Improvement the Flame Retardancy and Thermal Conductivity of Epoxy Composites via Melamine Polyphosphate-Modified Carbon Nanotubes
Abstract
:1. Introduction
2. Experiment Part
2.1. Materials
2.2. Preparation of Composites
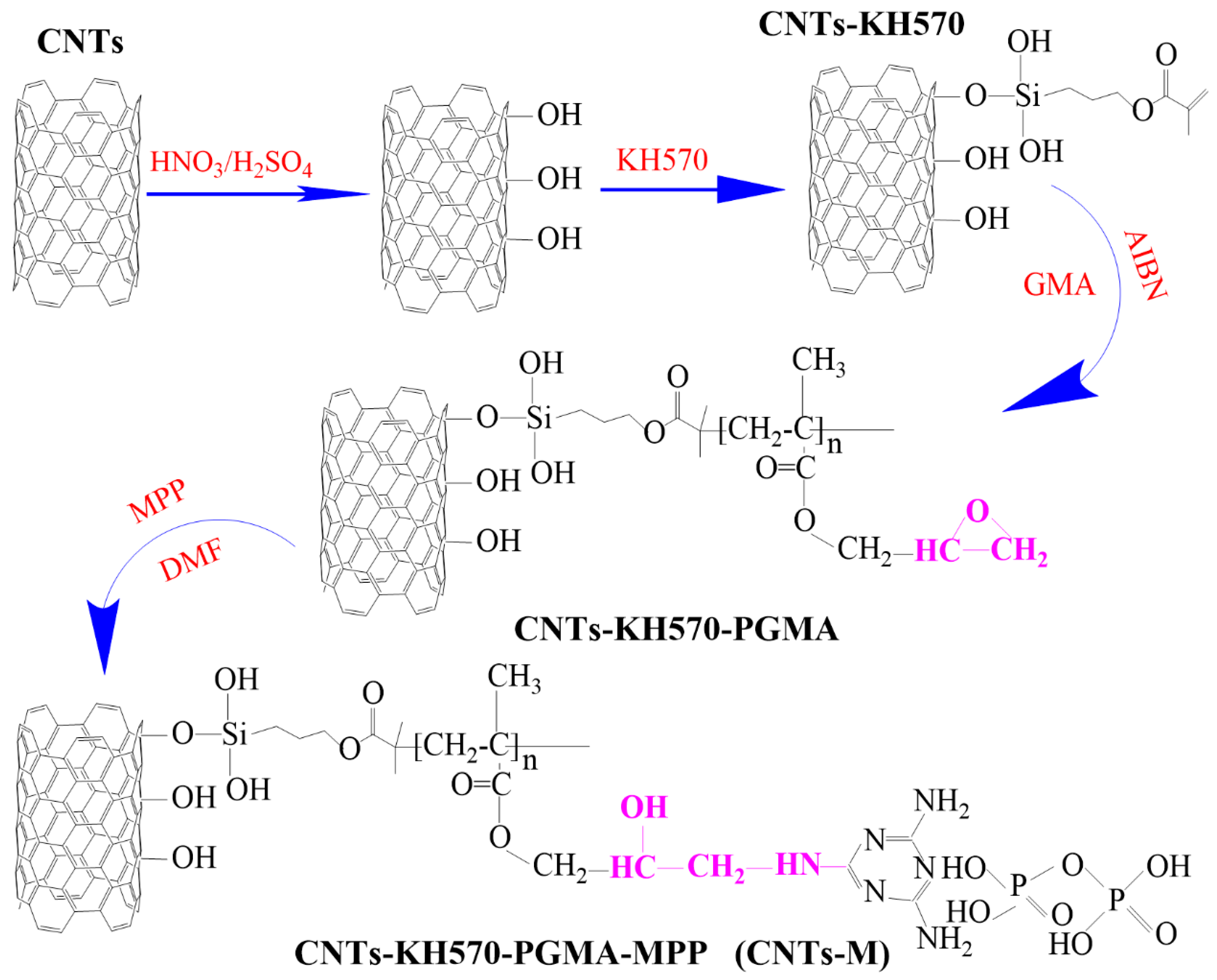
2.2.1. Carbon Nanotubes Modified by Silane Coupling Agent KH570
2.2.2. GMA Modified the CNTs-KH570
2.2.3. Flame Retardant MPP-Modified CNTs-KH570-PGMA
2.2.4. Preparation of EP/CNTs-M Composites
2.3. Measurements and Characterization
3. Results and Discussion
3.1. Characterization of Flame Retardant Molecular Modification on the Surface of CNTs
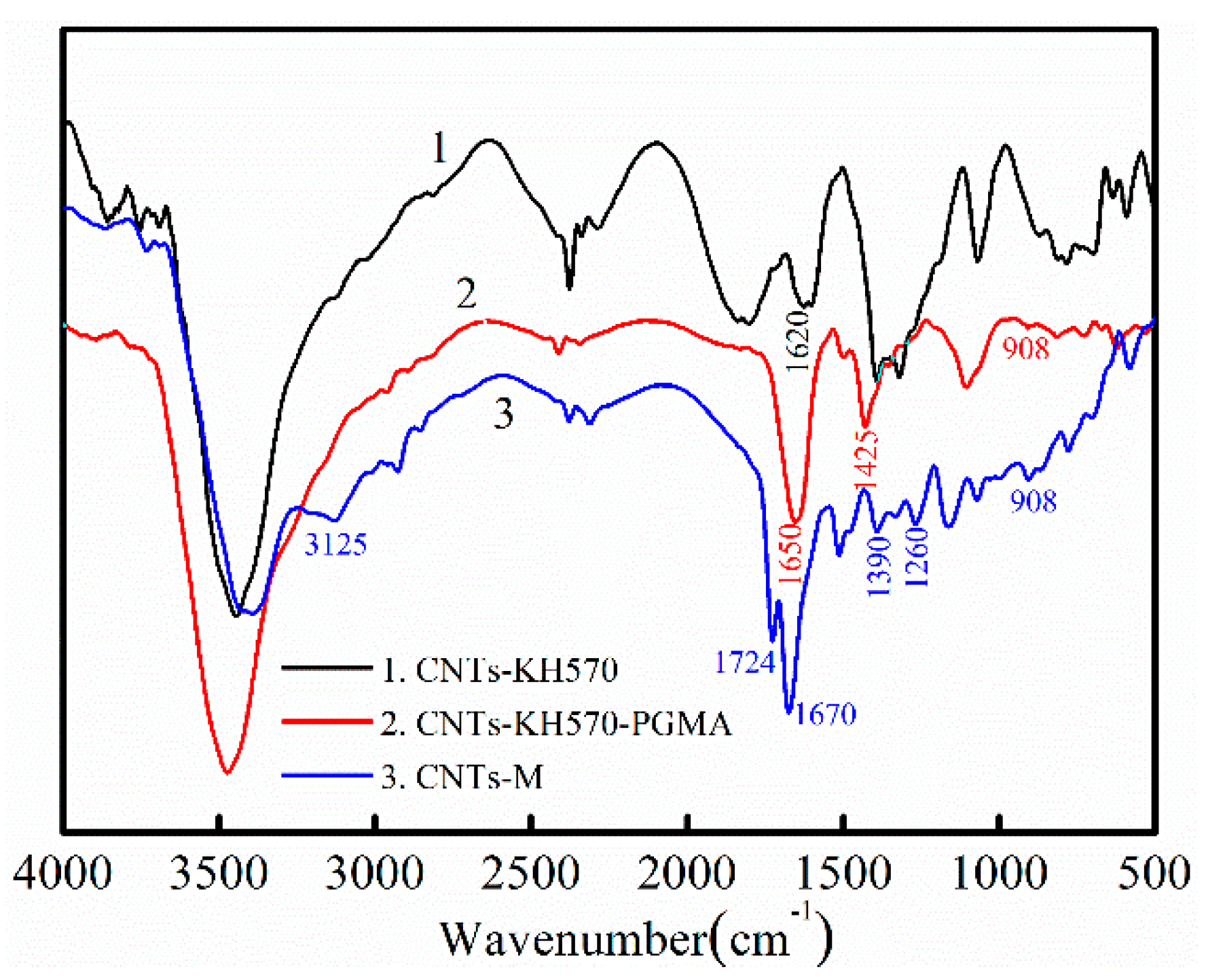
3.1.1. Infrared Spectrum
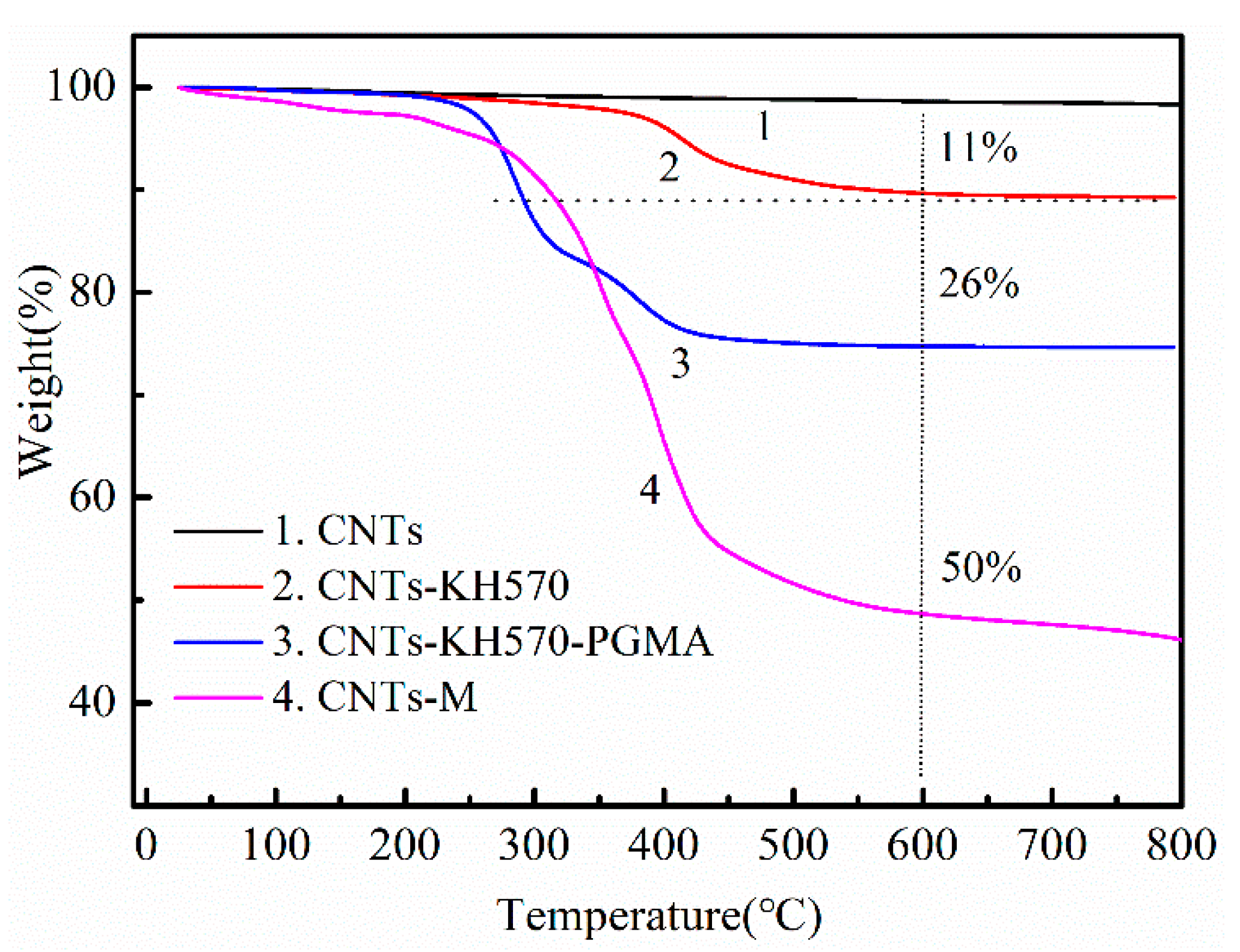
3.1.2. Thermogravimetric Analysis
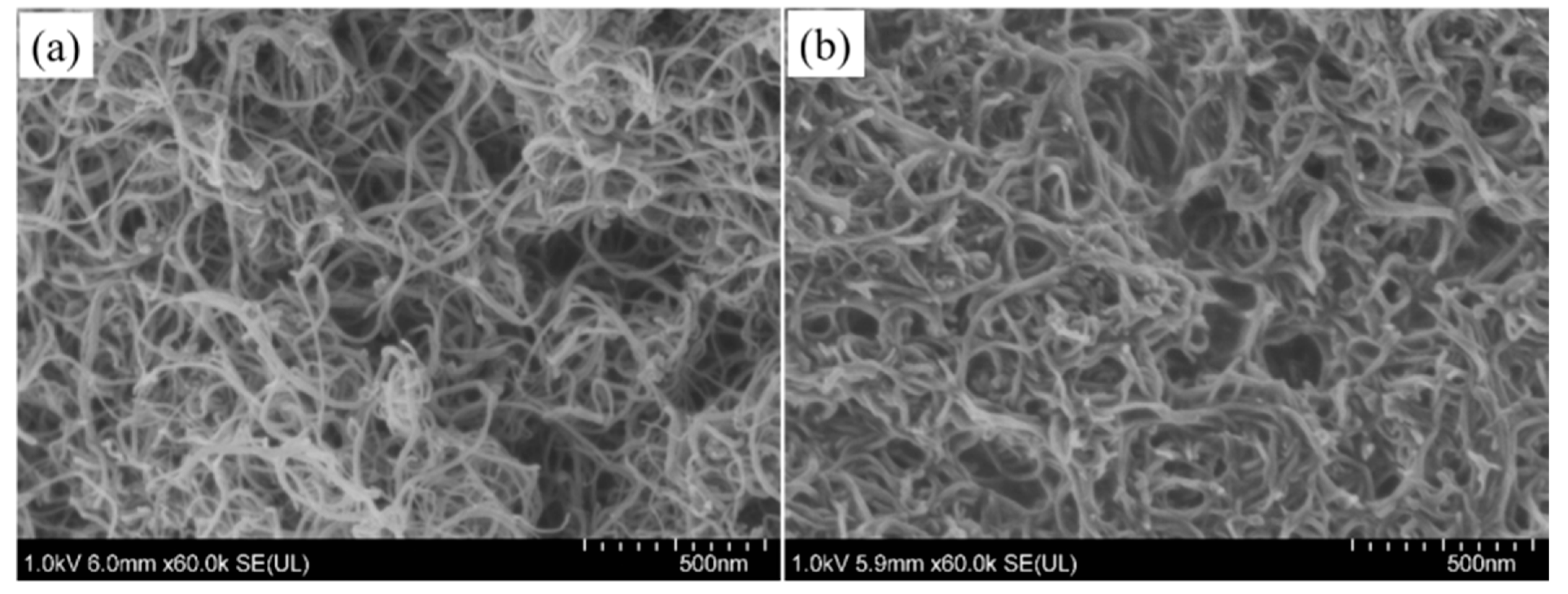
3.1.3. Morphology of CNTs and Modified CNTs
3.2. Thermal Stability of Composites
3.3. Combustion Performance of Composites
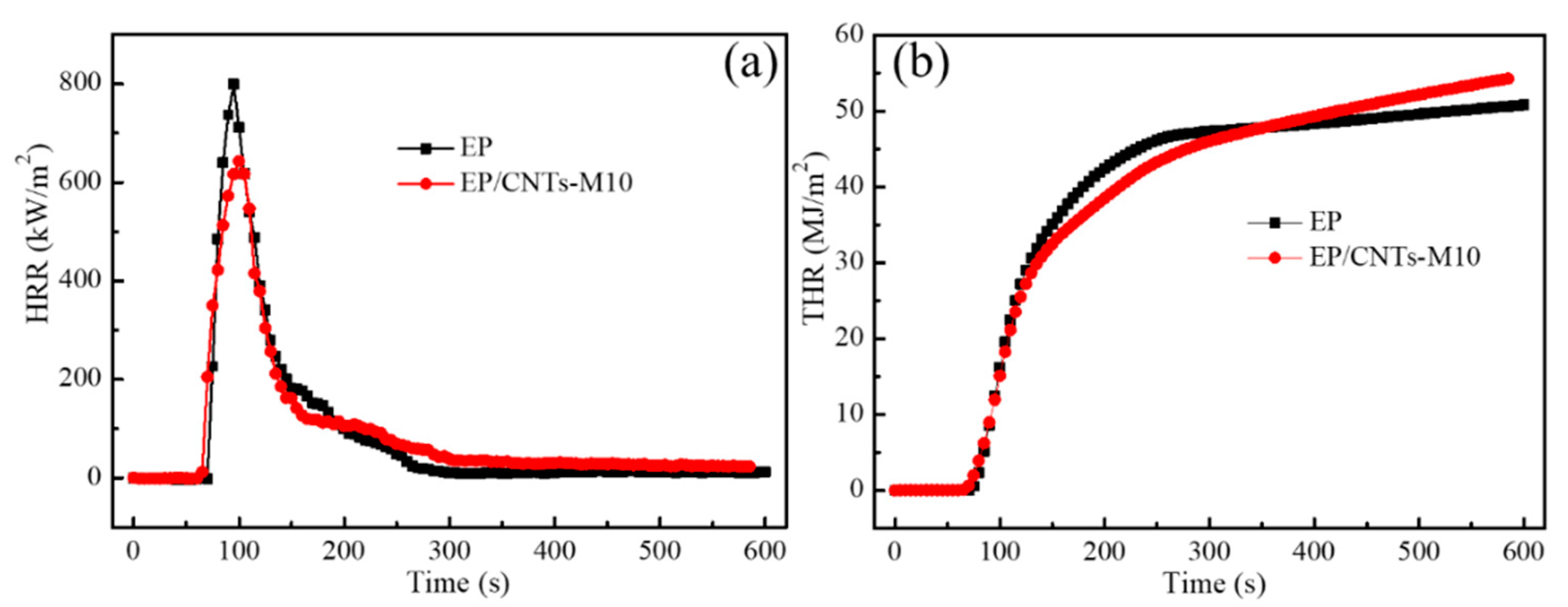
3.3.1. Study from the Heat Release
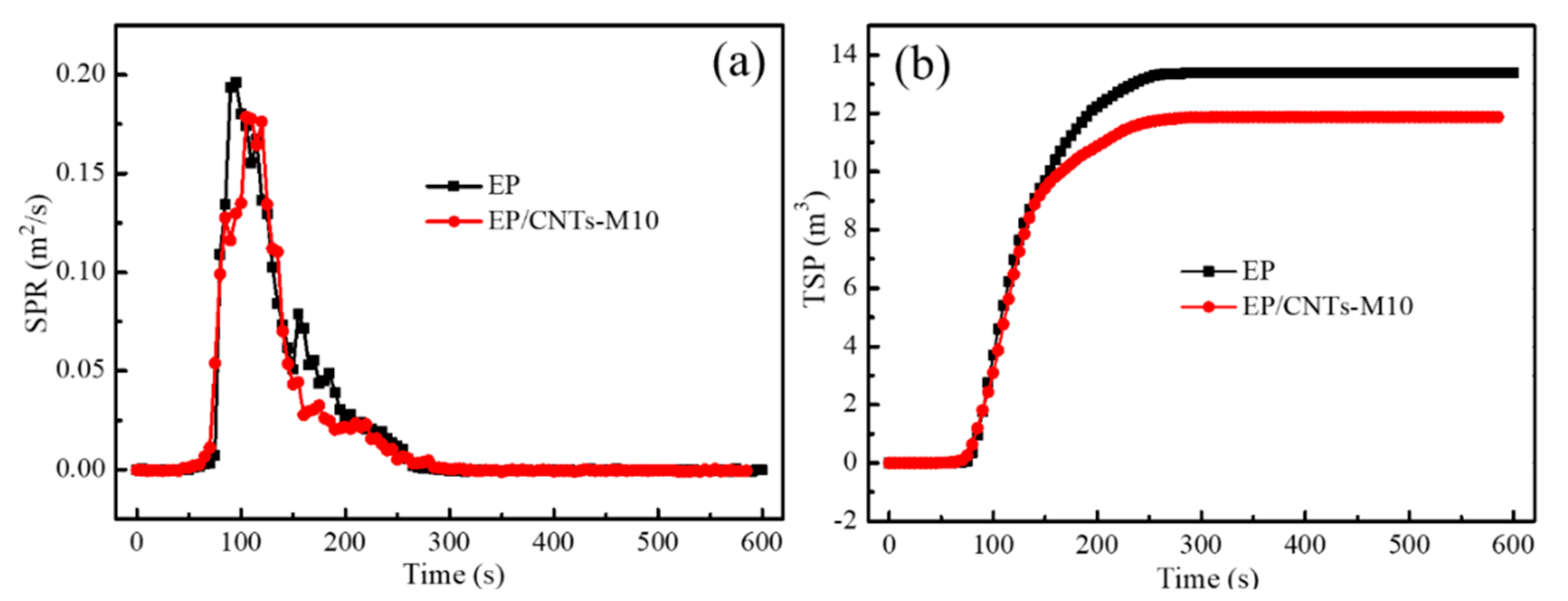
3.3.2. Study in Terms of Smoke Production
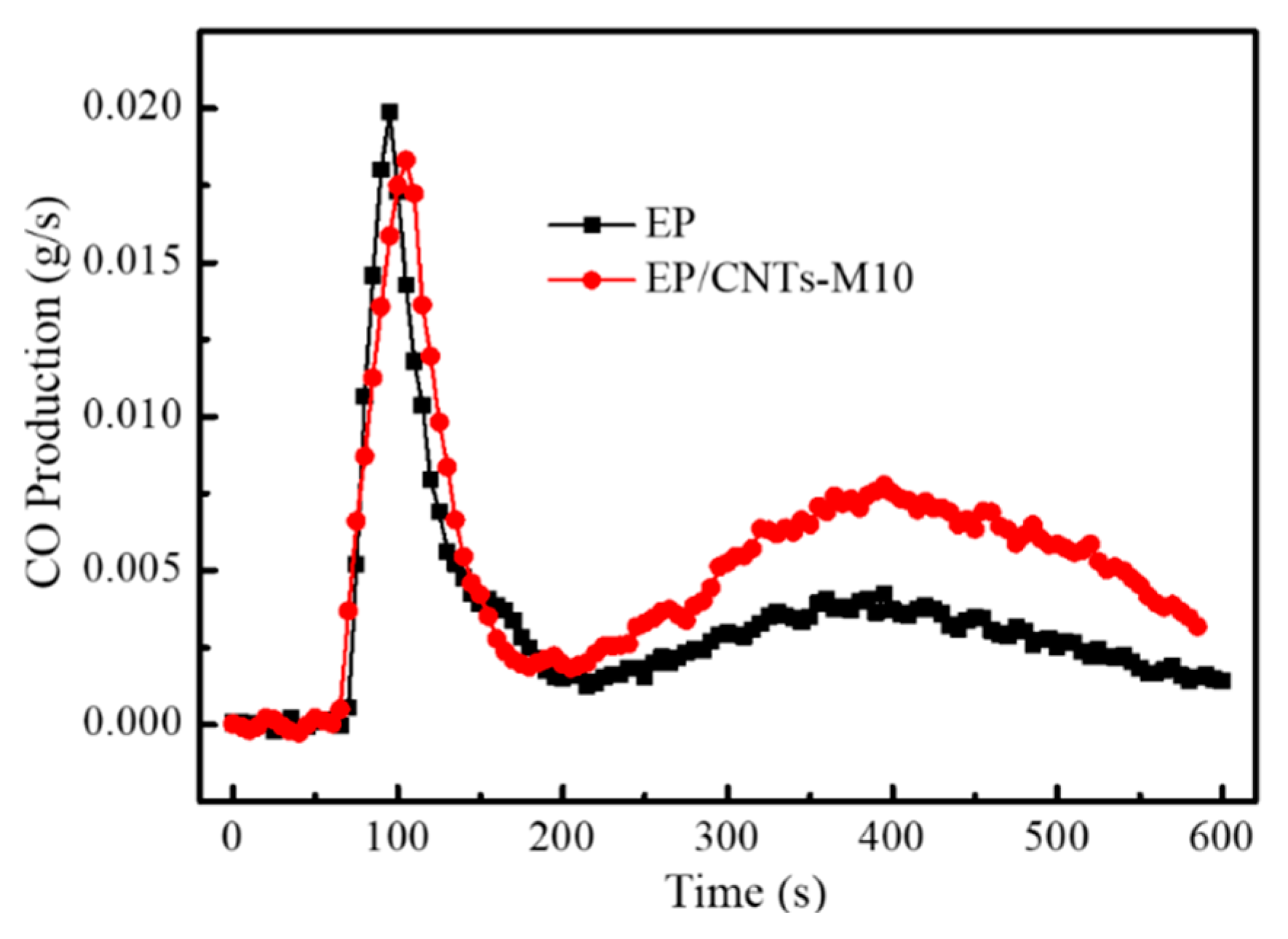
3.3.3. Study from Harmful Gases
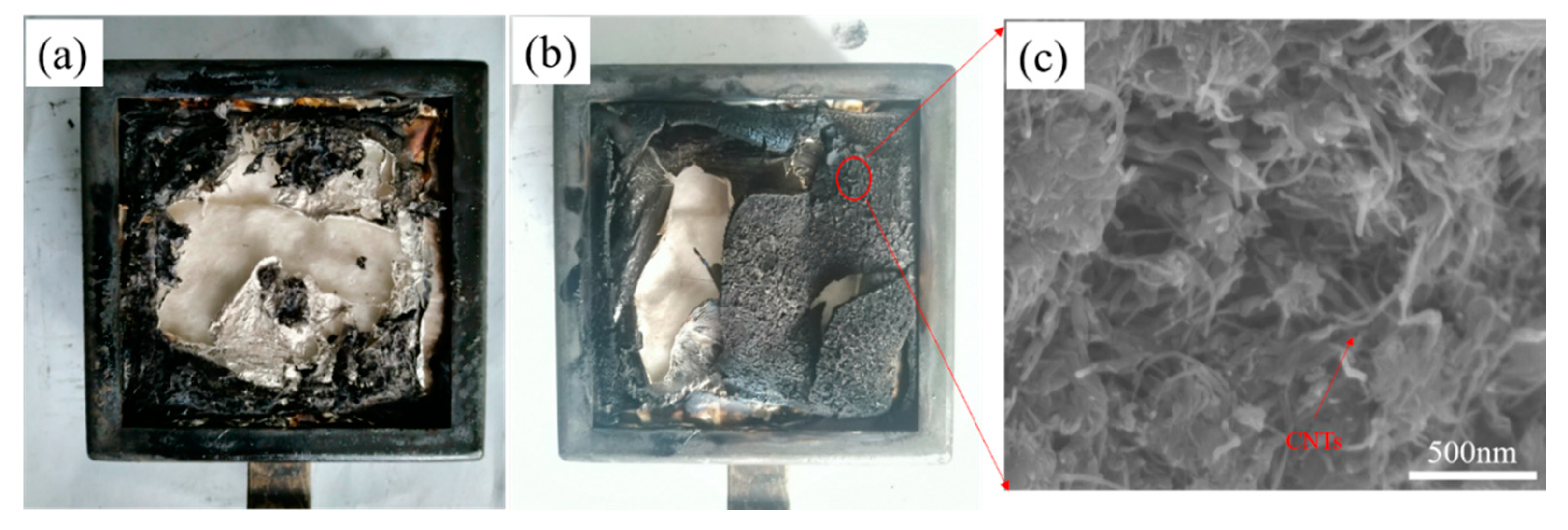
3.4. Carbon Residue Diagram and SEM of EP and Composites
3.5. Flame Retardant Performance Test of Composites
