Pilot-Scale Processing and Functional Properties of Antifungal EVOH-Based Films Containing Methyl Anthranilate Intended for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pilot Scale Production of Active EVOH-Based Films by Extrusion Processing
2.3. Characterization of Active Films
2.3.1. Identification and Quantification of Methyl Anthranilate in EVOH-Based Films
2.3.2. Morphological Characterization
2.3.3. Thermal Analysis
2.3.4. Mechanical Properties
2.3.5. Film Thickness, Colour Tests and Transparency
2.3.6. Barrier Properties
2.3.7. Influence of Environmental Humidity on the Release of Methyl Anthranilate
2.4. Efficacy of EVOH Films Containing Methyl Anthranilate against Fungal Growth In Vitro
2.4.1. Microbiological Studies
2.4.2. Antifungal Activity of Methyl Anthranilate
2.4.3. Antifungal Activity of Cast-Extruded EVOH Films Incorporated with Methyl Anthranilate
3. Results and Discussion
3.1. Identification and Quantification of Methyl Anthranilate in EVOH-Based Films
3.2. SEM Analysis
3.3. Thermal Properties
3.4. Mechanical Properties
3.5. Water Vapour Permeability
3.6. Optical Properties
3.7. Effect of Environmental Humidity on Methyl Anthranilate Release from EVOH-Based Films
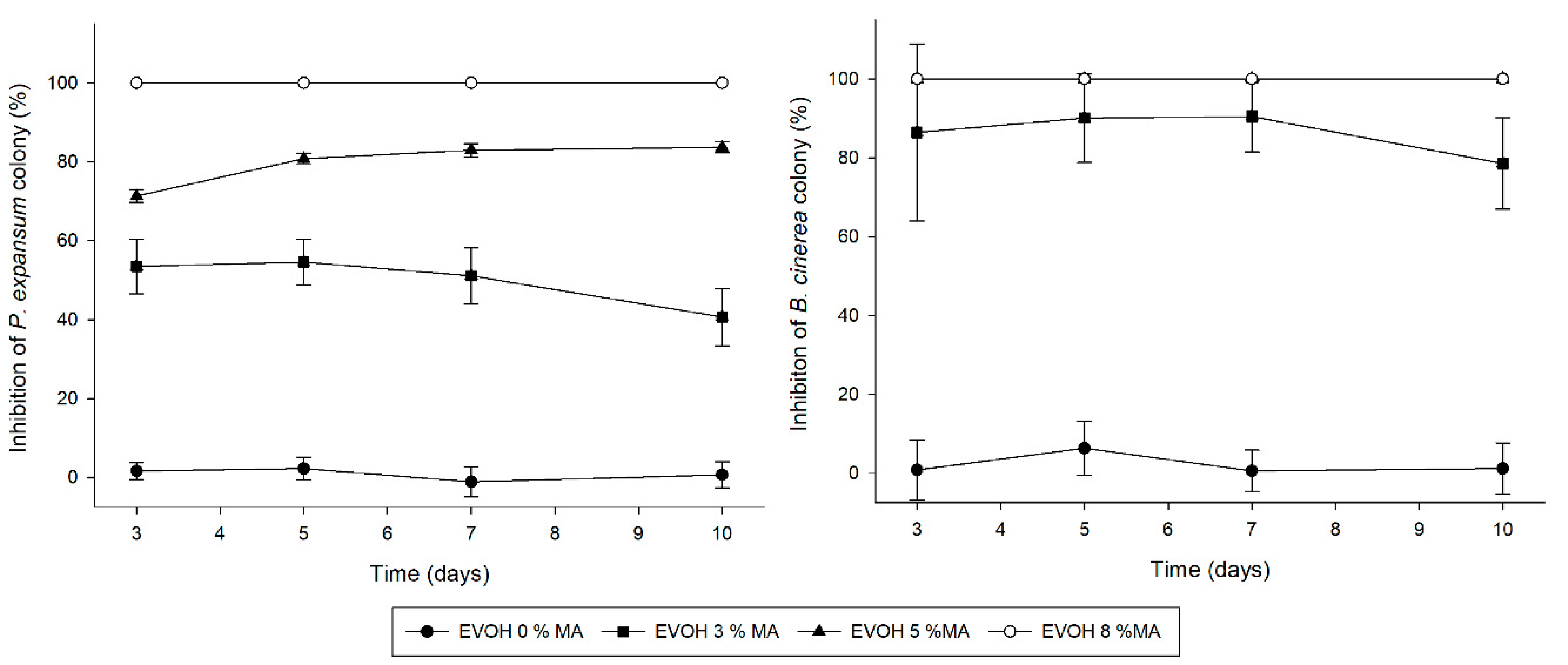
3.8. Antifungal Activity
3.8.1. Methyl Anthranilate Vapour Inhibition Assay
3.8.2. Antifungal Activity of Cast-Extruded Active EVOH-Based Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Definitional Framework of Food Loss—Save Food: Global Initiative on Food Loss and Waste Reduction. In Definitional Framework of Food Loss Working Papers; FAO: Rome, Italy, 2014; Volume 18. [Google Scholar]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Gavara, R.; López-Carballo, G.; Hernández-Muñoz, P.; Catalá, R.; Muriel-Galet, V.; Cerisuelo, J.P.; Dominguez, I. Practical Guide to Antimicrobial Active Packaging; iSmithers Rapra Publishing: Surrey, UK, 2015. [Google Scholar]
- Manfredi, M.; Fantin, V.; Vignali, G.; Gavara, R. Environmental assessment of antimicrobial coatings for packaged fresh milk. J. Clean. Prod. 2015, 95, 291–300. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; Lopez-Carballo, G.; Lara, M.; Gavara, R.; Hernandez-Munoz, P. Development of antimicrobial films for microbiological control of packaged salad. Int. J. Food Microbiol. 2012, 157, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Heras-Mozos, R.; Gavara, R.; Hernández-Muñoz, P. Development of antifungal biopolymers based on dynamic imines as responsive release systems for the postharvest preservation of blackberry fruit. Food Chem. 2021, 357, 129838. [Google Scholar] [CrossRef]
- Ettinger, D.J. Active and Intelligent Packaging: A U.S. and EU Perspective. Available online: https://www.packaginglaw.com/special-focus/active-and-intelligent-packaging-us-and-eu-perspective (accessed on 17 June 2002).
