Research Progress on Emerging Polysaccharide Materials Applied in Tissue Engineering
Abstract
1. Introduction
2. Polysaccharide Materials and Derivatives
2.1. Sodium Alginate
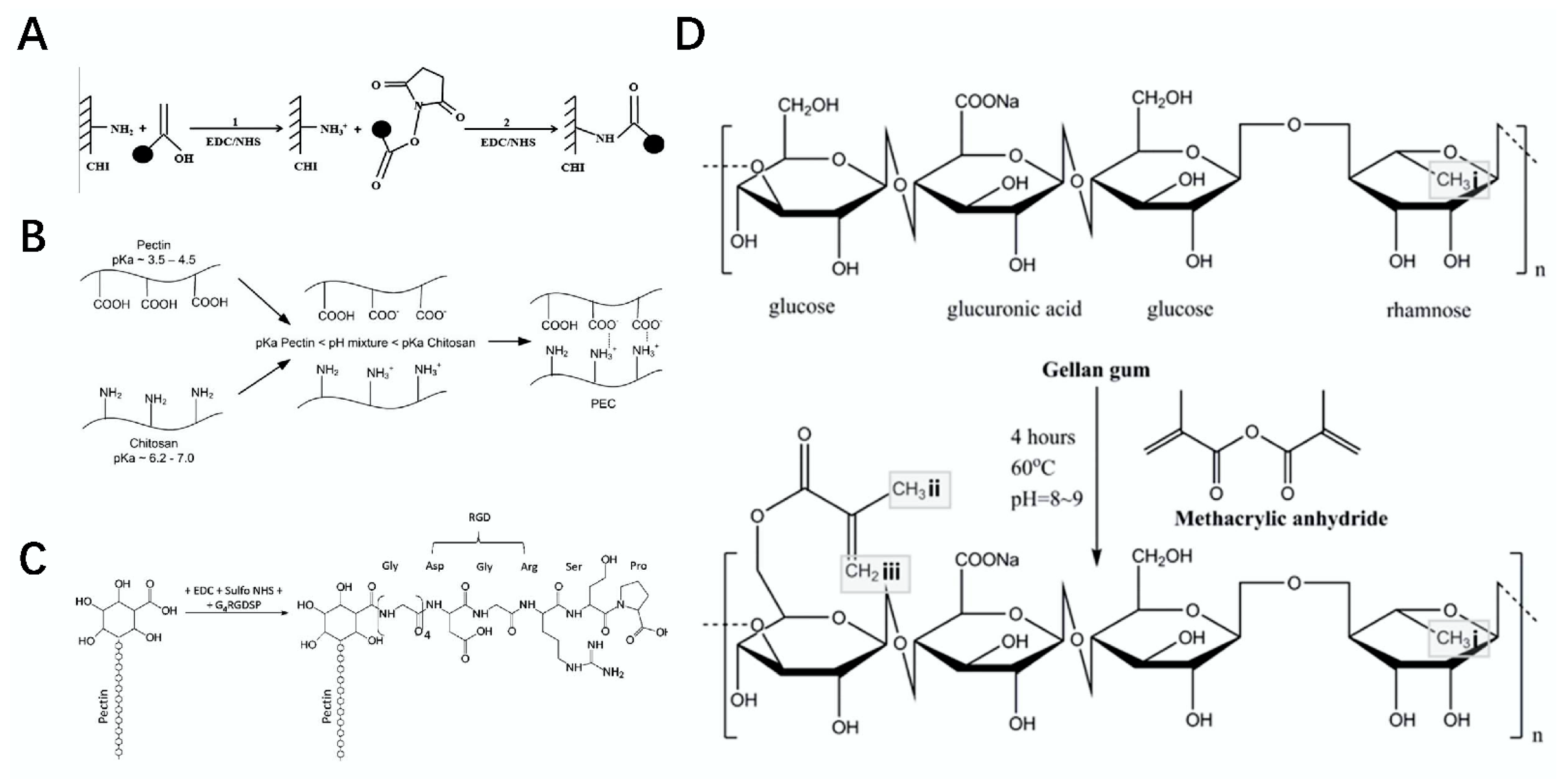
2.2. Chitosan
2.3. Hyaluronic Acid
2.4. Chondroitin Sulfate
2.5. Carrageenan
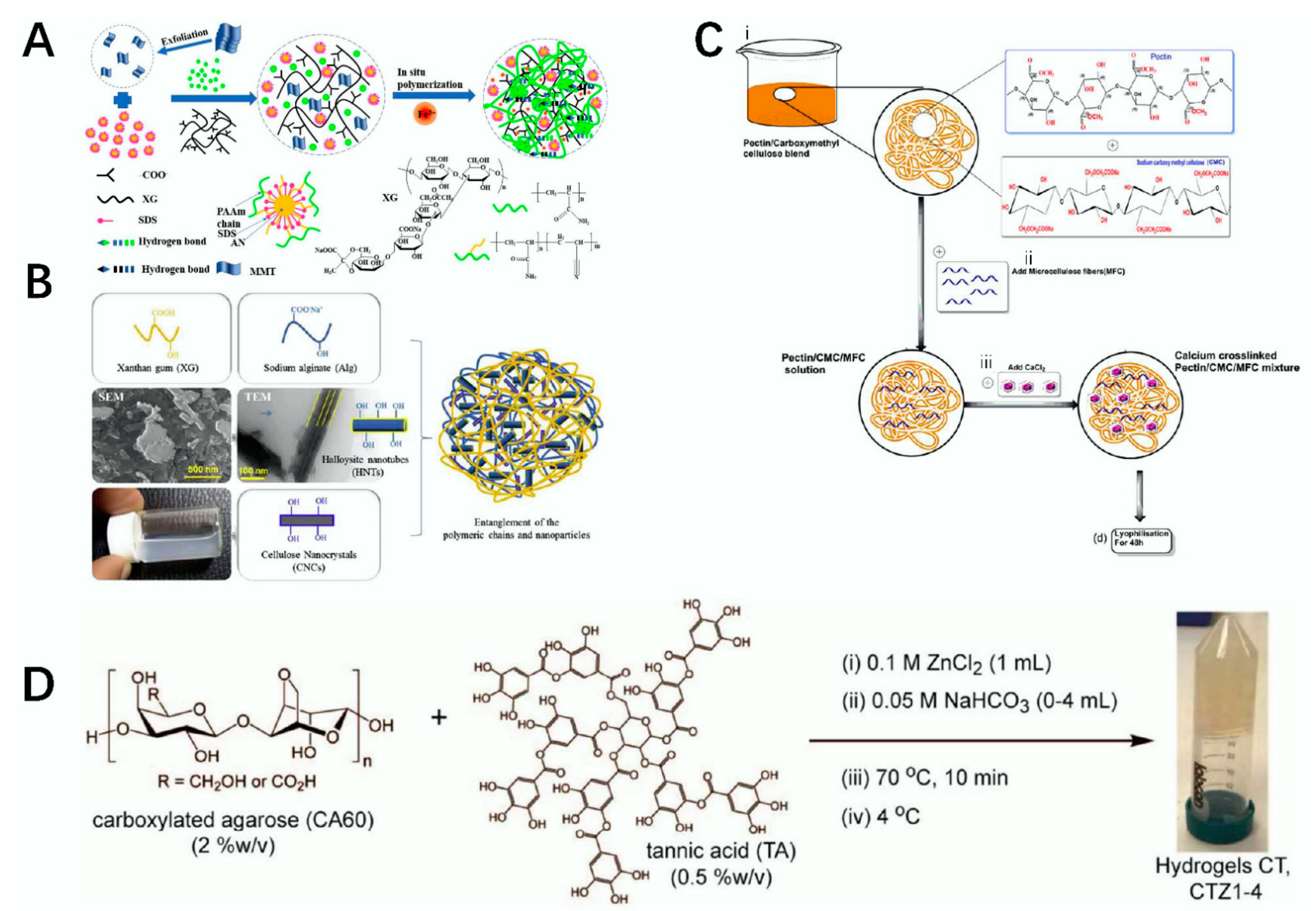
2.6. Xanthan Gum
2.7. Cellulose
2.8. Agarose
2.9. Heparin
2.10. Pectin
2.11. Gellan Gum
3. Processing Method of Polysaccharide Material
3.1. Amorphous Hydrogel
3.2. Microsphere
3.3. Membrane
3.4. Fiber
3.5. Microneedle
4. Tissue Engineering Application of Polysaccharide Material
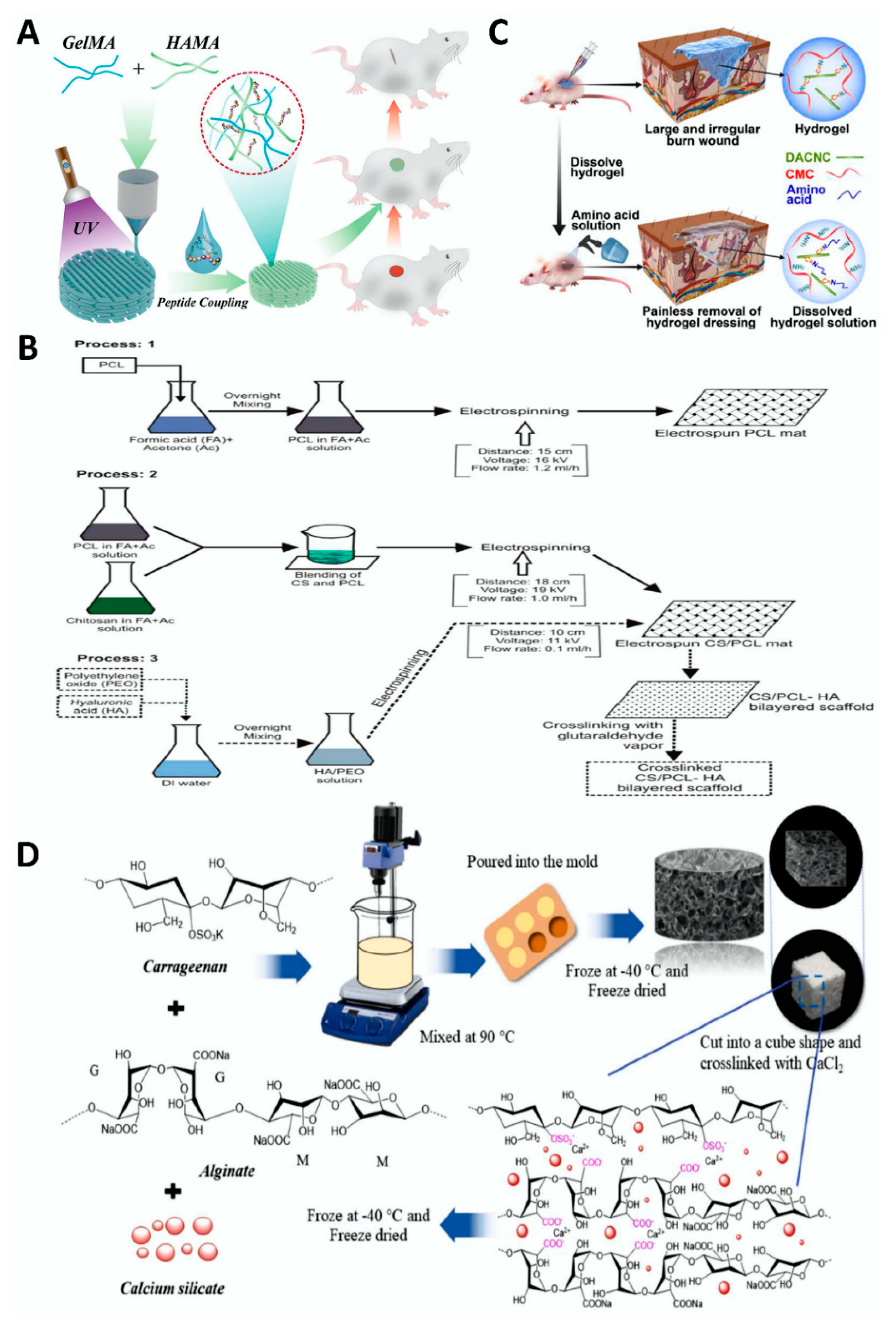
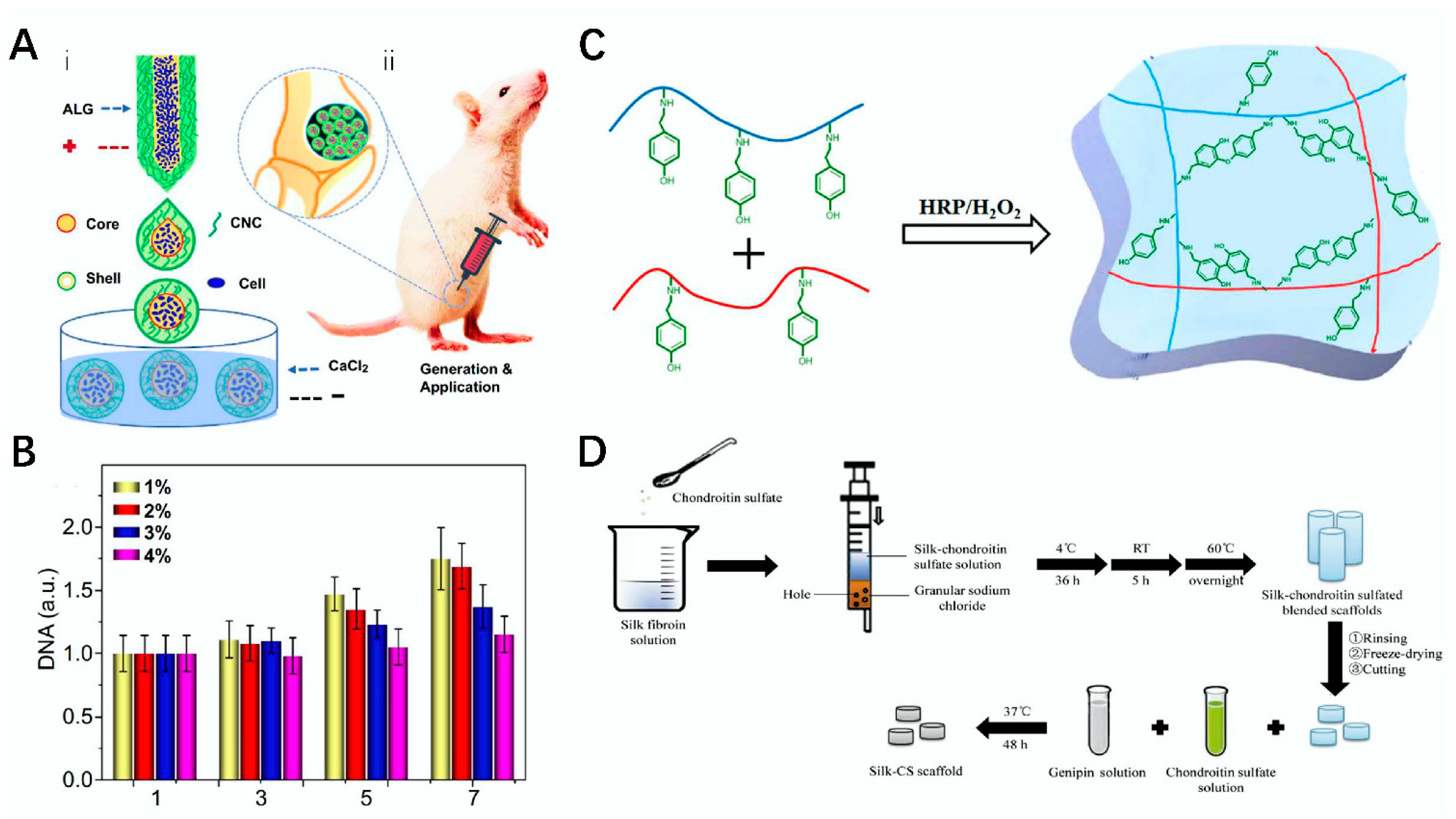
4.1. Skin Trauma
4.2. Bone Defect
4.3. Cartilage Repair
4.4. Arthritis
4.5. Cardiovascular System
4.6. Nervous System
4.7. Reproductive System
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cedric, D.; Andrée, F.; Philippe, M. Galactans: An overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Fang, D.; Yang, L.; Zhu, X.; Nie, J. Electrospun sodium alginate/poly(ethylene oxide) core–shell nanofibers scaffolds potential for tissue engineering applications. Carbohydr. Polym. 2012, 87, 737–743. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Cao, S.; Xu, G.; Li, Q.; Zhang, S.; Yang, Y.; Chen, J. Double crosslinking chitosan sponge with antibacterial and hemostatic properties for accelerating wound repair. Compos. B Eng. 2022, 234, 109746. [Google Scholar] [CrossRef]
- Pz, A.; Xp, B.; Bl, A.; Yl, A.; Hs, A.; Xl, A. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate—ScienceDirect. Carbohydr. Polym. 2022, 230, 115650. [Google Scholar] [CrossRef]
- Muzzarelli, R. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sakamoto, J.; Kimura, S. In vitro synthesis of cellulose and related polysaccharides. Prog. Polym. Sci. 2001, 26, 1525–1560. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Boddohi, S.; Kipper, M.J. Engineering Nanoassemblies of Polysaccharides. Adv. Mater. 2010, 22, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F. Xanthan gum: A versatile biopolymer for biomedical and technological applications. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Fuchs, S.; Ernst, A.U.; Wang, L.H.; Shariati, K.; Ma, M. Hydrogels in Emerging Technologies for Type 1 Diabetes. Chem. Rev. 2020, 121, 11458–11526. [Google Scholar] [CrossRef]
- Safikhani, M.M.; Zamanian, A.; Ghorbani, F.; Asefnejad, A.; Shahrezaee, M. Bi-layered electrospun nanofibrous polyurethane-gelatin scaffold with targeted heparin release profiles for tissue engineering applications. J. Polym. Eng. 2017, 37, 933–941. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Granja, P.L. Pectin-Based Injectable Biomaterials for Bone Tissue Engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Selvi, R.T.; Prakash, J.; Venkataprasanna, K.S.; Prema, D.; Pannerselvam, B.; Venkatasubbu, G.D. PVA/SA/TiO2-CUR patch for enhanced wound healing application: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2019, 138, 704–717. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 2019, 130, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kim, S.H.; Thambi, T.; You, D.G.; Jeon, J.; Lee, J.O.; Chung, B.Y.; Jo, D.G.; Park, J.H. A hyaluronic acid–methotrexate conjugate for targeted therapy of rheumatoid arthritis. Chem. Commun. 2014, 50, 7632–7635. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Raza, M.A.; Kadir, M.R.A.K.; Razak, S.I.A.; Shah, S.A.; Iqbal, Z.M.; Amin, R. Development and in vitro evaluation of κ-carrageenan based polymeric hybrid nanocomposite scaffolds for bone tissue engineering. RSC Adv. 2020, 10, 40529. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Kumar, M.; Muzzarelli, R.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Suzuki, M.; Itoh, S.; Yamaguchi, I.; Takakuda, K.; Kobayashi, H.; Shinomiya, K.; Tanaka, J. Tendon chitosan tubes covalently coupled with synthesized laminin peptides facilitate nerve regeneration in vivo. J. Neurosci. Res. 2003, 72, 646–659. [Google Scholar] [CrossRef]
