Recent Progresses in Adsorption Mechanism, Architectures, Electrode Materials and Applications for Advanced Electrosorption System: A Review
Abstract
:1. Introduction
2. Adsorption Mechanism and Cell Architectures of CDI
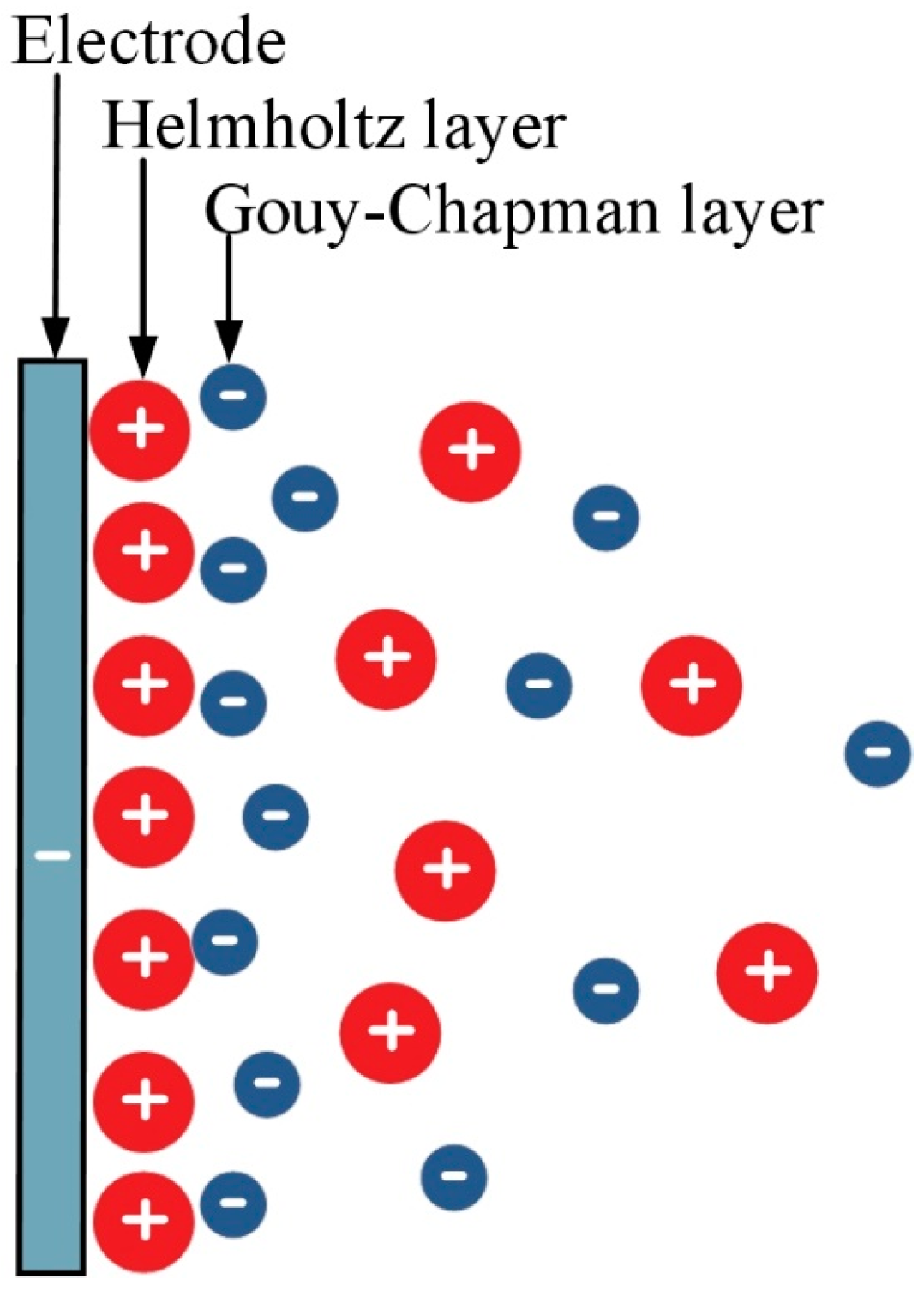
2.1. Adsorption Mechanism
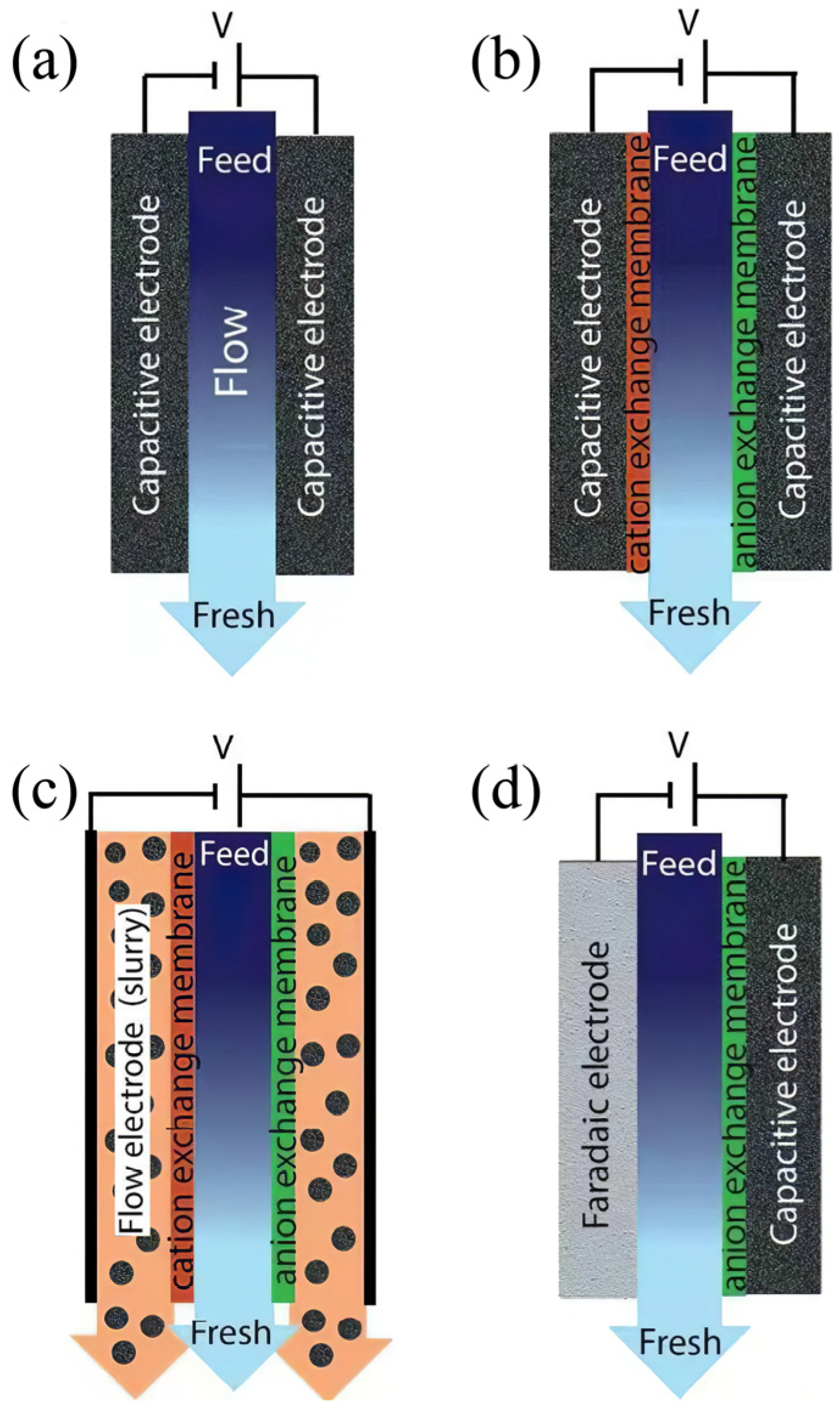
2.2. Cell Architectures of CDI
2.2.1. Membrane Capacitive Deionization
2.2.2. Flow Capacitive Deionization
2.2.3. Hybrid Capacitive Deionization
3. Electrode Materials of CDI
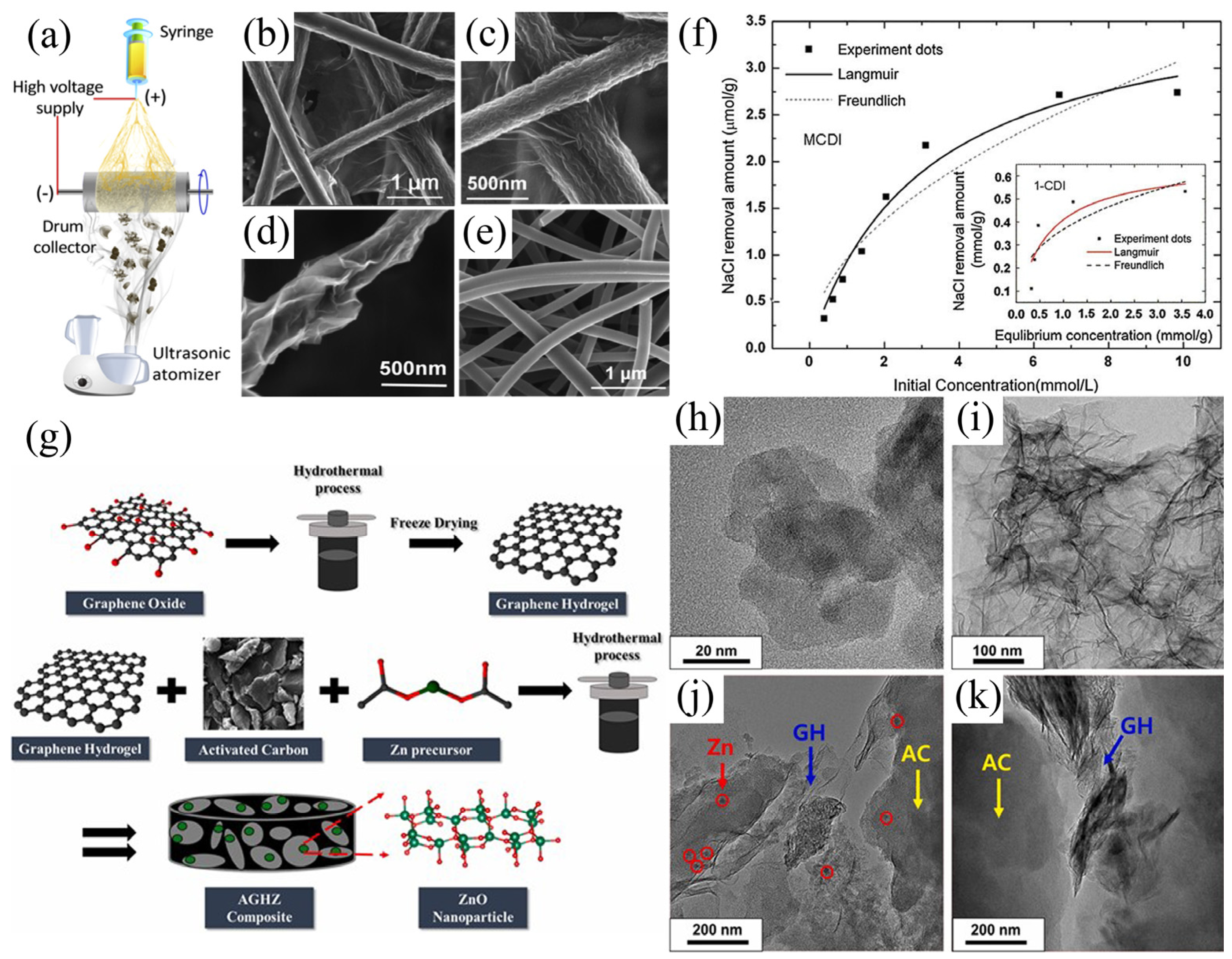
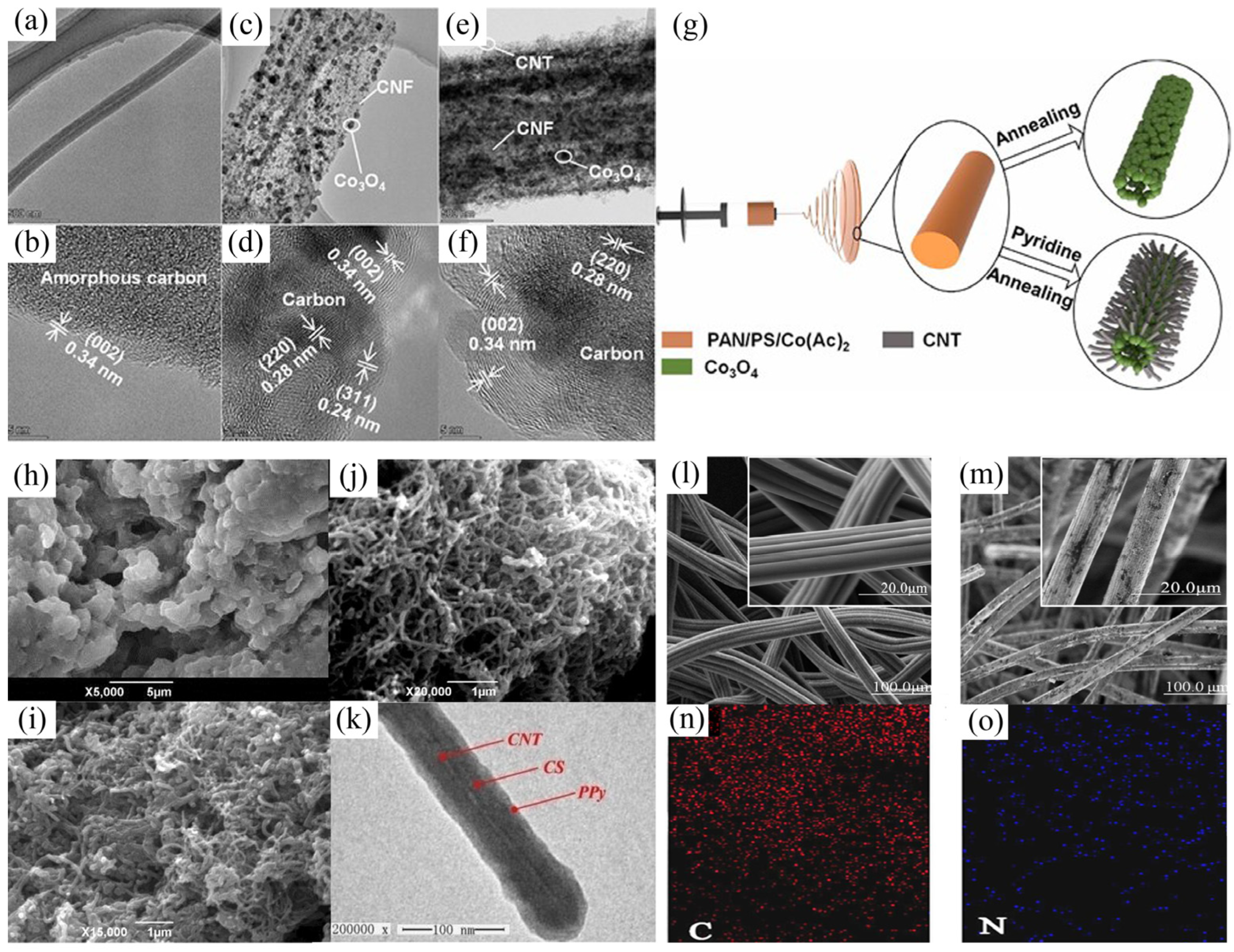
3.1. Carbon-Carbon Composite Materials
3.2. Metal-Oxide and Carbon Composite Materials
3.3. Conductive Polymers and Carbon Composite Materials
4. Applications of Electrosorption Technology in Water Treatment
4.1. Removal of Salt Ions from Water by Electrosorption
4.2. Removal of Heavy Metal Ions and Other Harmful Ions from Wastewater by Electrosorption
4.3. Removal of Various Organic Compounds from Wastewater by Electrosorption
5. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosegrant, M.W.; Cline, S.A. Global food security: Challenges and policies. Science 2003, 302, 1917–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinnis, R.L.; Elimelech, M. Global challenges in energy and water supply: The promise of engineered osmosis. Environ. Sci. Technol. 2008, 42, 8625–8629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.L.; Castrillón, S.R.V.; Shaffer, D.L.; Ma, J.; Elimelech, M. In situ surface chemical modification of thin-film composite forward osmosis membranes for enhanced organic fouling resistance. Environ. Sci. Technol. 2013, 47, 12219–12228. [Google Scholar] [CrossRef]
- Hu, Y.S.; Hu, B.; Ge, Y.X.; Nie, P.F.; Yang, J.M.; Huang, M.H.; Liu, J.Y. In-situ synthesis of UiO-66-NH2 on porous carbon nanofibers for high performance defluoridation by capacitive deionization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 129020. [Google Scholar] [CrossRef]
- Zhu, M.F.; Wang, Y.Y.; Zhang, W.C.; Luo, Y.J.; Sun, Z.M. Evolution of capacitive deionization devices configuration and research progress in fluoride ion removal electrodes. Chin. J. Nonferrous Met. 2021, 31, 3362–3379. [Google Scholar]
- Zhang, Q.L.; Cheng, Y.L.; Fang, C.Q.; Shi, J.Y.; Han, H.Z. Functionalized waste cellulose with metal-organic-frameworks as the adsorbent with adjustable pore size: Ultralight, effective, and selective removal of organic dyes. J. Solid State Chem. 2021, 302, 122361. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Cheng, Y.L.; Fang, C.Q.; Shi, J.Y.; Chen, J.; Han, H.Z. Novel and multifunctional adsorbent fabricated by Zeolitic imidazolate framworks-8 and waste cigarette filters for wastewater treatment: Effective adsorption and photocatalysis. J. Solid State Chem. 2021, 299, 122190. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Cheng, Y.L.; Fang, C.Q.; Shi, J.Y. Construction of novel regenerated cellulose based foam derived from waste cigarette filters as effective oil adsorbent. J. Appl. Polym. Sci. 2021, 139, 51900. [Google Scholar] [CrossRef]