- Gavara, R.; Catalá, R.; López Carballo, G.; Cerisuelo, J.P.; Dominguez, I.; Muriel-Galet, V.; Hernandez-Muñoz, P. Use of EVOH for Food Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Zavagna, L.; Sammon, C.; Cabedo, L.; Prieto, C.; Lagaron, J.M. Development and Characterization of Electrospun Fiber-Based Poly(ethylene-co-vinyl Alcohol) Films of Application Interest as High-Gas-Barrier Interlayers in Food Packaging. Polymers 2021, 13, 2061. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.M.A.; Ewender, J.; Welle, F. Recyclable multilayer packaging by means of thermoreversibly crosslinking adhesive in the context of food law. Polymers 2020, 12, 2988. [Google Scholar] [CrossRef]
- Arboleda, C.E.; Mejía, A.I.G.; López, B.L.O. Poly (vinylalcohol-co-ethylene) biodegradation on semi solid fermentation by Phanerochaete chrysosporium. Acta Farm. Bonaer. 2004, 23, 123–128. [Google Scholar]
- Mejía, G.A.I.; López, O.B.L.; Sierra, L. Biodegradation of poly(vinylalcohol-co-ethylene) with the fungus phanerochaete chrysosporium. Mater. Res. Innov. 2001, 4, 148–154. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; Mcdonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Wang, J.; De Luca, V. The biosynthesis and regulation of biosynthesis of Concord grape fruit esters, including “foxy” methylanthranilate. Plant J. 2005, 44, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.D.; Krishnan, M.S. An Ecofriendly Catalytic Route for the Preparation of Perfumery Grade Methyl Anthranilate from Anthranilic Acid and Methanol. Org. Process Res. Dev. 1998, 2, 86–95. [Google Scholar] [CrossRef]
- Luo, Z.W.; Cho, J.S.; Lee, S.Y. Microbial production of methyl anthranilate, a grape flavor compound. Proc. Natl. Acad. Sci. USA 2019, 116, 201903875. [Google Scholar] [CrossRef] [PubMed]
- Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M.C. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Korma, S.A.; Khan, I.M.; Mohsin, A.; Manzoor, M.F.; Ashraf, W.; Mushtaq, B.S.; Zainab, S.; et al. Spray dried nanoemulsions loaded with curcumin, resveratrol, and borage seed oil: The role of two different modified starches as encapsulating materials. Int. J. Biol. Macromol. 2021, 186, 820–828. [Google Scholar] [CrossRef]
