Effect of the Application of a Coating Native Potato Starch/Nopal Mucilage/Pectin on Physicochemical and Physiological Properties during Storage of Fuerte and Hass Avocado (Persea americana)
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Material
2.2. Preparation of the Emulsion
2.3. Determination of Transparency and Water Activity of Coatings
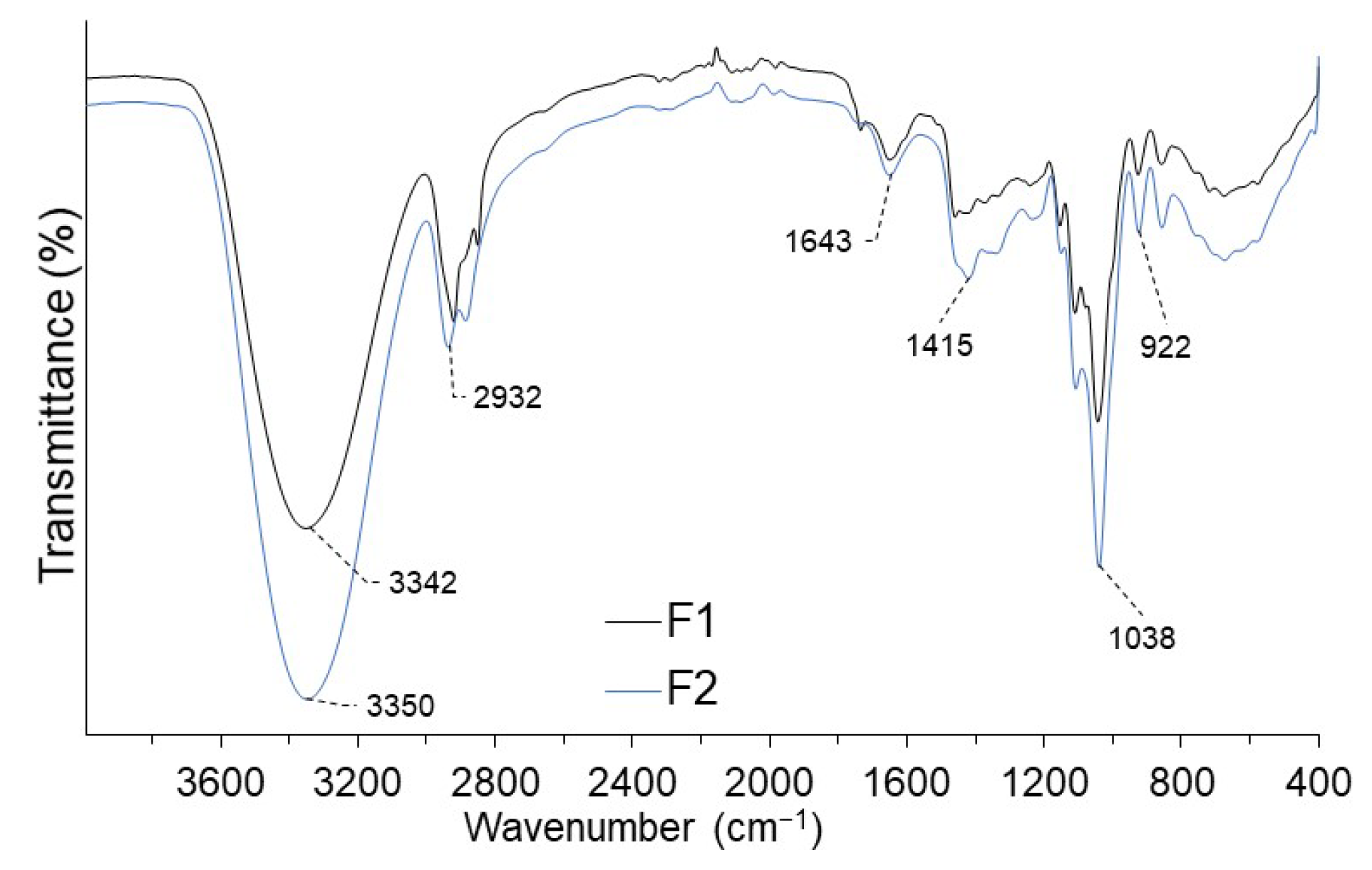
2.4. IR Analysis of the Coating
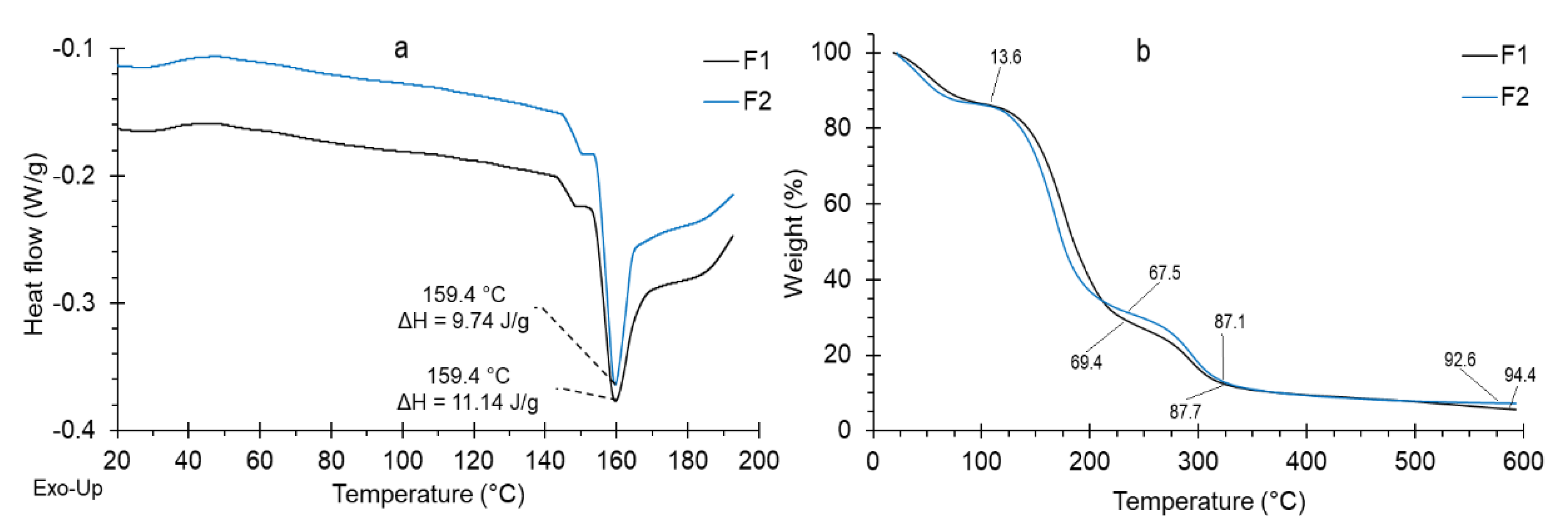
2.5. Thermal Analysis of the Coating
2.6. Coating Application
2.7. Determination of Physicochemical Properties
2.8. Determination of Physiological Properties
- If CI* −40 to −20, colors range from blue-violet to deep green.
- If CI* −20 to −2, colors range from deep green to yellowish-green.
- If CI* −2 to +2, represents greenish-yellow.
- If CI* +2 to +20, colors range from pale yellow to deep orange.
- If CI* +20 to +40, colors range from deep orange to deep red.
