Bioactive Interpenetrating Hydrogel Networks Based on 2-Hydroxyethyl Methacrylate and Gelatin Intertwined with Alginate and Dopped with Apatite as Scaffolding Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Syntheses
2.3. Hydrogel Scaffold Characterization
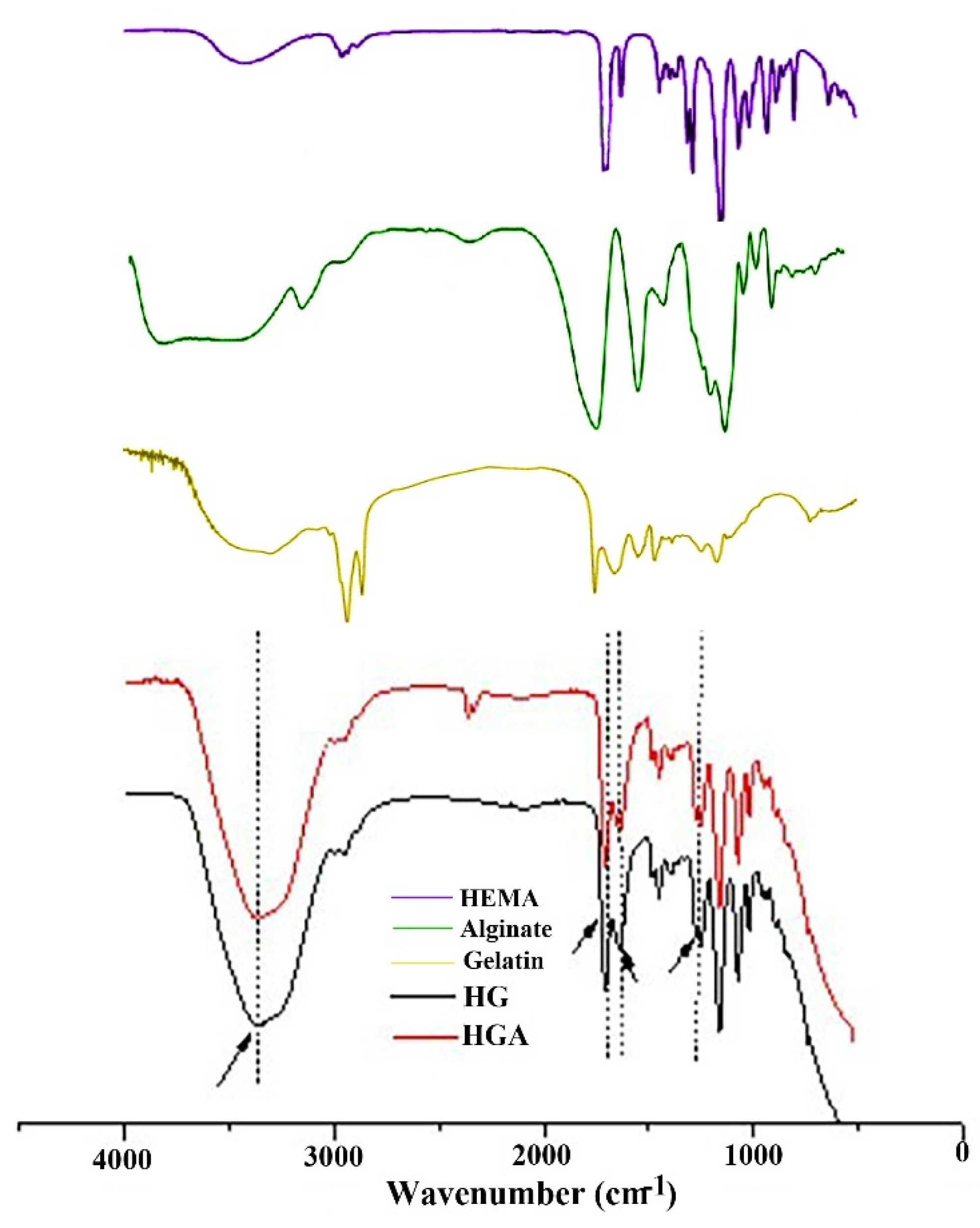
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
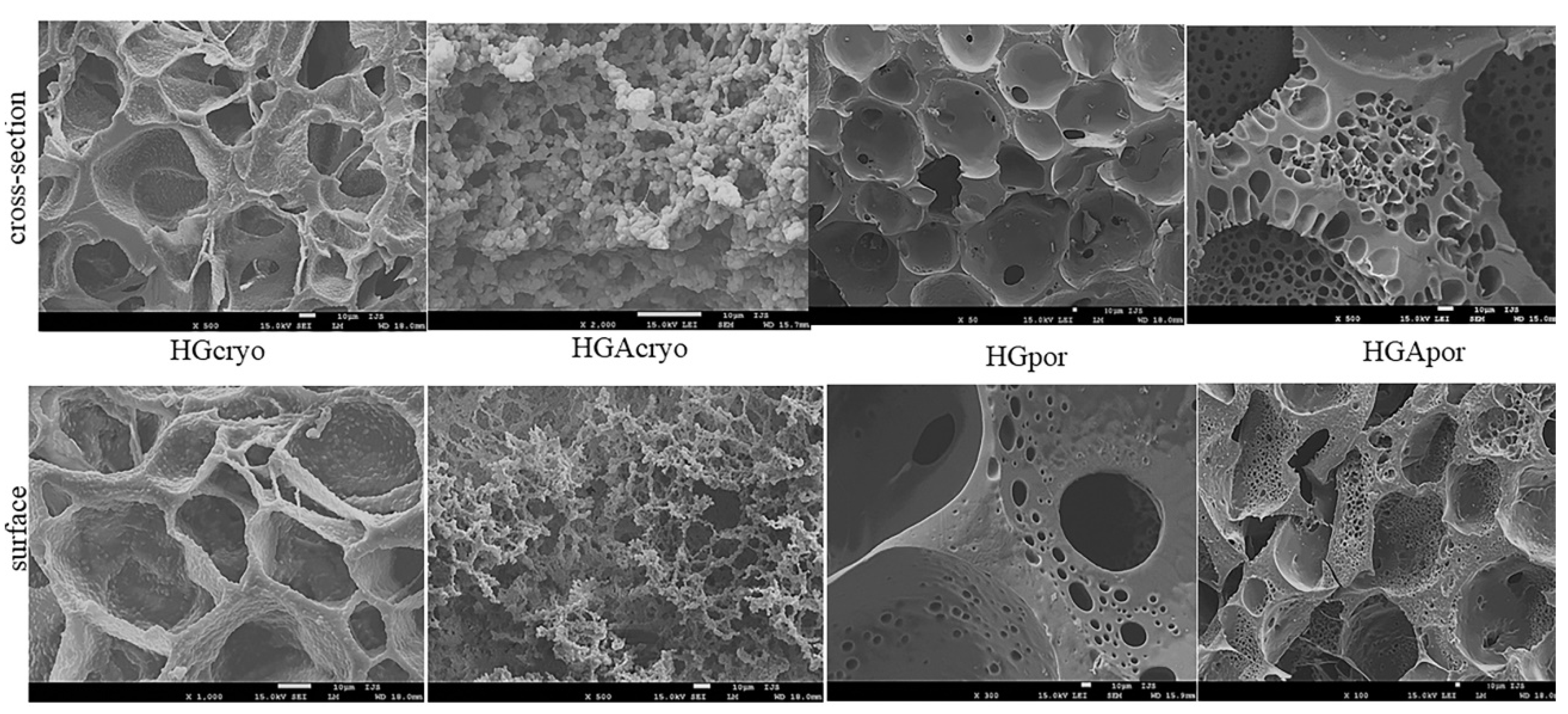
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Porosity Measurements