3.6. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Xue, Y.; Li, Z.; Wen, Y.; Li, X.; Wu, F.; Li, X.; Shi, D.; Xue, Z.; Xie, X. Construction of 3D boron nitride nanosheets/silver networks in epoxy-based composites with high thermal conductivity via in-situ sintering of silver nanoparticles. Chem. Eng. J. 2019, 369, 1150–1160. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhao, X.; Ye, Y.; Zhou, X.; Liu, H.; Liu, C.; Xie, X. Synergetic Improvement in Thermal Conductivity and Flame Retardancy of Epoxy/Silver Nanowires Composites by Incorporating “Branch-Like” Flame-Retardant Functionalized Graphene. ACS Appl. Mater. Interfaces 2018, 10, 21628–21641. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos. Part A Appl. Sci. Manuf. 2017, 103, 74–83. [Google Scholar] [CrossRef]
- Yang, S.; Xue, B.; Li, Y.; Li, X.; Xie, L.; Qin, S.; Xu, K.; Zheng, Q. Controllable Ag-rGO heterostructure for highly thermal conductivity in layer-by-layer nanocellulose hybrid films. Chem. Eng. J. 2020, 383, 123072. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, M.; Chen, C.; Xue, Z.; Xie, X.; Zhou, X.; Mai, Y.-W. Effect of elastic modulus mismatch of epoxy/titanium dioxide coated silver nanowire composites on the performance of thermal conductivity. Compos. Sci. Technol. 2018, 165, 206–213. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhan, C.; You, Y.; Tong, L.; Wei, R.; Liu, X. Preparation and thermal conductivity of copper phthalocyanine grafted boron nitride nanosheets. Mater. Lett. 2018, 227, 33–36. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P. Melamine foam-supported 3D interconnected boron nitride nanosheets network encapsulated in epoxy to achieve significant thermal conductivity enhancement at an ultralow filler loading. Chem. Eng. J. 2018, 348, 723–731. [Google Scholar] [CrossRef]