- Zhao, J.R.; Cheng, Y.L.; Fang, C.Q.; Wang, Z.; Zhang, Q.L.; Li, Y. Template-free synthesis of hollow yolk shelled VO2(M) microspheres and their application in effective removal of methylene blue. Ceram. Int. 2022, 48, 5655–5662. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Cheng, Y.L.; Fang, C.Q.; Chen, J.; Chen, H.P.; Li, H.; Yao, Y.T. Facile synthesis of porous carbon/Fe3O4 composites derived from waste cellulose acetate by one-step carbothermal method as a recyclable adsorbent for dyes. J. Mater. Sci. Technol. 2020, 9, 3384–3393. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Lee, J.; Kim, S.; Yoon, J. TiO2 sol-gel spray method for carbon electrode fabrication to enhance desalination efficiency of capacitive deionization. Desalination 2014, 342, 70–74. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Yoon, J. Rocking chair desalination battery based on prussian blue electrodes. ACS Omega 2017, 2, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hu, X. Electrosorption of thiocyanate anion son active carbon felt electrode in dilute solution. J. Colloid Interface Sci. 2005, 290, 190–195. [Google Scholar]
- Jia, B.; Zhang, W. Preparation and Application of Electrodes in Capacitive Deionization (CDI): A State-of-Art Review. Nanoscale Res. Lett. 2016, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Bae, W.S.; Choi, J.H. Electrode reactions and adsorption/desorption performance related to the applied potential in a capacitive deionization process. Desalination 2010, 258, 159–163. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive deionization (CDI) for desalination and water treatment-past, present and future (a review). Desalination 2008, 8, 10–29. [Google Scholar] [CrossRef]
- Li, X.; Close, T.; Pustulka, S.; Pedu, S.; Xue, Y.; Richter, C.; Taboada-Serrano, P. Electrosorption of monovalent alkaline metal ions onto highly ordered mesoporous titanium dioxide nanotube electrodes. Electrochim. Acta 2017, 231, 632–640. [Google Scholar] [CrossRef]
- Quan, X.P.; Fu, Z.B.; Yuan, L.; Zhong, M.L.; Mi, R.; Yang, X.; Yi, Y.; Wang, C. Capacitive deionization of NaCl solutions with ambient pressure dried carbon aerogel microsphere electrodes. RSC Adv. 2017, 7, 35875–35882. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.H.; Sun, K.W.; Zhang, Y.H.; Qian, C.F.; Bao, W.Z. Dimensional optimization enables high-performance capacitive deionization. J. Mater. Chem. A 2022, 10, 6414–6441. [Google Scholar] [CrossRef]
- Zhang, M.; Kong, W.Q. Recent progress in graphene-based and ion-intercalation electrode materials for capacitive deionization. J. Electroanal. Chem. 2020, 878, 114703. [Google Scholar] [CrossRef]
- Bian, Y.H.; Yang, X.F.; Liang, P.; Jiang, Y.; Zhang, C.Y.; Huang, X. Enhanced desalination performance of membrane capacitive deionization cells by packing the flow chamber with granular activated carbon. Water Res. 2015, 85, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Zuo, K.C.; Zhang, X.R.; Zhang, C.Y.; Liang, P. Selective ion separation by capacitive deionization (CDI) based technologies: A state-of-the-art review. Environ. Sci. Water Res. Technol. 2020, 6, 243–257. [Google Scholar] [CrossRef]
- Damaskin, B.B.; Petrii, O.A. Historical development of theories of the electrochemical double layer. J. Solid State Electrochem. 2011, 15, 1317–1334. [Google Scholar] [CrossRef]
- Wu, X.R.; Jia, Z.J.; Ma, H.Y.; Liao, S.D.; Wang, B.G. Fundamentals of electrochemistry(III): Electrical double layer model and its development. Energy Storage Sci. Technol. 2013, 2, 152–156. [Google Scholar]
- Bockris, J.; Reddy, A.K.N. Modern Electrochemistry 2B: Electrodics in Chemistry, Engineering, Biology and Environmental Science, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Wu, J.Z. Understanding the Electric Double-Layer Structure, Capacitance, and Charging Dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef]