2.3.4. Mechanical Testing
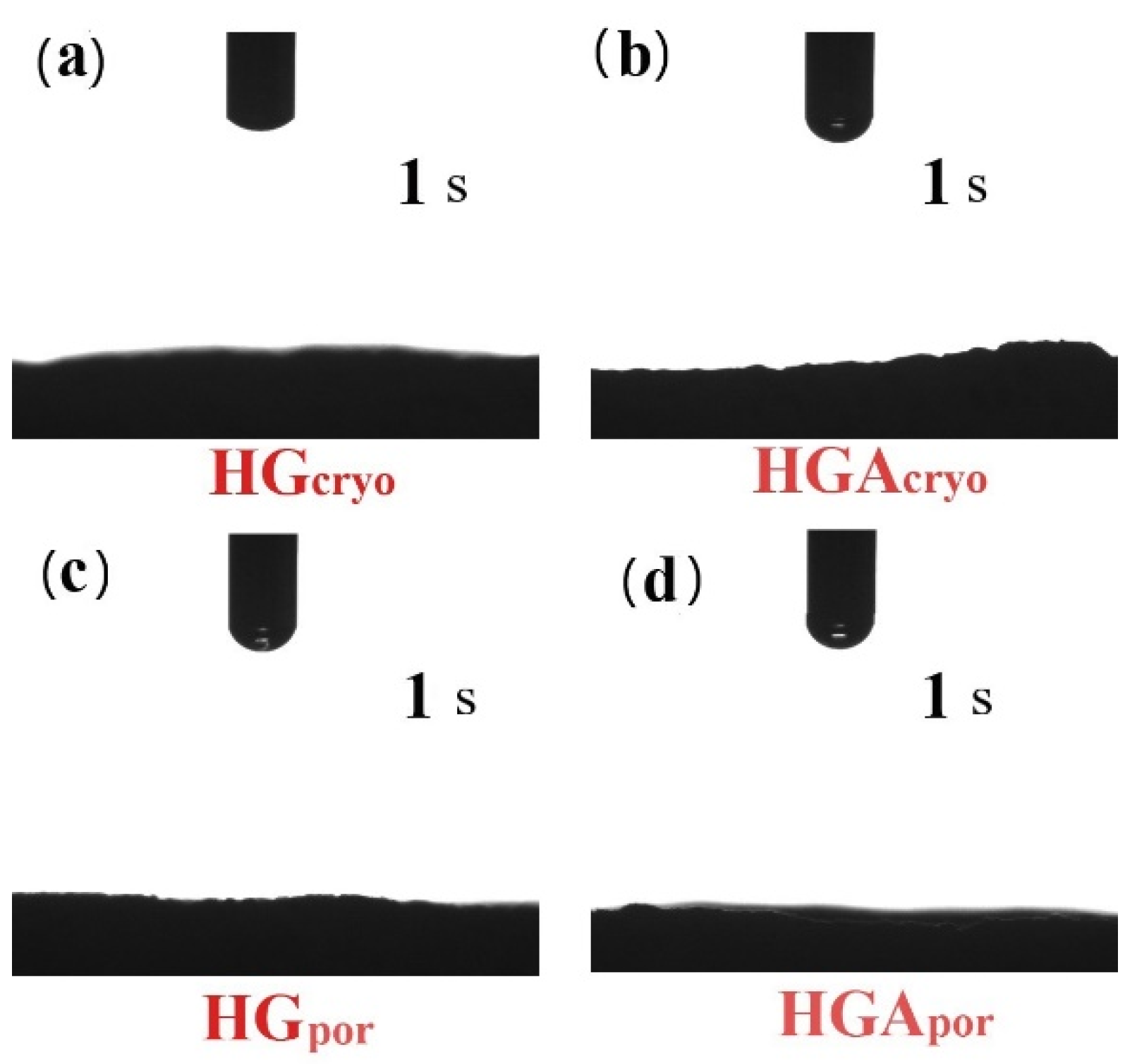
2.3.5. Water Contact Angle Measurement
2.3.6. In Vitro Degradation Study
2.4. Biological Activity Studies
2.4.1. Lactate Dehydrogenase Activity (LDH) Assay
2.4.2. Cell Expansion and Culture
2.4.3. Cell Adhesion to the Hydrogel Scaffolds
2.4.4. AlamarBlue® Assay
2.4.5. RNA Isolation and Real-Time PCR Analysis
2.4.6. Statistics