- Gnavi, S.; Barwig, C.; Freier, T.; Haastert-Talini, K.; Geuna, S. The Use of Chitosan-Based Scaffolds to Enhance Regeneration in the Nervous System. Int. Rev. Neurobiol. 2013, 109, 1–62. [Google Scholar]
- Shao, J.; Ding, Z.; Li, L.; Chen, Y.; Zhu, J.; Qian, Q. Improved accumulation of TGF-β by photopolymerized chitosan/silk protein bio-hydrogel matrix to improve differentiations of mesenchymal stem cells in articular cartilage tissue regeneration—ScienceDirect. J. Photochem. Photobiol. B 2020, 203, 111744. [Google Scholar] [CrossRef]
- Yu, L.M.; Kazazian, K.; Shoichet, M.S. Peptide surface modification of methacrylamide chitosan for neural tissue engineering applications. J. Biomed. Mater. Res. Part A 2007, 82, 243–255. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, M.T.Z.; Zia, S.; Khalid, S.; Islam, A.; Sajid, I.; Khan, R.U.; King, M.W. Hybrid cross-linked hydrogels as a technology platform for in vitro release of cephradine. Polym. Adv. Technol. 2019, 30, 2414–2424. [Google Scholar] [CrossRef]
- Chang, K.Y.; Hung, L.H.; Chu, I.M.; Ko, C.S.; Lee, Y.D. The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part A. 2010, 92, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Vogus, D.; Zhao, Z.; He, W.; Krishnan, V.; Kim, J.; Shi, Y.; Sarode, A.; Ukidve, A.; Mitragotri, S. Injectable hyaluronic acid hydrogels encapsulating drug nanocrystals for long-term treatment of inflammatory arthritis. Bioeng. Transl. Med. 2022, 7, e10245. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, B.; Taraballi, F.; Minardi, S.; Van Eps, J.; Cabrera, F.; Francis, L.W.; Gazze, S.A.; Ferrari, M.; Weiner, B.K.; Tasciotti, E. Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Transl. Med. 2016, 5, 670–682. [Google Scholar] [CrossRef]
- Liu, J.; Su, C.; Chen, Y.; Tian, S.; Lu, C.; Huang, W.; Lv, Q. Current understanding of the Applications of photocrosslinked hydrogels in biomedical engineering. Gels 2022, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.H.; Kienitz, B.L.; Penick, K.J.; Welter, J.F.; Zawodzinski, T.A.; Baskaran, H. Concentrated collagen-chondroitin sulfate scaffolds for tissue engineering applications. J. Biomed. Mater. Res. Part A. 2010, 94, 1050–1060. [Google Scholar] [CrossRef]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; Williams, H.J. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef]
- Bauerova, K.; Ponist, S.; Kuncirova, V.; Mihalova, D.; Paulovicova, E.; Volpi, N. Chondroitin sulfate effect on induced arthritis in rats. Osteoarthr. Cartil. 2011, 19, 1373–1379. [Google Scholar] [CrossRef][Green Version]
- Ramanathan, Y.; Vignesh, S.; Sivashanmugam, A.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Moorthy, N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2017, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Hua, D.; Gao, S.; Zhang, M.; Ma, W.; Huang, C. A Novel Xanthan Gum-Based Conductive Hydrogel with Excellent Mechanical, Biocompatible, and Self-Healing Performances. Carbohydr. Polym. 2020, 247, 116743. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.-K.; Elain, A.; Thomas, S.; Grohens, Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013, 98, 877–885. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Anti-bacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Wu, L.; He, Y.; Mao, H.; Gu, Z. Bioactive hydrogels based on polysaccharides and peptides for soft tissue wound management. J. Mater. Chem. B. 2022, in press. [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Composites with Graphene for Tissue Engineering Applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef]