- Tominaga, Y.; Sato, K.; Hotta, Y.; Shibuya, H.; Sugie, M.; Saruyama, T. Effect of the addition of Al2O3 and h-BN fillers on the thermal conductivity of a cellulose nanofiber/nanodiamond composite film. Cellulose 2019, 26, 5281–5289. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, L.; Tang, Y.; Zhang, X.; Zheng, K.; Tian, X. Enhanced thermal conductivity of silicon carbide nanowires (SiCw)/epoxy resin composite with segregated structure. Compos. Part A Appl. Sci. Manuf. 2019, 116, 98–105. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Wang, B.; Zhou, X.; Ma, J.; Ye, Y.; Liu, C.; Xie, X. Multiple synergistic effects of graphene-based hybrid and hexagonal born nitride in enhancing thermal conductivity and flame retardancy of epoxy. Chem. Eng. J. 2020, 379, 122402. [Google Scholar] [CrossRef]
- Mai, V.D.; Lee, D.I.; Park, J.H.; Lee, D.S. Rheological Properties and Thermal Conductivity of Epoxy Resins Filled with a Mixture of Alumina and Boron Nitride. Polymers 2019, 11, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. Novel Multifunctional Organic–Inorganic Hybrid Curing Agent with High Flame-Retardant Efficiency for Epoxy Resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Shao, Z.B.; Yu, L.X.; Long, J.W.; Qi, M.; Chen, L.; Wang, Y.Z. Piperazine-modified ammonium polyphosphate as mono component flame-retardant hardener for epoxy resin: Flame retardance, curing behavior and mechanical property. Polym Chem. 2016, 7, 3003–3012. [Google Scholar] [CrossRef]
