Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review
Abstract
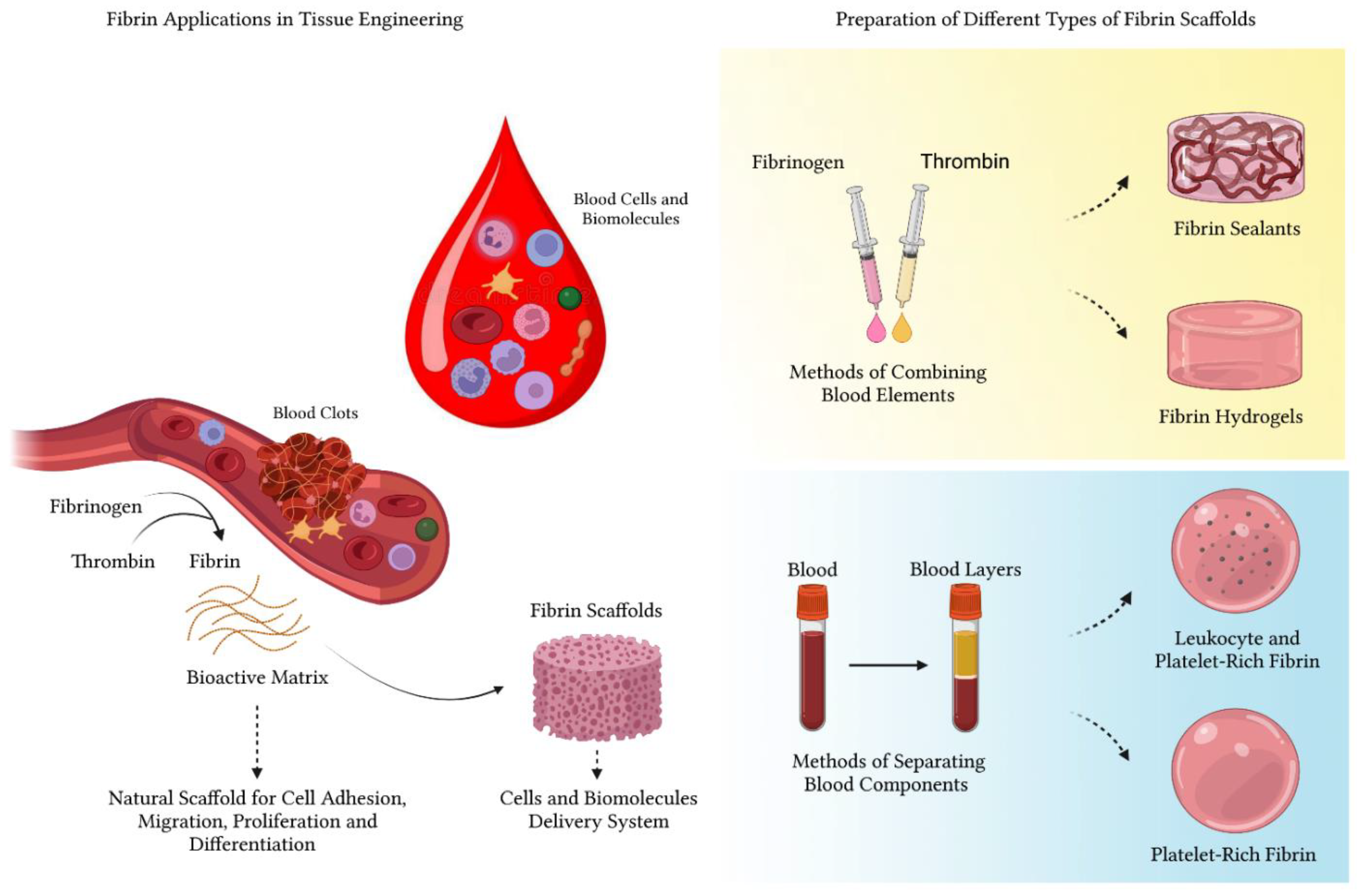
:1. Introduction
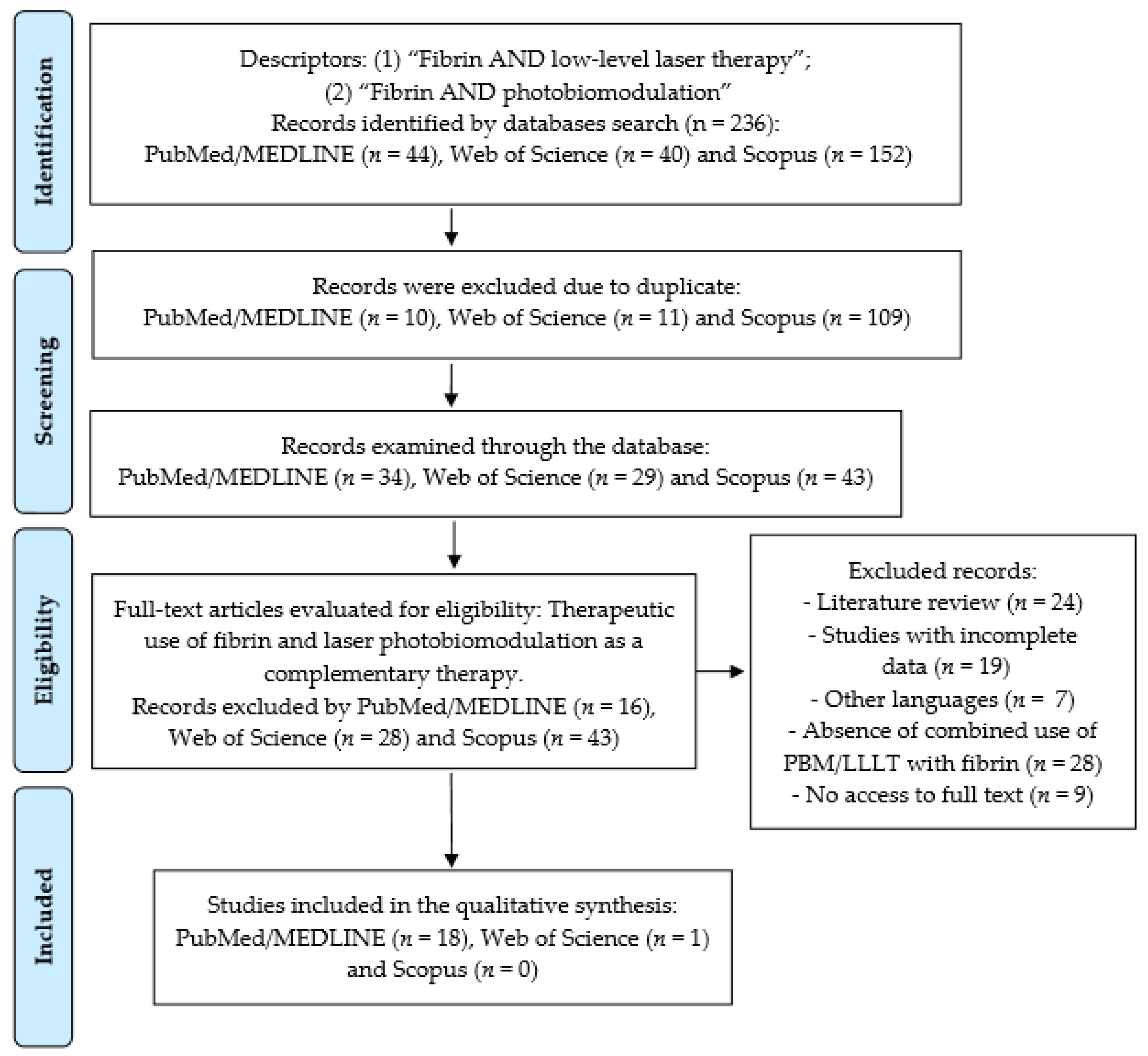
2. Materials and Methods
- -
- Eligibility Criteria:The inclusion criteria were:
- Therapeutic use of fibrin and PBM therapy as complementary therapy;
- Studies in humans;
- Studies in animals;
- In vivo studies;
- Case reports;
- Publications only in English and that allowed full access to the text;
- Each article included must present data on the PBM protocol.
- -
- The exclusion criteria were:
- Articles that were duplicated;
- When the title had no connection to the objective;
- Did not use fibrin;
- Did not use photobiomodulation;
- Used high power laser;
- Other languages (except English);
- When access to the full text was not obtained;
- Incomplete data on the type of fibrin used.