- Kornyshev, A.A. Double-layer in ionic liquids: Paradigm change? J. Phys. Chem. B 2007, 111, 5545–5557. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Storey, B.D.; Kornyshev, A.A. Double Layer in Ionic Liquids: Overscreening versus Crowding. Phys. Rev. Lett. 2011, 106, 046102. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, Z.A.H.; Feng, G.; Kornyshev, A.A. Mean-Field Theory of Electrical Double Layer In Ionic Liquids with Account of Short-Range Correlations. Electrochim. Acta 2017, 225, 190–197. [Google Scholar] [CrossRef] [Green Version]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of Capacitive Deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Li, H.; Nie, C.Y.; Pan, L.K.; Sun, Z. The study of membrane capacitive deionization from charge efficiency. Desalination Water Treat. 2012, 42, 210–215. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zou, L. Ion-exchange membrane capacitive deionization: A new strategy for brackish water desalination. Desalination 2011, 275, 62–66. [Google Scholar] [CrossRef]
- Kwak, N.S.; Koo, J.S.; Hwang, T.S.; Choi, E.M. Synthesis and electrical properties of NaSS–MAA–MMA cation exchange membranes for membrane capacitive deionization (MCDI). Desalination 2011, 285, 138–146. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, M.; Van der Wal, B. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 2012, 5, 9520–9527. [Google Scholar] [CrossRef] [Green Version]
- Li, H.B.; Gao, Y.; Pan, L.K.; Zhang, Y.P.; Chen, Y.W.; Sun, Z. Electrosorptive desalination by carbon nanotubes and nanofibres electrodes and ion-exchange membranes. Water Res. 2008, 42, 4923–4928. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, J.H. The production of ultrapure water by membrane capacitive deionization (MCDI) technology. J. Membr. Sci. 2012, 409–410, 251–256. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Towards Electrochemical Water Desalination Techniques: A Review on Capacitive Deionization, Membrane Capacitive Deionization and Flow Capacitive Deionization. Membranes 2020, 10, 96. [Google Scholar] [CrossRef]
- Shahmirzadi, M.A.A.; Hosseini, S.S.; Luo, J.Q.; Ortiz, I. Significance, evolution and recent advances in adsorption technology, materials and processes for desalination, water softening and salt removal. J. Environ. Manage. 2018, 215, 324–344. [Google Scholar] [CrossRef]
- Xu, X.T.; Wang, M.; Liu, Y.; Liu, T.; Pan, L.K. Ultrahigh Desalinization Performance of Asymmetric Flow-Electrode Capacitive Deionization Device with an Improved Operation Voltage of 1.8 V. ACS Sustain. Chem. Eng. 2017, 5, 189–195. [Google Scholar] [CrossRef]
- Shin, Y.U.; Lim, J.; Boo, C.; Hong, S. Improving the feasibility and applicability of flow-electrode capacitive deionization (FCDI): Review of process optimization and energy efficiency. Desalination 2021, 502, 114930. [Google Scholar] [CrossRef]
- Jeon, S.I.; Park, H.R.; Yeo, J.G.; Yang, S.; Cho, C.H.; Han, M.N.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Yang, F.; He, Y.F.; Rosentsvit, L.; Suss, M.E.; Zhang, X.R.; Gao, T.; Liang, P. Flow-electrode capacitive deionization: A review and new perspectives. Water Res. 2021, 15, 117222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Wei, H.X.; Zhao, H.C.; Wang, Y.F.; Tang, N. Electrode materials for capacitive deionization: A review. J. Electroanal. Chem. 2020, 873, 114416. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ma, J.X.; Wu, L.; Sun, J.Y.; Wang, L.; Li, T.Y.; Waite, T.D. Flow Electrode Capacitive Deionization (FCDI): Recent Developments, Environmental Applications, and Future Perspectives. Environ. Sci. Technol. 2021, 55, 4243–4267. [Google Scholar] [CrossRef]
