD, 100 days, 1% gel implantation 20 days, SEC | 6 | 5.91 ± 1.03 | 30.06 ± 4.35 | 3.00 ± 0.74 | 24.36 ± 3.51 | 2.68 ± 0.74 | 10.51 ± 2.49 |
| 4. | ChD, 110 days, 1% gel implantation 30 days, SEC | 6 | 3.69 ± 0.85 | 33.52 ± 1.7 | 0.69 ± 0.09 | 31.16 ± 1.83 | 1.68 ± 0.39 | 50.80 ± 9.59 |
| 5. | ChD, 100 days, 1% gel implantation 20 days, ChAsc | 6 | 7.72 ± 1.75 | 34.56 ± 3.05 | 3.13 ± 0.88 | 27.94 ± 2.98 | 3.15 ± 0.79 | 14.61 ± 4.80 |
| 6. | ChD, 110 days, 1% gel implantation 30 days, ChAsc | 6 | 3.36 ± 0.74 | 35.78 ± 1.63 | 0.49 ± 0.09 | 23.76 ± 1.68 | 1.53 ± 0.34 | 54.60 ± 9.43 |
| 7. | ChDf, 100 days, 1% gel implantation 20 days, SEC | 6 | 0.69 ± 0.14 | 0.77 ± 0.14 | 0.37 ± 0.02 | 0.25 | 0.31 ± 0.06 | 0.6 |
| 8. | ChDf, 110 days, 1% gel implantation 30 days, SEC | 6 | 1.35 ± 0.17 | 0.65 ± 0.26 | 0.39 ± 0.07 | - | 0.61 ± 0.08 | - |
| 9. | ChDf, 100 days, 1% gel implantation 20 days, ChAsc | 6 | 0.99 ± 0.32 | 0.70 ± 0.24 | 0.38 ± 0.16 | - | 0.45 ± 0.14 | - |
| 10. | ChDf, 110 days, 1% gel implantation 30 days, ChAsc | 6 | 1.43 ± 0.16 | 0.06 ± 0.03 | 0.16 ± 0.06 | - | 0.65 ± 0.07 | - |
| Animal Group | Limb Shin | Diet | n | Vvwa (%) | Vvla (%) | Vvma (%) | Para-Adventitial Space Vessels | Subintimal Myocyte Ratio |
|---|---|---|---|---|---|---|---|---|
| 1% gel of SEC | Right rear | ChD | 6 | 82.04 ± 8.10 # | 16.47 ± 2.48 + | 76.82 ± 4.71 + | 18.42 ± 2.99 | 85.56 ± 9.34 # |
| ChDf | 6 | 52.46 ± 4.73 | 25.69 ± 3.25 | 53.18 ± 3.46 | 15.89 ± 5.83 | 50.39 ± 6.32 | ||
| Left rear | ChD | 6 | 60.12 ± 3.58 * | 30.87 ± 3.4 * | 60.13 ± 3.22 * | 28.75 ± 3.11 * # | 62.76 ± 4.71 * | |
| ChDf | 6 | 46.57 ± 4.42 | 30.52 ± 3.89 | 52.41 ± 3.84 | 31.22 ± 3.43 * # | 50.89 ± 5.38 | ||
| 1% gel of ChAsc | Right rear | ChD | 6 | 82.86 ± 6.09 + | 16.47 ± 2.48 + | 76.82 ± 4.71 + | 18.42 ± 2.99 | 85.56 ± 9.34 + |
| ChDf | 6 | 52.46 ± 4.73 | 25.69 ± 3.25 | 53.18 ± 3.46 | 15.89 ± 5.83 | 50.39 ± 6.32 | ||
| Left rear | ChD | 6 | 70.29 ± 8.86 + | 15.81 ± 9.10 + | 69.65 ± 6.13 | 30.67 ± 5.89 * + | 74.22 ± 8.22 + | |
| ChDf | 6 | 49.21 ± 3.29 | 28.52 ± 4.23 | 49.87 ± 4.28 | 29.48 ± 4.46 * + | 53.98 ± 3.47 | ||
| Intact animals | Right and left rear | ChDf | 12 | 50.17 ± 5.80 | 27.69 ± 4.65 | 50.86 ± 5.68 | 17.38 ± 6.28 | 52.09 ± 7.04 |