3. Results and Discussion
3.1. Structural Characteristics of Hydrogel Scaffolds
3.2. Hydrogel Scaffold Morphology
3.3. Hydrogel Scaffold Porosity
3.4. Hydrophilicity of Hydrogel Scaffolds
3.5. Mechanical Properties of Hydrogel Scaffolds
3.6. In Vitro Degradation Behavior of Hydrogel Scaffolds
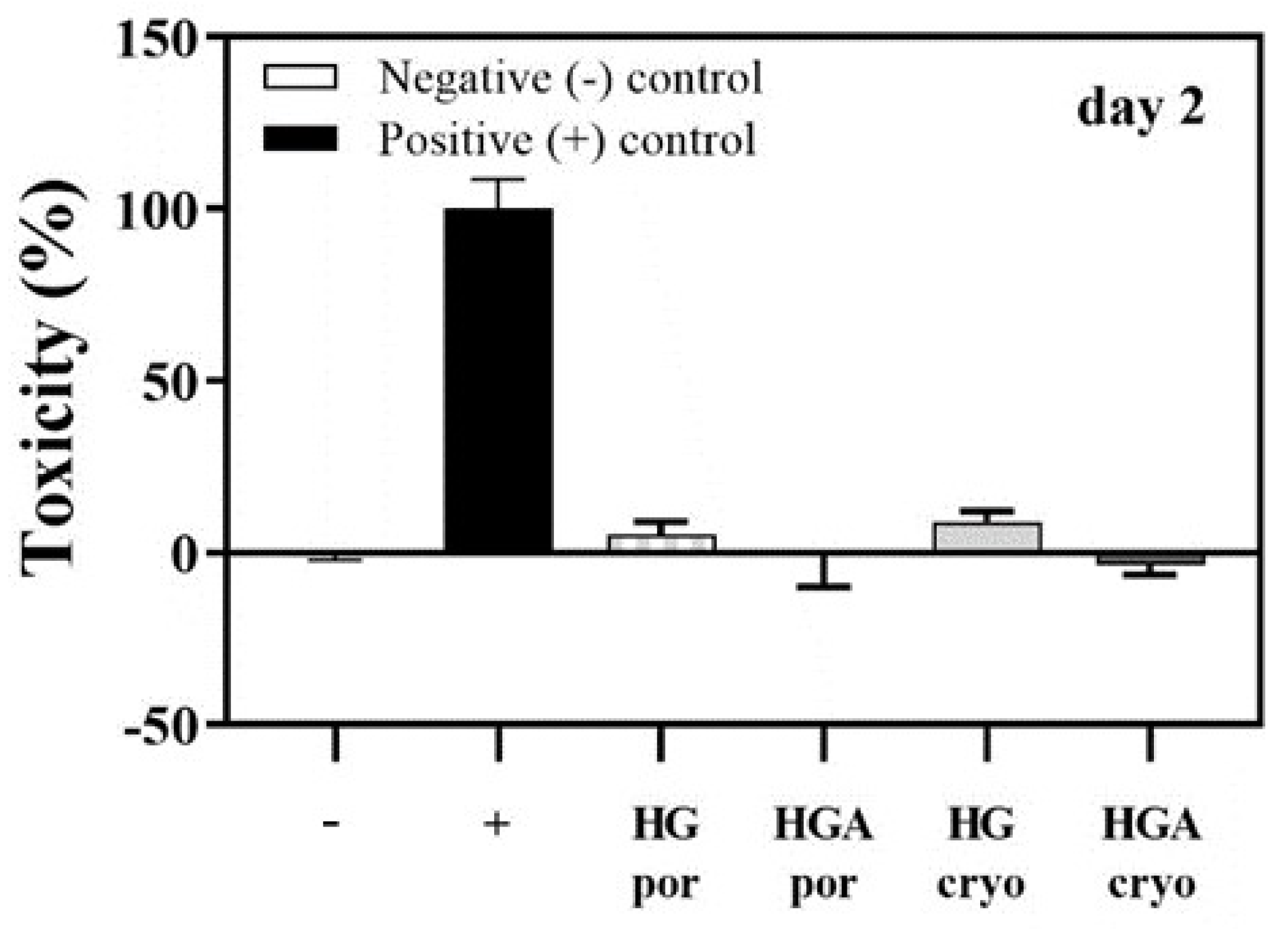
3.7. Cytotoxicity of Hydrogel Scaffolds: Lactate Dehydrogenase Activity (LDH) Assay