- Pooyan, P.; Tannenbaum, R.; Garmestani, H. Mechanical behavior of a cellulose-reinforced scaffold in vascular tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 7, 50–59. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Xiong, C. Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J. Biomed. Sci. 2009, 16, 65. [Google Scholar]
- Eeeeee Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ji, C.; Fu, R.; Yang, X.; Wan, Z.; Wen, L.; Song, Q.; Liu, Y.; Wang, Y.; Sai, H. Robust SiO2–Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue. Gels 2022, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Khanarian, N.T.; Haney, N.M.; Burga, R.A.; Lu, H.H. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials 2012, 33, 5247–5258. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Mojian, S.; Hongmei, W.; Lijuan, Z.; Wei, W. Low molecular weight heparins and their clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 163, 21–39. [Google Scholar]
- Aslani, S.; Kabiri, M.; Hosseinzadeh, S.; Hanaee-Ahvaz, H.; Soleimani, M. The applications of heparin in vascular tissue engineering. Microvasc. Res. 2020, 131, 104027. [Google Scholar] [CrossRef]
- Seib, F.P.; Herklotz, M.; Burke, K.A.; Maitz, M.F.; Werner, C.; Kaplan, D.L. Multifunctional silk–heparin biomaterials for vascular tissue engineering applications. Biomaterials 2014, 35, 83–91. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Fang, Z.; Xiao, R.; Yuan, W.; Khan, M. Fabrication and characterization of electrospun gelatin-heparin nanofibers as vascular tissue engineering. Macromol. Res. 2013, 21, 860–869. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, X.; Wang, X. Heparin inhibits the inflammation and proliferation of human rheumatoid arthritis fibroblastlike synoviocytes through the NFκB pathway. Mol. Med. Rep. 2016, 14, 3743–3748. [Google Scholar] [CrossRef]
- Gümüşderelioğlu, M.; Aday, S. Heparin-functionalized chitosan scaffolds for bone tissue engineering. Carbohydr. Res. 2011, 346, 606–613. [Google Scholar] [CrossRef]
- Coimbra, P.; Ferreira, P.; Sousa, H.; Batista, P.; Rodrigues, M.A.; Correia, I.J.; Gil, M.H. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef]
- Zihao, X.; Zhuqing, L.; Shan, J.; Bratlie, K.M. Chemically Modified Gellan Gum Hydrogels with Tunable Properties for Use as Tissue Engineering Scaffolds. ACS Omega 2018, 3, 6998–7007. [Google Scholar]
- Voragen, A.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Lerouge, P.; Quéméner, B. Mass spectrometry for pectin structure analysis. Carbohydr. Res. 2009, 344, 1798–1807. [Google Scholar] [CrossRef]
- Laurent, M.; Boulenguer, P. Stabilization mechanism of acid dairy drinks (ADD) induced by pectin. Food Hydrocolloid 2003, 17, 445–454. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Liu, L.S.; Won, Y.J.; Cooke, P.H.; Coffin, D.R.; Fishman, M.L.; Hicks, K.B.; Ma, P.X. Pectin/poly(lactide-co-glycolide) composite matrices for biomedical applications. Biomaterials 2004, 25, 3201–3210. [Google Scholar] [CrossRef]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; Panhuis, M. Tissue engineering with gellan gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Ma, B.; Xu, C.; Liu, H. Emerging Tumor-on-Chips with Electrochemical Biosensors. Trends Analyt Chem. 2022, 116640. [Google Scholar] [CrossRef]
