Thermally Activable Bistetrazoles for Elastomers Crosslinking
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Cross-Linkers 1–5
2.3. Mixing Procedure
2.4. Dynamic Mechanical Properties
2.4.1. Single Temperature Curing
2.4.2. Separate Temperature Curing
3. Results and Discussion
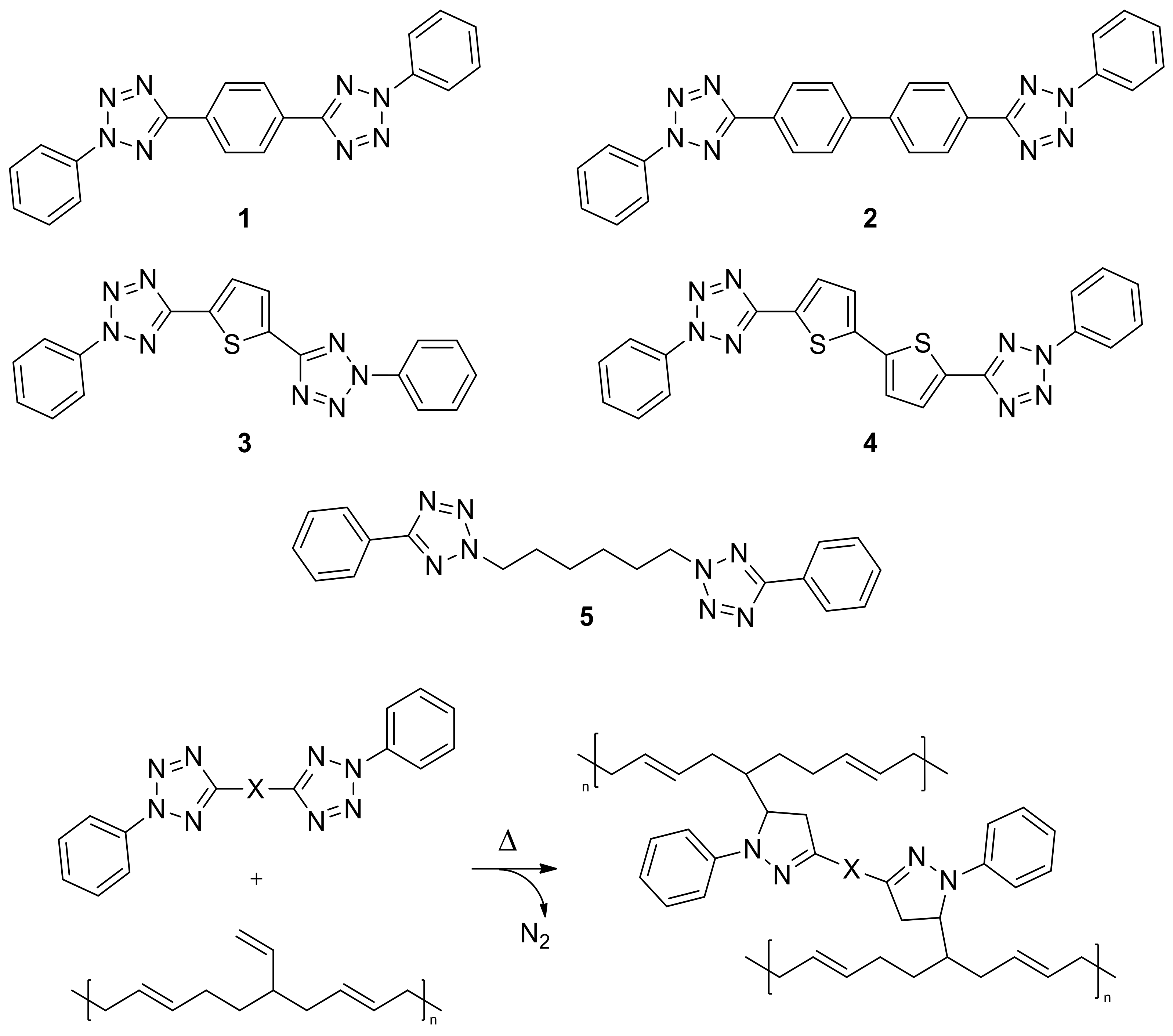
3.1. Synthesis
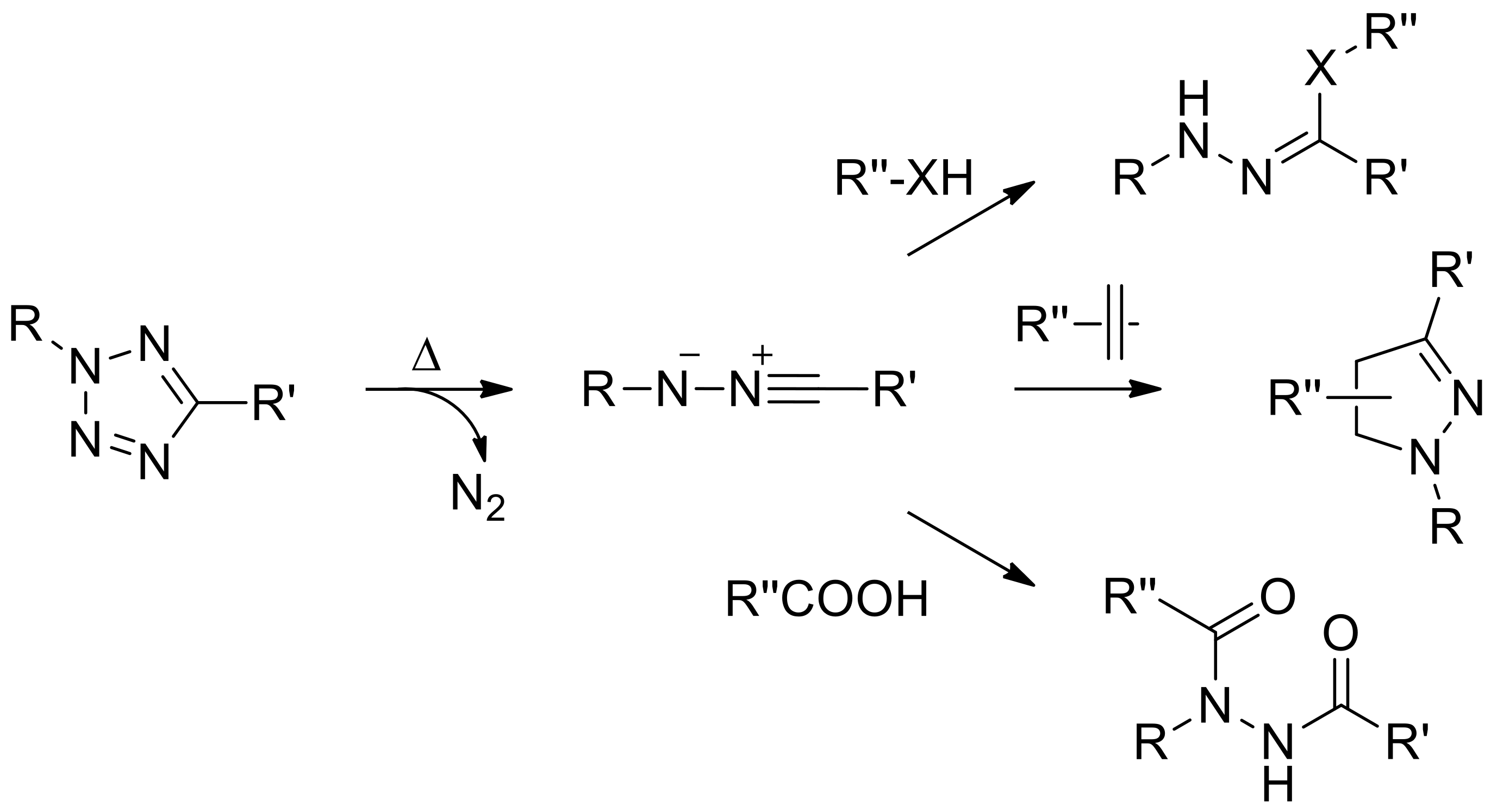