3.8. Influence of Hydrogel Scaffold Properties on Cell Behaviour
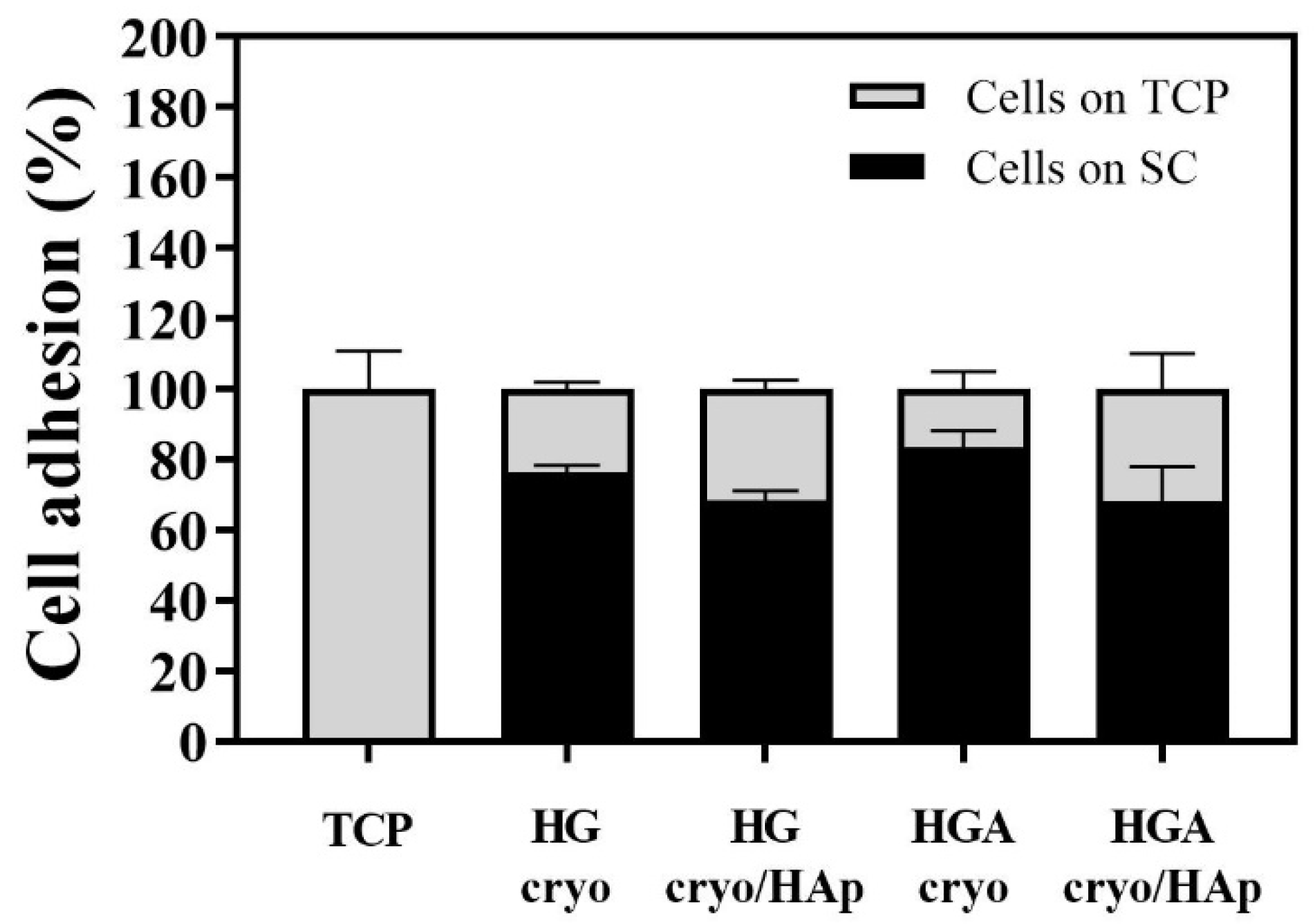
3.8.1. Cell Adhesion to the Hydrogel Scaffolds
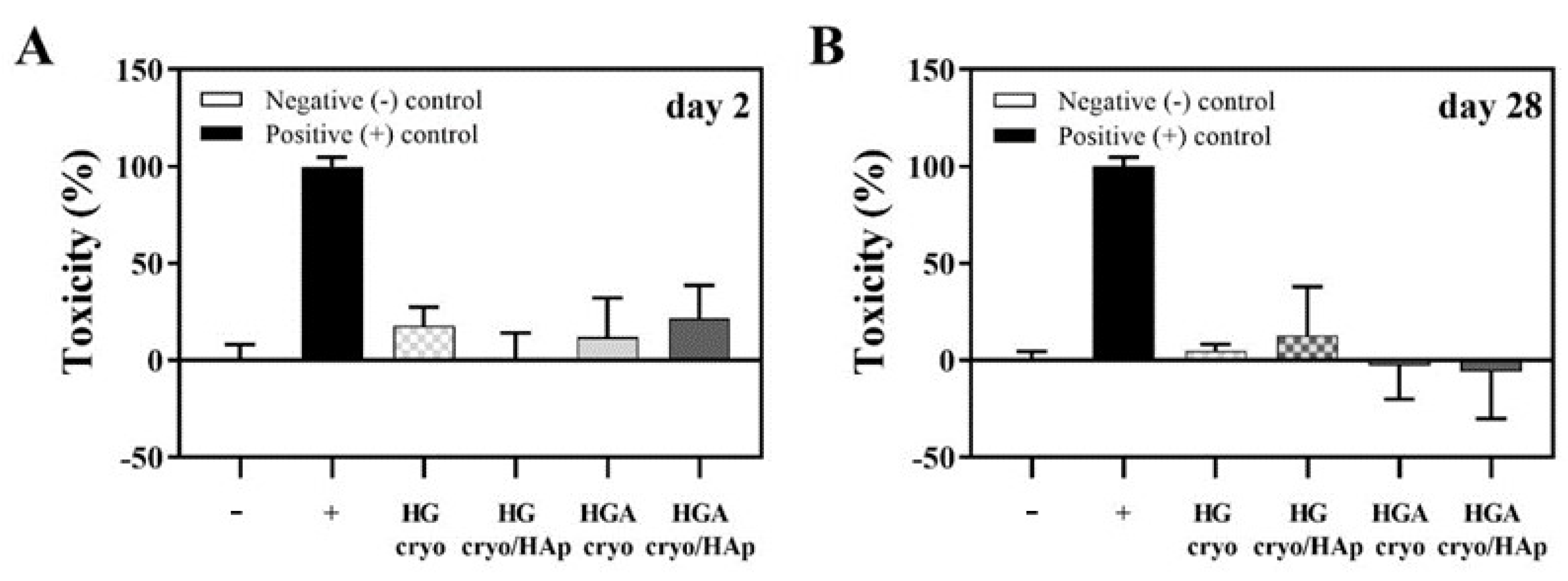
3.8.2. Cytotoxicity of Cryogelated Scaffolds with or without HAp on Seeded Cells
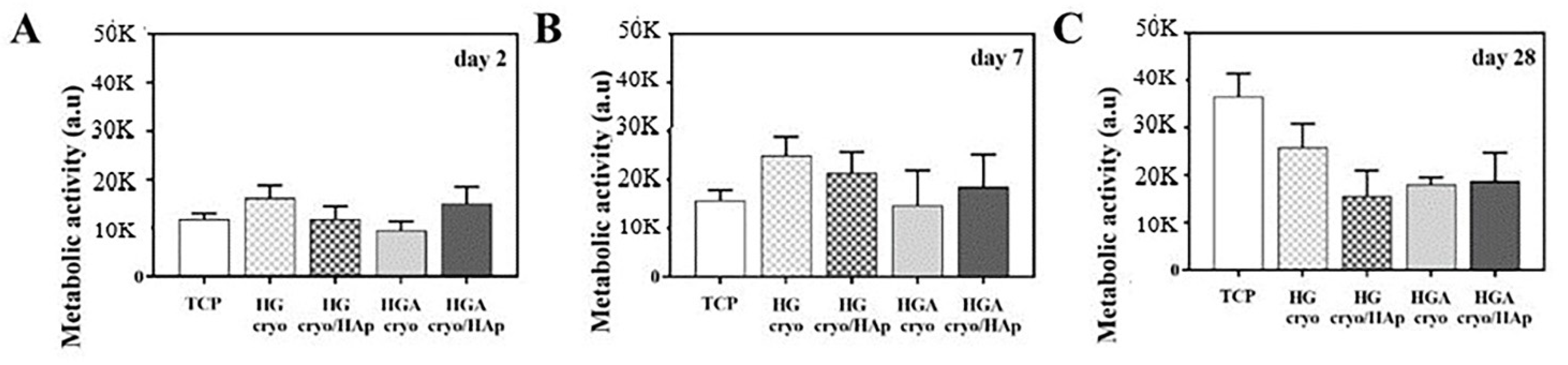
3.8.3. Cell Metabolic Activity
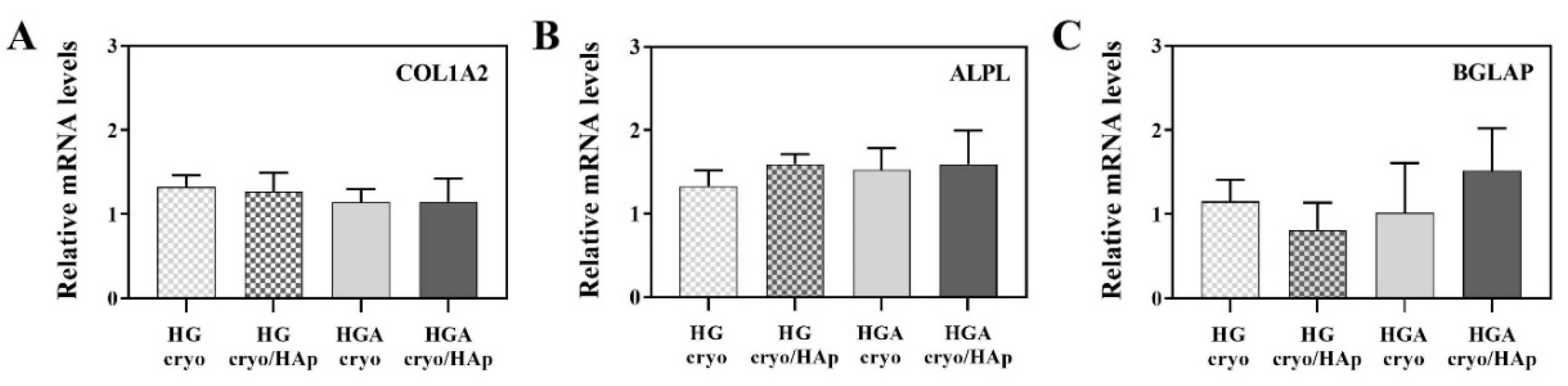
3.8.4. Expression of Osteogenesis-Related Genes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gavrilov, L.A.; Gavrilova, N.S. New developments in the biodemography of aging and longevity. Gerontology 2015, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Geris, L.; Papantoniou, I. The third era of tissue engineering: Reversing the innovation drivers. Tissue Eng.-Part A 2019, 25, 821–826. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2019; World Health Organization: Geneva, Switzerland, 2020.