- Amiri, F.; Babaei, M.; Jamshidi, N.; Agheb, M.; Rafienia, M.; Kazemi, M. Fabrication and assessment of a novel hybrid scaffold consisted of polyurethane-gellan gum-hyaluronic acid-glucosamine for meniscus tissue engineering. Int. J. Biol. Macromol. 2022, 203, 610–622. [Google Scholar] [CrossRef]
- Cerqueira, M.T.; da Silva, L.P.; Santos, T.C.; Pirraco, R.P.; Correlo, V.M.; Reis, R.L.; Marques, A.P. Gellan gum-hyaluronic acid spongy-like hydrogels and cells from adipose tissue synergize promoting neoskin vascularization. ACS Appl. Mater. Interfaces 2014, 6, 19668–19679. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Custódio, C.; Gasperini, L.; Reis, R.L.; Mano, J.F. Autonomous osteogenic differentiation of hASCs encapsulated in methacrylated gellan-gum hydrogels. Acta Biomater. 2016, 41, 119–132. [Google Scholar] [CrossRef]
- Almeida, H.V.; Eswaramoorthy, R.; Cunniffe, G.M.; Buckley, C.T.; O’Brien, F.J.; Kelly, D.J. Fibrin Hydrogels Functionalized with Particulated Cartilage Extracellular Matrix and Incorporating Freshly Isolated Stromal Cells as an Injectable for Cartilage Regeneration. Acta Biomater. 2016, 36, 55–62. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Yang, J.A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Meilander-Lin, N.J.; Cheung, P.J.; Wilson, D.L.; Bellamkonda, R.V. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Eng. 2005, 11, 546–555. [Google Scholar] [CrossRef]
- Nitta, S.; Kaketani, S.; Iwamoto, H. Development of chitosan-nanofiber-based hydrogels exhibiting high mechanical strength and pH-responsive controlled release. Eur. Polym. J. 2015, 67, 50–56. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, M.T.Z.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: A preclinical study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F.J. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 39, 1800069. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef]
- Lei, L.; Wang, X.; Zhu, Y.; Su, W.; Lv, Q.; Li, D. Antimicrobial hydrogel microspheres for protein capture and wound healing. Mater. Des. 2022, 215, 110478. [Google Scholar] [CrossRef]
- Stie, M.B.; Kalouta, K.; Vetri, V.; Foderà, V. Protein materials as sustainable non-and minimally invasive strategies for biomedical applications. J. Control. Release 2022, 344, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, G.; Wang, H.; Zhao, Y.; Chai, R. Bio-inspired intestinal scavenger from microfluidic electrospray for detoxifying lipopolysaccharide. Bioact. Mater. 2021, 6, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kong, T.; Yeung, K.; Shum, H.C.; Cheung, K.; Wang, L.; To, M. Fabrication and characterization of monodisperse PLGA–alginate core–shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013, 9, 7410–7419. [Google Scholar] [CrossRef]
- Wang, H.; Gu, H.; Chen, Z.; Shang, L.; Zhao, Z.; Gu, Z.; Zhao, Y. Enzymatic inverse opal hydrogel particles for biocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 12914–12918. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhu, Y.; Qin, X.; Chai, S.; Li, D. Magnetic biohybrid microspheres for protein purification and chronic wound healing in diabetic mice. Chem. Eng. J. 2021, 425, 130671. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Shi, H.; Gao, C.; Han, C. Chitosan-hyaluronic acid hybrid film as a novel wound dressing: In vitro and in vivo studies. Polym. Adv. Technol. 2007, 18, 869–875. [Google Scholar] [CrossRef]