- Wen, Y.; Cheng, Z.; Li, W.; Li, Z.; Liao, D.; Hu, X.; Pan, N.; Wang, D.; Hull, T.R. A novel oligomer containing DOPO and ferrocene groups: Synthesis, characterization, and its application in fire retardant epoxy resin. Polym. Degrad. Stab. 2018, 156, 111–124. [Google Scholar] [CrossRef]
- Rao, W.-H.; Hu, Z.-Y.; Xu, H.-X.; Xu, Y.-J.; Qi, M.; Liao, W.; Xu, S.; Wang, Y.-Z. Flame-Retardant Flexible Polyurethane Foams with Highly Efficient Melamine Salt. Ind. Eng. Chem. Res. 2017, 56, 7112–7119. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, X.; Yu, B.; Feng, X.; Mu, X.; Yuen, R.K.; Hu, Y. Flame-retardant-wrapped polyphosphazene nanotubes: A novel strategy for enhancing the flame retardancy and smoke toxicity suppression of epoxy resins. J. Hazard. Mater. 2017, 325, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Liao, S.F.; Shang, K.; Chen, M.J.; Huang, J.Q.; Wang, Y.Z.; Schiraldi, D.A. Efficient approach to improving the flame retardancy of poly (vinyl alcohol)/clay aerogels: Incorporating piperazine-modified ammonium polyphosphate. ACS Appl. Mater. Interfaces 2015, 7, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ye, Y.; Xue, Y.; Xie, X.; Mai, Y.-W. Recent advances in covalent functionalization of carbon nanomaterials with polymers: Strategies and perspectives. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Flame Retardancy and Dispersion of Functionalized Carbon Nanotubes in Thiolene Nanocomposites. Polymers 2021, 13, 3308. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, F.; Wang, J. Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers 2021, 13, 3332. [Google Scholar] [CrossRef]
- Anton, M.; Andrey, S.; Andrey, Y.; Sergey, A.; Marina, L. Epoxy nanocomposites reinforced with functionalized carbon nanotubes. Polymers 2020, 12, 1816. [Google Scholar]
- Anton, M.; Andrey, S.; Andrey, Y.; Sergey, A.; Marina, L. Reinforced epoxy composites modified with functionalized graphene oxide. Polymers 2022, 14, 338. [Google Scholar]
- Vázquez-Moreno, J.M.; Sánchez-Hidalgo, R.; Sanz-Horcajo, E.; Viña, J.; Verdejo, R.; López-Manchado, M.A. Preparation and Mechanical Properties of Graphene/Carbon Fiber-Reinforced Hierarchical Polymer Composites. J. Compos. Sci. 2019, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Kamedulski, P.; Kaczmarek-Kedziera, A.; Lukaszewicz, J.P. Influence of intermolecular interactions on the properties of carbon nanotubes. Bull. Mater. Sci. 2018, 41, 76. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Janani, M.; Srikrishnarka, P.; Nair, S.V.; Nair, A.S. An in-depth review on the role of carbon nanostructures in dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 17914–17938. [Google Scholar] [CrossRef]