- Letters to the editor;
- Review papers;
- Commentaries;
- Unpublished abstracts;
- Dissertations or theses from repositories
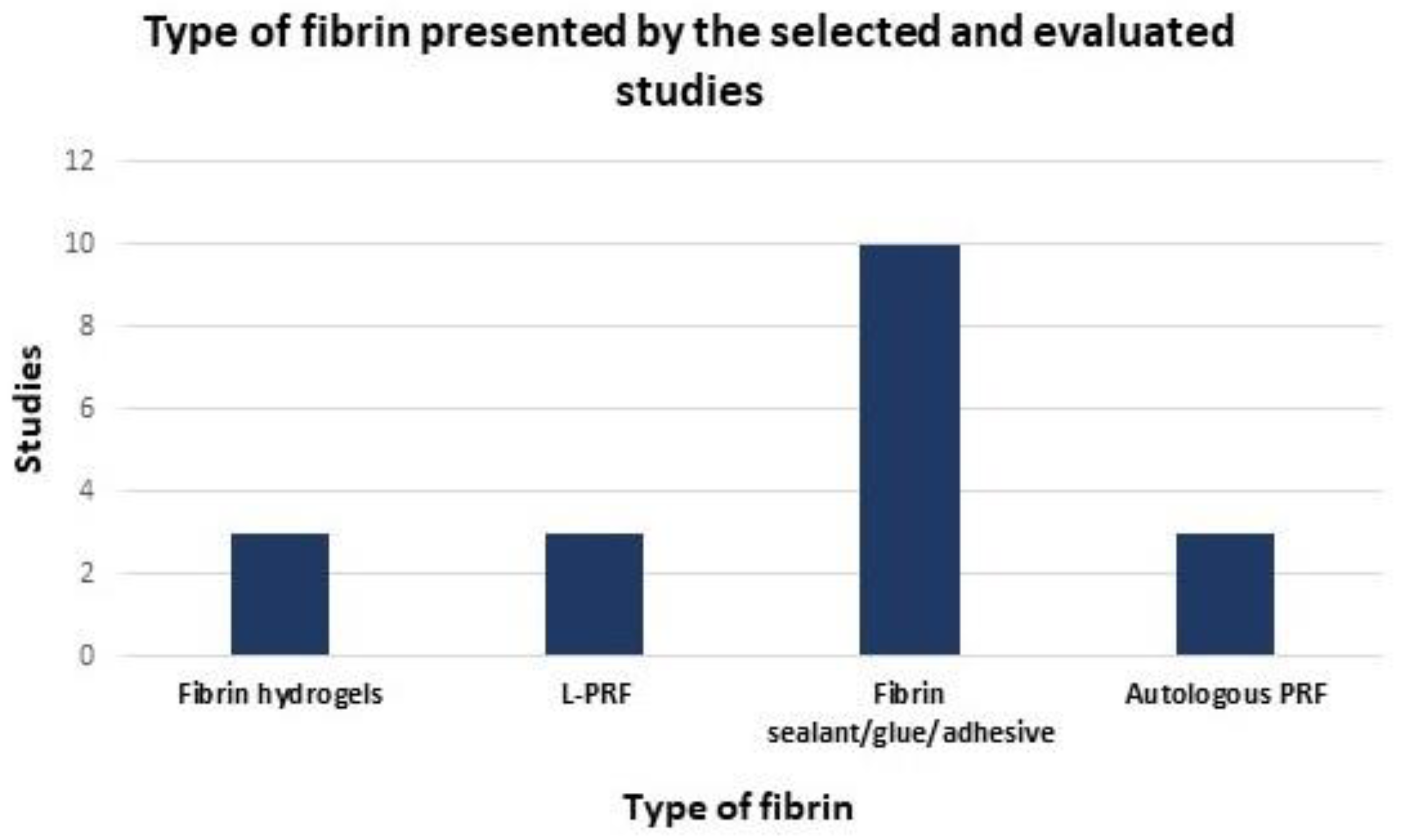
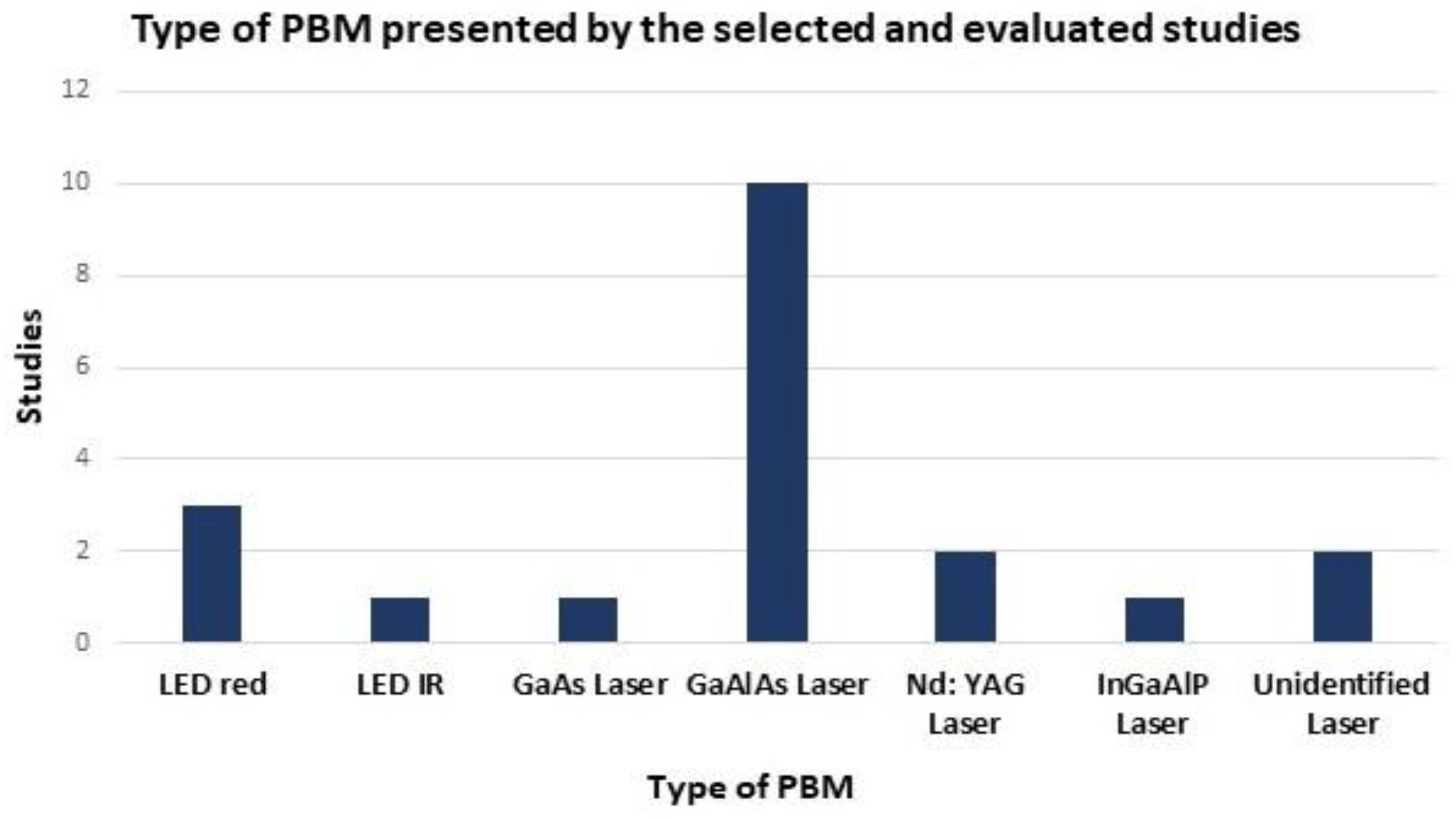
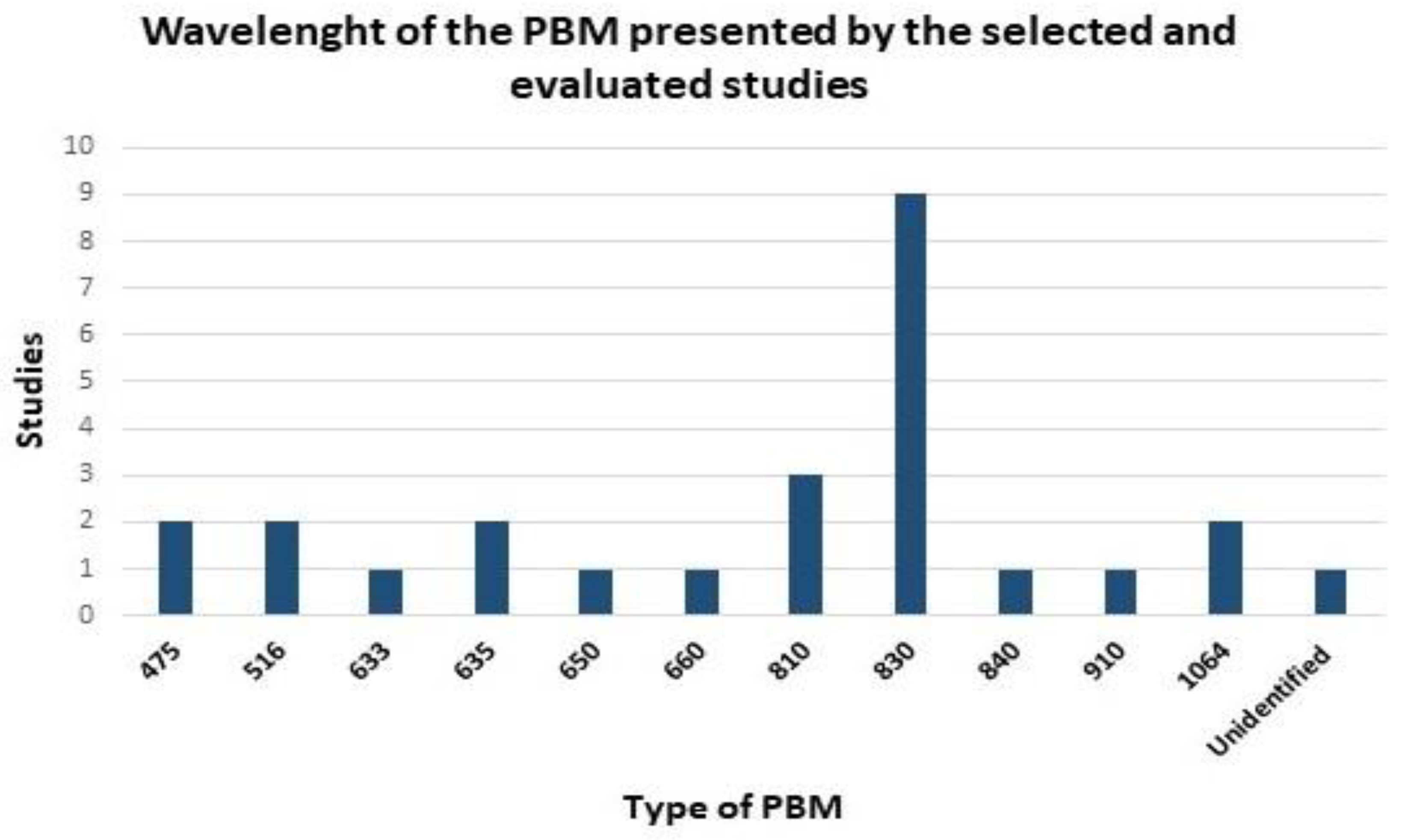
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin(ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahani-Sherafat, S.; Mokmeli, S.; Rostami-Nejad, M.; Razzaghi, Z.; Tavirani, M.R.; Razzaghi, M. The Effectiveness of Photobiomudulation Therapy (Pbmt) in COVID-19 Infection. J. Lasers Med. Sci. 2020, 11, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, R.I.; Pieters, M.; de Lange-Loots, Z.; Weisel, J.W. Fibrinogen and Fibrin. In Macromolecular Protein Complexes III: Structure and Function. Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2021; pp. 471–501. [Google Scholar]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, W. Fixation of platelet-rich plasma and fibrin gels on knee cartilage defects after microfracture with arthroscopy. Int. Orthop. 2022, 474. [Google Scholar] [CrossRef]
- Grecu, A.F.; Reclaru, L.; Ardelean, L.C.; Nica, O.; Ciucă, E.M.; Ciurea, M.E. Platelet-rich fibrin and its emerging therapeutic benefits for musculoskeletal injury treatment. Medicine 2019, 55, 141. [Google Scholar] [CrossRef] [Green Version]
- Vasilikos, I.; Beck, J.; Ghanaati, S.; Grauvogel, J.; Nisyrios, T.; Grapatsas, K.; Hubbe, U. Integrity of dural closure after autologous platelet rich fibrin augmentation: An in vitro study. Acta Neurochir. 2020, 162, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.V.T.C.; Coletta, B.B.D.; Pomini, K.T.; De Oliveira Rosso, M.P.; Campos, L.M.G.; Ferreira, R.S.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, 1–15. [Google Scholar] [CrossRef]
- Rosso, M.P.d.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation therapy (PBMT) in peripheral nerve regeneration: A systematic review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Dionisi, L.; Pizzicannella, J.; Kothari, A.; De Fazio, D.; Garcovich, S. Impact of the different preparation methods to obtain autologous non-activated platelet-rich plasma (A-PRP) and activated platelet-rich plasma (AA-PRP) in plastic surgery: Wound healing and hair regrowth evaluation. Int. J. Mol. Sci. 2020, 21, 431. [Google Scholar] [CrossRef] [Green Version]
- Bressan, E.; Favero, V.; Gardin, C.; Ferroni, L.; Iacobellis, L.; Favero, L.; Vindigni, V.; Berengo, M.; Sivolella, S.; Zavan, B. Biopolymers for Hard and Soft Engineered Tissues: Application in Odontoiatric and Plastic Surgery Field. Polymers 2011, 3, 509–526. [Google Scholar] [CrossRef]
- Pepelassi, E.; Deligianni, M. The Adjunctive Use of Leucocyte-and Platelet-Rich Fibrin in Periodontal Endosseous and Furcation Defects: A Systematic Review and Meta-Analysis. Materials 2022, 15, 2088. [Google Scholar] [CrossRef] [PubMed]
- Caramês, J.M.M.; Vieira, F.A.; Caramês, G.B.; Pinto, A.C.; Francisco, H.C.O.; Marques, D.N.D.S. Guided Bone Regeneration in the Edentulous Atrophic Maxilla Using Deproteinized Bovine Bone Mineral (DBBM) Combined with Platelet-Rich Fibrin (PRF)—A Prospective Study. J. Clin. Med. 2022, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Lee, J.H.; Wadhwa, P.; Jiang, H.B.; Jang, H.S.; Lee, E.S. Bone regeneration in peri-implant defect using autogenous tooth biomaterial enriched with platelet-rich fibrin in animal model. Appl. Sci. 2020, 10, 1939. [Google Scholar] [CrossRef] [Green Version]
- Tallarico, M.; Xhanari, E.; Lumbau, A.M.I.; Alushi, A.; Ieria, I.; Fiorillo, L.; Famà, F.; Meto, A.; Baldoni, E.; Meloni, S.M.; et al. Histological and histomorphometric evaluation of post-extractive sites filled with a new bone substitute with or without autologous plate concentrates: One-year randomized controlled trial. Materials 2022, 15, 254. [Google Scholar] [CrossRef]
- Li, Q.; Reed, D.A.; Min, L.; Gopinathan, G.; Li, S.; Dangaria, S.J.; Li, L.; Geng, Y.; Galang, M.T.; Gajendrareddy, P.; et al. Lyophilized Platelet-Rich Fibrin (PRF) promotes craniofacial bone regeneration through Runx2. Int. J. Mol. Sci. 2014, 15, 8509–8525. [Google Scholar] [CrossRef]
- Zumarán, C.C.; Parra, M.V.; Olate, S.A.; Fernández, E.G.; Muñoz, F.T.; Haidar, Z.S. The 3 R’s for platelet-rich fibrin: A “super” tri-dimensional biomaterial for contemporary naturally-guided oro-maxillo-facial soft and hard tissue repair, reconstruction and regeneration. Materials 2018, 11, 1239. [Google Scholar] [CrossRef] [Green Version]