| Animal Group | Limb Thigh | Diet | n | Vvwa (%) | Vvla (%) | Vvma (%) | Para-Adventitial Space Vessels | Subintimal Myocyte Ratio |
|---|---|---|---|---|---|---|---|---|
| 1% gel of SEC | Right rear | ChD | 6 | 75.08 ± 4.03 + | 13.07 ± 4.62 + | 72.27 ± 2.14 + | 14.06 ± 3.08 | 82.71 ± 5.69 + |
| ChDf | 50.17 ± 2.20 | 26.64 ± 2.31 | 58.46 ± 4.11 | 16.67 ± 4.16 | 54.24 ± 6.08 | |||
| Left rear | ChD | 51.75 ± 3.75 * | 24.98 ± 4.04 * | 64.13 ± 3.22 * | 18.0 ± 3.85 | 64.01 ± 6.41 * | ||
| ChDf | 54.32 ± 5.21 | 25.57 ± 5.32 | 48.64 ± 2.79 | 18.2 ± 2.87 | 54.79 ± 3.94 | |||
| 1% gel of ChAsc | Right rear | ChD | 6 | 75.08 ± 4.03 + | 13.07 ± 4.62 + | 72.27 ± 2.14 + | 14.06 ± 3.08 | 82.71 ± 5.69 + |
| ChDf | 50.17 ± 2.20 | 26.64 ± 2.31 | 58.46 ± 4.11 | 16.67 ± 4.16 | 54.24 ± 6.08 | |||
| Left rear | ChD | 75.18 ± 4.82 | 12.65 ± 1.54 | 76.39 ± 3.85 + | 12.17 ± 5.98 | 85.85 ± 9.83 + | ||
| ChDf | 53.28 ± 4.33 | 24.32 ± 5.12 | 57.45 ± 5.34 | 15.3 ± 2.87 | 61.32 ± 3.54 | |||
| Intact animals | Right and left rear | ChDf | 12 | 56.64 ± 3.49 | 29.83 ± 3.62 | 56.32 ± 2.39 | 15.29 ± 3.78 | 60.62 ± 6.80 |
| Plasma Parameters | Sorption Time at 22 °C | ||||||
|---|---|---|---|---|---|---|---|
| 0 min | 15 min | 30 min | 1 h | 2 h | 3 h | 4 h | |
| Total protein, g/L | 6.045 | 6.60 | 7.00 | 6.00 | 6.40 | 6.80 | 6.70 |
| Albumin, g/L | 3.08 | 2.50 | 2.50 | 2.50 | 2.40 | 2.50 | 2.60 |
| Total cholesterol, mmol/L | 0.49 | 0.46 | 0.46 | 0.45 | 0.46 | 0.47 | 0.45 |
| TG, mmol/L | 0.109 | 0.11 | 0.12 | 0.11 | 0.12 | 0.12 | 0.12 |
| Ch-LHL, mmol/L | 0.12 | 0.13 | 0.14 | 0.14 | 0.13 | 0.14 | 0.15 |
| Ch-vLDL, mmol/L | 0.052 | 0.05 | 0.06 | 0.05 | 0.06 | 0.06 | 0.06 |
| Ch-LDL, mmol/L | 0.32 | 0.28 | 0.26 | 0.26 | 0.27 | 0.27 | 0.25 |
| Atherogenic coefficient | 3.17 | 2.54 | 2.29 | 2.21 | 2.54 | 2.36 | 2.07 |
| Biopolymer | Before Biopolymer Implantation (80th Day of the Experiment) PU | 20th Day after Biopolymer Implantation (100th Day of the Experiment) PU | 30th Day after Biopolymer Implantation (110th Day of the Experiment) PU | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Limb | Diet | Point 1 (n = 12) | Point 2 (n = 12) | Point 3 (n = 12) | Point 1 (n = 6) | Point 2 (n = 6) | Point 3 (n = 6) | Point 1 (n = 6) | Point 2 (n = 6) | Point 3 (n = 6) | |