- Li, R.M.; Deng, C.; Deng, C.L.; Dong, L.P.; Di, H.W.; Wang, Y.Z. An efficient method to improve simultaneously the water resistance, flame retardancy and mechanical properties of POE intumescent flame-retardant systems. RSC Adv. 2015, 5, 16328–16339. [Google Scholar] [CrossRef]
- Yang, J.C.; Cao, Z.J.; Wang, Y.Z.; Schiraldi, D.A. Ammonium polyphosphate-based nanocoating for melamine foam towards high flame retardancy and anti-shrinkage in fire. Polymer 2015, 66, 86–93. [Google Scholar] [CrossRef]
- Dong, L.P.; Deng, C.; Li, R.M.; Cao, Z.J.; Lin, L.; Chen, L.; Wang, Y.Z. Poly (piperazinyl phosphamide): A novel highly efficient charring agent for an EVA/APP intumescent flame retardant system. RSC Adv. 2016, 6, 30436–30444. [Google Scholar] [CrossRef]
- Hassanzadeh-Aghdam, M.K.; Mahmoodi, M.J.; Ansari, R. Creep performance of CNT polymer nanocomposites—An emphasis on viscoelastic interphase and CNT agglomeration. Compos. Part B Eng. 2019, 168, 274–281. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Feng, Y.; Li, S.; Zhou, X.; Xie, X. Enhanced thermal conductivity and ideal dielectric properties of epoxy composites containing polymer modified hexagonal boron nitride. Compos. Part A Appl. Sci. Manuf. 2018, 107, 657–664. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.-W. Multi-functional interface tailoring for enhancing thermal conductivity, flame retardancy and dynamic mechanical property of epoxy/Al2O3 composites. Compos. Sci. Technol. 2018, 160, 42–49. [Google Scholar] [CrossRef]