- Almeida Barros Mourão, C.F.D.; Valiense, H.; Melo, E.R.; Freitas Mourão, N.B.M.; Maia, M.D.C. Obtenção da fibrina rica em plaquetas injetável (I-PRF) e sua polimerização com enxerto ósseo: Nota técnica. Rev. Col. Bras. Cir. 2015, 42, 421–423. [Google Scholar] [CrossRef] [Green Version]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. Prp and prf—Subgroups and divisions when used in dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Ikumi, A.; Gingery, A.; Toyoshima, Y.; Zhao, C.; Moran, S.L.; Livia, C.; Rolland, T.; Peterson, T.; Sabbah, M.S.; Boroumand, S.; et al. Administration of Purified Exosome Product in a Rat Sciatic Serve Reverse Autograft Model. Plast. Reconstr. Surg. 2021, 148, 200e–211e. [Google Scholar] [CrossRef]
- Ardjomandi, N.; Duttenhoefer, F.; Xavier, S.; Oshima, T.; Kuenz, A.; Sauerbier, S. In vivo comparison of hard tissue regeneration with ovine mesenchymal stem cells processed with either the FICOLL method or the BMAC method. J. Craniomaxillofac. Surg. 2015, 43, 1177–1183. [Google Scholar] [CrossRef]
- Mittermayr, R.; Branski, L.; Moritz, M.; Jeschke, M.G.; Herndon, D.N.; Traber, D.; Schense, J.; Gampfer, J.; Goppelt, A.; Redl, H. Fibrin biomatrix-conjugated platelet-derived growth factor AB accelerates wound healing in severe thermal injury. J. Tissue Eng. Regen. Med. 2016, 10, E275–E285. [Google Scholar] [CrossRef]
- Hidd, S.M.C.M.; Tim, C.R.; Dutra, E.F., Jr.; Maia Filho, A.L.M.; Assis, L.; Ferreira, R.S., Jr.; Barraviera, B.; Silva, J.F.; Amaral, M.M. Fibrin biopolymer sealant and aquatic exercise association for calcaneal tendon repair. Acta Cir. Bras. 2021, 36, e360407. [Google Scholar] [CrossRef]
- Canonico, S. The use of human fibrin glue in the surgical operations. Acta Biomed. 2003, 74, 21–25. [Google Scholar]
- Su, Y.Y.; Lin, Y.S.; Yang, L.Y.; Pan, Y.B.; Huang, Y.T.; Weng, C.H.; Wu, K.Y.; Wang, C.J. Use of human fibrin glue (Tisseel) versus suture during transvaginal natural orifice ovarian cystectomy of benign and non-endometriotic ovarian tumor: A retrospective comparative study. BMC Surg. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Ferreira, R.S.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Pontes, L.G.; dos Santos, L.D.; Barraviera, B. Heterologous fibrin sealant derived from snake venom: From bench to bedside—An overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Photobiomodulation or low-level laser therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; De Oliveira, A.L.R.; De Castro Rodrigues, A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef]
- Iatecola, A.; Barraviera, B.; Ferreira, R.S., Jr.; dos Santos, G.R.; Neves, J.I.; da Cunha, M.R. Use of a new fibrin sealant and laser irradiation in the repair of skull defects in rats. Braz. Dent. J. 2013, 24, 456–461. [Google Scholar] [CrossRef]
- Santos, C.M.D.C.; Pimenta, C.A.D.M.; Nobre, M.R.C. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enfermagem 2007, 15, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Garcovich, S. Systematic review—The potential implications of different platelet-rich plasma (Prp) concentrations in regenerative medicine for tissue repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef]
- Rosso, M.P.D.O.; Buchaim, D.V.; Pomini, K.T.; Coletta, B.B.D.; Reis, C.H.B.; Pilon, J.P.G.; Júnior, G.D.; Buchaim, R.L. Photobiomodulation therapy (PBMT) applied in bone reconstructive surgery using bovine bone grafts: A systematic review. Materials 2019, 12, 4051. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.d.C.; Fideles, S.O.M.; Pomini, K.T.; Bellini, M.Z.; Pereira, E.S.B.M.; Reis, C.H.B.; Pilon, J.P.G.; de Marchi, M.Â.; Trazzi, B.F.d.M.; da Silva, W.S.; et al. Potential of Fibrin Glue and Mesenchymal Stem Cells (MSCs) to Regenerate Nerve Injuries: A Systematic Review. Cells 2022, 11, 221. [Google Scholar] [CrossRef]
- Ortiz, A.d.C.; Fideles, S.O.M.; Pomini, K.T.; Reis, C.H.B.; Bueno, C.R.d.S.; Pereira, E.d.S.B.M.; Rossi, J.d.O.; Novais, P.C.; Pilon, J.P.G.; Rosa Junior, G.M.; et al. Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review. Cells 2021, 10, 2323. [Google Scholar] [CrossRef]
- Bikmulina, P.Y.; Kosheleva, N.V.; Shpichka, A.I.; Efremov, Y.M.; Yusupov, V.I.; Timashev, P.S.; Rochev, Y.A. Beyond 2D: Effects of photobiomodulation in 3D tissue-like systems. J. Biomed. Opt. 2020, 25, 048001. [Google Scholar] [CrossRef]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of medication-related osteonecrosis of the jaw (MRONJ) using leukocyte-and platelet-rich fibrin (l-PRF) and photobiomodulation: A retrospective study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, J.B.; Buchaim, D.V.; de Souza Bueno, C.R.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; de Castro Rodrigues, A.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2016, 162, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, D.V.; Andreo, J.C.; Ferreira Junior, R.S.; Barraviera, B.; De Castro Rodrigues, A.; De Cássia MacEdo, M.; Rosa Junior, G.M.; Shinohara, A.L.; German, I.J.S.; Pomini, K.T.; et al. Efficacy of Laser Photobiomodulation on Morphological and Functional Repair of the Facial Nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef]