2.9. Statistical Analysis
3. Results and Discussion
3.1. Coating Characteristics
3.2. Physicochemical Properties
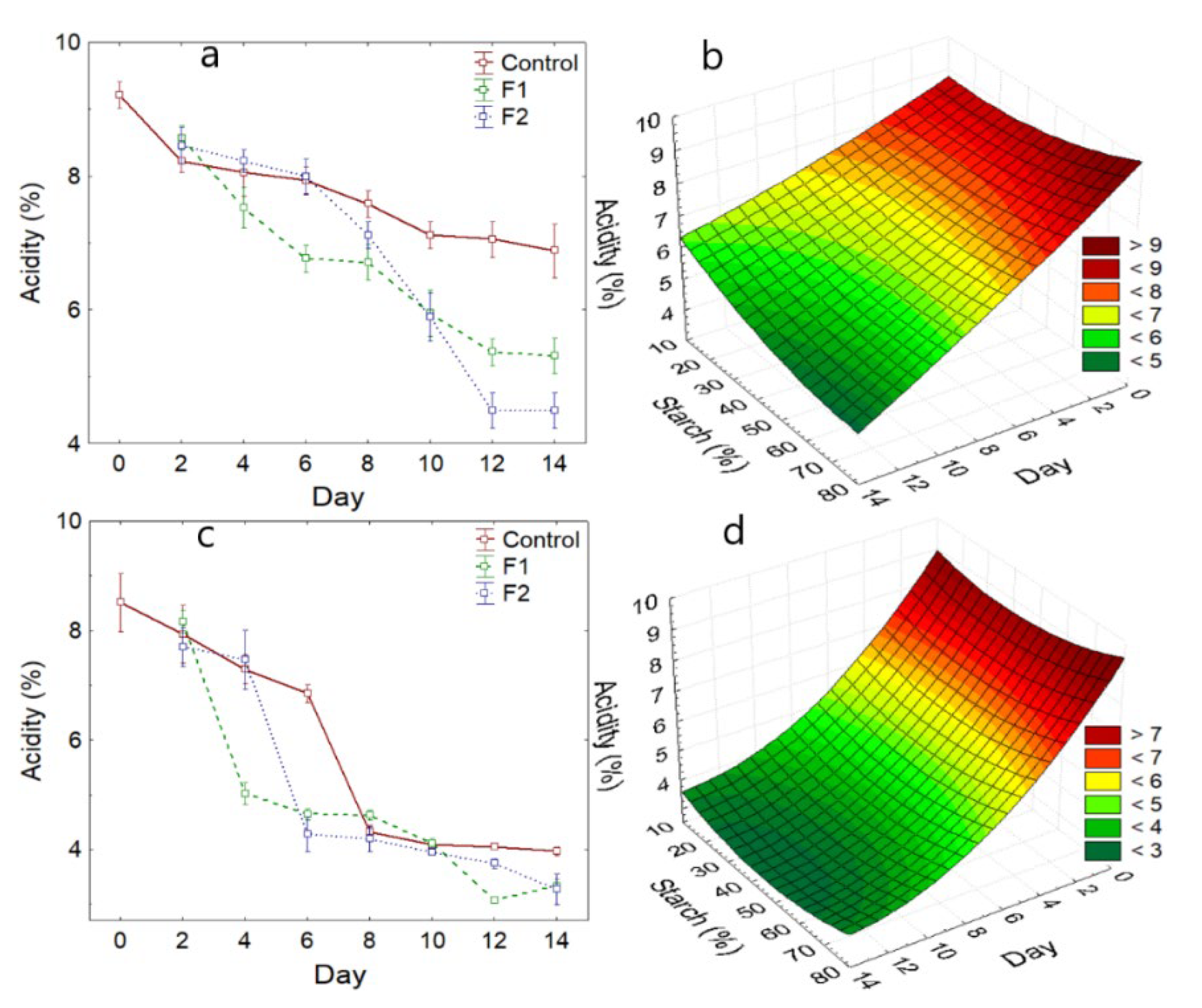
3.2.1. Acidity
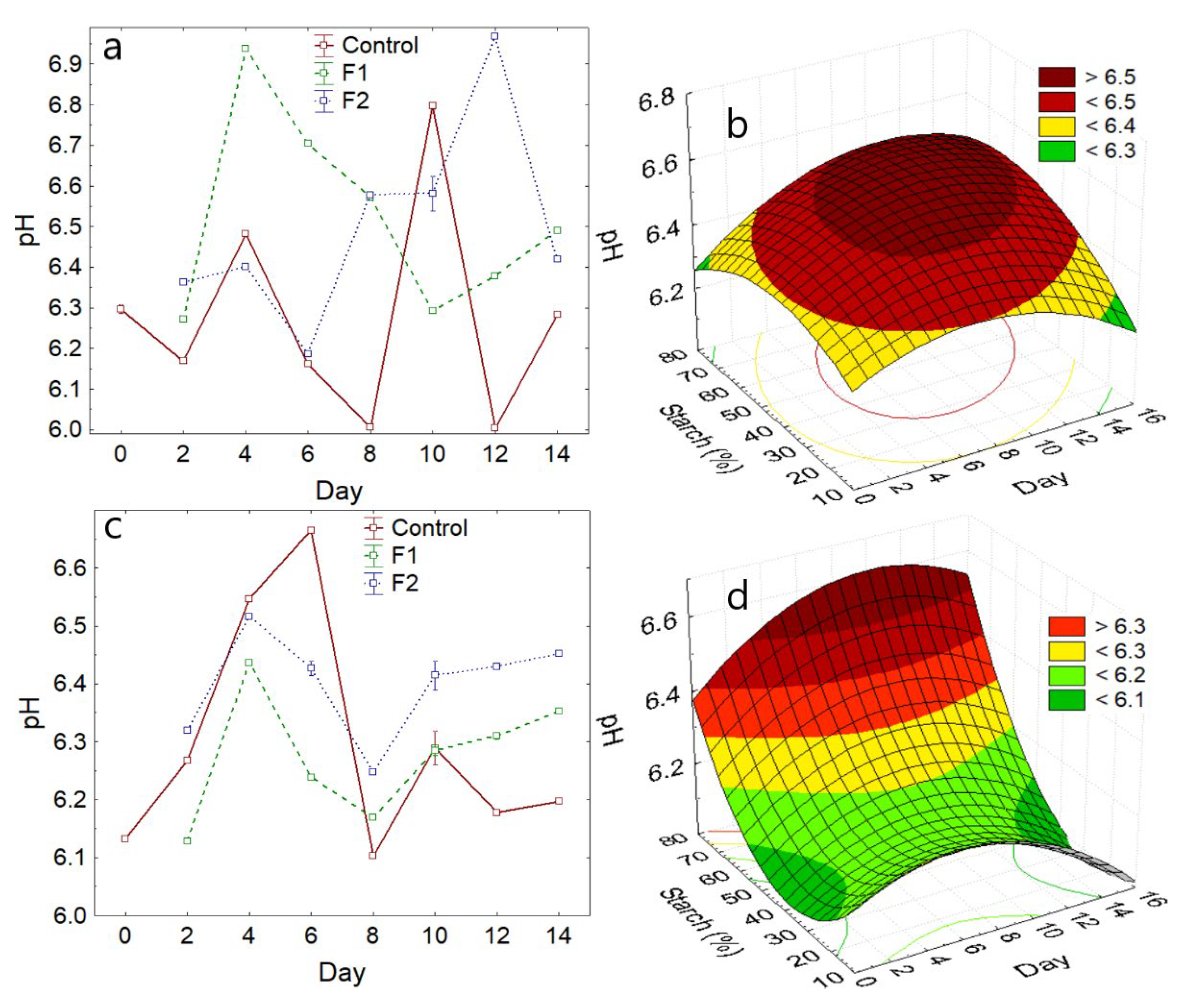
3.2.2. pH
3.2.3. Soluble Solids
3.3. Evaluation of Physiological Properties
3.3.1. Avocado Weight Loss
3.3.2. Avocado Color
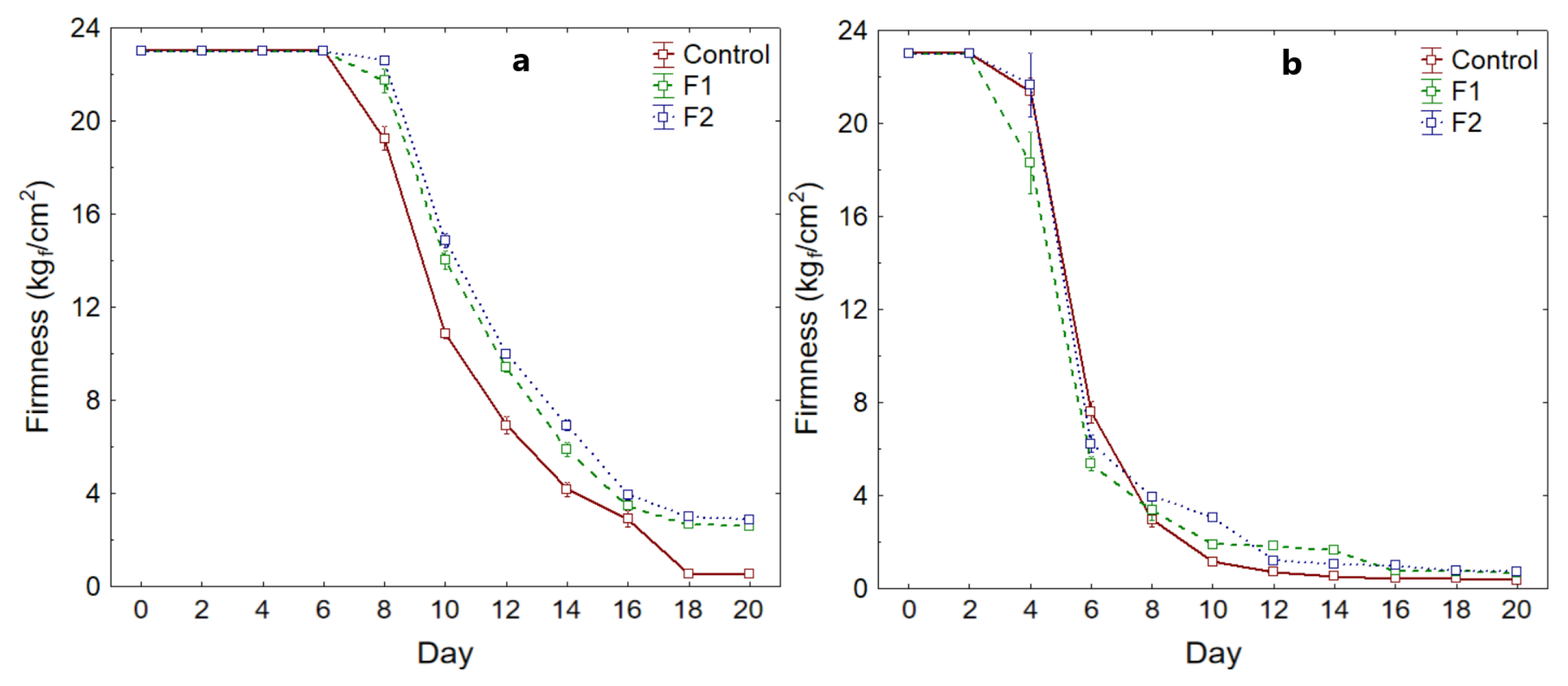
3.3.3. Fruit Firmness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benítez, J.; Sánchez, A.; Bolaños, C.; Bernal, L.; Ochoa-Martínez, C.; Vélez, C.; Sandoval, A. Physicochemical changes of avocado Hass during cold storage and accelerated ripening. Biotecnol. Sect. Agropecu. Agroind. 2021, 19, 41–56. [Google Scholar] [CrossRef]
- Park, Y.S.; Jung, S.T.; Gorinstein, S. Ethylene treatment of ‘Hayward’ kiwifruits (Actinidia deliciosa) during ripening and its influence on ethylene biosynthesis and antioxidant activity. Sci. Hortic. 2006, 108, 22–28. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Yahia, E.M.; González-León, A.; Ifie, I.; Robles-Zepeda, R.E.; Domínguez-Avila, J.A.; González-Aguilar, G.A. Ripening of “Hass” avocado mesocarp alters its phytochemical profile and the in vitro cytotoxic activity of its methanolic extracts. South Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Escobar, J.; Rodríguez, P.; Cortés, M.; Correa, G. Influence of dry matter as a harvest index and cold storage time on cv. Hass avocado quality produced in high tropic region. Inf. Tecnol. 2019, 30, 199–210. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Ferreyra, R.; Sellés, G.; Saavedra, J.; Ortiz, J.; Zúñiga, C.; Troncoso, C.; Rivera, S.; González-Agüero, M.; Defilippi, B. Identification of pre-harvest factors that affect fatty acid profiles of avocado fruit (Persea americana Mill) cv. ‘Hass’ at harvest. S. Afr. J. Bot. 2016, 104, 15–20. [Google Scholar] [CrossRef]