- Pansuwan, J.; Chaiyasat, A. Innovative and high performance synthesis of microcapsules containing methyl anthranilate by microsuspension iodine transfer polymerization. Polym. Int. 2017, 66, 1921–1927. [Google Scholar] [CrossRef]
- Buendía-Moreno, L.; Soto-Jover, S.; Ros-Chumillas, M.; Antolinos, V.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; López-Gómez, A. Innovative cardboard active packaging with a coating including encapsulated essential oils to extend cherry tomato shelf life. LWT 2019, 116, 108584. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Production of liposomes loaded with antioxidants using a supercritical CO2 assisted process. Powder Technol. 2018, 323, 155–162. [Google Scholar] [CrossRef]
- Chaudhary, V.; Thakur, N.; Kajla, P.; Thakur, S.; Punia, S. Application of Encapsulation Technology in Edible Films: Carrier of Bioactive Compounds. Front. Sustain. Food Syst. 2021, 5, 374. [Google Scholar] [CrossRef]
- Antosik, A.K.; Kowalska, U.; Stobińska, M.; Dzięcioł, P.; Pieczykolan, M.; Kozłowska, K.; Bartkowiak, A. Development and characterization of bioactive polypropylene films for food packaging applications. Polymers 2021, 13, 3478. [Google Scholar] [CrossRef] [PubMed]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef]
- Nobile, M.A.; Conte, A.; Buonocore, G.G.; Anna Lucia, I.; Massaro, A.; Panza, O. Active packaging by extrusión processing of reciclable and biodegradable polymer. J. Food Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- Nam, S.; Scanlon, M.G.; Han, J.; Izydorczyk, M. Extrusion of Pea Starch Containing Lysozyme and Determination of Antimicrobial Activity. J. Food Sci. 2007, 72, E477–E484. [Google Scholar] [CrossRef]
- Aragón-Gutiérrez, A.; Heras-Mozos, R.; Gallur, M.; López, D.; Hernández-Muñoz, P. Hot-Melt-Extruded Active Films Prepared from Packaging Applications. Foods 2021, 10, 1591. [Google Scholar] [CrossRef]
- Beltrán Sanahuja, A.; Valdés García, A. New Trends in the Use of Volatile Compounds in Food Packaging. Polymers 2021, 13, 1053. [Google Scholar] [CrossRef]
- Faisant, J.B.; Aït-Kadi, A.; Bousmina, M.; Deschênes, L. Morphology, thermomechanical and barrier properties of polypropylene-ethylene vinyl alcohol blends. Polymer 1998, 39, 533–545. [Google Scholar] [CrossRef]