- Rohringer, S.; Holnthoner, W.; Chaudary, S.; Slezak, P.; Priglinger, E.; Strassl, M.; Pill, K.; Mühleder, S.; Redl, H.; Dungel, P. The impact of wavelengths of LED light-therapy on endothelial cells. Sci. Rep. 2017, 7, 11061. [Google Scholar] [CrossRef]
- Priglinger, E.; Maier, J.; Chaudary, S.; Lindner, C.; Wurzer, C.; Rieger, S.; Redl, H.; Wolbank, S.; Dungel, P. Photobiomodulation of freshly isolated human adipose tissue-derived stromal vascular fraction cells by pulsed light-emitting diodes for direct clinical application. J. Tissue Eng. Regen. Med. 2018, 12, 1352–1362. [Google Scholar] [CrossRef]
- Pomini, K.T.; Buchaim, D.V.; Andreo, J.C.; Rosso, M.P.; Della Coletta, B.B.; German, Í.J.S.; Biguetti, A.C.C.; Shinohara, A.L.; Rosa Júnior, G.M.; Shindo, J.V.T.C.; et al. Fibrin sealant derived from human plasma as a scaffold for bone grafts associated with photobiomodulation therapy. Int. J. Mol. Sci. 2019, 20, 1761. [Google Scholar] [CrossRef] [Green Version]
- Hemaid, S.; Saafan, A.; Hosny, M.; Wimmer, G. Enhancement of healing of periodontal intrabony defects using 810 nm diode laser and different advanced treatment modalities: A blind experimental study. Open Access Maced. J. Med. Sci. 2019, 7, 1847–1853. [Google Scholar] [CrossRef] [Green Version]
- Şahin, O.; Tatar, B.; Ekmekcioğlu, C.; Aliyev, T.; Odabaşi, O. Prevention of medication related osteonecrosis of the jaw after dentoalveolar surgery: An institution’s experience. J. Clin. Exp. Dent. 2020, 12, e771–e776. [Google Scholar] [CrossRef]
- Thalaimalai, D.B.R.; Victor, D.J.; Prakash, P.S.G.; Subramaniam, S.; Cholan, P.K. Effect of Low-Level Laser Therapy and Platelet-Rich Fibrin on the Treatment of Intra-bony Defects. J. Lasers Med. Sci. 2020, 11, 456–463. [Google Scholar] [CrossRef]
- Della Coletta, B.B.; Jacob, T.B.; Moreira, L.A.d.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; Pereira, E.d.S.B.M.; Roque, D.D.; Rosso, M.P.d.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. [Google Scholar] [CrossRef]
- Şahin, O.; Akan, E.; Tatar, B.; Ekmekcioğlu, C.; Ünal, N.; Odabaşı, O. Combined approach to treatment of advanced stages of medication-related osteonecrosis of the jaw patients. Braz. J. Otorhinolaryngol. 2021, 88, 613–620. [Google Scholar] [CrossRef]
- de Freitas Dutra Júnior, E.; Hidd, S.M.C.M.; Amaral, M.M.; Filho, A.L.M.M.; Assis, L.; Ferreira, R.S.; Barraviera, B.; Martignago, C.C.S.; Figueredo-Silva, J.; de Oliveira, R.A.; et al. Treatment of partial injury of the calcaneus tendon with heterologous fibrin biopolymer and/or photobiomodulation in rats. Lasers Med. Sci. 2022, 37, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Andreo, J.C.; Pomini, K.T.; Barraviera, B.; Ferreira, R.S.; Duarte, M.A.H.; Alcalde, M.P.; Reis, C.H.B.; de Bortoli Teixeira, D.; de Souza Bueno, C.R.; et al. A biocomplex to repair experimental critical size defects associated with photobiomodulation therapy. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.P.d.O.; Rosa Júnior, G.M.; Buchaim, D.V.; German, I.J.S.; Pomini, K.T.; de Souza, R.G.; Pereira, M.; Favaretto Júnior, I.A.; Bueno, C.R.d.S.; Gonçalves, J.B.d.O.; et al. Stimulation of morphofunctional repair of the facial nerve with photobiomodulation, using the end-to-side technique or a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2017, 175, 20–28. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Rosso, M.P.; Oyadomari, A.T.; Pomini, K.T.; Coletta, B.B.D.; Shindo, J.V.T.C.; Júnior, R.S.F.; Barraviera, B.; Cassaro, C.V.; Buchaim, D.V.; Teixeira, D.d.B.; et al. Photobiomodulation therapy associated with heterologous fibrin biopolymer and bovine bone matrix helps to reconstruct long bones. Biomolecules 2020, 10, 383. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, D.V.; Rodrigues, A.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.F.; Junior, G.M.R.; Bueno, C.R.S.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, N.V.; Huynh, T.Q.; Tran, S.; Wang, G.; Hamlet, S.; Dau, V.; Dao, D.; Nguyen, N.T.; Nguyen, H.T.; Doan, J.; et al. Multidisciplinary approach to maximize angiogenesis and wound healing using piezoelectric surgery, concentrated growth factors and photobiomodulation for dental implant placement surgery involving lateral wall sinus lift: Two case reports. Vasc. Cell 2020, 12, 2. [Google Scholar] [CrossRef]
- Macfarlane, R.G. An Enzyme Cascade in the Blood Clotting Mechanism, and its Function as a Biochemical Amplifier. Nature 1964, 202, 498–499. [Google Scholar] [CrossRef]