- Donetti, M.; Terry, L.A. Biochemical markers defining growing area and ripening stage of imported avocado fruit cv. Hass. J. Food Compos. Anal. 2014, 34, 90–98. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Pedreschi, R.; Uarrota, V.; Fuentealba, C.; Alvaro, J.E.; Olmedo, P.; Defilippi, B.G.; Meneses, C.; Campos-Vargas, R. Primary Metabolism in Avocado Fruit. Front. Plant Sci. 2019, 10, 795. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.A.; Aguilera-Aguirre, S.; López-García, U.M.; Gutiérrez-Martínez, P.; Chacón-López, M.A. Effect of chitosan on the in vitro control of Colletotrichum sp., and its influence on post-harvest quality in Hass avocado fruits. Rev. Bio Cienc. 2019, 5, e355. [Google Scholar] [CrossRef]
- Tochihuitl-Martiñón, A.; Chávez-Franco, S.H.; Saucedo-Veloz, C.; Suarez-Espinosa, J.; Guerra-Ramírez, D. Extracts of Persea americana Mill. that delay ripeningin avocado fruits. Rev. Mex. Cienc. Agríc. 2018, 9, 1639–1650. [Google Scholar] [CrossRef]
- Arpaia, M.L.; Collin, S.; Sievert, J.; Obenland, D. ‘Hass’ avocado quality as influenced by temperature and ethylene prior to and during final ripening. Postharvest Biol. Technol. 2018, 140, 76–84. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and Characterization of Coating Based on Protein Nanofibers and Polyphenol and Application for Salted Duck Egg Yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Dhall, R. Advances in edible coatings for fresh fruits and vegetables: A Review. Crit Rev. Food Sci Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Sandoval-Contreras, T.; Calderón-Santoyo, M. Sodium alginate coatings added with Meyerozyma caribbica: Postharvest biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass). Postharvest Biol. Technol. 2020, 163, 111123. [Google Scholar] [CrossRef]
- Arpaia, M.; Requejo-Jackman, C.; Wollf, A.; Thompson, J.; Slaughter, D.; Tokar, V. Avocado Postharvest quality. In Proceedings of the California Avocado Research Symposium, Riverside, CA, USA, 4 November 2006; University of California: Riverside, CA, USA, 2006; pp. 143–155. Available online: http://www.avocadosource.com/ARAC/SUM_1999/SYMP_1999_PG_1.pdf.S. (accessed on 13 May 2022).