- Aragón-Gutiérrez, A.; Rosa, E.; Gallur, M.; López, D.; Hernández-Muñoz, P.; Gavara, R. Melt-Processed Bioactive EVOH Films Incorporated with Ferulic Acid. Polymers 2021, 13, 68. [Google Scholar] [CrossRef]
- ISO 527:2012; lastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2006.
- Hernández-García, E.; Vargas, M.; Chiralt, A. Starch-polyester bilayer films with phenolic acids for pork meat preservation. Food Chem. 2022, 385, 132650. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, S.; Cruz Romero, M.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Figura, L.O.; Teixeira, A.A. (Eds.) Permeability. In BT—Food Physics: Physical Properties—Measurement and Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 233–255. [Google Scholar]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; Kenny, J.; Garrigós, M. Characterization and disintegrability under composting conditions of PLA-based nanocomposite films with thymol and silver nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef]
- Kurek, M.; Guinault, A.; Voilley, A.; Galić, K.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Khoomsab, R.; Rodkate, N. Establishment of Methyl Anthranilate Quantification by UV-Spectroscopy and Identification Using FTIR. EAU Herit. J. Sci. Technol. 2019, 13, 94–105. [Google Scholar]
- Ramos, M.; Beltrán, A.; Peltzer, M.; Valente, A.J.M.; del Carmen Garrigós, M. Release and antioxidant activity of carvacrol and thymol from polypropylene active packaging films. LWT Food Sci. Technol. 2014, 58, 470–477. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.A.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled release of thymol from poly(Lactic acid)-based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef]
- Galotto, M.J.; Valenzuela, X.; Rodriguez, F.; Bruna, J.; Guarda, A. Evaluation of the effectiveness of a new antimicrobial active packaging for fresh atlantic salmon (Salmo Salar L.) shelf life. Packag. Technol. Sci. 2012, 25, 363–372. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Strategy To Modify the Crystallization Behavior of EVOH32 through Interactions with Low-Molecular-Weight Molecules. Ind. Eng. Chem. Res. 2016, 55, 3517–3524. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Puglia, D. Antioxidant Packaging Films Based on Ethylene Vinyl Alcohol Copolymer (EVOH) and Caffeic Acid. Molecules 2020, 25, 3953. [Google Scholar] [CrossRef] [PubMed]