- Davie, E.W.; Ratnoff, O.D. Waterfall sequence for intrinsec blood clotting. Science 1964, 145, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.N.; Sousa, M.O.; Dusse, L.M.S.; Carvalho, M.G. A cell-based model of coagulation and its implications. Rev. Bras. Hematol. Hemoter. 2010, 32, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol Endodontology 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.M.; Collen, A.; Koolwijk, P. Role of fibrin matrix in angiogenesis. Ann. N. Y. Acad. Sci. 2001, 936, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Fabris, V.; Mallmann, F.; Rech, C.A.; Carvalho, R.V.; Ruschel, G.H. Fibrinas Ricas em Plaquetas, Uma Alternativa para Regeneração Tecidual: Revisão de Literatura. J. Oral Investig. 2015, 4, 57–62. [Google Scholar] [CrossRef]
- Khurshid, Z.; Asiri, F.Y.I.; Najeeb, S.; Ratnayake, J. The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review. Materials 2022, 15, 2776. [Google Scholar] [CrossRef]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. An opportunity in perio-implantology: The PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Sahu, K.; Jadhav, S.; Khan, S.S.n.; Singh, N.; Khan, M.; Agarwal, A. Choukroun’s platelet-rich fibrin (L-PRF): A benevolence to surgical and reconstructive dentistry. Int. J. Oral Care Res. 2020, 8, 45. [Google Scholar] [CrossRef]
- da Silva, L.M.P.; Sávio, D.D.S.F.; de Ávila, F.C.; Vicente, R.M.; Reis, G.G.D.; Denardi, R.J.; da Costa, N.M.M.; Silva, P.H.F.; de Almeida Barros Mourão, C.F.; Miron, R.J.; et al. Comparison of the effects of platelet concentrates produced by high and low-speed centrifugation protocols on the healing of critical-size defects in rat calvaria: A microtomographic and histomorphometric study low-speed centrifugation protocols on the h. Platelets 2022, 1–10. [Google Scholar] [CrossRef]
- Maaruf, N.A.; Jusoh, N. Angiogenic and Osteogenic Properties of Fibrin in Bone Tissue Engineering. Mal. J. Med. Health Sci. 2022, 18, 85–94. [Google Scholar]
- do Lago, E.S.; Ferreira, S.; Garcia, I.R.; Okamoto, R.; Mariano, R.C. Improvement of bone repair with l-PRF and bovine bone in calvaria of rats. histometric and immunohistochemical study. Clin. Oral Investig. 2020, 24, 1637–1650. [Google Scholar] [CrossRef]
- Crisci, A.; Marotta, G.; Licito, A.; Serra, E.; Benincasa, G.; Crisci, M. Use of Leukocyte Platelet (L-PRF) Rich Fibrin in Diabetic Foot Ulcer with Osteomyelitis (Three Clinical Cases Report). Diseases 2018, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Medina, T.; Vaquette, C.; Ivanovski, S. Systematic comparison of the effect of four clinical-grade platelet rich hemoderivatives on osteoblast behaviour. Int. J. Mol. Sci. 2019, 20, 6243. [Google Scholar] [CrossRef] [Green Version]
- Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A.; Kaneno, R.; De Assis Golim, M.; Dos Santos, D.C.; Creste, C.F.Z.; Oba, E.; Maia, L.; Barraviera, B.; et al. A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res. Ther. 2017, 8, 654. [Google Scholar] [CrossRef] [Green Version]
- Cassaro, C.V.; Justulin, L.A.; De Lima, P.R.; De Assis Golim, M.; Biscola, N.P.; De Castro, M.V.; De Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J.; Ferreira, R.S.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, R.; Kyrylenko, S.; Castro, M.V.; Ferreira, R.S.; Barraviera, B.; Oliveira, A.L.R. Combination of heterologous fibrin sealant and bioengineered human embryonic stem cells to improve regeneration following autogenous sciatic nerve grafting repair. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.H.B.; Buchaim, R.L.; Pomini, K.T.; Hamzé, A.L.; Zattiti, I.V.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Ferreira Júnior, R.S.; Pontes, F.M.L.; et al. Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers 2022, 14, 2075. [Google Scholar] [CrossRef] [PubMed]
- Venante, H.S.; Chappuis-Chocano, A.P.; Marcillo-Toala, O.O.; Da Silva, R.A.; Da Costa, R.M.B.; Pordeus, M.D.; Barraviera, B.; Junior, R.S.F.; Lara, V.S.; Neppelenbroek, K.H.; et al. Fibrin biopolymer incorporated with antimicrobial agents: A proposal for coating denture bases. Materials 2021, 14, 1618. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; Rodrigues, A.d.C. Biocompatibility of anionic collagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Buchaim, D.V. Laser Therapy Together with a Fibrin Biopolymer Improves Nerve and Bone Tissue Regeneration. SciELO Perspect. 2022. P2022. Available online: https://pressreleases.scielo.org/en/2022/06/06/laser-therapy-together-with-a-fibrin-biopolymer-improves-nerve-and-bone-tissue-regeneration/ (accessed on 12 June 2022).
