Polymorphic Phases of Supramolecular Liquid Crystal Complexes Laterally Substituted with Chlorine
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Supramolecular Complexes
2.2. Characterizations
3. Results and Discussion
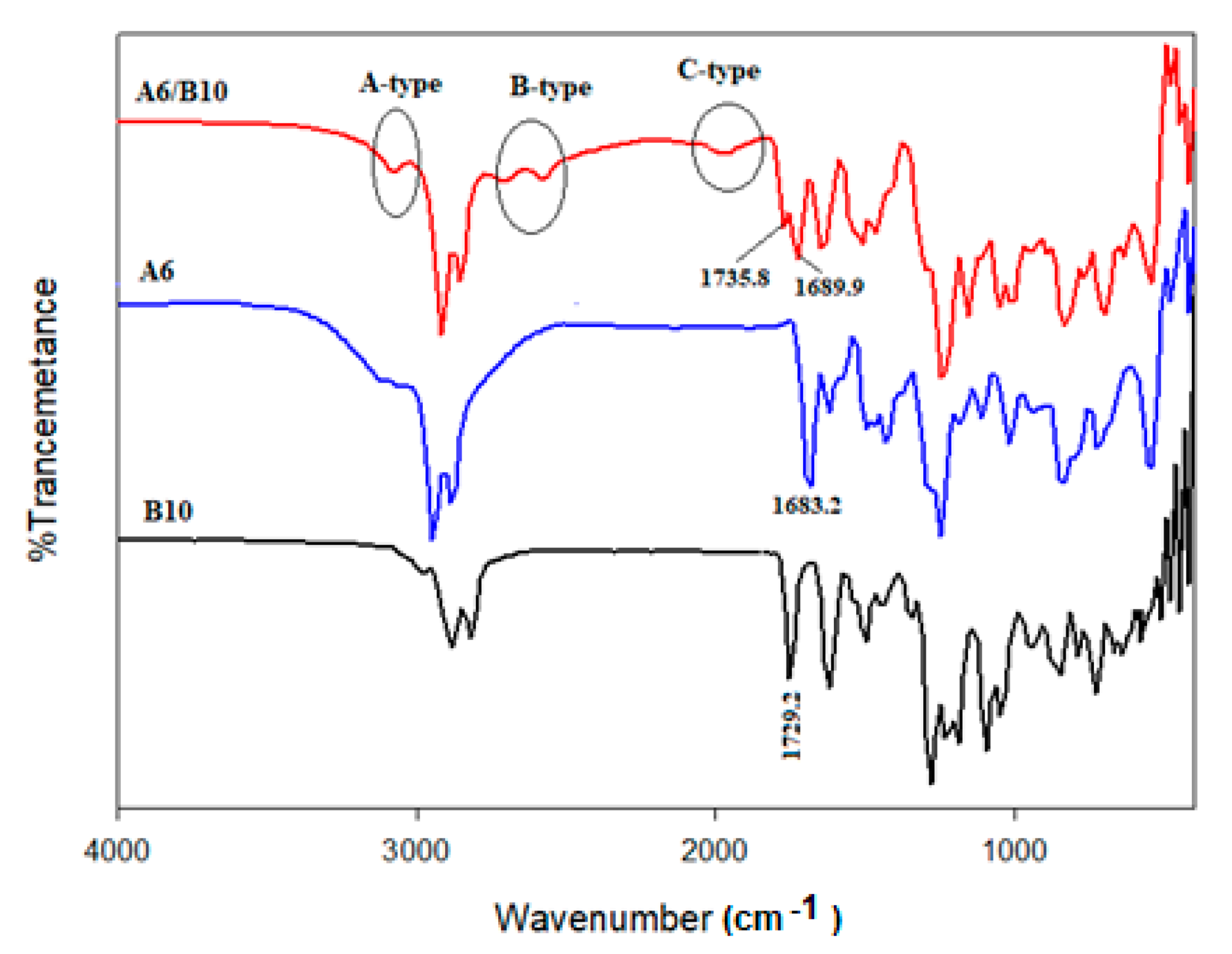
3.1. Spectroscopic Confirmation of SMHBCs Interactions
3.2. Mesomorphic Study
3.3. Effect of Position and Role of Lateral Polar Group on the SMHBCs Stability
3.4. Entropy Change of SMHB Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shen, P.; Zhang, X.; Lu, H.; Su, Z.; Zhou, Y.; Song, B.; Li, X.; Yang, X.; Tu, Y.; Li, C.Y. Effect of Fullerene Volume Fraction on Two-Dimensional Crystal-Constructed Supramolecular Liquid Crystals. Chem. Asian J. 2019, 14, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; DeChant, M.; Gerbig, L.; Baumann, M. Supramolecular click procedures in liquid crystals. Liq. Cryst. 2019, 46, 1985–1994. [Google Scholar] [CrossRef]
- Saccone, M.; Pfletscher, M.; Kather, S.; Wölper, C.; Daniliuc, C.; Mezger, M.; Giese, M. Improving the mesomorphic behaviour of supramolecular liquid crystals by resonance-assisted hydrogen bonding. J. Mater. Chem. C 2019, 7, 8643–8648. [Google Scholar] [CrossRef]
- Sharma, V.S.; Shah, A.P.; Sharma, A.S. A new class of supramolecular liquid crystals derived from azo calix [4] arene functionalized 1,3,4-thiadiazole derivatives. New J. Chem. 2019, 43, 3556–3564. [Google Scholar] [CrossRef]
- Wang, X.; Bai, L.; Kong, S.; Song, Y.; Meng, F. Star-shaped supramolecular ionic liquid crystals based on pyridinium salts. Liq. Cryst. 2019, 46, 512–522. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Fréchet, J.M.J. Supramolecular Liquid-Crystalline Networks Built by Self-Assembly of Multifunctional Hydrogen-Bonding Molecules. Chem. Mater. 1996, 8, 961–968. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Fréchet, J.M.J. Induction of a cholesteric phase via self-assembly in supramolecular networks built of non-mesomorphic molecular components. Liq. Cryst. 1998, 24, 413–418. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4″-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Gowda, A.; Jacob, L.; Joy, N.; Philip, R.; Pratibha, R.; Kumar, S. Thermal and nonlinear optical studies of newly synthesized EDOT based bent-core and hockey-stick like liquid crystals. New J. Chem. 2018, 42, 2047–2057. [Google Scholar] [CrossRef]
- Zaki, A.A. Optical measurements of phase transitions in difluorophenylazophenyl benzoate thermotropic liquid crystal with specific orientated fluorine atoms. Phase Transit. 2018, 92, 135–148. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff base/ester liquid crystals with different lateral substituents: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Eltawab, M.M.A.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behaviour of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Ahmed, H.; Saad, G. Mesophase behaviour of laterally di-fluoro-substituted four-ring compounds. Liq. Cryst. 2015, 42, 1765–1772. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Ahmed, H.A. Effect of the relative orientation of the two fluoro-substituents on the mesophase behavior of phenylazophenyl benzoates. Mol. Cryst. Liq. Cryst. 