- Duan, B.; Yuan, X.; Yi, Z.; Zhang, Y.; Li, X.; Yang, Z.; Yao, K. A nanofibrous composite membrane of PLGA–chitosan/PVA prepared by electrospinning. Eur. Polym. J. 2006, 42, 2013–2022. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater. Sci. Eng. C 2017, 80, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Despang, F.; Lode, A.; Gelinsky, M. Cell-laden biphasic scaffolds with anisotropic structure for the regeneration of osteochondral tissue. J. Tissue Eng. Regen. Med. 2016, 10, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H.; Zaidi, A.A. Natural fiber reinforced composites: Sustainable materials for emerging applications. Results Eng. 2021, 11, 100263. [Google Scholar] [CrossRef]
- Maeda, N.; Miao, J.; Simmons, T.J.; Dordick, J.S.; Linhardt, R.J. Composite polysaccharide fibers prepared by electrospinning and coating. Carbohydr. Polym. 2014, 102, 950–955. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of chitosan-containing nanofibres by electrospinning of chitosan/poly(ethylene oxide) blend solutions. e-Polym. 2004, 4, 624. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun Non-Woven Nanofibrous Hybrid Mats Based on Chitosan and PLA for Wound-Dressing Applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef]
- Iwasaki, N.; Yamane, S.-T.; Majima, T.; Kasahara, Y.; Minami, A.; Harada, K.; Nonaka, S.; Maekawa, N.; Tamura, H.; Tokura, S. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: Evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules 2004, 5, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, K.; Minato, K.-I.; Kumagai, G.; Hayashi, S.; Yamamoto, H. Chitosan nanofiber. Biomacromolecules 2006, 7, 3291–3294. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.-H.; Shi, X.-L.; Feng, Z.-Q.; Gu, Z.-Z.; Ding, Y.-T. Chitosan nanofiber scaffold enhances hepatocyte adhesion and function. Biotechnol. Lett. 2009, 31, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Su, B.B.; Jeong, J.E.; Su, A.P.; Park, W.H. Dual-crosslinked silk fibroin hydrogels with elasticity and cytocompatibility for the regeneration of articular cartilage. Polymer 2021, 224, 123739. [Google Scholar]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound dressings: A comprehensive review. Curr. Rheumatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhang, D.; Wu, X.; Zhao, Y.; Shang, L.; Ren, J.; Zhao, Y. Natural polysaccharide based complex drug delivery system from microfluidic electrospray for wound healing. Appl. Mater. Today 2021, 23, 101000. [Google Scholar] [CrossRef]
- Guan, G.; Lv, Q.; Liu, S.; Jiang, Z.; Zhou, C.; Liao, W. 3D-bioprinted peptide coupling patches for wound healing. Mater. Today Bio 2022, 13, 100188. [Google Scholar] [CrossRef]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef] [PubMed]
- Sathain, A.; Monvisade, P.; Siriphannon, P. Bioactive alginate/carrageenan/calcium silicate porous scaffolds for bone tissue engineering. Mater. Today Commun. 2021, 26, 102165. [Google Scholar] [CrossRef]
- Jing, W.; Smith, A.A.; Liu, B.; Li, J.; Helms, J.A. Reengineering autologous bone grafts with the stem cell activator WNT3A. Biomaterials 2015, 47, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Injectable and 3D bioprinted polysaccharide hydrogels: From cartilage to osteochondral tissue engineering. Biomacromolecules. 2017, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Basu, B.; Kumar, A. Physical and cytocompatibility properties of bioactive glass–polyvinyl alcohol–sodium alginate biocomposite foams prepared via sol–gel processing for trabecular bone regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Martino, A.D.