- Huang, H.N.; Li, F.Y.; Yu, C.L.; Fang, H.S.; Guo, X.C.; Li, D.P. Anion-/cationic compounds enhance the dispersion of flow electrodes to obtain high capacitive deionization performance. Desalination 2021, 515, 115182. [Google Scholar] [CrossRef]
- Biesheuvel, P.; Van der Wal, A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, K.K.; Eum, H.M.; Lee, C.W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Li, H.B.; Lu, T.; Pan, L.K.; Zhang, Y.P.; Sun, Z. Electrosorption behavior of graphene in NaCl solutions. J. Mater. Chem. 2009, 19, 6773–6779. [Google Scholar] [CrossRef]
- Tang, K.X.; Kim, Y.H.; Chang, J.J.; Mayes, R.T.; Gabitto, J.; Yiacoumi, S.; Tsouris, C. Seawater desalination by over-potential membrane capacitive deionization: Opportunities and hurdles. Chem. Eng. J. 2019, 357, 103–111. [Google Scholar] [CrossRef]
- Qiu, Q.; Cha, J.H.; Choi, Y.M.; Choi, J.H.; Shin, J.W.; Lee, Y.S. Preparation of polyethylene membranes filled with crosslinked sulfonated polystyrene for cation exchange and transport in membrane capacitive deionization process. Desalination 2017, 417, 87–93. [Google Scholar] [CrossRef]
- Omosebi, A.; Gao, X.; Landon, J.; Liu, K. Asymmetric electrode configuration for enhanced membrane capacitive deionization. ACS Appl. Mater. Interfaces 2014, 6, 12640–12649. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jo, J.; Kim, K.; Lee, T.; Yoon, J. Temporal and spatial distribution of pH in flowmode capacitive deionization and membrane capacitive deionization. Desalination 2018, 439, 188–195. [Google Scholar] [CrossRef]
- Tang, W.W.; He, D.; Zhang, C.Y.; Kovalsky, P.; Waite, T.D. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes. Water Res. 2017, 120, 229–237. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Li, Y.Q.; Ding, Z.B.; Li, J.F.; Li, J.B.; Lu, T.; Pan, L.K. Highly efficient and stable desalination via novel hybrid capacitive deionization with redox-active polyimide cathode. Desalination 2019, 469, 114098. [Google Scholar] [CrossRef]
- Zhao, C.J.; Wang, X.L.; Zhang, S.B.; Sun, N.; Zhou, H.J.; Wang, G.Z.; Zhang, Y.X.; Zhang, H.M.; Zhao, H.J. Porous Carbon Nanosheets Functionalized with Fe3O4 Nanoparticles for Capacitive Removal of Heavy Metal Ions from Water. Environ. Sci. Water Res. Technol. 2019, 6, 331–340. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Xu, X.T.; Ding, Z.B.; Wang, K.; Sun, X.; Lu, T.; Konarova, M.; Eguchi, M.; Shapter, J.G.; Pan, L.; et al. Ti3C2 MXenes-Derived NaTi2(PO4)3/MXene Nanohybrid for Fast and Efficient Hybrid Capacitive Deionization Performance. Chem. Eng. J. 2020, 407, 127148. [Google Scholar] [CrossRef]
- Elisadiki, J.; King’ondu, C.K. Performance of ion intercalation materials in capacitive deionization/electrochemical deionization: A review. J. Electroanal. Chem. 2020, 878, 114588. [Google Scholar] [CrossRef]