- Perkins, M.L.; Joyce, D.C.; Coates, L.M. Possible contribution of impact injury at harvest to anthracnose expression in ripening avocado: A review. Sci. Hortic. 2019, 246, 785–790. [Google Scholar] [CrossRef]
- Careli-Gondim, I.; Mesquita, T.C.; Boas, E.V.D.B.V.; Caliari, M.; Júnior, M.S.S. The effect of active coating and refrigerated storage on the quality of avocado cultivar, Quintal. J. Food Sci. Technol. 2020, 57, 143–151. [Google Scholar] [CrossRef]
- Landero-Valenzuela, N.; Lara-Viveros, F.M.; Andrade-Hoyos, P.; Aguilar-Pérez, L.A.; Aguado Rodríguez, G.J. Alternatives for the control of Colletotrichum spp. Rev. Mex. Cienc. Agríc. 2016, 7, 1189–1198. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydr. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef]
- Fuentealba, C.; Vidal, J.; Zulueta, C.; Ponce, E.; Uarrota, V.; Defilippi, B.G.; Pedreschi, R. Controlled Atmosphere Storage Alleviates Hass Avocado Black Spot Disorder. Horticulturae 2022, 8, 369. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Moalemiyan, M.; Kushalappa, A.C. Effect of pectin-based edible emulsion coating on changes in quality of avocado exposed to Lasiodiplodia theobromae infection. Carbohydr. Polym. 2007, 68, 341–349. [Google Scholar] [CrossRef]
- Kubheka, S.F.; Tesfay, S.Z.; Mditshwa, A.; Magwaza, L.S. Evaluating the efficacy of edible coatings incorporated with moringa leaf extract on postharvest of “maluma” avocado fruit quality and its biofungicidal effect. HortScience 2020, 55, 410–415. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Sridhar, R.; Immanuel, V.P. Development of model for mechanical properties of tapioca starch based edible films. Ind. Crops Prod. 2013, 42, 159–168. [Google Scholar] [CrossRef]
- Quintão, S.D.P.; Oshiro, A.M.; Mugnol, D. Postharvest conservation of guavira (Campomanesia adamantium Camb.) under different coating and temperatures of storage. Rev. Bras. Frutic. Jaboticabal 2012, 34, 1022–1029. [Google Scholar] [CrossRef][Green Version]
- Scalon, S.D.; Oshiro, A.M.; Dresch, D.M. Coating effect of Modified cassava starch in Hass avocado. Prod. Limpia 2015, 10, 31–37. [Google Scholar]
- Aguilar-Méndez, M.A.; Martín-Martínez, E.S.; Tomás, S.A.; Cruz-Orea, A.; Jaime-Fonseca, M.R. Gelatine–starch films: Physicochemical properties and their application in extending the post-harvest shelf life of avocado (Persea americana). J. Sci. Food Agric. 2008, 88, 185–193. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H. Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT-Food Sci. Technol. 2005, 38, 617–624. [Google Scholar] [CrossRef]
- Granada, D.; López-Lujan, L.; Ramírez-Restrepo, S.; Morales, J.; Peláez-Jaramillo, C.; Andrade, G.; Bedoya-Pérez, J.C. Bacterial extracts and bioformulates as a promising control of fruit body rot and root rot in avocado cv. Hass. J. Integr. Agric. 2020, 19, 748–758. [Google Scholar] [CrossRef]
- Olivares, D.; Alvarez, E.; Véliz, D.; García-Rojas, M.; Díaz, C.; Defilippi, B.G. Effects of 1-Methylcyclopropene and Controlled Atmosphere on Ethylene Synthesis and Quality Attributes of Avocado cvs. Edranol and Fuerte. J. Food Qual. 2020, 2020, 5075218. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S.; Mbili, N.; Mditshwa, A. Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Sci. Hortic. 2017, 226, 201–207. [Google Scholar] [CrossRef]
- Kader, A. Increasing food availability by reducing potharvest losses of fresh produce. Int. Postharvest Symp. 2004, 682, 2169–2176. [Google Scholar] [CrossRef]
- Osondu, H.A.A.; Akinola, S.A.; Shoko, T.; Pillai, S.K.; Sivakumar, D. Coating properties, resistance response, molecular mechanisms and anthracnose decay reduction in green skin avocado fruit (‘Fuerte’) coated with chitosan hydrochloride loaded with functional compounds. Postharvest Biol. Technol. 2022, 186, 111812. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Froehner, S.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Choque-Quispe, Y.; Solano-Reynoso, A.M.; Taipe-Pardo, F.; Zamalloa-Puma, L.M.; Calla-Florez, M.; et al. Preparation and Chemical and Physical Characteristics of an Edible Film Based on Native Potato Starch and Nopal Mucilage. Polymers 2021, 13, 3719. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Solís-Pacheco, J.R.; Calderón-Santoyo, M. Characterization of sodium alginate coatings with Meyerozyma caribbica and impact on quality properties of avocado fruit. LWT-Food Sci. Technol. 2021, 152, 112346. [Google Scholar] [CrossRef]
- Garcia, F.; Davidov-Pardo, G. Recent advances in the use of edible coatings for preservation of avocados: A review. J. Food Sci. 2021, 86, 6–15. [Google Scholar] [CrossRef]
- Valdés, A.; Martínez, C.; Garrigos, M.C.; Jimenez, A. Multilayer Films Based on Poly(lactic acid)/Gelatin Supplemented with Cellulose Nanocrystals and Antioxidant Extract from Almond Shell By-Product and Its Application on Hass Avocado Preservation. Polymers 2021, 13, 3615. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Ventura-Sobrevilla, J.; Martínez-Vazquez, G.; Ruelas-Chacón, X.; Rojas, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Effects of a natural bioactive coating on the quality and shelf life prolongation at different storage conditions of avocado (Persea americana Mill.) cv. Hass. Food Packag. Shelf Life 2017, 14, 102–107. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ramos-Pacheco, B.S.; Ligarda-Samanez, C.A.; Barboza-Palomino, G.I.; Kari-Ferro, A.; Taipe-Pardo, F.; Choque-Quispe, Y. Heavy metal removal by biopolymers-based formulations with native potato starch/nopal mucilage. Rev. Fac. Ing. Univ. Antioq. 2022, 103, 44–50. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 20th ed.; AOAC International: Washington, DC, USA, 2016; pp. 1–3172. [Google Scholar]