- Simões, T.M.S.; Fernandes Neto, J.d.A.; de Oliveira, T.K.B.; Nonaka, C.F.W.; Catão, M.H.C.d.V. Photobiomodulation of red and green lights in the repair process of third-degree skin burns. Lasers Med. Sci. 2020, 35, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Jayme, C.C.; Rezende, N.; Tedesco, A.C. Synergistic effect of photobiomodulation and phthalocyanine photosensitizer on fibroblast signaling responses in an in vitro three-dimensional microenvironment. J. Photochem. Photobiol. B Biol. 2021, 222, 112256. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum-Livnat, M.M.; Almog, M.; Nissan, M.; Loeb, E.; Shapira, Y.; Rochkind, S. Photobiomodulation triple treatment in peripheral nerve injury: Nerve and muscle response. Photomed. Laser Surg. 2016, 34, 638–645. [Google Scholar] [CrossRef]
- Della Santa, G.M.L.; Ferreira, M.C.; Machado, T.P.G.; Oliveira, M.X.; Santos, A.P. Effects of Photobiomodulation Therapy (LED 630 nm) on Muscle and Nerve Histomorphometry after Axonotmesis. Photochem. Photobiol. 2021, 97, 1116–1122. [Google Scholar] [CrossRef]
- Tim, C.R.; Martignago, C.C.S.; Assis, L.; Neves, L.M.; Andrade, A.L.; Silva, N.C.; Parizotto, N.; Pinto, K.Z.; Rennó, A.C. Effects of photobiomodulation therapy in chondrocyte response by in vitro experiments and experimental model of osteoarthritis in the knee of rats. Lasers Med. Sci. 2022, 37, 1677–1686. [Google Scholar] [CrossRef]
- Gonçalves, A.B.; Bovo, J.L.; Goines, B.S.; Pigoso, A.A.; Felonato, M.; Esquisatto, M.A.M.; de Jesus Lopes Filho, G.; do Bomfim, F.R.C. Photobiomodulation (λ = 808nm) and Platelet-Rich Plasma (PRP) for the Treatment of Acute Rheumatoid Arthritis in Wistar Rats. J. Lasers Med. Sci. 2021, 12, e60. [Google Scholar] [CrossRef]
- Hendler, K.G.; Canever, J.B.; de Souza, L.G.; das Neves, L.M.S.; de Cássia Registro Fonseca, M.; Kuriki, H.U.; da Silva Aguiar Junior, A.; Barbosa, R.I.; Marcolino, A.M. Comparison of photobiomodulation in the treatment of skin injury with an open wound in mice. Lasers Med. Sci. 2021, 36, 1845–1854. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.-P.; Tan, R.K.; Lee, H.-W.; Han, S.-K. Effect of Low-Level Laser Therapy on Proliferation and Collagen Synthesis of Human Fibroblasts in Vitro. J. Wound Manag. Res. 2018, 14, 1–6. [Google Scholar] [CrossRef]
- Mosca, R.C.; Ong, A.A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv. Ski. Wound Care 2019, 32, 157–167. [Google Scholar] [CrossRef]
- Escudero, J.S.B.; Perez, M.G.B.; de Oliveira Rosso, M.P.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Injury 2019, 50, 1853–1867. [Google Scholar] [CrossRef] [PubMed]
- Nica, D.F.; Riviș, M.; Roi, C.I.; Todea, C.D.; Duma, V.F.; Sinescu, C. Complementarity of photo-biomodulation, surgical treatment, and antibiotherapy for medication-related osteonecrosis of the jaws (Mronj). Medicine 2021, 57, 145. [Google Scholar] [CrossRef] [PubMed]
- Mosca, R.C.; Santos, S.N.; Nogueira, G.E.C.; Pereira, D.L.; Costa, F.C.; Pereira, J.X.; Zeituni, C.A.; Arany, P.R. The Efficacy of Photobiomodulation Therapy in Improving Tissue Resilience and Healing of Radiation Skin Damage. Photonics 2022, 9, 10. [Google Scholar] [CrossRef]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.-F.; Todea, C.D. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef] [Green Version]
- Borges, R.M.M.; Cardoso, D.S.; Flores, B.C.; da Luz, R.D.; Machado, C.R.; Cerveira, G.P.; Daitx, R.B.; Dohnert, M.B. Effects of different photobiomodulation dosimetries on temporomandibular dysfunction: A randomized, double-blind, placebo-controlled clinical trial. Lasers Med. Sci. 2018, 33, 1859–1866. [Google Scholar] [CrossRef]
- Pinheiro, A.L.; Gerbi, M.E. Photoengineering of bone repair processes. Photomed Laser Surg. 2006, 24, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Dos Santos Haupenthal, D.P.; Zaccaron, R.P.; de Bem Silveira, G.; Corrêa, M.E.A.B.; de Roch Casagrande, L.; de Sousa Mariano, S.; de Souza Silva, J.I.; de Andrade, T.A.M.; Feuser, P.E.; et al. Effects of the Association between Photobiomodulation and Hyaluronic Acid Linked Gold Nanoparticles in Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5132–5144. [Google Scholar] [CrossRef]
- Tripodi, N.; Corcoran, D.; Antonello, P.; Balic, N.; Caddy, D.; Knight, A.; Meehan, C.; Sidiroglou, F.; Fraser, S.; Kiatos, D.; et al. The effects of photobiomodulation on human dermal fibroblasts in vitro: A systematic review. J. Photochem. Photobiol. B 2021, 214, 112100. [Google Scholar] [CrossRef]
- Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Litscher, G. Curcumin and Photobiomodulation in Chronic Viral Hepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 7150. [Google Scholar] [CrossRef] [PubMed]
- Kumar Rajendran, N.; George, B.P.; Chandran, R.; Tynga, I.M.; Houreld, N.; Abrahamse, H. The Influence of Light on Reactive Oxygen Species and NF-kB in Disease Progression. Antioxidants 2019, 8, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spannbauer, A.; Mester-Tonczar, J.; Traxler, D.; Kastner, N.; Zlabinger, K.; Hašimbegović, E.; Riesenhuber, M.; Pavo, N.; Goliasch, G.; Gyöngyösi, M. Large Animal Models of Cell-Free Cardiac Regeneration. Biomolecules 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef] [PubMed]
- Chaudary, S.; Karner, L.; Weidinger, A.; Meixner, B.; Rieger, S.; Metzger, M.; Zipperle, J.; Dungel, P. In vitro effects of 635 nm photobiomodulation under hypoxia/reoxygenation culture conditions. J. Photochem. Photobiol. B 2020, 209, 111935. [Google Scholar] [CrossRef]
- Dos Santos Ferreira, F.; Cadoná, F.C.; Aurélio, A.R.; de Oliveira Martins, T.N.; Pivetta, H.M.F. Photobiomodulation-blue and red LED: Protection or cellular toxicity? In vitro study with human fibroblasts. Lasers Med. Sci. 2022, 37, 523–530. [Google Scholar] [CrossRef]
- Robijns, J.; Censabella, S.; Bulens, P.; Maes, A.; Mebis, J. The use of low-level light therapy in supportive care for patients with breast cancer: Review of the literature. Lasers Med. Sci. 2017, 32, 229–242. [Google Scholar] [CrossRef]


| Reference (Database) | Type of Laser/LED (Manufacturer) | Wavelength (nm) and Output Power (mW) | Power Density (mW/cm2) | Energy Density (J/cm2) | Objective | Fibrin | Intervention | Outcome/Results | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Bikmulina et al., 2020 [37] (PubMed) | LED light red and infrared (IR) (Original apparatus LDM-07) | Red: 633 IR: 840 and Red: 160 ± 20 IR: 320 ± 40 | Red: 1.8 ± 0.2 IR: 3.6 ± 0.4 | Red and IR: 2.2 ± 0.2 | Evaluation of PBM therapy for cell stimulation in hydrogels | Mesenchymal stromal cells (MSCs) obtained from human gingiva mucosa were encapsulated in fibrin (hydrogels) | A single exposure was made to low-intensity light, both red and infrared. After three days of culture, the physiological activity and viability of the cells were verified | The authors observed a dependence on cell viability in relation to the concentration of gel-forming proteins and the thickness of the hydrogels | Infrared light can be indicated for stimulation of MSCs proliferation and metabolism, in hydrogels with thicknesses of up to 3 mm |
| Tenore et al., 2020 [38] (PubMed) | Red and Infrared Gallium-Arsenide laser (GaAs) (Fisioline; Lumix® C.P.S. Dental Multidiodic laser) | Three wavelengths: 650, 810, 910 and G1: total power of 600 mW; G3 total power of 1100 mW | -/- | -/- | To evaluate the effect of three different protocols on the healing outcome in patients with established medication-related osteonecrosis of the jaw (MRONJ) | Leukocyte- and platelet-rich fibrin (L-PRF) | G1 was treated with antibiotic therapy, surgery, L-PRF and PBM; G2 with antibiotic therapy and surgery; G3 with antibiotic and PBM | There was no significant association between MRONJ results and location, stage, duration of drug treatment, diabetes, smoking, corticosteroid therapy, underlying disease, sex, and chemotherapy history at three and six months | The combination of antibiotic therapy, L-PRF, surgery and PBM can effectively contribute to the treatment of MRONJ |