- Han, L.C.; Karthikeyan, K.G.; Anderson, M.A.; Wouters, J.J.; Gregory, K.B. Mechanistic insights into the use of oxide nanoparticles coated asymmetric electrodes for capacitive deionization. Electrochim. Acta 2013, 90, 573–581. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. A short review of activated carbon assisted electrosorption process: An overview, current stage and future prospects. J. Hazard. Mater. 2009, 170, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Thamilselvan, A.; Nesaraj, A.S.; Noel, M.; Noel, M. Review on carbon-based electrode materials for application in capacitive deionization process. Int. J. Environ. Sci. Technol. 2016, 13, 2961–2976. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Q.; Wu, T.T.; Zhan, F.; Zhou, M.; Qiu, J.S. Ultrasound-assisted preparation of electrospun carbon fiber/graphene electrodes for capacitive deionization: Importance and unique role of electrical conductivity. Carbon 2016, 103, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.L.; Wang, Y.; Chen, C.Y.; Yu, F.; Ma, J. A Comparison of graphene hydrogels modified with single-walled/multi-walled carbon nanotubes as electrode materials for capacitive deionization. J. Colloid Interface Sci. 2018, 518, 69–75. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J. Electro-assisted adsorption of Cs(I) and Co (II) from aqueous solution by capacitive deionization with activated carbon cloth/graphene oxide composite electrode. Sci. Total Environ. 2020, 749, 141524. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.M.; Kim, H.Y. Graphene wrapped MnO2- nanostructures as effective and stable electrode materials for capacitive deionization desalination technology. Desalination 2014, 344, 289–298. [Google Scholar] [CrossRef]
- Myint, M.T.Z.; Dutta, J. Fabrication of zinc oxide nanorods modified activated carbon cloth electrode for desalination of brackish water using capacitive deionization approach. Desalination 2012, 05, 24–30. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, H.O.; Mohamed, I.M.A.; Mousa, H.M.; Barakat, N.A.M. Enhanced desalination performance of capacitive deionization using zirconium oxide nanoparticles-doped graphene oxide as a novel and effective electrode. Sep. Purif. Technol. 2016, 171, 34–43. [Google Scholar] [CrossRef]
- Prasanna, B.P.; Avadhani, D.N.; Muralidhara, H.B.; Chaitra, K.; Thomas, V.R.; Revanasiddappa, M.; Kathyayini, N. Synthesis of polyaniline/ZrO2 nanocomposites and their performance in AC conductivity and electrochemical supercapacitance. Bull. Mater. Sci. 2016, 39, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Khan, S.J.; Jamal, Y.; Kamran, H.; Ahsan, A.; Ahmad, M. Desalination of brackish water using capacitive deionization (CDI) technology. Desalination Water Treat. 2016, 57, 7659–7666. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bail´on-García, E.; Perez-Cadenas, A.F.; Maldonado-Hodar, F.J.; Castelo-Quiben, J.; Carrasco-Marin, F. Electrochemical performances of supercapacitors from carbon-ZrO2 composites. Electrochim. Acta 2018, 259, 803–814. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, I.M.A.; Mousa, H.M.; Park, C.H.; Kim, C.S. Facile synthesis of TiO2/ZrO2 nanofibers/nitrogen co-doped activated carbon to enhance the desalination and bacterial inactivation via capacitive deionization. Sci. Rep. 2018, 8, 541. [Google Scholar] [CrossRef]