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Fan, C.; Wang, D.-A. Macroporous hydrogel scaffolds for three-dimensional cell culture and tissue engineering. Tissue Eng. Part B Rev. 2017, 23, 451–461. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Yahia, L.; Chirani, N.; Yahia, L.; Gritsch, L.; Motta, F.; Chirani, S.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Neumann, M.; Fu, T.; Li, W.; Cheng, X.; Su, B.L. Porous and responsive hydrogels for cell therapy. Curr. Opin. Colloid Interface Sci. 2018, 38, 135–157. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 3, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Griesbach, J.; Ganeyev, M.; Zehnder, A.-K.; Zeng, P.; Schädli, G.N.; de Leeuw, A.; Lai, Y.; Rubert, M.; Müller, R. Long-term mechanical loading is required for the formation of 3D bioprinted functional osteocyte bone organoids. Biofabrication 2022, 14, 035018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Eyisoylu, X.; Qin, X.-H.; Rubert, M.; Müller, R. 3D bioprinting of graphene oxide-incorporated cell-laden bone mimicking scaffolds for promoting scaffold fidelity, osteogenic differentiation and mineralization. Acta Biomater. 2021, 121, 637–652. [Google Scholar] [CrossRef]
- Urruela-Barrios, R.; Ramírez-Cedillo, E.; de León, A.D.; Alvarez, A.; Ortega-Lara, W. Alginate/gelatin hydrogels reinforced with TiO2 and β-TCP fabricated by microextrusion-based printing for tissue regeneration. Polymers 2019, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.; Gough, J. Tissue Engineering Using Ceramics and Polymers; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 1–587. [Google Scholar]
- Bhatia, S. Natural Polymer Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–225. [Google Scholar]
- Kumbar, S.; Laurencin, C.; Deng, M. Natural and Synthetic Biomedical Polymers; Elsevier: Berlin/Heidelberg, Germany, 2014; pp. 1–402. [Google Scholar]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing porous bone tissue engineering scaffolds with enhanced mechanical properties from composite hydrogels composed of modified alginate, gelatin and bioactive glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef] [PubMed]
- Harini, B.; Shadamarshan, R.P.K.; Rao, S.H.; Selvamurugan, N.; Balagangadharan, K. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2017, 110, 88–96. [Google Scholar]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.; Malda, J.; Melchels, F.P. Gelatin-methacryloyl hydrogels: Towards biofabrication-based tissue repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhao, F.; Zhang, W.; Mo, Y.; Zeng, L.; Li, X. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Mater. Sci. Eng. C 2018, 89, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Rottensteiner, U.; Sarker, B.; Heusinger, D.; Dafinova, D.; Rath, S.N.; Beier, J.P.; Kneser, U.; Horch, R.E.; Detsch, R.; Boccaccini, A.R.; et al. In vitro and in vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials 2014, 7, 1957. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Zehnder, T.; Rath, S.N.; Horch, R.E.; Kneser, U.; Detsch, R.; Boccaccini, A.R. Oxidized alginate-gelatin hydrogel: A favorable matrix for growth and osteogenic differentiation of adipose-derived stem cells in 3D. ACS Biomater. Sci. Eng. 2017, 3, 1730–1737. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Zhao, X.; Wang, H.; Yin, H. Preparation of alginate oligosaccharides and their biological activities in plants: A review. Carbohydr. Res. 2020, 494, 108056. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Gao, X.; Cheng, C.; Liu, C.; Wang, Q.; Han, X. The Structural Characteristics of Seaweed Polysaccharides and Their Application in Gel Drug Delivery Systems. Mar. Drugs 2020, 18, 658. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, A.C.; Tellez-Jurado, L.; Rodriguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Saltz, A.; Kandalam, U. Mesenchymal stem cells and alginate microcarriers for craniofacial bone tissue engineering: A review. J. Biomed. Mater. Res. A 2016, 104, 1276–1284. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Tshai, K.Y.; Nordin, N.; Prasad, V. Gelatin based scaffolds for tissue engineering-a review. Polym Res. J. 2015, 9, 15–32. [Google Scholar]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhossein, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutic. Bioeng.Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Horák, D. Application of poly(2-hydroxyethyl methacrylate) in medicine. In Polymers and Composites: Synthesis, Properties, and Applications, Polymer Yearbook; Pethrick, R.A., Zaikov, G.E., Horák, D., Eds.; Nova Science Publishers: New York, NY, USA, 2007; Volume 21, pp. 1–33. [Google Scholar]