2012, 562, 43–65. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Alhaddad, O.A.; Ahmed, H.A. Experimental and geometrical structure characterizations of new synthesized laterally fluorinated nematogenic system. Liq. Cryst. 2021, 1–11. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; El-Atawy, M.A.; Al-Zahrani, S.A.; Omar, A.Z. Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives. Molecules 2021, 26, 4546. [Google Scholar] [CrossRef]
- Naoum, M.M.; Mohammady, S.Z.; Ahmed, H. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-)methyl phenyl-4′-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, H. Different morphologies of self-assembled nanofibers fabricated with amphiphilic low-molecular-weight azopyridinium salts. RSC Adv. 2013, 3, 22155–22159. [Google Scholar] [CrossRef]
- Zhou, W.; Kobayashi, T.; Zhu, H.; Yu, H. Electrically conductive hybrid nanofibers constructed with two amphiphilic salt components. Chem. Commun. 2011, 47, 12768–12770. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, R.; Jackson, J.K.; Chiao, M.; Yu, H. Janus ultrathin film from multi-level self-assembly at air–water interfaces. Chem. Commun. 2014, 50, 14843–14846. [Google Scholar] [CrossRef]
- Mamiya, J.-I.; Yoshitake, A.; Kondo, M.; Yu, Y.; Ikeda, T. Is chemical crosslinking necessary for the photoinduced bending of polymer films? J. Mater. Chem. 2008, 18, 63–65. [Google Scholar] [CrossRef]
- Aoki, K.; Nakagawa, M.; Ichimura, K. Self-assembly of amphoteric azopyridine carboxylic acids: Organized structures and macroscopic organized morphology influenced by heat, pH change, and light. J. Am. Chem. Soc. 2000, 122, 10997–11004. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2016, 43, 222–234. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Refaie, A.A.; Alaasar, M.A. Novel hydrogen-bonded angular supramolecular liquid crystals. Liq. Cryst. 2012, 39, 47–61. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular Hydrogen-Bonded Liquid Crystals Formed from 4-(4′-Pyridylazophenyl)-4″-Substituted Benzoates and 4-Alkoxybenzoic Acids. Mol. Cryst. Liq. Cryst. 2008, 482, 57–70. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A. Supramolecular hydrogen-bonded liquid crystals formed from 4-(4′-pyridylazophenyl)-4″-alkoxy benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2008, 487, 74–91. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A. Supramolecular liquid crystals induced by hydrogen-bonding interactions between non-mesomorphic compounds. I. 4-(4′-Pyridylazophenyl)-4″-substituted benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2009, 506, 22–33. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.G.A.; Almllal, W.A. Supramolecular Liquid Crystals Induced by Hydrogen-Bonding Interactions between Non-Mesomorphic Compounds. II. Effect of Lateral Substitution. Mol. Cryst. Liq. Cryst. 2010, 518, 109–128. [Google Scholar] [CrossRef]
- Chen, K.Y. Crystal Structure, Hydrogen-Bonding Properties, and DFT Studies of 2-((2-(2-Hydroxyphenyl) benzo [d] thiazol-6-yl) methylene) malononitrile. Mol. Cryst. Liq. Cryst. 2015, 623, 285–296. [Google Scholar] [CrossRef]