- Lado, J.J.; P´erez-Roa, R.E.; Wouters, J.J.; Tejedor-Tejedor, M.I.; Federspill, C.; Ortiz, J.M.; Anderson, M.A. Removal of nitrate by asymmetric capacitive deionization. Sep. Purif. Technol. 2017, 183, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Yasin, A.S.; Mohamed, A.Y.; Kim, D.H.; Doan, T.L.L.; Chougule, S.S.; Jung, N.; Nam, S.; Lee, K. Design of zinc oxide nanoparticles and graphene hydrogel co-incorporated activated carbon for efficient capacitive deionization. Sep. Purif. Technol. 2021, 277, 119428. [Google Scholar] [CrossRef]
- Kyaw, H.H.; Myint, M.T.Z.; Al-Harthi, S.; Al-Muhtaseb, A.H.; Al-Abri, M. Electric field enhanced in situ silica nanoparticles grafted activated carbon cloth electrodes for capacitive deionization. Sep. Purif. Technol. 2022, 281, 119888. [Google Scholar] [CrossRef]
- Divyapriya, G.; Vijayakumar, K.K.; Nambi, I. Development of a novel graphene/Co3O4 composite for hybrid capacitive deionization system. Desalination 2019, 451, 102–110. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, J.T.; Ding, M.; Gu, C.D.; Vafakhah, S.; Zhang, W.; Li, D.S.; Alvarado, P.V.Y.; Yang, H.Y. Hierarchical Co3O4/CNT decorated electrospun hollow nanofiber for efficient hybrid capacitive deionization. Sep. Purif. Technol. 2021, 266, 118593. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Lee, S.Y.; Wei, T.; Li, J.; Fan, Z.J. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Fu, Q.J.; Bai, W.W.; Ning, X.; Yao, C.L. Construction and Application of nanocellulose/graphene/MnO2 three-dimensional composites as potential electrode materials for supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 31, 1236–1246. [Google Scholar] [CrossRef]
- Li, L.M.; Liu, E.H.; Li, J.; Yang, Y.J.; Shen, H.J.; Huang, Z.Z.; Xiang, X.X.; Li, W. A doped activated carbon prepared from polyaniline for high performance supercapacitors. J. Power Sources 2010, 195, 1516–1521. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Zornitta, R.L.; García-Mateos, F.J.; Lado, J.J.; Rodriguez-Mirasol, J.; Cordero, T.; Hammer, P.; Ruotolo, L.A.M. High-performance activated carbon from polyaniline for capacitive deionization. Carbon 2017, 123, 318–333. [Google Scholar] [CrossRef] [Green Version]
- Nie, P.F.; Yan, J.B.; Zhu, G.D.; Liu, J.Y. Inverted hybrid-capacitive deionization with polyaniline nanotubes doped activated carbon as an anode. Electrochim. Acta 2020, 339, 135920. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xue, J.Q.; Li, F.; Dai, J.Z. Preparation of Polypyrrole/Chitosan/Carbon Nanotube Composite Nano-electrode and Application to Capacitive Deionization Process for removing Cu2+. Chem Eng Process 2019, 139, 121–129. [Google Scholar] [CrossRef]
- Tian, S.C.; Zhang, Z.H.; Zhang, X.H.; Ostrikov, K. Capacitative deionization using commercial activated carbon fiber decorated with polyaniline. J. Colloid Interface Sci. 2018, 537, 247–255. [Google Scholar] [CrossRef]
- Sun, J.Q.; Liu, L.F.; Yanf, F.L. A WO3/PPy/ACF modified electrode in electrochemical system for simultaneous removal of heavy metal ion Cu2+ and organic acid. J. Hazard. Mater. 2020, 394, 122534. [Google Scholar] [CrossRef]
- Farmer, J.; Fix, D.V.; Mack, G.V.; Pekala, R.W. Capacitive deionization of NaCl and NaNO3 solution with carbon aerogel electrode. J. Electrochem. Soc. 1996, 143, 159–169. [Google Scholar] [CrossRef]