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Poly (propylene fumarate)/polyethylene glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, R.; Shamekhi, M.A.; Mahmoudi, R.; Zolfagharian, A.; Asgari, F.; Mousavizadeh, A.; Bodaghi, M.; Hadi, A.; Haghighipour, N. In vitro static and dynamic cell culture study of novel bone scaffolds based on 3D-printed PLA and cell-laden alginate hydrogel. Biomed. Mater. 2022, 17, 045024. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Srinivasan, B.; Anbu, J.; Azamthulla, M.; Banala, V.T.; Ramachandra, S. Improvement of bone microarchitecture in methylprednisolone induced rat model of osteoporosis by using thiolated chitosan-based risedronate mucoadhesive film. Drug Dev. Ind. Pharm. 2018, 44, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Sun, L.; Zhao, C.; Chen, G.; Zhao, Y. Biomass microcapsules with stem cell encapsulation for bone repair. Nanomicro Lett. 2022, 14, 4. [Google Scholar] [CrossRef]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci. Rep. 2016, 6, 20014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S.; Jiang, Y.; Shen, W.; Zhang, S. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.; Wong, D.D.; Chao, P.-H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Mccullen, S.D.; Callanan, A.; Autefage, H.; Accardi, M.A.; Dini, D.; Stevens, M.M. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater. 2013, 10, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Dausse, Y.; Grossin, L.; Miralles, G.; Pelletier, S.; Mainard, D.; Hubert, P.; Baptiste, D.; Gillet, P.; Dellacherie, E.; Netter, P. Cartilage repair using new polysaccharidic biomaterials: Macroscopic, histological and biochemical approaches in a rat model of cartilage defect. Osteoarthr. Cartil. 2003, 11, 16–28. [Google Scholar] [CrossRef]
- KhaliliJafarabad, N.; Behnamghader, A.; Khorasani, M.T.; Mozafari, M. Platelet-rich plasma-hyaluronic acid/chondrotin sulfate/carboxymethyl chitosan hydrogel for cartilage regeneration. Biotechnol. Appl. Biochem. 2022, 69, 534–547. [Google Scholar] [CrossRef]
- Bauer, C.; Berger, M.; Baumgartner, R.R.; Höller, S.; Zwickl, H.; Niculescu-Morzsa, E.; Halbwirth, F.; Nehrer, S. A novel cross-linked hyaluronic acid porous scaffold for cartilage repair: An in vitro study with osteoarthritic chondrocytes. Cartilage 2016, 7, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yan, S.; Li, G.; Cui, L.; Yin, J. In-situ birth of MSCs multicellular spheroids in poly (L-glutamic acid)/chitosan scaffold for hyaline-like cartilage regeneration. Biomaterials 2015, 71, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of three-dimensional chitosan-agarose-gelatin cryogel scaffold for the repair of subchondral cartilage defects: An in vivo study in a rabbit model. Tissue Eng. Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Cho, M.-L.; Kim, K.-W.; Juhn, J.-Y.; Hwang, S.-Y.; Yoon, C.-H.; Park, S.-H.; Lee, S.-H.; Kim, H.-Y. Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF-κB-and p38 MAPK-dependent signalling pathways. Rheumatology 2007, 46, 57–64. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J.; Liang, J.; Xu, X.; Cui, W.; Deng, L.; Zhang, H. Bioinspired hyaluronic acid/phosphorylcholine polymer with enhanced lubrication and anti-inflammation. Biomacromolecules 2019, 20, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Shou, X.; Ni, D.; Kong, T.; Zhao, Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale 2020, 12, 17093–17102. [Google Scholar] [CrossRef]
- Yang, L.; Sun, L.; Zhang, H.; Bian, F.; Zhao, Y. Ice-inspired lubricated drug delivery particles from microfluidic electrospray for osteoarthritis treatment. ACS Nano 2021, 15, 20600–20606. [Google Scholar] [CrossRef]
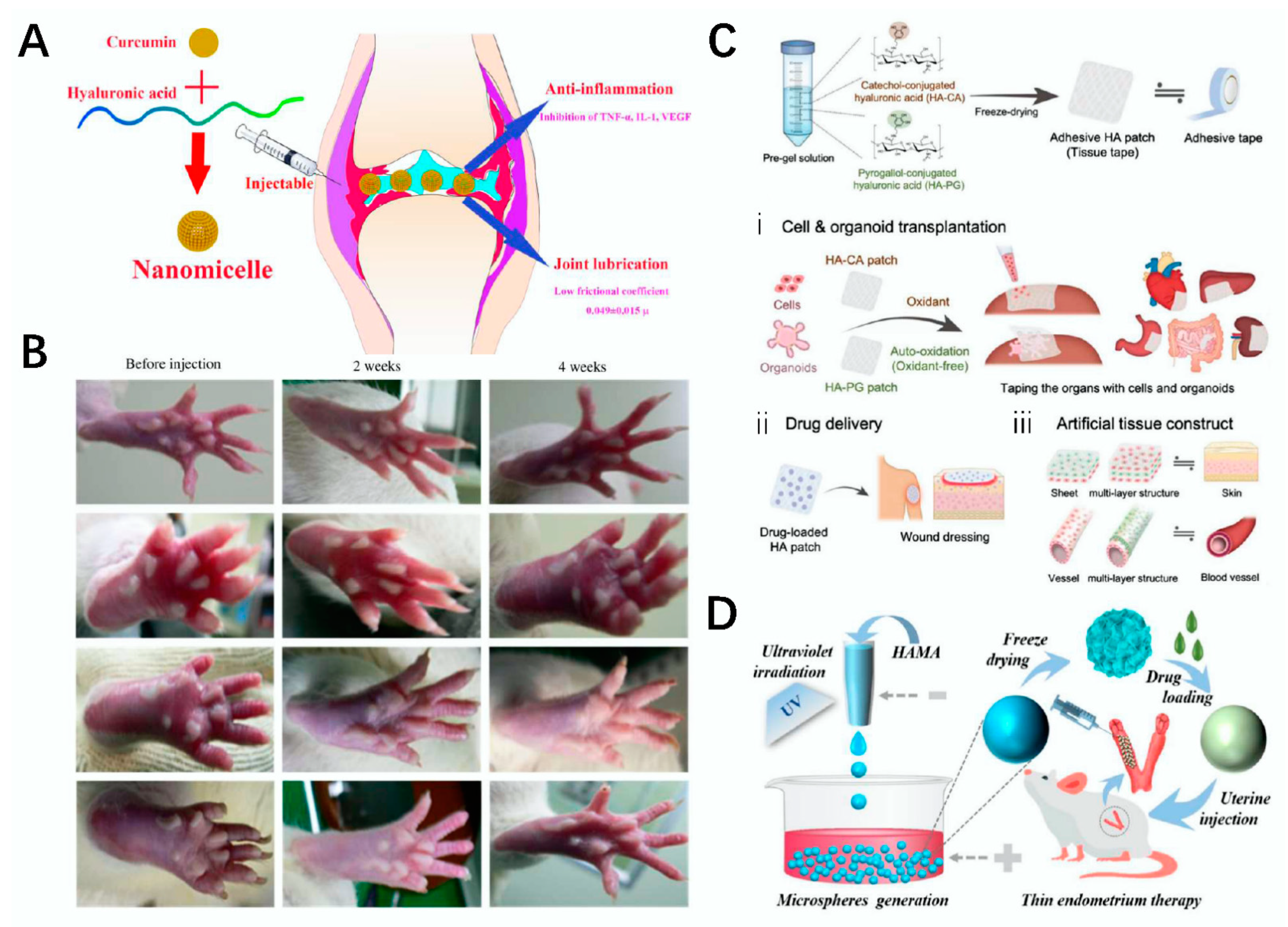