- Kopeček, J. Hydrogels from soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. A 2009, 47, 5929–5946. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Nam, S.H.; Koh, W.-G. Preparation of collagen-immobilized poly(ethylene glycol)/poly(2-hydroxyethyl methacrylate) interpenetrating network hydrogels for potential application of artificial cornea. J. Appl. Polym. Sci. 2012, 123, 637–645. [Google Scholar] [CrossRef]
- Hidzir, N.M.; Radzali, N.A.M.; Rahman, I.A.; Shamsudin, S.A. Gamma irradiation-induced grafting of 2-hydroxyethyl methacrylate (HEMA) onto ePTFE for implant applications. Nucl. Eng. Technol. 2020, 52, 2320–2327. [Google Scholar] [CrossRef]
- Passos, M.F.; Dias, D.R.C.; Bastos, G.N.T.; Jardini, A.L.; Benatti, A.C.B.; Dias, C.G.B.T.; Filho, R.M. pHEMA hydrogels: Synthesis, kinetics and in vitro tests. J. Therm. Anal. Calorim. 2016, 125, 361–368. [Google Scholar] [CrossRef]
- Dobić, S.N.; Filipović, J.M.; Tomić, S.Lj. Synthesis and characterization of poly(2-hydroxyethyl methacrylate/itaconic acid)/poly(ethyleneglycol dimethacrylate) hydrogels. Chem. Eng. J. 2012, 179, 372–380. [Google Scholar] [CrossRef]
- Filipović, V.V.; Bozić Nedeljković, B.D.; Vukomanović, M.; Tomić, S.Lj. Biocompatible and degradable scaffolds based on 2-hydroxyethyl methacrylate, gelatin and poly(beta amino ester) crosslinkers. Polym. Test. 2018, 68, 270–278. [Google Scholar] [CrossRef]
- Filipović, V.V.; Babić Radić, M.M.; Vuković, J.S.; Vukomanović, M.; Rubert, M.; Hofmann, S.; Müller, R.; Tomić, S.Lj. Biodegradable hydrogel scaffolds based on 2-hydroxyethyl methacrylate, gelatin, poly(β-amino esters), and hydroxyapatite. Polymers 2022, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Takić Miladinov, D.; Tomić, S.; Stojanović, S.; Najdanović, J.; Filipović, J.; Trajanović, M.; Najman, S. Synthesis, Swelling properties and evaluation of genotoxicity of hydrogels based on (meth)acrylates and itaconic acid. Mater. Res. 2016, 19, 1070–1079. [Google Scholar] [CrossRef][Green Version]
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Hagenmüller, H.; Koch, A.M.; Müller, R.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 2007, 28, 1152–1162. [Google Scholar] [CrossRef]
- Tomić, S.Lj.; Babić, M.M.; Antić, K.M.; Vuković, J.S.; Malešić, N.B.; Filipović, J.M. pH-sensitive hydrogels based on (meth)acrylates and itaconic acid. Macromol. Res. 2014, 22, 1203–1213. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhao, Y.; Ding, J.; Lin, S. Investigation on complex coacervation between fish skin gelatin from cold-water fish and gum arabic: Phase behavior, thermodynamic, and structural properties. Food Res. Int. 2018, 107, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhang, C.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Cestari, A.R.; Airoldi, C.; Loh, W. Polysaccharide-based hydrogels: Preparation, characterization, and drug interaction behavior. Biomacromolecules 2009, 9, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Leon, C.A. New perspectives in mercury porosimetry. Adv. Colloid Interface Sci. 1998, 76–77, 341–371. [Google Scholar] [CrossRef]
- Rill, R.L.; Van Winkle, D.H.; Locke, B.R. Templated pores in hydrogels for improved size selectivity in Gel Permeation Chromatography. Anal. Chem. 1998, 70, 2433–2438. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.T.; Jańczewski, D.; Neoh, K.G. Parallel control over surface charge and wettability using polyelectrolyte architecture: Effect on protein adsorption and cell adhesion. ACS Appl. Mater. Interf. 2016, 8, 30552–30563. [Google Scholar] [CrossRef] [PubMed]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell–material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Idaszek, J.; Kijeńska, E.; Łojkowski, M.; Swieszkowski, W. How important are scaffolds and their surface properties in regenerative medicine. Appl. Surf. Sci. 2016, 388, 762–774. [Google Scholar] [CrossRef]