- Salari, K.; Zarafshan, P.; Khashehchi, M.; Chegini, G.; Etezadi, H.; Karami, H.; Szulzyk-Cieplak, J.; Lagod, G. Knowledge and Technology Used in Capacitive Deionization of Water. Membranes 2022, 12, 459. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.M.; Khalia, K.A.; Motlak, M.; Kim, H.Y. Graphene/SnO2 nanocomposite as an effective electrode material for saline water desalination using capacitive deionization. Ceram. Int. 2014, 40, 14627–14634. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.D.; Wang, H.Q.; Wu, X.L.; Shen, J. Ambient Pressure-Dried Graphene–Composite Carbon Aerogel for Capacitive Deionization. Processes 2019, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.M.; Wang, Y.Z.; Gao, L.X.; Zhang, D.Q.; Lin, T. Graphene bonded carbon nanofiber aerogels with high capacitive deionization capability. Electrochim. Acta 2018, 260, 656–663. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Lu, T.; Liu, Y.; Xu, X.T.; Pan, L.K.; Alothman, A.A.; Shapter, J.; Wang, Y.; Yamauchi, Y. Highly efficient water desalination by capacitive deionization on biomass-derived porous carbon nanoflakes. Sep. Purif. Technol. 2021, 256, 117771. [Google Scholar] [CrossRef]
- Li, Y.J.; Liu, Y.; Wang, M.; Xu, X.T.; Lu, T.; Sun, C.Q.; Pan, L.K. Phosphorus-doped 3D carbon nanofiber aerogels derived from bacterial-cellulose for highly-efficient capacitive deionization. Carbon 2018, 130, 377–383. [Google Scholar] [CrossRef]
- Wang, H.; Yan, T.T.; Shen, J.J.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Efficient removal of metal ions by capacitive deionization with straw waste derived graphitic porous carbon nanosheets. Environ. Sci. Nano 2020, 7, 317–326. [Google Scholar] [CrossRef]
- Huang, C.C.; He, J.C. Electrosorptive removal of copper ions from wastewater by using ordered mesoporous carbon electrodes. Chem. Eng. J. 2013, 221, 469–475. [Google Scholar] [CrossRef]
- Oda, H.; Nakagawa, Y. Removal of ionic substances from dilute solution using activated carbon electrodes. Carbon 2003, 41, 1037–1047. [Google Scholar] [CrossRef]
- Huang, C.C.; Su, Y.J. Removal of copper ions from waste water by adsorption/electrosorption on modified activated carbon cloths. J. Hazard. Mater. 2010, 175, 477–483. [Google Scholar] [CrossRef]
- Dai, M.; Xia, L.; Song, S.X.; Peng, C.S.; Rene, J. Electrosorption of As (III) in aqueous solutions with activated carbon as the electrode. Appl. Surf. Sci. 2018, 434, 816–821. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, L.; Cai, Z.X.; Jason Ren, Z.Y. Individual and competitive removal of heavy metals using capacitive deionization. J. Hazard. Mater. 2016, 302, 323. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Z.; Liu, F.Y.; Lan, H.C.; Lu, H.J.; Qu, J.H. Preparation of a manganese dioxide/carbon fiber electrode for electrosorptive removal of copper ions from water. J. Colloid Interface Sci. 2015, 446, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gui, Y.; Blackwood, D.J. Development of a nanostructured alpha-MnO2/carbon paper composite for removal of Ni2+/Mn2+ ions by electrosorption. ACS Appl. Mater. Interfaces 2018, 10, 19615–19625. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Zhang, Q.; Xu, L.J.; Zheng, Y.Q.; Yao, C.X.; Jia, J.N.; Leng, W.N.; Hong, S.F. Porous Reduced Graphene Oxide/Single-Walled Carbon Nanotube Film as Freestanding and Flexible Electrode Materials for Electrosorption of Organic Dye. ACS Appl. Nano Mater. 2019, 2, 6258–6267. [Google Scholar] [CrossRef]