- Márquez Cardozo, C.J.; Yepes Betancur, D.P.; Sanchez Giraldo, L.; Osorio Saraz, J.A. Changes physical-chemical of avocado (Persea americana Mill. cv. “Hass”) in postharvest for two municipalities of Antioquia. Rev. Temas Agrar. 2014, 19, 32–47. [Google Scholar]
- Choque-Quispe, D.; Ramos-Pacheco, B.S.; Solano-Reynoso, A.M.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Quispe-Quispe, Y. Drying and color in punamuña leaves (Satureja boliviana). Dyna 2021, 88, 31–37. [Google Scholar] [CrossRef]
- Hadimani, L.; Mittal, N. Development of a computer vision system to estimate the colour indices of Kinnow mandarins. J. Food Sci Technol. 2019, 56, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Adekunte, A.; Tiwari, B.; Cullen, P.; Scannell, A.; O’Donnell, C. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Sängerlaub, S.; Wege, L.; Stäbler, A. Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Guldas, M.; Bayizit, A.A.; Yilsay, T.O.; Yilmaz, L. Effects of edible film coatings on shelf-life of mustafakemalpasa sweet, a cheese based dessert. J. Food Sci. Technol. 2010, 47, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Guillard, V.; Broyart, B.; Bonazzi, C.; Guilbert, S.; Gontard, N. Effect of Temperature on Moisture Barrier Efficiency of Monoglyceride Edible Films in Cereal-Based Composite Foods. Cereal Chem. 2004, 81, 767–771. [Google Scholar] [CrossRef]
- Kibar, E.A.A.; Us, F. Thermal, mechanical and water adsorption properties of corn starch–carboxymethylcellulose/methylcellulose biodegradable films. J. Food Eng. 2013, 114, 123–131. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Botía-Niño, Y.C.; Almanza-Merchán, P.; Balaguera-López, H.E. Temperature effect on the complementary madurity in banana passion fruit (Passiflora mollissima Bailey). Rev. UDCA Actual. Divulg. Científica 2008, 11, 187–196. [Google Scholar]
- Cenobio-Galindo, A.D.J.; Ocampo-López, J.; Reyes-Munguía, A.; Carrillo-Inungaray, M.L.; Cawood, M.; Medina-Pérez, G.; Fernández-Luqueño, F.; Campos-Montiel, R.G. Influence of bioactive compounds incorporated in a nanoemulsion as coating on avocado fruits (Persea americana) during postharvest storage: Antioxidant activity, physicochemical changes and structural evaluation. Antioxidants 2019, 8, 500. [Google Scholar] [CrossRef]
- Astudillo-Ordóñez, C.E.; Rodríguez, P. Physicochemical parameters of avocado Persea americana Mill. cv. Hass (Lauraceae) grown in Antioquia (Colombia) for export. Cienc. Tecnol. Agropecu. 2018, 19, 383–392. [Google Scholar] [CrossRef]
- Buelvas-Salgado, G.; Patiño-Gómez, J.; Cano-Salazar, J. Evaluation of the oil extraction from hasavocado (Persea americana Mill) by the use of an enzymatic treatment. Rev. Lasallista Investig. 2012, 9, 138–150. [Google Scholar]
- Saucedo-Pompa, S.; Rojas-Molina, R.; Aguilera-Carbó, A.F.; Saenz-Galindo, A.; de La Garza, H.; Jasso-Cantú, D.; Aguilar, C.N. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Res. Int. 2009, 42, 511–515. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Opara, O.; Mditshwa, A. A review on the role of packaging in securing food system: Adding value to food products and reducing losses and waste. Afr. J. Agric. Res. 2013, 8, 2621–2630. [Google Scholar]
- Vincent, C.; Mesa, T.; Munne-Bosch, S. Identification of a new variety of avocados (Persea americana Mill. CV. Bacon) with high vitamin E and impact of cold storage on tocochromanols composition. Antioxidants 2020, 9, 403. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Joyce, D.; Huang, X.; Xu, L.; Pang, X. Characterization of chlorophyll degradation in banana and plantain during ripening at high temperature. Food Chem. 2009, 114, 383–390. [Google Scholar] [CrossRef]
- Garcia, F.; Lin, W.J.; Mellano, V.; Davidov-Pardo, G. Effect of biopolymer coatings made of zein nanoparticles and ε-polylysine as postharvest treatments on the shelf-life of avocados (Persea americana Mill. Cv. Hass). J. Agric. Food Res. 2022, 7, 100260. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Islam, S.; Matsui, T.; Yoshida, Y. Effect of carbon dioxide enrichment on physico-chemical and enzymatic changes in tomato fruits at various stages of maturity. Sci. Hortic. 1996, 65, 137–149. [Google Scholar] [CrossRef]
- De La Vega, J.; Cañarejo, M.; Pinto, N. Avances en tecnología de atmósferas controladas y sus aplicaciones en la industria. Una revisión. Cienc. Agropecu. Ambient. 2017, 28, 75–86. [Google Scholar] [CrossRef]
- Dhalsamant, K.; Mangaraj, S.; Bal, L.M. Modified atmosphere packaging for mango and tomato: An appraisal to improve shelf life. J. Packag. Technol. Res. 2017, 1, 127–133. [Google Scholar] [CrossRef]