- Molyneux, S.; Stec, A.A.; Hull, T.R. The effect of gas phase flame retardants on fire effluent toxicity. Polym. Degrad. Stab. 2014, 106, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Improving flame retardancy of in-situ silica-epoxy nanocomposites cured with aliphatic hardener: Combined effect of DOPO-based flame-retardant and melamine. Compos. Part C Open Access 2020, 2, 100022. [Google Scholar] [CrossRef]
- Marset, D.; Dolza, C.; Fages, E.; Gonga, E.; Gutierrez, O.; Gomez-Caturla, J.; Ivorra-Martinez, J.; Sanchez-Nacher, L.; Quiles-Carrillo, L. The effect of halloysite nanotubes on the fire retardancy properties of partially biobased polyamide 610. Polymers 2020, 12, 3050. [Google Scholar] [CrossRef] [PubMed]
- Mostovoy, A.S.; Nurtazina, A.S.; Kadykova, Y.A.; Bekeshev, A.Z. Highly Efficient Plasticizers-Antipirenes for Epoxy Polymers. Inorg. Mater. Appl. Res. 2019, 10, 1135–1139. [Google Scholar] [CrossRef]
- Hassanzadeh-Aghdam, M.K.; Mahmoodi, M.J. Micromechanical modeling of thermal conducting behavior of general carbon nanotube-polymer nanocomposites. Mater. Sci. Eng. B 2018, 229, 173–183. [Google Scholar] [CrossRef]
- Kundalwal, S.I.; Suresh Kumar, R.; Ray, M.C. Effective thermal conductivities of a novel fuzzy carbon fiber heat exchanger containing wavy carbon nanotubes. Int. J. Heat Mass Tran. 2014, 72, 440–451. [Google Scholar] [CrossRef]


| The Qualitative Characteristics of CNTs | Value |
|---|---|
| Internal diameter (nm) | 4~10 |
| External diameter (nm) | 10~30 |
| Length (µm) | 30~50 |
| The Qualitative Characteristics of E-51 | Value |
|---|---|