- Duraccio, D.; Mauriello, A.; Cimmino, S.; Silvestre, C.; De Rosa, C.; Pirozzi, B.; Mitchell, G. Structure-property relationships in polyethylene based films obtained by blow molding as model system of industrial relevance. Eur. Polym. J. 2014, 62, 97–107. [Google Scholar] [CrossRef]
- Bunn, C.W. Crystal Structure of Polyvinyl Alcohol. Nature 1948, 161, 929–930. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Pérez, E.; Pereña, J.M.; Benavente, R. Wide-angle X-ray diffraction study of the phase behavior of vinyl alcohol-ethylene copolymers. Macromolecules 1998, 31, 2559–2564. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.M.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Barrera, J.; Rodríguez, J.; Perilla, J.; Algecira, N. A study of poly(vinyl alcohol) thermal degradation by thermogravimetry and differential thermogravimetry. Ing. Investig. 2007, 27, 100–105. [Google Scholar]
- Alvarez, V.A.; Ruseckaite, R.A.; Vázquez, A. Kinetic analysis of thermal degradation in poly(ethylene–vinyl alcohol) copolymers. J. Appl. Polym. Sci. 2003, 90, 3157–3163. [Google Scholar] [CrossRef]
- Krepker, M.; Zhang, C.; Nitzan, N.; Prinz-Setter, O.; Massad-Ivanir, N.; Olah, A.; Baer, E.; Segal, E. Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion. Polymers 2018, 10, 864. [Google Scholar] [CrossRef]
- Kendall, M.; Siviour, C. Rate dependence of poly(vinyl chloride), the effects of plasticizer and time-temperature superposition. Proc. R. Soc. A Math. Phys. Eng. Sci. 2014, 470, 20140012. [Google Scholar] [CrossRef]
- Gaucher-Miri, V.; Jones, G.K.; Kaas, R.; Hiltner, A.; Baer, E. Plastic deformation of EVA, EVOH and their multilayers. J. Mater. Sci. 2002, 37, 2635–2644. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermudez, J.M.; Baños, A.; Ariza, J.J.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Characterisation and antimicrobial activity of active polypropylene films containing oregano essential oil and Allium extract to be used in packaging for meat products. Food Addit. Contam. Part A 2018, 35, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermúdez, J.M.; Baños, A.; Núñez, C.