- Kyriakides, T.R. Molecular events at tissue/biomaterial interface. In Host Response to Biomaterials; Badylak, S.F., Ed.; Academic Press: Oxford, UK, 2015; pp. 81–116. [Google Scholar]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Prasitsilp, M.; Siriwittayakorn, T.; Molloy, R.; Suebsanit, N.; Siriwittayakorn, P.; Veeranondha, S. Cytotoxicity study of homopolymers and copolymers of 2-hydroxyethyl methacrylate and some alkyl acrylates for potential use as temporary skin substitutes. J. Mater. Sci. Mater. Med. 2003, 14, 595–600. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Krajnc, A.; Kramer, L.; Turk, B.; Suvorov, D. Designing Ga(III)-containing hydroxyapatite with antibacterial activity. RSC Adv. 2016, 6, 112839–112852. [Google Scholar] [CrossRef]
- Tomić, S.Lj.; Nikodinović-Runić, J.; Vukomanović, M.; Babić, M.M.; Vuković, J.S. Novel hydrogel scaffolds based on alginate, gelatin, 2-hydroxyethyl methacrylate, and hydroxyapatite. Polymers 2021, 13, 932. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Kołbuk, D.; Heljak, M.; Choinska, E.; Urbanek, O. Novel 3D hybrid nanofiber scaffolds for bone regeneration. Polymers 2020, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Yang, Z. Short-peptide-based molecular hydrogels: Novel gelation strategies and applications for tissue engineering and drug delivery. Nanoscale 2012, 4, 5259–5267. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, S.; Cao, X.; Yuan, L.; Wang, Y.; Yin, Y.; Qiu, T.; Dai, H.; Wang, X. Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo. Sci. Rep. 2014, 20, 7134. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, 9–19. [Google Scholar] [CrossRef]
- Fields, R.D.; Lancaster, M.V. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 1993, 11, 48–50. [Google Scholar]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In Vitro Uptake of Hydroxyapatite Nanoparticles and Their Effect on Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 2036176. [Google Scholar] [CrossRef] [PubMed]
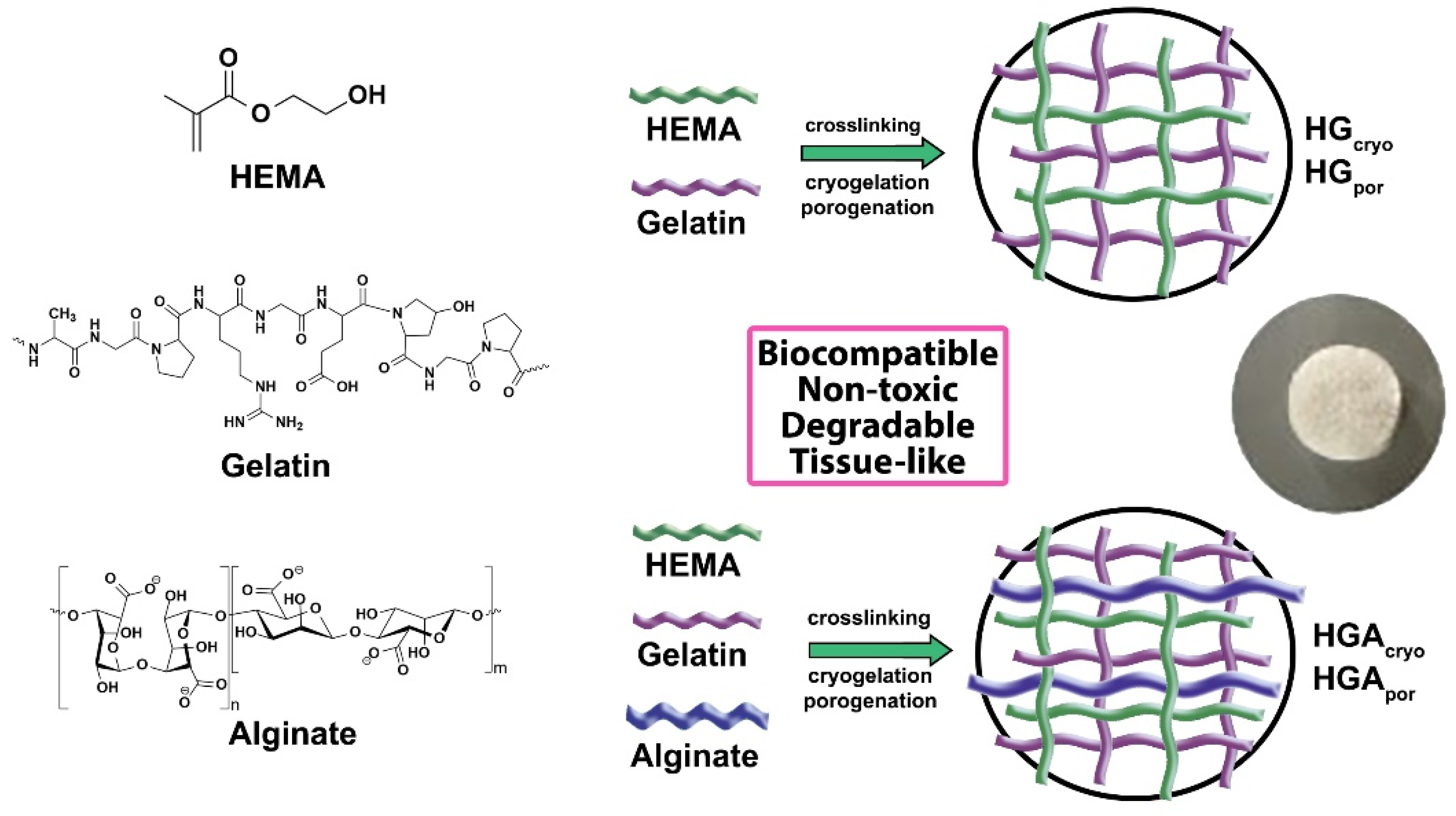
| Sample | Component 1 | Component 2 | Component 3 | Cross-Linker for HEMA | Cross-Linker for Gelatin | Initiator/Activator | Pore Formation Method |
|---|---|---|---|---|---|---|---|
| HGcryo | HEMA | Gelatin | - | EGDMA | EDC | PPS/TEMED | Cryogelation |
| HGAcryo | HEMA | Gelatin | Alginate | EGDMA | EDC | PPS/TEMED | Cryogelation |
| HGpor | HEMA | Gelatin | - | EGDMA | EDC | PPS/TEMED | Porogenation |
| HGApor | HEMA | Gelatin | Alginate | EGDMA | EDC | PPS/TEMED | Porogenation |
| Sample | Porosity (%) | Young’s Modulus (MPa) | Mass Loss (%) |
|---|---|---|---|
| HGcryo | 84.25 ± 3.58 | 2.08 ± 0.08 | 3.65 ± 0.10 |
| HGAcryo | 82.26 ± 3.51 | 9.75 ± 0.24 | 5.04 ± 0.12 |
| HGpor | 66.38 ± 3.02 | 4.61 ± 0.15 | 2.28 ± 0.07 |
| HGApor | 69.92 ± 3.14 | 0.99 ± 0.04 | 1.59 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babić Radić, M.M.; Filipović, V.V.; Vuković, J.S.; Vukomanović, M.; Rubert, M.; Hofmann, S.; Müller, R.; Tomić, S.L. Bioactive Interpenetrating Hydrogel Networks Based on 2-Hydroxyethyl Methacrylate and Gelatin Intertwined with Alginate and Dopped with Apatite as Scaffolding Biomaterials. Polymers 2022, 14, 3112. https://doi.org/10.3390/polym14153112
Babić Radić MM, Filipović VV, Vuković JS, Vukomanović M, Rubert M, Hofmann S, Müller R, Tomić SL. Bioactive Interpenetrating Hydrogel Networks Based on 2-Hydroxyethyl Methacrylate and Gelatin Intertwined with Alginate and Dopped with Apatite as Scaffolding Biomaterials. Polymers. 2022; 14(15):3112. https://doi.org/10.3390/polym14153112
Chicago/Turabian StyleBabić Radić, Marija M., Vuk V. Filipović, Jovana S. Vuković, Marija Vukomanović, Marina Rubert, Sandra Hofmann, Ralph Müller, and Simonida Lj. Tomić. 2022. "Bioactive Interpenetrating Hydrogel Networks Based on 2-Hydroxyethyl Methacrylate and Gelatin Intertwined with Alginate and Dopped with Apatite as Scaffolding Biomaterials" Polymers 14, no. 15: 3112. https://doi.org/10.3390/polym14153112
APA StyleBabić Radić, M. M., Filipović, V. V., Vuković, J. S., Vukomanović, M., Rubert, M., Hofmann, S., Müller, R., & Tomić, S. L. (2022). Bioactive Interpenetrating Hydrogel Networks Based on 2-Hydroxyethyl Methacrylate and Gelatin Intertwined with Alginate and Dopped with Apatite as Scaffolding Biomaterials. Polymers, 14(15), 3112. https://doi.org/10.3390/polym14153112