| Epoxy equivalent (g/mol) | 192~216 |
| Density at 25 °C (kg/m3) | 1167 |
| Molecular weight | 375.86 |
| Viscosity (Pa·s) | 13~20 |
| The Qualitative Characteristics of EMI-2,4 | Value |
|---|---|
| Melting point (°C) | 47~54 |
| Density at 25 °C (kg/m3) | 975 |
| Molecular weight | 110.16 |
| Samples | EP | EP/CNTs-M5 | EP/CNTs-M10 |
|---|---|---|---|
| LOI (vol%) | 25.1 | 26.4 | 28.3 |
| UL-94 rating | NR | NR | V2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Luo, S.; Du, X.; Li, Q.; Cheng, S. Improvement the Flame Retardancy and Thermal Conductivity of Epoxy Composites via Melamine Polyphosphate-Modified Carbon Nanotubes. Polymers 2022, 14, 3091. https://doi.org/10.3390/polym14153091
Shi X, Luo S, Du X, Li Q, Cheng S. Improvement the Flame Retardancy and Thermal Conductivity of Epoxy Composites via Melamine Polyphosphate-Modified Carbon Nanotubes. Polymers. 2022; 14(15):3091. https://doi.org/10.3390/polym14153091
Chicago/Turabian StyleShi, Xuejun, Shiying Luo, Xiangxiang Du, Qingbin Li, and Shiping Cheng. 2022. "Improvement the Flame Retardancy and Thermal Conductivity of Epoxy Composites via Melamine Polyphosphate-Modified Carbon Nanotubes" Polymers 14, no. 15: 3091. https://doi.org/10.3390/polym14153091
APA StyleShi, X., Luo, S., Du, X., Li, Q., & Cheng, S. (2022). Improvement the Flame Retardancy and Thermal Conductivity of Epoxy Composites via Melamine Polyphosphate-Modified Carbon Nanotubes. Polymers, 14(15), 3091. https://doi.org/10.3390/polym14153091