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Development of PLA films containing oregano essential oil (Origanum vulgare L. virens) intended for use in food packaging. Food Addit. Contam. 2016, 33, 1374–1386. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- López De Dicastillo, C.; Nerín, C.; Alfaro, P.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J. Agric. Food Chem. 2011, 59, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- López-de-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Aucejo, S.; Marco, C.; Gavara, R. Water effect on the morphology of EVOH copolymers. J. Appl. Polym. Sci. 1999, 74, 1201–1206. [Google Scholar] [CrossRef]
- Nidiry, E.S.J.; Babu, C.S.B. Antifungal activity of tuberose absolute and some of its constituents. Phytother. Res. 2005, 19, 447–449. [Google Scholar] [CrossRef]
- Xing, M.; Zheng, L.; Deng, Y.; Xu, D.; Xi, P.; Li, M.; Kong, G.; Jiang, Z. Antifungal Activity of Natural Volatile Organic Compounds against Litchi Downy Blight Pathogen Peronophythora litchii. Molecules 2018, 23, 358. [Google Scholar] [CrossRef]
- Chambers, A.H.; Evans, S.A.; Folta, K.M. Methyl Anthranilate and γ-Decalactone Inhibit Strawberry Pathogen Growth and Achene Germination. J. Agric. Food Chem. 2013, 61, 12625–12633. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Zhao, W.; Xie, Y. (E)-2-Hexenal, as a Potential Natural Antifungal Compound, Inhibits Aspergillus flavus Spore Germination by Disrupting Mitochondrial Energy Metabolism. J. Agric. Food Chem. 2019, 67, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- González-Estrada, R.R.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A.; Peyron, S.; Chalier, P. Antimicrobial soy protein isolate-based films: Physical characterisation, active agent retention and antifungal properties against Penicillium italicum. Int. J. Food Sci. Technol. 2018, 53, 921–929. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Yang, F.; Chen, M.; Zhou, D.; Li, L. Active Packaging Films from Ethylene Vinyl Alcohol Copolymer and Clove Essential Oil as Shelf Life Extenders for Grass Carp Slice. Packag. Technol. Sci. 2016, 29, 383–396. [Google Scholar] [CrossRef]