- Wu, B.; Quilot, B.; Génard, M.; Kervella, J.; Li, S. Changes in sugar and organic acid concentrations during fruit maturation in peaches, P. davidiana and hybrids as analyzed by principal component analysis. Sci. Hortic. 2005, 103, 429–439. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Pastrana-Castro, L.; Rua-Rodríguez, M.L.; Alvarez-Pérez, O.B.; Rodríguez-Herrera, R.; Aguilar, C.N. Experimental protocol for the recovery and evaluation of bioactive compounds of tarbush against postharvest fruit fungi. Food Chem. 2016, 198, 62–67. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf Life 2017, 11, 40–48. [Google Scholar] [CrossRef]




| Formulation | PS% (at 3%) | NM% (at 2%) | G% | PC% (at 2%) |
|---|---|---|---|---|
| F1 | 60.0 | 4.0 | 4.0 | 32.0 |
| F2 | 70.0 | 4.0 | 4.0 | 22.0 |
| Parameter | F1 | F2 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ± | SD | CV (%) | ± | SD | CV (%) | ||||
| aw | 0.422 | ± | 0.006 | 1.517 | 0.404 | ± | 0.005 | 1.151 | <0.05 |
| Transmittance (%) | 81.315 | ± | 2.617 | 3.219 | 83.187 | ± | 1.564 | 1.880 | 0.26 |
| Transparency (nm/mm) | 6.939 | ± | 0.276 | 3.971 | 7.332 | ± | 0.194 | 2.643 | 0.06 |
| Hass | Fuerte | |||||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | Control | F1 | F2 | |
| Maximum | 9.45 | 8.75 | 8.75 | 9.10 | 8.40 | 8.05 |
| Minimum | 6.65 | 5.08 | 4.20 | 3.88 | 3.03 | 3.05 |
| **** | 7.76 | 6.60 | 6.67 | 5.88 | 4.71 | 4.94 |
| DS | 0.77 | 1.14 | 1.64 | 1.89 | 1.60 | 1.76 |
| CV(%) | 9.90 | 17.33 | 24.64 | 32.09 | 33.98 | 35.61 |
| p-value * | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| p-value ** | <0.05 | <0.05 | ||||
| p-value *** | <0.05 | <0.05 | ||||
| Hass | Fuerte | |||||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | Control | F1 | F2 | |
| Maximum | 6.80 | 6.94 | 6.97 | 6.67 | 6.44 | 6.52 |
| Minimum | 6.00 | 6.27 | 6.18 | 6.10 | 6.13 | 6.24 |
| **** | 6.27 | 6.52 | 6.50 | 6.30 | 6.27 | 6.40 |
| DS | 0.25 | 0.23 | 0.23 | 0.19 | 0.10 | 0.08 |
| CV(%) | 3.98 | 3.45 | 3.57 | 3.06 | 1.59 | 1.32 |
| p-value * | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| p-value ** | <0.05 | <0.05 | ||||
| p-value *** | <0.05 | <0.05 | ||||
| Hass | Fuerte | |||||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | Control | F1 | F2 | |
| Maximum | 12.00 | 8.50 | 11.00 | 8.00 | 8.00 | 8.50 |
| Minimum | 7.00 | 7.00 | 7.00 | 2.50 | 2.75 | 2.50 |
| **** | 8.99 | 7.63 | 8.61 | 4.73 | 4.46 | 4.41 |
| DS | 1.49 | 0.43 | 1.09 | 1.63 | 1.47 | 1.63 |
| CV (%) | 16.55 | 5.58 | 12.68 | 34.56 | 32.92 | 36.89 |
| p-value * | <0.05 | 0.1 | <0.05 | <0.05 | <0.05 | <0.05 |
| p-value ** | <0.05 | <0.05 | ||||
| p-value *** | <0.05 | 0.928 | ||||
| Hass | Fuerte | |||||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | Control | F1 | F2 | |
| Maximum | 7.25 | 5.81 | 5.67 | 5.10 | 4.16 | 4.04 |
| Minimum | 6.03 | 4.85 | 4.71 | 4.56 | 3.62 | 3.55 |
| **** | 6.64 | 5.33 | 5.19 | 4.83 | 3.89 | 3.79 |
| DS | 0.66 | 0.53 | 0.53 | 0.30 | 0.30 | 0.26 |
| CV (%) | 9.98 | 9.90 | 10.12 | 6.18 | 7.65 | 6.97 |
| p-value * | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| p-value ** | 0.150 | 0.817 | ||||
| p-value *** | <0.05 | <0.05 | ||||
| Day | L* | a* | b* | CI* | ΔE * | Referential Color | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | CV | ± | SD | CV | ± | SD | CV | ± | SD | CV | * | ± | SD | CV | |||||||
| Control | ||||||||||||||||||||||
| 0 | 32.90 | ± | 0.78 | 2.37 | −17.30 | ± | 1.15 | 6.67 | 55.77 | ± | 0.21 | 0.37 | −9.42 | ± | 0.45 | 4.79 | a | |||||
| 2 | 32.73 | ± | 0.32 | 0.98 | −18.73 | ± | 0.70 | 3.75 | 57.10 | ± | 0.56 | 0.98 | −10.03 | ± | 0.54 | 5.40 | a | 2.34 | ± | 0.52 | 22.25 | |
| 4 | 24.97 | ± | 0.21 | 0.83 | −16.23 | ± | 0.15 | 0.94 | 61.83 | ± | 0.31 | 0.49 | −10.52 | ± | 0.17 | 1.62 | a,b | 10.09 | ± | 0.98 | 9.68 | |
| 6 | 22.23 | ± | 0.38 | 1.70 | −13.83 | ± | 0.25 | 1.82 | 56.97 | ± | 0.29 | 0.51 | −10.93 | ± | 0.40 | 3.65 | a,b | 11.33 | ± | 0.80 | 7.03 | |
| 8 | 21.07 | ± | 0.25 | 1.19 | −13.67 | ± | 0.51 | 3.75 | 53.87 | ± | 0.47 | 0.88 | −12.05 | ± | 0.61 | 5.06 | b,c | 12.57 | ± | 1.15 | 9.18 | |
| 10 | 19.80 | ± | 0.70 | 3.54 | −13.93 | ± | 0.25 | 1.81 | 51.07 | ± | 0.35 | 0.69 | −13.79 | ± | 0.27 | 1.95 | c,d | 14.35 | ± | 0.65 | 4.55 | |
| 12 | 17.83 | ± | 0.49 | 2.77 | −12.60 | ± | 0.44 | 3.46 | 50.67 | ± | 1.06 | 2.09 | −13.95 | ± | 0.38 | 2.71 | d | 16.67 | ± | 1.22 | 7.29 | |
| 14 | 17.63 | ± | 0.50 | 2.85 | −12.93 | ± | 0.15 | 1.18 | 51.83 | ± | 0.50 | 0.97 | −14.16 | ± | 0.60 | 4.22 | d | 16.39 | ± | 1.19 | 7.25 | |
| 16 | 15.37 | ± | 0.31 | 1.99 | −11.59 | ± | 0.50 | 4.28 | 52.07 | ± | 0.40 | 0.78 | −14.48 | ± | 0.40 | 2.76 | d | 18.81 | ± | 0.81 | 4.31 | |
| 18 | 12.93 | ± | 0.38 | 2.93 | −9.93 | ± | 0.51 | 5.17 | 53.23 | ± | 0.42 | 0.78 | −14.45 | ± | 1.25 | 8.63 | d | 21.45 | ± | 1.17 | 5.47 | |
| 20 | 10.87 | ± | 0.21 | 1.92 | −8.40 | ± | 0.36 | 4.29 | 53.37 | ± | 0.67 | 1.25 | −14.50 | ± | 0.94 | 6.51 | d | 23.90 | ± | 0.97 | 4.08 | |
| F1 | ||||||||||||||||||||||
| 0 | 36.17 | ± | 0.75 | 2.08 | −17.77 | ± | 1.38 | 7.77 | 55.00 | ± | 0.78 | 1.42 | −8.92 | ± | 0.40 | 4.44 | a | |||||
| 2 | 36.27 | ± | 0.42 | 1.15 | −17.87 | ± | 0.40 | 2.26 | 51.70 | ± | 0.40 | 0.77 | −9.53 | ± | 0.08 | 0.82 | a,b | 3.73 | ± | 0.62 | 16.71 | |