- Fan, Z.; Li, J.; Liu, J.; Jiao, H.; Liu, B. Anti-inflammation and joint lubrication dual effects of a novel hyaluronic acid/curcumin nanomicelle improve the efficacy of rheumatoid arthritis therapy. ACS Appl. Mater. Interfaces 2018, 10, 23595–23604. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.; Yang, J.-A.; Jeon, J.-H.; Bhang, S.; Kim, B.-S.; Hahn, S. Injectable hyaluronic acid–tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater. 2011, 7, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.; Kim, J.H.; Cho, J.H.; Jin, Y.; Kim, S.; Min, S.; Kim, S.K.; Choi, D.; Cho, S.W. Tissue Tapes—Phenolic hyaluronic acid hydrogel patches for off-the-shelf therapy. Adv. Funct. Mater. 2019, 29, 1903863. [Google Scholar] [CrossRef]
- Lei, L.; Lv, Q.; Jin, Y.; An, H.; Shi, Z.; Hu, G.; Yang, Y.; Wang, X.; Yang, L. Angiogenic microspheres for the treatment of a thin endometrium. ACS Biomater. Sci. Eng. 2021, 7, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef]
- Han, F.; Jia, X.; Dai, D.; Yang, X.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 2013, 34, 7302–7313. [Google Scholar] [CrossRef]
- Choi, W.S.; Joung, Y.K.; Lee, Y.; Bae, J.W.; Park, H.K.; Park, Y.H.; Park, J.-C.; Park, K.D. Enhanced patency and endothelialization of small-caliber vascular grafts fabricated by coimmobilization of heparin and cell-adhesive peptides. ACS Appl. Mater. Interfaces 2016, 8, 4336–4346. [Google Scholar] [CrossRef]
- Mukherjee, D.; Topol, E.J. The role of low-molecular–weight heparin in cardiovascular diseases. Prog. Cardiovasc. Dis. 2002, 45, 139–156. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, X.; Shen, J.; Jiang, B.; Hu, D.; Zhao, M. Heparin-binding protein: A novel biomarker linking four different cardiovascular diseases. Cardiol. Res. Pract. 2020, 2020, 9575373. [Google Scholar] [CrossRef]
- Ye, L.; Wu, X.; Duan, H.Y.; Geng, X.; Chen, B.; Gu, Y.Q.; Zhang, A.Y.; Zhang, J.; Feng, Z.G. The in vitro and in vivo biocompatibility evaluation of heparin–poly (ε-caprolactone) conjugate for vascular tissue engineering scaffolds. J. Biomed. Mater. Res. Part A 2012, 100, 3251–3258. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Yang, C.; Shao, C.; Shi, K.; Shang, L.; Ye, F.; Zhao, Y. Microfluidic 3D printing responsive scaffolds with biomimetic enrichment channels for bone regeneration. Adv. Funct. Mater. 2021, 31, 2105190. [Google Scholar] [CrossRef]
- Shaw, D.; Shoichet, M.S. Toward spinal cord injury repair strategies: Peptide surface modification of expanded poly (tetrafluoroethylene) fibers for guided neurite outgrowth in vitro. J. Craniofacial Surg. 2003, 14, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Böcker, A.; Däschler, S.C.; Kneser, U.; Harhaus, L. Relevance and recent developments of chitosan in peripheral nerve surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Matsumoto, I.; Kaneko, M.; Oda, M.; Watanabe, G. Repair of intra-thoracic autonomic nerves using chitosan tubes. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Matsumoto, I.; Suzuki, M.; Kaneko, M.; Nitta, K.; Seguchi, R.; Ooi, A.; Takemura, H. Chitosan tubes can restore the function of resected phrenic nerves. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 8–13. [Google Scholar] [CrossRef]
- Dai, C.; Hu, L.; Gong, F.; Tan, Y.; Cai, S.; Zhang, S.; Dai, J.; Lu, C.; Chen, J.; Chen, Y. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet. Med. 2019, 21, 431–440. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaman, S.; Sajjad, M.; Shoaib, M.; Gilani, G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int. J. Appl. Basic Med. Res. 2011, 1, 93. [Google Scholar] [CrossRef]