| 1% gel implantation SEC | Left rear | ChD | 2.79 ± 0.33 * | 3.43 ± 0.35 * | 3.22 ± 0.11 * | 2.48 ± 0.21 | 6.16 ± 0.51 # | 3.04 ± 0.31 | 2.11 ± 0.16 | 4.36 ± 0.25 # | 2.71 ± 0.12 |
| ChDf | 5.32 ± 0.56 | 7.88 ± 0.78 | 3.76 ± 0.18 | 5.59 ± 0.87 | 9.70 ± 0.27 # | 3.60 ± 0.63 | 5.39 ± 0.67 | 9.87 ± 0.45 # | 3.81 ± 0.35 | ||
| Right rear | ChD | 2.79 ± 0.33 * | 3.43 ± 0.35 * | 3.22 ± 0.11 * | 2.01 ± 0.15 | 3.31 ± 0.53 | 2.79 ± 0.16 | 1.74 ± 0.29 | 3.25 ± 0.29 | 2.58 ± 0.27 | |
| ChDf | 5.32 ± 0.56 | 7.88 ± 0.78 | 3.76 ± 0.18 | 5.69 ± 0.26 | 7.67 ± 0.34 | 3.24 ± 0.23 | 4.90 ± 0.41 | 7.52 ± 0.61 | 3.17 ± 0.14 | ||
| 1% gel ChAsc | Left rear | ChD | 2.79 ± 0.33 * | 3.43 ± 0.35 * | 3.22 ± 0.11 * | 2.51 ± 0.66 | 5.64 ± 0.76 | 2.98 ± 0.59 | 2.15 ± 0.33 | 4.41 ± 0.59 | 2.60 ± 0.40 |
| ChDf | 5.32 ± 0.56 | 7.88 ± 0.78 | 3.76 ± 0.18 | 3.97 ± 0.35 | 9.89 ± 0.54 | 4.25 ± 0.94 | 3.97 ± 0.46 | 9.77 ± 0.19 | 3.45 ± 0.30 | ||
| Right rear | ChD | 2.79 ± 0.33 * | 3.43 ± 0.35 * | 3.22 ± 0.11 * | 2.75 ± 0.30 | 3.36 ± 0.63 | 3.01 ± 0.13 | 2.46 ± 0.71 | 3.24 ± 0.60 | 2.77 ± 0.57 | |
| ChDf | 5.32 ± 0.56 | 7.88 ± 0.78 | 3.76 ± 0.18 | 4.63 ± 0.25 | 6.99 ± 0.76 | 3.30 ± 0.27 | 4.12 ± 0.38 | 7.08 ± 0.58 | 3.00 ± 0.44 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolshakov, I.N.; Gornostaev, L.M.; Fominykh, O.I.; Svetlakov, A.V. Synthesis, Chemical and Biomedical Aspects of the Use of Sulfated Chitosan. Polymers 2022, 14, 3431. https://doi.org/10.3390/polym14163431
Bolshakov IN, Gornostaev LM, Fominykh OI, Svetlakov AV. Synthesis, Chemical and Biomedical Aspects of the Use of Sulfated Chitosan. Polymers. 2022; 14(16):3431. https://doi.org/10.3390/polym14163431
Chicago/Turabian StyleBolshakov, I. N., L. M. Gornostaev, O. I. Fominykh, and A. V. Svetlakov. 2022. "Synthesis, Chemical and Biomedical Aspects of the Use of Sulfated Chitosan" Polymers 14, no. 16: 3431. https://doi.org/10.3390/polym14163431
APA StyleBolshakov, I. N., Gornostaev, L. M., Fominykh, O. I., & Svetlakov, A. V. (2022). Synthesis, Chemical and Biomedical Aspects of the Use of Sulfated Chitosan. Polymers, 14(16), 3431. https://doi.org/10.3390/polym14163431