| 4 | 34.83 | ± | 0.31 | 0.88 | −17.77 | ± | 0.40 | 2.27 | 50.97 | ± | 0.32 | 0.63 | −10.01 | ± | 0.37 | 3.73 | a,b | 4.46 | ± | 1.03 | 23.16 | |
| 6 | 33.77 | ± | 0.31 | 0.90 | −17.80 | ± | 0.26 | 1.49 | 50.70 | ± | 0.36 | 0.71 | −10.40 | ± | 0.21 | 2.01 | a,b | 5.13 | ± | 0.80 | 15.52 | |
| 8 | 33.13 | ± | 0.59 | 1.77 | −15.83 | ± | 0.25 | 1.59 | 43.73 | ± | 0.45 | 1.03 | −10.93 | ± | 0.08 | 0.69 | b.c | 11.87 | ± | 1.08 | 9.09 | |
| 10 | 30.13 | ± | 0.60 | 2.00 | −14.67 | ± | 0.46 | 3.15 | 40.77 | ± | 0.46 | 1.13 | −11.95 | ± | 0.54 | 4.55 | c,d | 15.80 | ± | 1.83 | 11.59 | |
| 12 | 27.27 | ± | 0.45 | 1.65 | −13.13 | ± | 0.21 | 1.59 | 38.17 | ± | 0.40 | 1.06 | −12.62 | ± | 0.27 | 2.11 | d | 19.62 | ± | 1.06 | 5.40 | |
| 14 | 25.27 | ± | 0.57 | 2.25 | −12.00 | ± | 0.44 | 3.63 | 37.23 | ± | 0.64 | 1.73 | −12.77 | ± | 0.83 | 6.53 | d | 21.68 | ± | 0.90 | 4.13 | |
| 16 | 22.23 | ± | 0.45 | 2.03 | −10.67 | ± | 0.51 | 4.81 | 37.03 | ± | 0.67 | 1.80 | −12.96 | ± | 0.65 | 5.02 | d | 23.85 | ± | 1.63 | 6.85 | |
| 18 | 18.13 | ± | 0.55 | 3.04 | −8.90 | ± | 0.26 | 2.97 | 37.03 | ± | 0.21 | 0.56 | −13.27 | ± | 0.76 | 5.71 | d | 26.98 | ± | 1.38 | 5.11 | |
| 20 | 18.00 | ± | 0.26 | 1.47 | −9.00 | ± | 0.36 | 4.01 | 37.13 | ± | 0.35 | 0.95 | −13.47 | ± | 0.78 | 5.78 | d | 26.97 | ± | 1.49 | 5.53 | |
| F2 | ||||||||||||||||||||||
| 0 | 36.80 | ± | 0.40 | 1.09 | −19.00 | ± | 0.26 | 1.39 | 57.03 | ± | 0.67 | 1.17 | −9.05 | ± | 0.19 | 2.07 | a | |||||
| 2 | 36.20 | ± | 0.44 | 1.20 | −18.97 | ± | 0.21 | 1.10 | 56.73 | ± | 0.21 | 0.37 | −9.24 | ± | 0.15 | 1.58 | a | 1.08 | ± | 0.21 | 19.81 | |
| 4 | 34.23 | ± | 0.46 | 1.35 | −18.80 | ± | 0.53 | 2.81 | 57.73 | ± | 0.60 | 1.04 | −9.51 | ± | 0.29 | 3.05 | a,b | 2.84 | ± | 0.89 | 31.39 | |
| 6 | 33.70 | ± | 0.26 | 0.79 | −15.77 | ± | 0.29 | 1.83 | 46.70 | ± | 1.73 | 3.71 | −10.03 | ± | 0.55 | 5.44 | a,b | 11.32 | ± | 1.58 | 13.93 | |
| 8 | 33.20 | ± | 0.36 | 1.09 | −15.47 | ± | 0.32 | 2.08 | 46.37 | ± | 0.59 | 1.26 | −10.05 | ± | 0.36 | 3.54 | a,b | 11.80 | ± | 0.30 | 2.56 | |
| 10 | 31.60 | ± | 0.44 | 1.38 | −14.87 | ± | 0.21 | 1.40 | 45.83 | ± | 0.55 | 1.20 | −10.27 | ± | 0.23 | 2.24 | b,c | 13.03 | ± | 0.37 | 2.82 | |
| 12 | 29.30 | ± | 0.66 | 2.24 | −14.23 | ± | 0.51 | 3.61 | 43.67 | ± | 0.90 | 2.07 | −11.13 | ± | 0.52 | 4.69 | c,d | 16.07 | ± | 1.28 | 7.99 | |
| 14 | 26.60 | ± | 0.40 | 1.50 | −13.70 | ± | 0.35 | 2.53 | 45.53 | ± | 1.05 | 2.31 | −11.31 | ± | 0.07 | 0.62 | d,e | 16.28 | ± | 1.19 | 7.30 | |
| 16 | 20.93 | ± | 0.32 | 1.54 | −10.90 | ± | 0.26 | 2.43 | 44.13 | ± | 1.27 | 2.88 | −11.80 | ± | 0.24 | 2.00 | d,e | 22.00 | ± | 1.41 | 6.39 | |
| 18 | 18.77 | ± | 0.31 | 1.63 | −9.83 | ± | 0.06 | 0.59 | 43.30 | ± | 0.46 | 1.06 | −12.10 | ± | 0.28 | 2.29 | d,e | 24.45 | ± | 0.46 | 1.87 | |
| 20 | 18.17 | ± | 0.42 | 2.29 | −9.67 | ± | 0.42 | 4.31 | 43.70 | ± | 0.61 | 1.39 | −12.18 | ± | 0.56 | 4.58 | e | 24.76 | ± | 0.47 | 1.91 | |
| Day | L* | a* | b* | CI* | ΔE* | Referential Color | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | CV | ± | SD | CV | ± | SD | CV | ± | SD | CV | * | ± | SD | CV | |||||||
| Control | ||||||||||||||||||||||
| 0 | 29.73 | ± | 0.51 | 1.73 | −15.77 | ± | 0.45 | 2.86 | 55.10 | ± | 0.61 | 1.10 | −9.62 | ± | 0.20 | 2.11 | f,g | |||||
| 2 | 26.60 | ± | 0.46 | 1.72 | −15.80 | ± | 0.20 | 1.27 | 50.07 | ± | 0.15 | 0.31 | −11.86 | ± | 0.09 | 0.77 | g | 5.99 | ± | 0.48 | 8.01 | |
| 4 | 22.83 | ± | 0.21 | 0.91 | −13.50 | ± | 0.61 | 4.51 | 46.60 | ± | 0.30 | 0.64 | −12.69 | ± | 0.72 | 5.64 | g | 11.21 | ± | 0.76 | 6.79 | |
| 6 | 19.83 | ± | 0.55 | 2.78 | −10.63 | ± | 0.55 | 5.18 | 43.53 | ± | 0.45 | 1.04 | −12.31 | ± | 0.42 | 3.41 | g | 16.10 | ± | 1.02 | 6.35 | |
| 8 | 19.73 | ± | 0.80 | 4.06 | −8.77 | ± | 0.50 | 5.74 | 43.77 | ± | 0.35 | 0.80 | −10.15 | ± | 0.27 | 2.62 | g | 16.70 | ± | 1.20 | 7.21 | |
| 10 | 18.70 | ± | 0.50 | 2.67 | −4.07 | ± | 0.25 | 6.19 | 42.33 | ± | 0.47 | 1.12 | −5.13 | ± | 0.13 | 2.47 | e,f | 20.54 | ± | 0.78 | 3.77 | |
| 12 | 14.17 | ± | 0.35 | 2.48 | −0.53 | ± | 0.06 | 10.83 | 22.17 | ± | 0.71 | 3.20 | −1.70 | ± | 0.10 | 6.16 | e | 39.49 | ± | 0.61 | 1.54 | |
| 14 | 12.10 | ± | 0.20 | 1.65 | 1.47 | ± | 0.12 | 7.87 | 11.40 | ± | 0.46 | 4.02 | 10.63 | ± | 0.67 | 6.31 | d | 50.18 | ± | 0.66 | 1.32 | |
| 16 | 6.93 | ± | 0.35 | 5.07 | 1.33 | ± | 0.12 | 8.66 | 11.10 | ± | 0.17 | 1.56 | 17.34 | ± | 1.50 | 8.67 | c | 52.43 | ± | 0.64 | 1.22 | |
| 18 | 5.80 | ± | 0.40 | 6.90 | 1.47 | ± | 0.12 | 7.87 | 6.60 | ± | 0.36 | 5.46 | 38.44 | ± | 3.42 | 8.89 | b | 56.77 | ± | 0.75 | 1.32 | |
| 20 | 6.00 | ± | 0.17 | 2.89 | 1.63 | ± | 0.15 | 9.35 | 6.07 | ± | 0.25 | 4.15 | 44.89 | ± | 3.80 | 8.47 | a | 57.19 | ± | 0.53 | 0.93 | |
| F1 | ||||||||||||||||||||||
| 0 | 29.80 | ± | 0.52 | 1.74 | −18.63 | ± | 0.40 | 2.17 | 55.20 | ± | 0.56 | 1.01 | −11.33 | ± | 0.48 | 4.22 | f | |||||
| 2 | 26.50 | ± | 0.26 | 1.00 | −17.27 | ± | 0.45 | 2.61 | 55.47 | ± | 0.25 | 0.45 | −11.75 | ± | 0.40 | 3.38 | f | 3.70 | ± | 0.27 | 7.35 | |
| 4 | 25.33 | ± | 0.06 | 0.23 | −16.67 | ± | 0.55 | 3.30 | 51.23 | ± | 0.47 | 0.92 | −12.84 | ± | 0.56 | 4.34 | f,g | 6.32 | ± | 0.39 | 6.17 | |
| 6 | 21.63 | ± | 0.64 | 2.97 | −14.10 | ± | 0.40 | 2.84 | 43.83 | ± | 0.42 | 0.95 | −14.88 | ± | 0.70 | 4.69 | h | 14.72 | ± | 0.49 | 3.36 | |