- Yao, C.X.; Zhang, W.Q.; Xu, L.J.; Cheng, M.M.; Su, Y.; Xue, J.; Liu, J.L.; Hou, S.F. A facile synthesis of porous MXene-based freestanding film and its spectacular electrosorption performance for organic dyes. Sep. Purif. Technol. 2021, 263, 118365. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Cheng, Y.L.; Fang, C.Q.; Shi, J.Y.; Han, H.Z.; Li, M.Y.; Zhao, J.R. Electrochemically enhanced adsorption of organic dyes from aqueous using a freestanding metal–organic frameworks/cellulose-derived porous monolithic carbon foam. Bioresour. Technol. 2022, 347, 126424. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Liu, L.; Zhang, J.; Meng, Q.H. Capacitive deionization and methyl orange removal of holey graphene hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126463. [Google Scholar] [CrossRef]
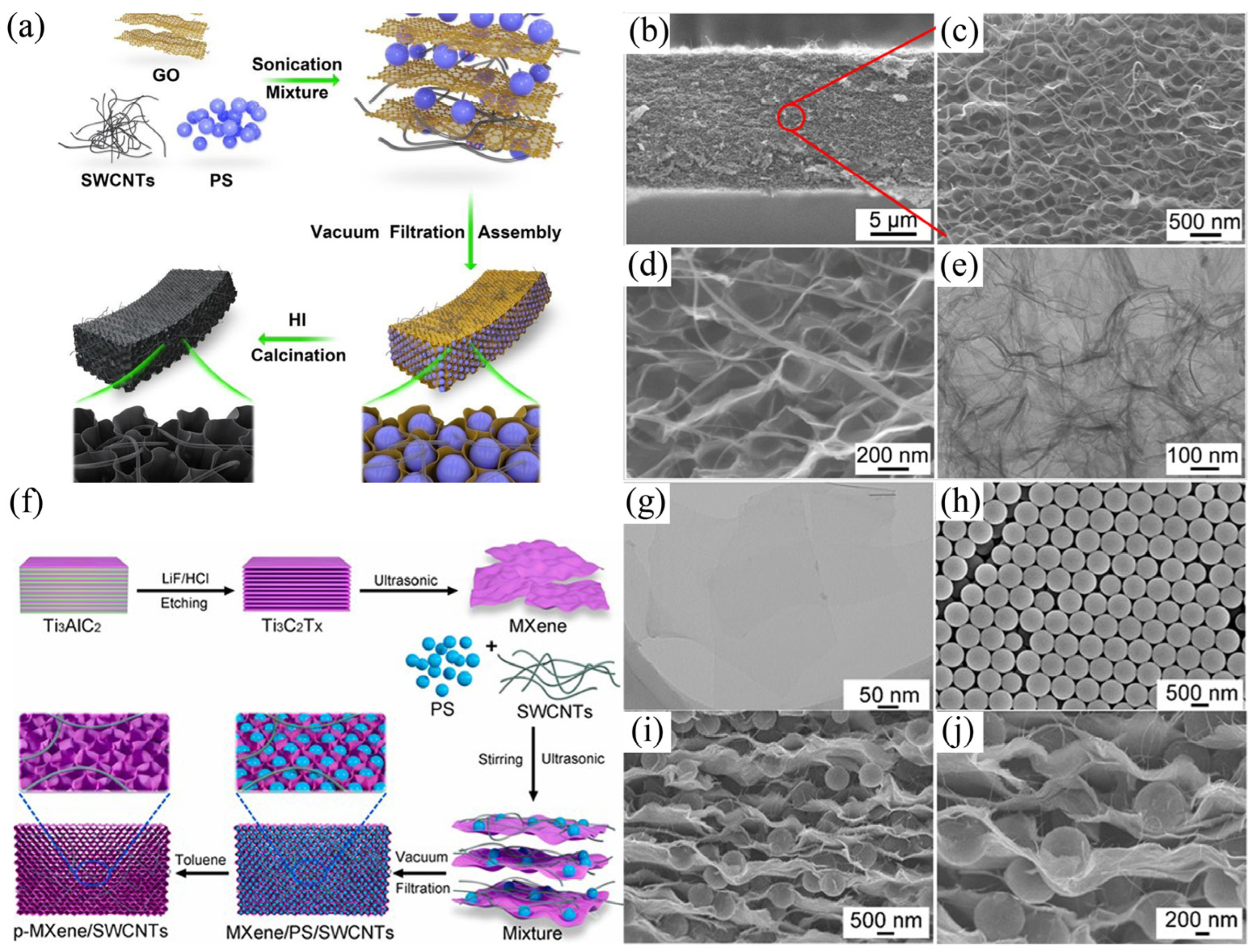
| Solution (mg/L) | Electrodes | Specific Surface Area (m2/g) | Electrical Conductivity | Applied Voltage (V) | Ion Capacity (mg/g) | Removal Efficiency (%) | Operation Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| NaCl (100) | ACF/rGO | 649 | 42.6 (S/m) | 1.2 | 9.2 | - | 0.5 | [65] |
| ACF | 630 | 2.1 (S/m) | 1.2 | ~4 | - | |||
| NaCl (300) | SWCNTs/rGO | 308.37 | Specific capacitance 36.35 F/g | 2 | 48.73 | - | 10 | [66] |
| MWCNTs/rGO | 262.44 | - | 2 | 39.53 | - | |||
| NaCl (50) | AGHZ * | - | Specific capacitance 746.5 F/g | 1.2 | 9.95 | 83.65 | 2 | [76] |
| NaCl (1500) | Co3O4@CNF@CNT | 320.8 | Specific capacitance 395 F/g | 1.4 | 58.6 | - | - | [79] |
| NaCl (200) | ACF/PANI | 415 | - | 2 | 19.9 | - | 2 | [87] |
| NaCl (500) | Graphene/carbon aerogel | 546.2 | - | - | 26.9 | - | 10 | [92] |
| PANI/GO carbon aerogel | 542.8 | Specific capacitance 220 F/g | 1.2 | 15.7 | - | 37 min | [93] | |
| NaCl (1000) | Porous carbon nanoflake | 408.1 | Specific capacitance 187.6 F/g | 1.2 | 16.29 | - | 0.5 | [95] |
| Cu2+ (100) | PPy/CS/CNT composite | 33.51 | Specific capacitance 103.19 F/g | 0.8 | 16.83 | 80.08 | 0.5 | [86] |
| WO3/PPy/ACF // ACF | 788.27 | Areal capacitance (2.58 F/cm2) | 1 | - | 97.8 | 5 | [88] | |
| Chitosan impregnated ACF cloth | 1123.3 | - | 0.3 | 0.380 mmol/g | - | 12 | [100] | |
| Cu (NO3)2 (-) | Two MnO2/CF electrodes | - | Specific capacitance 387 F/g | 0.8 | 172.88 | - | - | [103] |
| MB (30) | prGO/SWCNTs film | 93.8 | ~74 (S/cm) | 1.2 | 730.6 | 95 | 24 | [105] |
| p-MXene/SWCNTs film | 42.9 | - | 1.2 | 1068.8 | 95 | 24 | [106] | |
| 0 | 55.8 | - | 24 | |||||
| MB (-) | Carbon foam | 1457 | - | 1.2 | - | 98 | 12 | [107] |
| - | 0 | - | 63 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Shi, J.; Zhang, Q.; Fang, C.; Chen, J.; Li, F. Recent Progresses in Adsorption Mechanism, Architectures, Electrode Materials and Applications for Advanced Electrosorption System: A Review. Polymers 2022, 14, 2985. https://doi.org/10.3390/polym14152985
Cheng Y, Shi J, Zhang Q, Fang C, Chen J, Li F. Recent Progresses in Adsorption Mechanism, Architectures, Electrode Materials and Applications for Advanced Electrosorption System: A Review. Polymers. 2022; 14(15):2985. https://doi.org/10.3390/polym14152985
Chicago/Turabian StyleCheng, Youliang, Jiayu Shi, Qingling Zhang, Changqing Fang, Jing Chen, and Fengjuan Li. 2022. "Recent Progresses in Adsorption Mechanism, Architectures, Electrode Materials and Applications for Advanced Electrosorption System: A Review" Polymers 14, no. 15: 2985. https://doi.org/10.3390/polym14152985
APA StyleCheng, Y., Shi, J., Zhang, Q., Fang, C., Chen, J., & Li, F. (2022). Recent Progresses in Adsorption Mechanism, Architectures, Electrode Materials and Applications for Advanced Electrosorption System: A Review. Polymers, 14(15), 2985. https://doi.org/10.3390/polym14152985