| Buchaim et al., 2015 [29] (PubMed) | Gallium-Aluminum-Arsenide (GaAlAs) (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | -/- | 4 | To analyze whether the fibrin adhesive allows, through end-to-side neurorrhaphy, the collateral growth of axons without an epineural window of the vagus nerve into a sural nerve graft and whether laser therapy contributes to the regeneration process | Fibrin glue derived from snake venom | Experimental Group (EG; n = 12 rats), sural nerve graft was coapted to the vagus nerve with fibrin glue; and experimental group laser (EGL; n = 12 rats), EG + LLLT and control group (CG; n = 8 rats), the intact sural nerve was collected | There was sprouting of axons from the vagus nerve into the autologous graft in the EG and EGL, and in the CG all of the dimensions measured were better, with a significant difference in relation to the EG and EGL, except for the area and thickness of the myelin sheath, which showed a significant difference only in relation to the EG | LLLT potentiates nerve regeneration and fibrin glue provided conditions for axonal regeneration in peripheral nerve injuries |
| de Oliveira Gonçalves et al., 2016 [39] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 258.6 | 6 | To evaluate the effects of LLLT on an autogenous bone graft integration process stabilized with a new heterologous fibrin sealant (NHFS) | Heterologous fibrin sealant | Autogenous bone graft from rat calvaria, removed from the right parietal bone, with a 5 mm osteotomy, was adhered on the left side with fibrin sealant; groups: autogenous Fibrin graft (AFG) and autogenous fibrin graft laser (AFGL), with the same procedures as the AFG, plus LLLT | The bone regeneration process was not complete, with new bone tissue partially integrating the graft into the recipient bed, with some areas of connective tissue. Morphometrically, minor interfaces occurred in the AFGL group, with significant differences in all analyzed periods | LLLT stimulated bone neoformation and improved the process of integration of autogenous bone graft |
| Buchaim et al., 2017 [40] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 258.6 | 6.2 | To analyze the efficacy of LLLT on quantitative, qualitative and functional aspects in the facial nerve regeneration | NHFS derived from snake venom | Suture experimental (SEG) and fibrin experimental (FEG) groups, the buccal branch of the facial nerve was sectioned, end-to-end epineural suture on the right side, and a NHFS on the left side; laser suture experimental (LSEG) and laser fibrin experimental (LFEG) groups, the same procedures as SEG and FEG with the addition of LLLT; control group (CG), facial nerve intact | LLLT resulted in a significant increase in the density and number of new axons. The LSEG and LFEG presented better scores in functional analysis in comparison with the SEG and FEG | Both repair techniques were effective in promoting axonal growth and LLLT improved these results, in addition to accelerating the functional recovery of whiskers |
| Rohringer et al., 2017 [41] (PubMed) | LED lamps were provided by Repuls Lichtmedizintechnik GmbH, Vienna, Austria | Pulsed LED light of either 475 nm (blue), 516 nm (green), 635 nm (red) or remained unstimulated (control) | Peak irradiance intensity of 80 mW/cm2 on all LED devices; average irradiance intensity of 40 mW/cm2 | Dose 24 J/cm2 (daily) | To compare the effects of PBM using light-emitting diodes (LED) with different wavelengths on endothelial cells in vitro | 3D fibrin matrices and fibrin gels | Migration and proliferation tests were performed in 2D and 3D. 3D fibrin gel co-culture model with human umbilical vein endothelial cells (HUVEC) and adipose-derived stem cells (ASC) was used to analyze early vasculogenic effects, continuous stimulation of LLLT, after one week of culture | Stimulation with green and red LED light increased 3D migration and proliferation of HUVEC. HUVEC also had greater potential for 2D migration with green light stimulation. Blue light was ineffective | Green light, in several parameters, has been shown to be more potent in stimulating endothelial cell migration and proliferation than red light |
| Priglinger et al., 2018 [42] (PubMed) | LED lamps were provided by Repuls Lichtmedizintechnik GmbH, Vienna, Austria | Pulsed LED light 475 nm (blue), 516 nm (green), 635 nm (red) | All LED devices had a peak irradiance intensity of 80 mW/cm2 | Fluence of 24 J/cm2 | To analyze the effects of green, blue and red light (RL) emitted by LEDs directly on freshly isolated SVF and analyzed cell phenotype, cell number, viability, ATP content, LDH cytotoxicity and proliferation, but also osteogenic, adipogenic and pro-angiogenic differentiation in vitro | 3D fibrin matrices | Pulsed blue (475 nm), green (516 nm) and RL (635 nm) from LEDs applied on freshly isolated Stromal Vascular Fraction (SVF) | LLLT increased, compared to untreated cells, the colony-forming unit fibroblast assay with RL. The frequency of colony forming cells was not affected. LLLT with green light and RL resulted in a better potential to form vascular tubes by SVF compared to untreated cells when grown in 3D fibrin matrices | LLLT has beneficial effects in relation to SVF cell proliferation and vascularization potential. LLLT may represent a good method for clinical practice in activating SVF cells |
| Pomini et al., 2019 [43] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 258.6 | 6 | In rat calvaria (critical size defect—CSD), to evaluate the scaffold formed by a fibrin sealant (FS) plus xenograft associated with PBM therapy | Tisseel Lyo® (Baxter Healthcare Ltd., Norfolk, UK) | CSD in calvaria, 36 rats: 4 groups: BC (n = 8), defect with blood clot; FSB (n = 10), FS and xenograft; BCPBMT (n = 8), blood clot and PBM; FSBPBMT (n = 10), FS, xenograft, and PBM | Bone neoformation was observed in all groups, limited to the defect margins. In the FSB group, new bone increased between periods (4.3 ± 0.46 to 6.01 ± 0.32), but with lower volume when compared to the FSBPBMT (5.6 ± 0.45 to 10.64 ± 0.97) | The biocomplex formed by the xenograft plus FS associated with the PBM therapy had a positive effect on the new bone formation |
| Hemaid et al., 2019 [44] (PubMed) | Diode Laser Gallium-Aluminum-Arsenide (GaAlAs) | 810 and 100 | -/- | 46.8 | To observe and compare the combined use of LLLT (810 nm), PRF and NanoHA in the healing of induced intraosseous periodontal defects | Autologous platelet-rich fibrin (PRF) | Sixteen defects in rabbits divided in four groups: laser irradiated control (CL); Control non-treated (C); PRF + NanoHA graft treated group and laser irradiated (NanoHA-Graft + PRF + L) | NanoHA-Graft + PRF + L showed significantly higher bone density in relation to the other groups | The best form of treatment was the combined use of LLLT + PRF + NanoHA as it presented the best results in the formation of new bone |
| Sahin et al., 2020 [45] (PubMed) | Nd: YAG laser (Fotona, Ljubljana Slovenia) | 1064 and 1250 | -/- | -/- | To analyze the surgical procedures used to prevent the development of MRONJ after dentoalveolar surgery in patients who received bisphosphonates | Leukocyte and platelet-rich fibrin (L-PRF) | Sixty-three surgeries were performed on forty-four patients taking bisphosphonate. Procedures: performed dentoalveolar surgical; antibiotics; fill the socket with L-PRF; LLLT (Nd: YAG laser) | There were no intercurrences until cure. Complete mucosal healing occurred in all patients within one month with no long-term failures | The surgical protocol demonstrates promising results for the protection of MRONJ after performing dentoalveolar surgeries |
| Thalaimalai et al., 2020 [46] (PubMed) | Diode laser | 810 and 500 | -/- | -/- | To evaluate the combined effect of LLLT and PRF, in site modulated intra-bony defects, which were accessed using a simplified papilla preservation flap (SPPF), on the periodontal disease | Autologous platelet-rich fibrin | Thirty patients with intra-bony defects (2 groups, n = 15 each). There was SPPF access at test group (TG) sites and defects received intramedullary penetration (IMP) after debridement, followed by LLLT and PRF grafting. In the control group (CG), the defects were accessed with SPPF and grafted only with PRF | TG showed a clinically relevant increase in mean probing pocket depth reduction, clinical attachment level gain, and radiographic bone fill compared to the CG, six months post-intervention | Together, LLLT with PRF caused an improvement in clinical and radiographic results within modulated intraosseous defects |