- Mateo, E.M.; Gómez, J.V.; Domínguez, I.; Gimeno-Adelantado, J.V.; Mateo-Castro, R.; Gavara, R.; Jiménez, M. Impact of bioactive packaging systems based on EVOH films and essential oils in the control of aflatoxigenic fungi and aflatoxin production in maize. Int. J. Food Microbiol. 2017, 254, 36–46. [Google Scholar] [CrossRef]
- Heras-Mozos, R.; Muriel-Galet, V.; López-Carballo, G.; Catalá, R.; Hernández-Muñoz, P.; Gavara, R. Active EVOH/PE bag for sliced pan loaf based on garlic as antifungal agent and bread aroma as aroma corrector. Food Packag. Shelf Life 2018, 18, 125–130. [Google Scholar] [CrossRef]
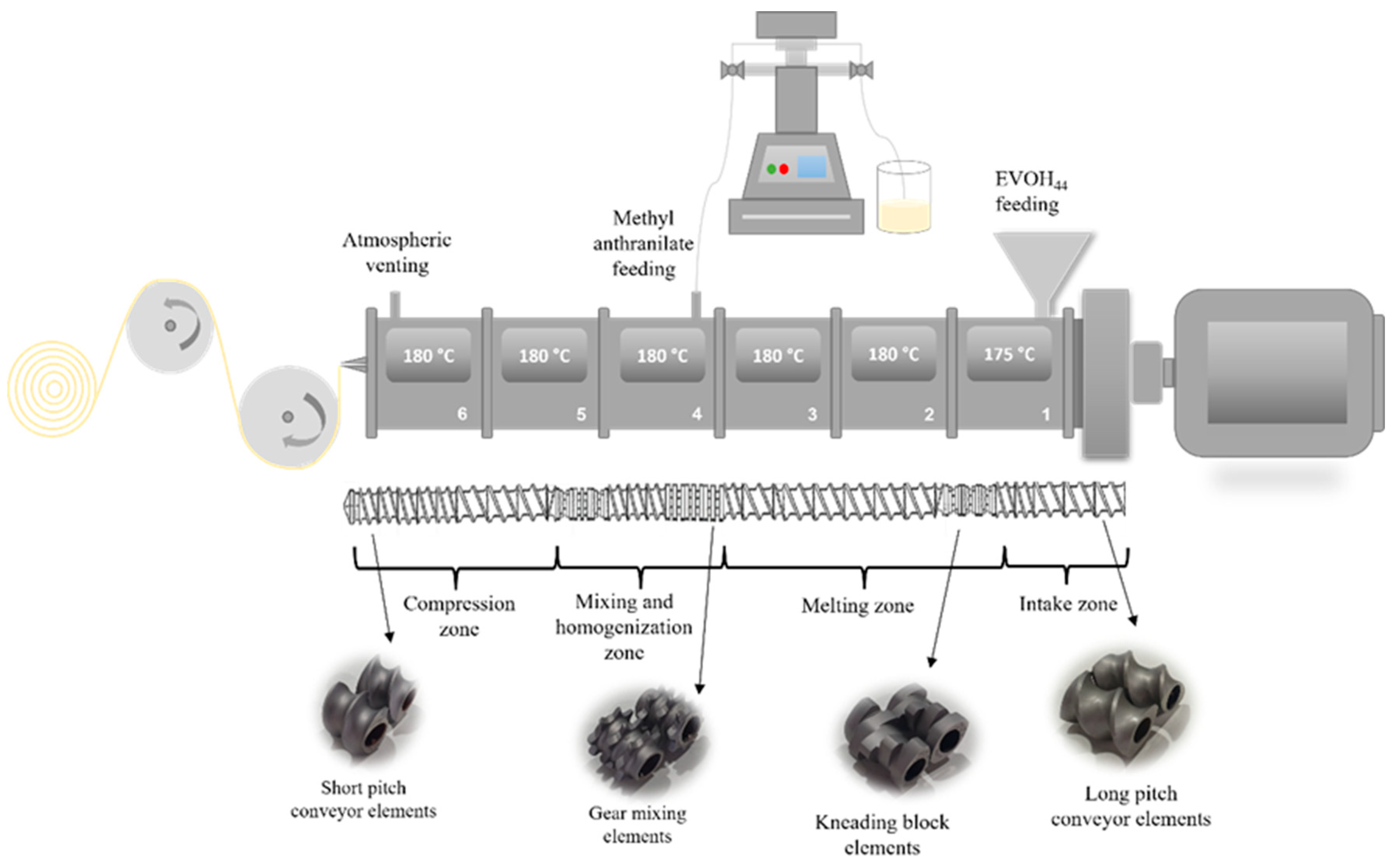

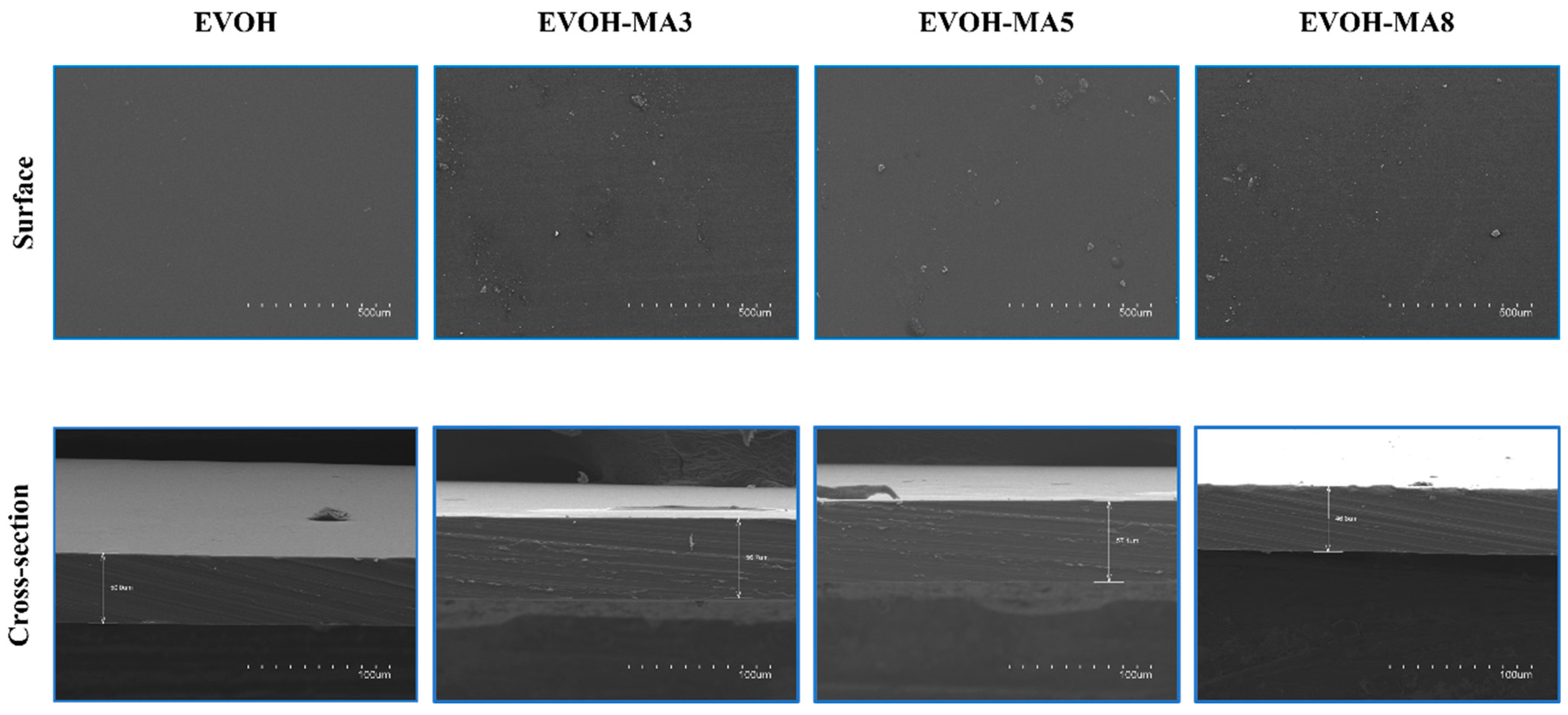

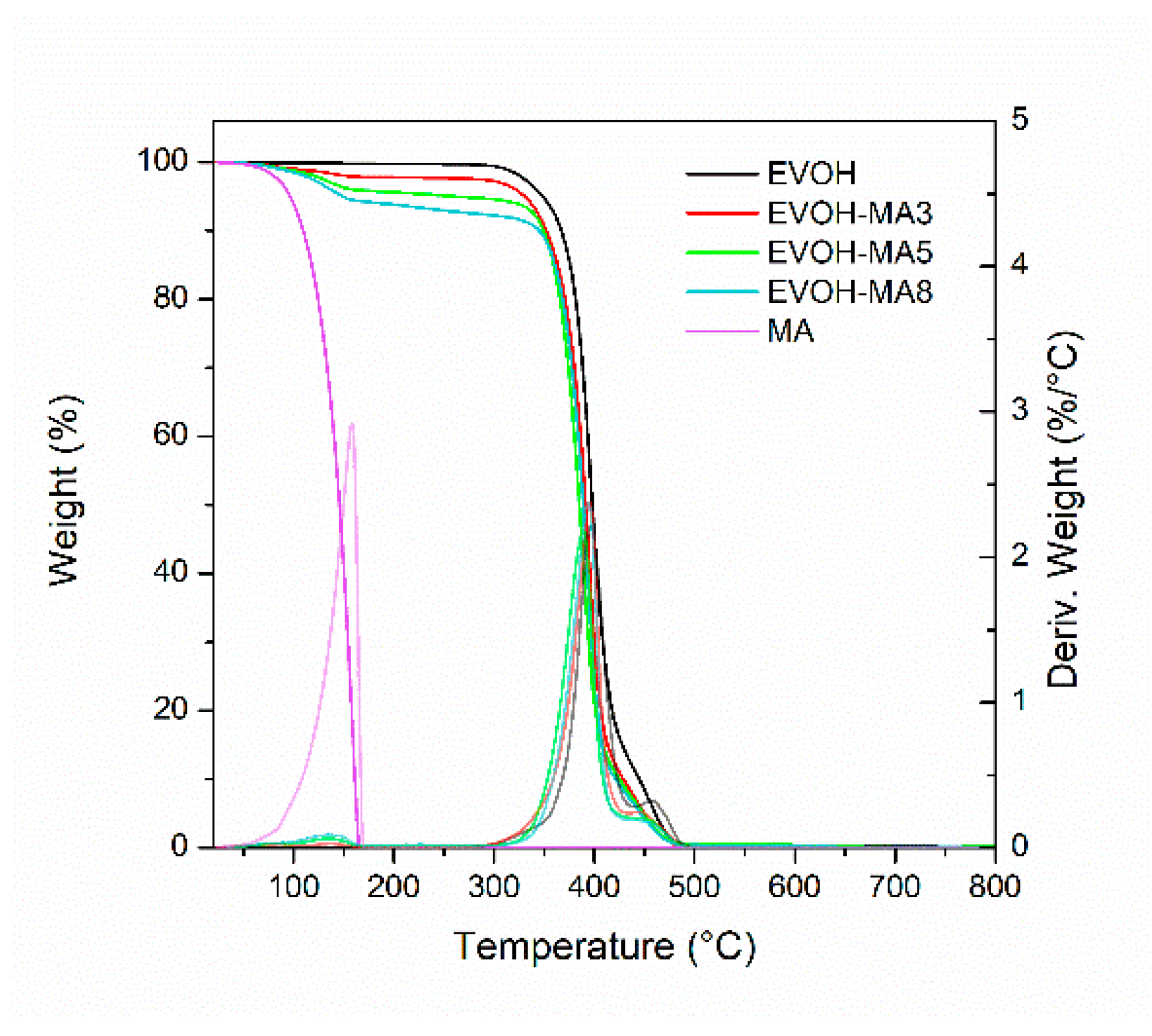

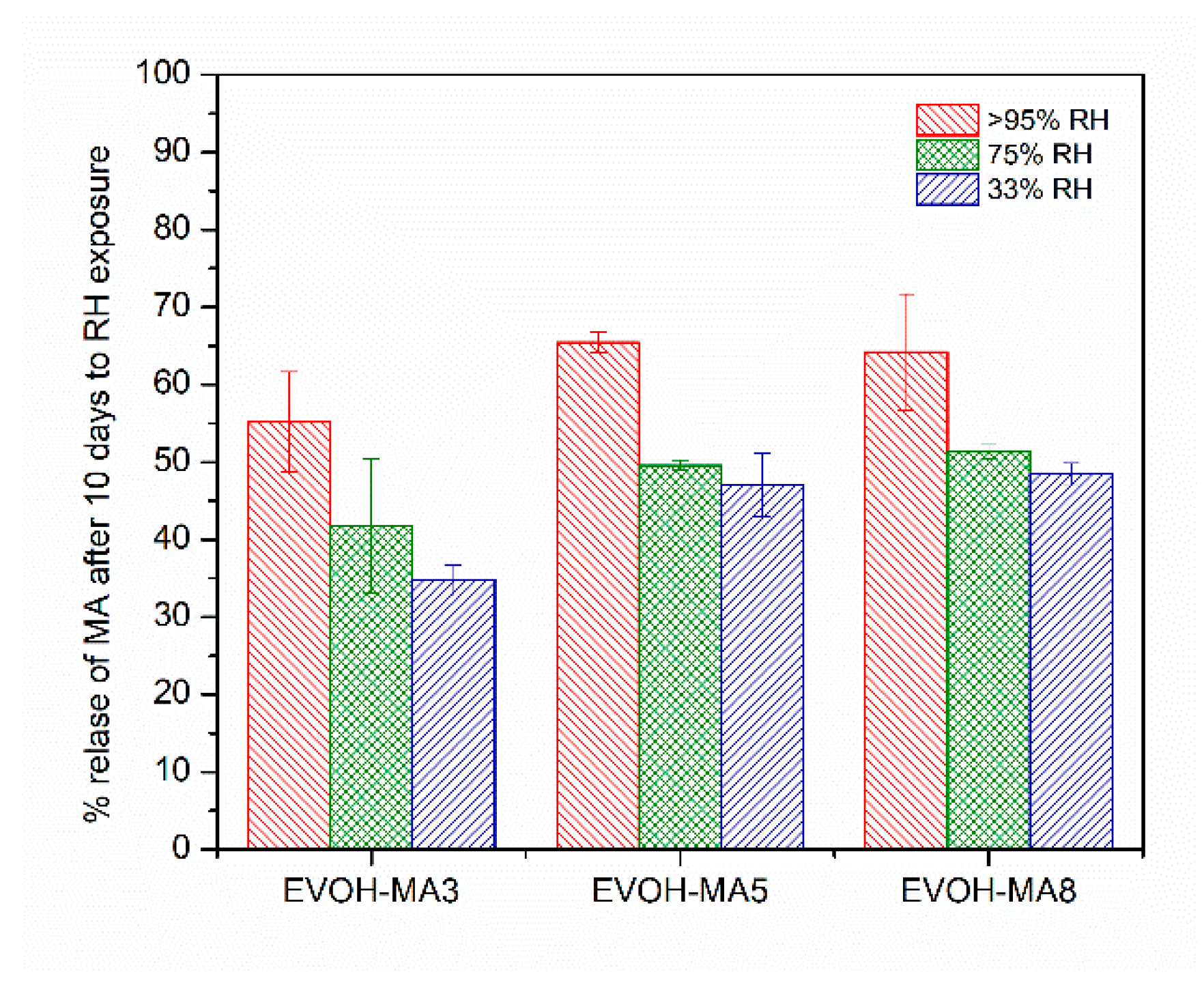

| Formulation | Code | Methyl Anthranilate (wt.%) | Retention Capacity (%) |
|---|---|---|---|
| Neat EVOH | EVOH | n.d. | - |
| EVOH + 3 wt.% methyl anthranilate | EVOH-MA3 | 2.52 ± 0.36 | 84 |
| EVOH + 5 wt.% methyl anthranilate | EVOH-MA5 | 3.84 ± 0.20 | 76 |
| EVOH + 8 wt.% methyl anthranilate | EVOH-MA8 | 6.42 ± 0.17 | 80 |
| First Heating Scan | Cooling Scan | Second Heating Scan | TGA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formulation | Tg (°C) | Tm (°C) | ∆Hm (J/g) | χc (%) | Tc (°C) | ∆Hc (J/g) | Tg (°C) | Tm (°C) | ∆Hm (J/g) | χc (%) | ΔW0–200 (%) | TmaxI (°C) | TmaxII (°C) |
| EVOH | 52 | 169 | 68.8 | 31.5 | 143 | 61.8 | 54 | 167 | 66.5 | 30.5 | 0.2 | 399 | 459 |
| EVOH-MA3 | 43 | 165 | 55.7 | 26.4 | 141 | 56.8 | 44 | 165 | 65.3 | 30.9 | 2.25 | 395 | 445 |
| EVOH-MA5 | 40 | 164 | 57.4 | 27.3 | 139 | 53.9 | 42 | 163 | 56.9 | 27.4 | 4.33 | 389 | 452 |
| EVOH-MA8 | 37 | 162 | 50.9 | 25.4 | 138 | 52.9 | 39 | 162 | 52.7 | 26.3 | 6.12 | 392 | 451 |
| Reference | E (GPa) | Stress @ Yield (MPa) | Stress @ Break (MPa) | Elongation @ Break (%) | WVP·1015 (kg·m/m2 s Pa) | |
|---|---|---|---|---|---|---|
| 33% RH | 75% RH | |||||
| EVOH | 2.5 ± 0.2 a | 46 ± 3 a | 62 ± 5 a | 174 ± 16 a | 0.12 ± 0.01 | 1.08 ± 0.02 |
| EVOH-MA3 | 2.4 ± 0.1 a | 41 ± 4 a | 54 ± 7 b | 224 ± 18 b | 0.11 ± 0.01 | 0.99 ± 0.04 |