- Tamadon, A.; Park, K.-H.; Kim, Y.Y.; Kang, B.-C.; Ku, S.-Y. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng. Regen. Med. 2016, 13, 447–454. [Google Scholar] [CrossRef]
- Paulini, F.; Vilela, J.M.; Chiti, M.C.; Donnez, J.; Jadoul, P.; Dolmans, M.-M.; Amorim, C.A. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. BioMed. Online 2016, 33, 425–432. [Google Scholar] [CrossRef]
- Mousset-Siméon, N.; Jouannet, P.; Le Cointre, L.; Coussieu, C.; Poirot, C. Comparison of three in vitro culture systems for maturation of early preantral mouse ovarian follicles. Zygote 2005, 13, 167–175. [Google Scholar] [CrossRef]
- Desai, N.; Abdelhafez, F.; Calabro, A.; Falcone, T. Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: A preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod. Biol. Endocrinol. 2012, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, D.; Li, W.; Yang, L. Emerging biomaterials for reproductive medicine. Eng. Regen. 2021, 2, 230–245. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jiang, H.-L.; Jere, D.; Park, I.-K.; Cho, M.-H.; Nah, J.-W.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog. Polym. Sci. 2007, 32, 726–753. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.l.; Zhang, Y.; Wang, Y.q.; Xu, W.m.; Yang, J. The Immunogenicity of CRISP 1 Plasmid-Based Contraceptive Vaccine can be Improved When Using Chitosan Nanoparticles as the Carrier. Am. J. Reprod. Immunol. 2016, 75, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermo-responsive polymers as novel biomaterials. Macromol. Res. 2006, 6, 991–1008. [Google Scholar] [CrossRef]




| Name of Polysaccharide | Structural Formula | References |
|---|---|---|
| Sodium alginate | 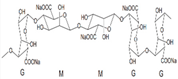 | [12] |
| Chitosan | 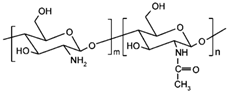 | [3] |
| Hyaluronic acid | 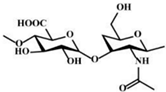 | [3] |
| 6-chondroitin sulfate | 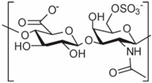 | [13] |
| κ-carrageenan |  | [5] |
| Xanthan gum | 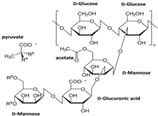 | [14] |
| Cellulose | 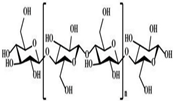 | [3] |
| Agarose |  | [15] |
| Heparin |  | [16] |
| Pectin |  | [17] |
| Gellan gum | 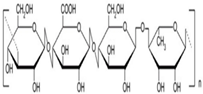 | [18] |
| Types | Amorphous Hydrogel | Microspheres | Membrane | Fiber | Microneedles |
|---|---|---|---|---|---|
| Schematic diagram |  |  |  |  |  |
| Security | Safety | Higher security | Safety | Low toxic | Safety |
| Processing difficulty | Easy | Easy | Easy | Easy | Easy |
| Biocompatibility | Good | Good | Good | Good | Aponia |
| Trauma | Small | Small | Small | Invasive small | Traumatic small |
| Other characteristics | Injectable | Low toxic | Selectivity | Workability | Invasive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Chen, Y.; Tian, S.; Lu, C.; Lv, Q. Research Progress on Emerging Polysaccharide Materials Applied in Tissue Engineering. Polymers 2022, 14, 3268. https://doi.org/10.3390/polym14163268
Su C, Chen Y, Tian S, Lu C, Lv Q. Research Progress on Emerging Polysaccharide Materials Applied in Tissue Engineering. Polymers. 2022; 14(16):3268. https://doi.org/10.3390/polym14163268
Chicago/Turabian StyleSu, Chunyu, Yutong Chen, Shujing Tian, Chunxiu Lu, and Qizhuang Lv. 2022. "Research Progress on Emerging Polysaccharide Materials Applied in Tissue Engineering" Polymers 14, no. 16: 3268. https://doi.org/10.3390/polym14163268
APA StyleSu, C., Chen, Y., Tian, S., Lu, C., & Lv, Q. (2022). Research Progress on Emerging Polysaccharide Materials Applied in Tissue Engineering. Polymers, 14(16), 3268. https://doi.org/10.3390/polym14163268