| Della Coletta et al., 2021 [47] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 258.6 | 6.2 | To evaluate the effects of PBM therapy on the guided bone regeneration process (GBR) in defects in the calvaria of rats filled with biphasic calcium phosphate (BCP) associated with fibrin | Fibrin biopolymer (FB) | Thirty Wistar rats: BMG, defects filled with biomaterial and covered by membrane; BFMG, biomaterial and fibrin biopolymer (FB) covered by membrane; and BFMLG, biomaterial and FB covered by membrane and biostimulated with PBM | There was more evident bone growth in the BFMLG, in addition to a progressive increase in new bone tissue in all groups, with a significant difference in the BFMLG, whose group presented greater bone neoformation in the periods of 14 and 42 days, followed by BFMG and BMG | PBM has been shown to be effective in improving and accelerating the GBR process when associated with BCP and FB |
| Sahin et al., 2021 [48] (PubMed) | Nd: YAG laser (Fotona, Ljubljana Slovenia) | 1064 and 1250 | -/- | -/- | To analyze the surgical technique described in the treatment of advanced stages of MRONJ patients | Autologous L-PRF concentrate | Twnty-one patients affected by Stage 2-3 MRONJ were treated with ultrasonic piezoelectric for bone surgery, with necrotic bone removing, L-PRF and LLLT | Two patients, who were Stage 3, had delayed healing at 1 month after surgery. Complete mucosal healing occurred in all patients in the third month | The surgical protocol shows promising results for surgical management of advanced stages of MRONJ patients |
| de Freitas Dutra Júnior et al., 2021 [49] PubMed | Indium-Gallium-Aluminum-Phosphide laser (InGaAlP) (MMOptics®, São Carlos, Brazil) | 660 and 40 | 1000 | 6 | To verify, in tendon injuries, the action of the new heterologous fibrin biopolymer (HFB) associated or not with PBM | Heterologous fibrin biopolymer | Partial transection calcaneus tendon (PTCT) was performed in 84 rats divided into 4 groups: control (CG); HFB; PBM; HFB + PBM. HFB was applied immediately after PTCT, while PBM started 24 h after injury and continued every 24 h for 7, 14 and 21 days. | It can be noted that the reduction of edema was effective in the treatment groups when compared to the CG. In the periods of 14 and 21 days, PBM had a better repair process compared to GC | The HFB and PBM treatments, associated or isolated, promoted a reduction in the edema volume, favoring the repair process. HFB alone contributed more in promoting the tendon repair process |
| Buchaim et al., 2022 [50] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 258.6 | 6.2 | To analyze the effects of PBM on CSD filled with xenogenic bone substitute associated with HFB | Heterologous fibrin biopolymer (HFB) | CSD in 36 Wistar rats, four groups: BC and BC-PBM (controls) with defects filled by a clot (without or with PBM); XS and XS-PBM, filled with biocomplex Bio-Oss® + HFB. PBM was applied transoperatively and continued three times a week | BC-PBM and XS-PBM had a higher density of the bone neoformation in relation to the groups without PBM. Significant vascular proliferation and new bone deposition around the XS particles were observed in the animals which biocomplex (XS and XS-PBM) | PBM allowed an improvement in none neoformation, with a more organized deposition of collagen fibers. Biocomplex favored the permanence and insertion of the particulate biomaterial in bone defect |
| Rosso et al., 2017 [51] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 260 | 6.2 | To evaluate the action of PBM on lesions of the facial nerve repaired with the end-to-side technique or coaptation with a NHFS | New Heterologous Fibrin Sealant | Thirty-two rats, five groups: control (CG); experimental suture (ESG) and experimental fibrin (EFG) groups, end-to-side sutured to the zygomatic branch on the right side of the face or NHFS on the left side; experimental suture laser (ESLG) and experimental fibrin laser (EFLG) groups, with PBM | There was a significant difference in the fiber nerve area between the EFG and the EFLG. There was also faster functional recovery of the whisker movement in the ESLG and EFLG, where PBM was used, with results closer to the CG | Photobiomodulation with LLLT accelerated functional and morphological nerve repair, in both techniques |
| Rosso et al., 2020 [52] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 258.6 | 6 | To evaluate the action of PBM on rat tibial defect filled with biomaterial of the lyophilized bovine bone matrix (BM) associated or not with HFB | Heterologous fibrin biopolymer (HFB) | Thirty rats, three groups. A noncritical bone defect of 2 mm was produced. Four Groups: (1) BM + PBMT; (2) BM + HFB; (3): BM + HFB + PBM. In Groups 1 and 3 the animals were submitted to intraoperative PBM and every 48 h until the period of euthanasia | Statistical difference in bone neoformation between Groups 3 and 2 (26.4% ± 1.03% and 20.0% ± 1.87%, respectively) at 14 days and 42 days (38.2% ± 1.59% and 31.6% ± 1.33%, respectively). In 42 days there was presence of new bone with mature characteristics | The combined use of PBM with HFB and BM contributed to the process of reconstruction of non-critical bone defects |
| Buchaim et al., 2016 [53] (PubMed) | GaAlAs (Laserpulse IBRAMED®, Amparo, Brazil) | 830 and 30 | 258.6 | 6 | To evaluate the effects of LLLT in the repair of the buccal branch of the facial nerve with two techniques: coaptation with HFS and end-to-end epineural suture | Heterologous fibrin sealant (HFS) | Forty-two rats, five groups: (1) control (CG), facial nerve (buccal branch) was collected without lesion; (2) experimental suture (EGS) and experimental fibrin (EGF) groups: end-to-end suture on the right side and HFS on the left side; (3) experimental suture laser (EGSL) and experimental fibrin laser (EGFL): plus LLLT | Axonal growth occurred in the distal stump of the facial nerve in all groups. The morphological aspect was similar to the GC fibers, with the majority of myelinated fibers. In the last period of the experiment, the EGSL presented the best results, being closer to the CG, in all measurements performed, except in the axon area | Laser therapy showed better results in facial nerve regeneration, being an effective technique to stimulate the repair process of peripheral nerve injuries |
| Doan et al., 2020 [54] (Scopus) | MLS laser (ASA laser, Vicenza, Italy) | -/- | -/- | 1.27 | Two clinical cases with piezoelectric surgery (PES), concentrated growth factors (CGF) and PBM, used in the search to increase the formation of new blood vessels and tissue repair after maxillary sinus lift surgeries with dental implants | Autologous concentrated growth factors (CGF) | The lateral sinus windows were created using PES. The implants were inserted in the same surgery and wrapped with CGF. A laser treatment of PBM was performed at the site, applied in the apical, buccal, lingual, coronal, mesial and distal regions of the surgical wound | Vascular budding and wound closure was observed after the first day. New bone formation was detected in the enlarged maxillary sinuses next to the implants, through radiographs and cone-beam computed tomography | PBM, PES, and CGF promoted the formation of new vessels, favored the approximation of the edges, closing the wound and reducing edema and bleeding. In addition, there was less postoperative pain, less use of analgesics and speech impairment, without trismus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.d.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.d.B.; Pereira, E.d.S.B.M.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers 2022, 14, 3150. https://doi.org/10.3390/polym14153150
Reis CHB, Buchaim DV, Ortiz AdC, Fideles SOM, Dias JA, Miglino MA, Teixeira DdB, Pereira EdSBM, da Cunha MR, Buchaim RL. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers. 2022; 14(15):3150. https://doi.org/10.3390/polym14153150
Chicago/Turabian StyleReis, Carlos Henrique Bertoni, Daniela Vieira Buchaim, Adriana de Cássia Ortiz, Simone Ortiz Moura Fideles, Jefferson Aparecido Dias, Maria Angelica Miglino, Daniel de Bortoli Teixeira, Eliana de Souza Bastos Mazuqueli Pereira, Marcelo Rodrigues da Cunha, and Rogerio Leone Buchaim. 2022. "Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review" Polymers 14, no. 15: 3150. https://doi.org/10.3390/polym14153150
APA StyleReis, C. H. B., Buchaim, D. V., Ortiz, A. d. C., Fideles, S. O. M., Dias, J. A., Miglino, M. A., Teixeira, D. d. B., Pereira, E. d. S. B. M., da Cunha, M. R., & Buchaim, R. L. (2022). Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers, 14(15), 3150. https://doi.org/10.3390/polym14153150