| 8 | 20.43 | ± | 1.07 | 5.23 | −11.90 | ± | 0.40 | 3.36 | 40.07 | ± | 0.55 | 1.37 | −14.55 | ± | 0.36 | 2.46 | g,h | 19.07 | ± | 0.29 | 1.50 | |
| 10 | 17.80 | ± | 0.30 | 1.69 | −8.20 | ± | 0.26 | 3.23 | 39.00 | ± | 0.46 | 1.18 | −11.81 | ± | 0.23 | 1.97 | f | 22.71 | ± | 0.69 | 3.03 | |
| 12 | 16.70 | ± | 0.46 | 2.74 | −3.97 | ± | 0.15 | 3.85 | 28.87 | ± | 0.81 | 2.82 | −8.24 | ± | 0.42 | 5.04 | e | 32.87 | ± | 0.78 | 2.37 | |
| 14 | 15.00 | ± | 0.36 | 2.40 | −2.07 | ± | 0.15 | 7.39 | 21.83 | ± | 0.55 | 2.52 | −6.30 | ± | 0.25 | 3.99 | d | 40.09 | ± | 0.54 | 1.34 | |
| 16 | 11.77 | ± | 0.67 | 5.66 | 0.93 | ± | 0.06 | 6.19 | 13.80 | ± | 0.56 | 4.03 | 5.75 | ± | 0.17 | 2.96 | c | 49.22 | ± | 0.83 | 1.68 | |
| 18 | 10.23 | ± | 0.40 | 3.95 | 1.17 | ± | 0.12 | 9.90 | 10.37 | ± | 0.15 | 1.47 | 11.00 | ± | 0.94 | 8.57 | b | 52.77 | ± | 0.58 | 1.09 | |
| 20 | 9.10 | ± | 0.20 | 2.20 | 1.20 | ± | 0.10 | 8.33 | 9.20 | ± | 0.26 | 2.88 | 14.35 | ± | 1.29 | 8.99 | a | 54.20 | ± | 0.46 | 0.84 | |
| F2 | ||||||||||||||||||||||
| 0 | 29.70 | ± | 0.26 | 0.89 | −18.17 | ± | 0.68 | 3.75 | 47.90 | ± | 0.44 | 0.91 | −12.78 | ± | 0.67 | 5.21 | e | ± | ||||
| 2 | 27.80 | ± | 0.26 | 0.95 | −18.40 | ± | 0.69 | 3.77 | 47.30 | ± | 0.46 | 0.97 | −13.99 | ± | 0.53 | 3.80 | e,f,g | 2.31 | ± | 0.16 | 7.06 | |
| 4 | 25.67 | ± | 0.21 | 0.81 | −16.20 | ± | 0.46 | 2.83 | 46.80 | ± | 0.82 | 1.75 | −13.49 | ± | 0.24 | 1.80 | e,f | 4.71 | ± | 0.23 | 4.90 | |
| 6 | 21.13 | ± | 0.21 | 0.99 | −14.73 | ± | 0.71 | 4.82 | 46.97 | ± | 0.61 | 1.30 | −14.84 | ± | 0.48 | 3.22 | f,g,h | 9.31 | ± | 0.42 | 4.54 | |
| 8 | 20.17 | ± | 0.93 | 4.61 | −13.63 | ± | 0.50 | 3.69 | 42.00 | ± | 0.46 | 1.09 | −16.15 | ± | 1.48 | 9.18 | h | 12.10 | ± | 0.60 | 4.95 | |
| 10 | 18.23 | ± | 0.42 | 2.28 | −11.97 | ± | 0.42 | 3.48 | 41.93 | ± | 0.49 | 1.18 | −15.66 | ± | 0.64 | 4.12 | g,h | 14.36 | ± | 0.46 | 3.22 | |
| 12 | 17.37 | ± | 0.51 | 2.95 | −8.13 | ± | 0.47 | 5.81 | 36.10 | ± | 0.53 | 1.47 | −12.98 | ± | 0.71 | 5.45 | e,f | 19.81 | ± | 0.44 | 2.20 | |
| 14 | 13.33 | ± | 0.15 | 1.15 | −2.93 | ± | 0.15 | 5.21 | 32.13 | ± | 0.25 | 0.78 | −6.85 | ± | 0.45 | 6.62 | d | 27.37 | ± | 0.31 | 1.13 | |
| 16 | 9.80 | ± | 0.60 | 6.12 | 0.63 | ± | 0.06 | 9.12 | 27.47 | ± | 0.65 | 2.37 | 2.35 | ± | 0.17 | 7.10 | c | 34.17 | ± | 0.18 | 0.52 | |
| 18 | 7.87 | ± | 0.31 | 3.88 | 0.93 | ± | 0.06 | 6.19 | 20.77 | ± | 0.31 | 1.47 | 5.71 | ± | 0.12 | 2.06 | b | 39.72 | ± | 0.40 | 1.00 | |
| 20 | 7.47 | ± | 0.15 | 2.05 | 1.33 | ± | 0.12 | 8.66 | 20.60 | ± | 0.66 | 3.18 | 8.67 | ± | 0.63 | 7.31 | a | 40.26 | ± | 0.54 | 1.35 | |
| Hass | Fuerte | |||||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | Control | F1 | F2 | |
| Maximum | 23.00 | 23.00 | 23.00 | 23.00 | 23.00 | 23.00 |
| Minimum | 0.50 | 2.50 | 2.80 | 0.30 | 0.60 | 0.70 |
| **** | 12.47 | 13.80 | 14.20 | 7.42 | 7.32 | 7.78 |
| SD | 9.53 | 8.88 | 8.72 | 9.58 | 8.96 | 9.34 |
| CV (%) | 76.41 | 64.34 | 61.39 | 129.22 | 122.28 | 119.98 |
| p-value * | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| p-value ** | <0.05 | <0.05 | ||||
| p-value *** | <0.05 | <0.05 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choque-Quispe, D.; Diaz-Barrera, Y.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Ramos-Pacheco, B.S.; Ligarda-Samanez, C.A.; Peralta-Guevara, D.E.; Martínez-Huamán, E.L.; Aguirre Landa, J.P.; Correa-Cuba, O.; et al. Effect of the Application of a Coating Native Potato Starch/Nopal Mucilage/Pectin on Physicochemical and Physiological Properties during Storage of Fuerte and Hass Avocado (Persea americana). Polymers 2022, 14, 3421. https://doi.org/10.3390/polym14163421
Choque-Quispe D, Diaz-Barrera Y, Solano-Reynoso AM, Choque-Quispe Y, Ramos-Pacheco BS, Ligarda-Samanez CA, Peralta-Guevara DE, Martínez-Huamán EL, Aguirre Landa JP, Correa-Cuba O, et al. Effect of the Application of a Coating Native Potato Starch/Nopal Mucilage/Pectin on Physicochemical and Physiological Properties during Storage of Fuerte and Hass Avocado (Persea americana). Polymers. 2022; 14(16):3421. https://doi.org/10.3390/polym14163421
Chicago/Turabian StyleChoque-Quispe, David, Yasmine Diaz-Barrera, Aydeé M. Solano-Reynoso, Yudith Choque-Quispe, Betsy S. Ramos-Pacheco, Carlos A. Ligarda-Samanez, Diego E. Peralta-Guevara, Edgar L. Martínez-Huamán, John Peter Aguirre Landa, Odilon Correa-Cuba, and et al. 2022. "Effect of the Application of a Coating Native Potato Starch/Nopal Mucilage/Pectin on Physicochemical and Physiological Properties during Storage of Fuerte and Hass Avocado (Persea americana)" Polymers 14, no. 16: 3421. https://doi.org/10.3390/polym14163421
APA StyleChoque-Quispe, D., Diaz-Barrera, Y., Solano-Reynoso, A. M., Choque-Quispe, Y., Ramos-Pacheco, B. S., Ligarda-Samanez, C. A., Peralta-Guevara, D. E., Martínez-Huamán, E. L., Aguirre Landa, J. P., Correa-Cuba, O., Agreda Cerna, H. W., Masco-Arriola, M. L., Lechuga-Canal, W. J., Loayza-Céspedes, J. C., & Álvarez-López, G. J. (2022). Effect of the Application of a Coating Native Potato Starch/Nopal Mucilage/Pectin on Physicochemical and Physiological Properties during Storage of Fuerte and Hass Avocado (Persea americana). Polymers, 14(16), 3421. https://doi.org/10.3390/polym14163421