- Shoji, M.; Tanaka, F. Theoretical study of hydrogen-bonded supramolecular liquid crystals. Macromolecules 2002, 35, 7460–7472. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Alamro, F.S.; Gomha, S.M.; Shaban, M.; Altowyan, A.S.; Abolibda, T.Z.; Ahmed, H.A. Optical investigations and photoactive solar energy applications of new synthesized Schiff base liquid crystal derivatives. Sci. Rep. 2021, 11, 6233. [Google Scholar]
- Gomha, S.M.; Ahmed, H.A.; Shaban, M.; Abolibda, T.Z.; Alharbi, K.A.; Alalawy, H.H. New nematogenic conical-shaped supramolecular H-bonded complexes for solar energy investigations. Sci. Rep. 2021, 11, 17622. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.; Yeap, G.-Y. Liquid crystal oligomers: Going beyond dimers. Liq. Cryst. 2009, 36, 755–777. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Popoola, S.A.; Aboelnaga, A. Synthesis, Phase Behavior and Computational Simulations of a Pyridyl-Based Liquid Crystal System. Molecules 2021, 26, 6416. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Naoum, M.M.; Al-Zahrani, S.A.; Ahmed, H.A. New Nitro-Laterally Substituted Azomethine Derivatives; Synthesis, Mesomorphic and Computational Characterizations. Molecules 2021, 26, 1927. [Google Scholar] [CrossRef] [PubMed]
- Gulbas, H.; Coskun, D.; Gursel, Y.; Bilgin-Eran, B. Synthesis, characterization and mesomorphic properties of side chain liquid crystalline oligomer having schiff base type mesogenic group. Adv. Mater. 2014, 5, 333–338. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Ahmed, H.A.; Hagar, M.; Abu Al-Ola, K.A.; Alrefay, B.S.; Haddad, B.A.; AlBalawi, R.F.; Aljuhani, R.H.; Aloqebi, L.D.; AlSenani, S.F. Induced phases of new H-bonded supramolecular liquid crystal complexes; mesomorphic and geometrical estimation. Molecules 2020, 25, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvarasu, C.; Kannan, P. Effect of azo and ester linkages on rod shaped Schiff base liquid crystals and their photophysical investigations. J. Mol. Struct. 2016, 1125, 234–240. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.; Alshabanah, L.; Ahmed, H.; Alalawy, H.; Al Alwani, M. Synthesis, Mesomorphic and Computational Characterizations of Nematogenic Schiff Base Derivatives in Pure and Mixed State. Molecules 2021, 26, 2038. [Google Scholar] [CrossRef] [PubMed]
- Alamro, F.S.; Ahmed, H.A.; Mostafa, A.M.; Naoum, M.M. Thermal and Mesomorphic Investigations of 1:1 Supramolecular Assemblies of 4-[(4-(n-Alkoxy)phenylimino)methyl]benzoic Acids Having Symmetrical and Un-Symmetrical Terminal Chain Lengths. Symmetry 2021, 13, 1785. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Ahmed, H.A.; Hagar, M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules 2020, 25, 365. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Ahmed, H.A.; Alhaddad, O.A. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. RSC Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.D.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Felipe, A.; Imrie, C. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Ghanem, A.; Noël, C. FTIR Investigation of Two Alkyl-p-Terphenyl-4,4″-Dicarboxylates in Their Crystalline, Smectic and Isotropic Phases. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1987, 150, 447–472. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; Marcelis, A.T.M.; Storey, J.M.D.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.P.; Martinez-Felipe, A.; Paterson, D.A.; Forsyth, E.; Lawrence, G.B.; Henderson, P.A.; Storey, J.M.D.; Gorecka, E.; et al. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Naoum, M.M.; Mostafa, A.M.; Alserehi, A.A. Induced Smectic Phases from Supramolecular H-Bonded Complexes Based on Non-Mesomorphic Components. Crystals 2021, 11, 940. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.; Alshabanah, L.A.; Ahmed, H.; Abu Al-Ola, K.; Hagar, M. New Rod-like H-bonded Assembly Systems: Mesomorphic and Geometrical Aspects. Crystals 2020, 10, 795. [Google Scholar] [CrossRef]
- Thaker, B.T.; Kanojiya, J.B.; Tandel, R.S. Effects of Different Terminal Substituents on the Mesomorphic Behavior of Some Azo-Schiff Base and Azo-Ester-Based Liquid Crystals. Mol. Cryst. Liq. Cryst. 2010, 528, 120–137. [Google Scholar] [CrossRef]
- Cleland, W.W.; Kreevoy, M.M. Low-Barrier Hydrogen Bonds and Enzymic Catalysis. Sci. 1994, 264, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.T. Laterally substituted dimeric liquid crystals. Liq. Cryst. 1989, 6, 391–396. [Google Scholar] [CrossRef]
- Schroeder, J.; Bristol, D. Liquid crystals. IV. Effects of terminal substituents on the nematic mesomorphism of p-phenylene dibenzoates. J. Org. Chem. 1973, 38, 3160–3164. [Google Scholar] [CrossRef]









| System | TCr-SmC | TSmC-SmA | TSmA-N | TN-I | ∆T | ∆S/RC-A | ∆S/RA-N | ∆S/RN-I |
|---|---|---|---|---|---|---|---|---|
| A6/B6 | 84.5 (35.30) | 128.3 (2.93) | 155.9 (1.38) | 216.1 (1.16) | 131.6 | 0.88 | 0.39 | 0.29 |
| A6/B8 | 68.9 (29.83) | 129.1 (2.29) | 156.3 (1.83) | 189.8 (1.54) | 120.9 | 0.68 | 0.51 | 0.40 |
| A6/B10 | 70.3 (52.93) | 129.3 (2.61) | 157.0 (1.65) | 187.4 (1.42) | 117.1 | 0.78 | 0.46 | 0.37 |
| A6/B12 | 75.1 (49.52) | 129.5 (2.52) | 157.3 (1.27) | 187.0 (1.09) | 111.9 | 0.75 | 0.35 | 0.28 |
| A6/B16 | 83.7 (40.06) | 130.1 (2.29) | 157.9 (1.63) | 186.9 (1.58) | 103.2 | 0.68 | 0.45 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, F.S.; Alhaddad, O.A.; Naoum, M.M.; Ahmed, H.A. Polymorphic Phases of Supramolecular Liquid Crystal Complexes Laterally Substituted with Chlorine. Polymers 2021, 13, 4292. https://doi.org/10.3390/polym13244292
Alamro FS, Alhaddad OA, Naoum MM, Ahmed HA. Polymorphic Phases of Supramolecular Liquid Crystal Complexes Laterally Substituted with Chlorine. Polymers. 2021; 13(24):4292. https://doi.org/10.3390/polym13244292
Chicago/Turabian StyleAlamro, Fowzia S., Omaima A. Alhaddad, Magdi M. Naoum, and Hoda A. Ahmed. 2021. "Polymorphic Phases of Supramolecular Liquid Crystal Complexes Laterally Substituted with Chlorine" Polymers 13, no. 24: 4292. https://doi.org/10.3390/polym13244292
APA StyleAlamro, F. S., Alhaddad, O. A., Naoum, M. M., & Ahmed, H. A. (2021). Polymorphic Phases of Supramolecular Liquid Crystal Complexes Laterally Substituted with Chlorine. Polymers, 13(24), 4292. https://doi.org/10.3390/polym13244292