| EVOH-MA5 | 2.3 ± 0.2 a | 41 ± 4 a | 46 ± 10 b,c | 260 ± 21 c | 0.15 ± 0.02 | 1.14 ± 0.02 |
| EVOH-MA8 | 2.2 ± 0.1 a | 40 ± 5 a | 43 ± 8 c | 253 ± 12 c | 0.18 ± 0.01 | 1.38 ± 0.06 |
| Reference | Thickness (µm) | L | a | b | ΔE | T (A600/t) |
|---|---|---|---|---|---|---|
| EVOH | 50 ± 2 | 93.1 | 0.04 | −0.49 | - | 0.89 ± 0.01 |
| EVOH-MA3 | 53 ± 3 | 92.6 ± 0.1 | −0.59 ± 0.02 | 3.6 ± 0.04 | 4.1 ± 0.1 | 0.88 ± 0.01 |
| EVOH-MA5 | 55 ± 3 | 92.5 ± 0.1 | −0.81 ± 0.01 | 4.12 ± 0.02 | 4.7 ± 0.2 | 0.84 ± 0.01 |
| EVOH-MA8 | 54 ± 4 | 92.6 ± 0.1 | −0.78 ± 0.05 | 4.31 ± 0.04 | 4.9 ± 0.1 | 0.77 ± 0.02 |
| MIC (μL/plate) | MFC (μL/plate) | Control | MIC | MFC | |
|---|---|---|---|---|---|
| P. expansum | 2.5 | 20 | 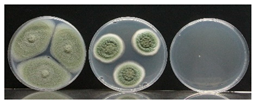 | ||
| B. cinerea | 1 | 5 |  | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Gutiérrez, A.; Heras-Mozos, R.; Montesinos, A.; Gallur, M.; López, D.; Gavara, R.; Hernández-Muñoz, P. Pilot-Scale Processing and Functional Properties of Antifungal EVOH-Based Films Containing Methyl Anthranilate Intended for Food Packaging Applications. Polymers 2022, 14, 3405. https://doi.org/10.3390/polym14163405
Aragón-Gutiérrez A, Heras-Mozos R, Montesinos A, Gallur M, López D, Gavara R, Hernández-Muñoz P. Pilot-Scale Processing and Functional Properties of Antifungal EVOH-Based Films Containing Methyl Anthranilate Intended for Food Packaging Applications. Polymers. 2022; 14(16):3405. https://doi.org/10.3390/polym14163405
Chicago/Turabian StyleAragón-Gutiérrez, Alejandro, Raquel Heras-Mozos, Antonio Montesinos, Miriam Gallur, Daniel López, Rafael Gavara, and Pilar Hernández-Muñoz. 2022. "Pilot-Scale Processing and Functional Properties of Antifungal EVOH-Based Films Containing Methyl Anthranilate Intended for Food Packaging Applications" Polymers 14, no. 16: 3405. https://doi.org/10.3390/polym14163405
APA StyleAragón-Gutiérrez, A., Heras-Mozos, R., Montesinos, A., Gallur, M., López, D., Gavara, R., & Hernández-Muñoz, P. (2022). Pilot-Scale Processing and Functional Properties of Antifungal EVOH-Based Films Containing Methyl Anthranilate Intended for Food Packaging Applications. Polymers, 14(16), 3405. https://doi.org/10.3390/polym14163405