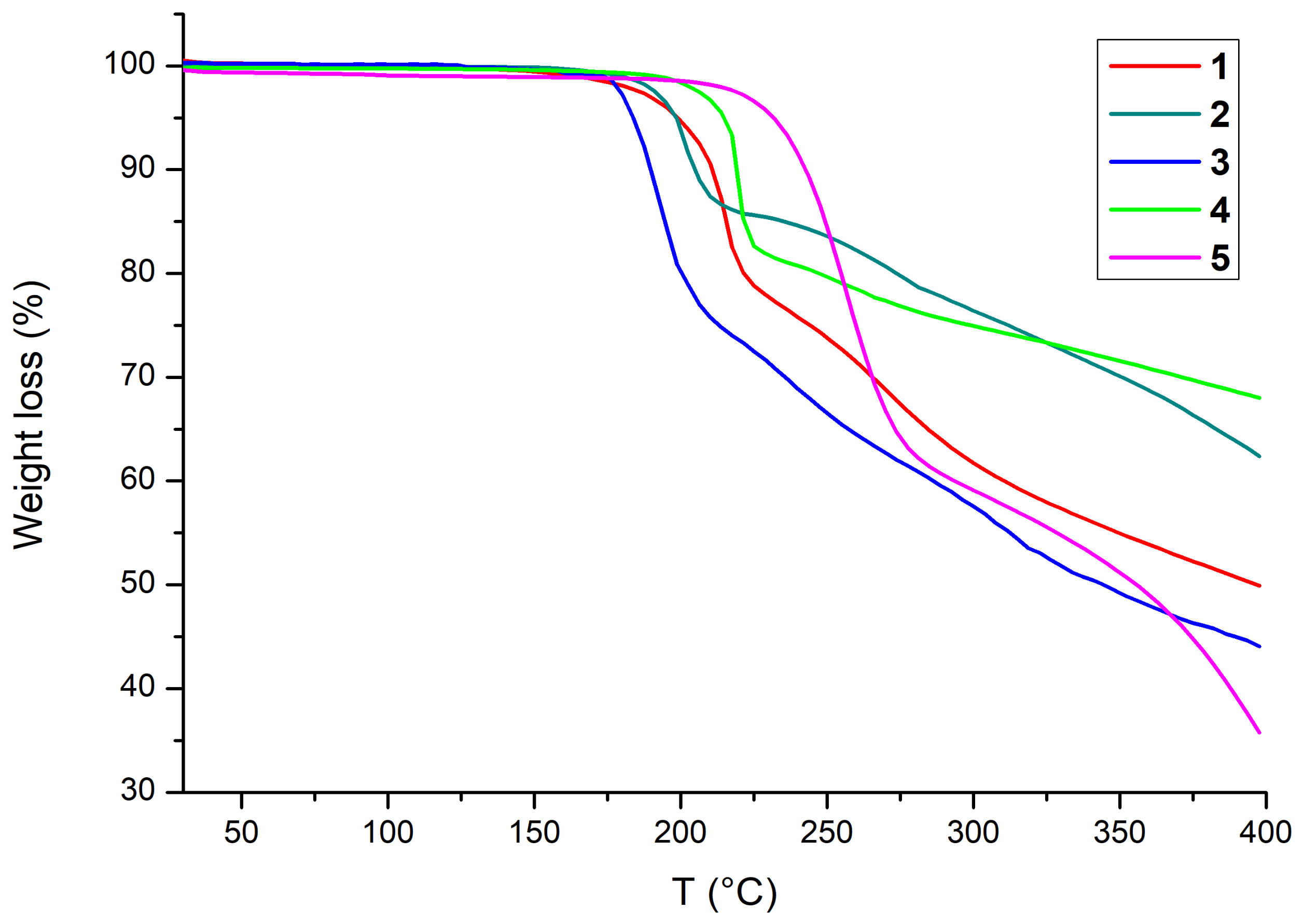
3.2. Thermal Activation
3.3. SBR Curing with Bis-Tetrazoles
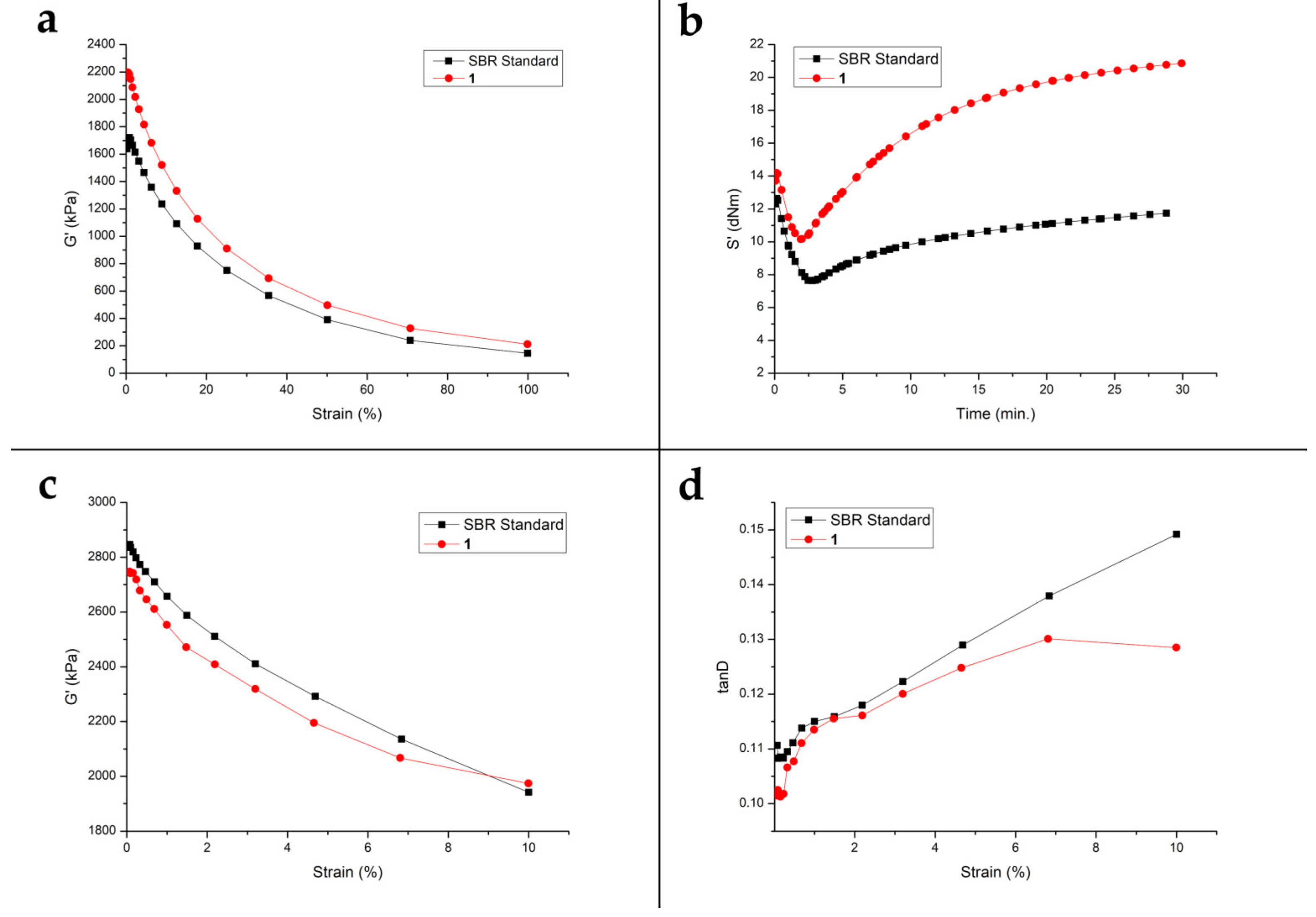
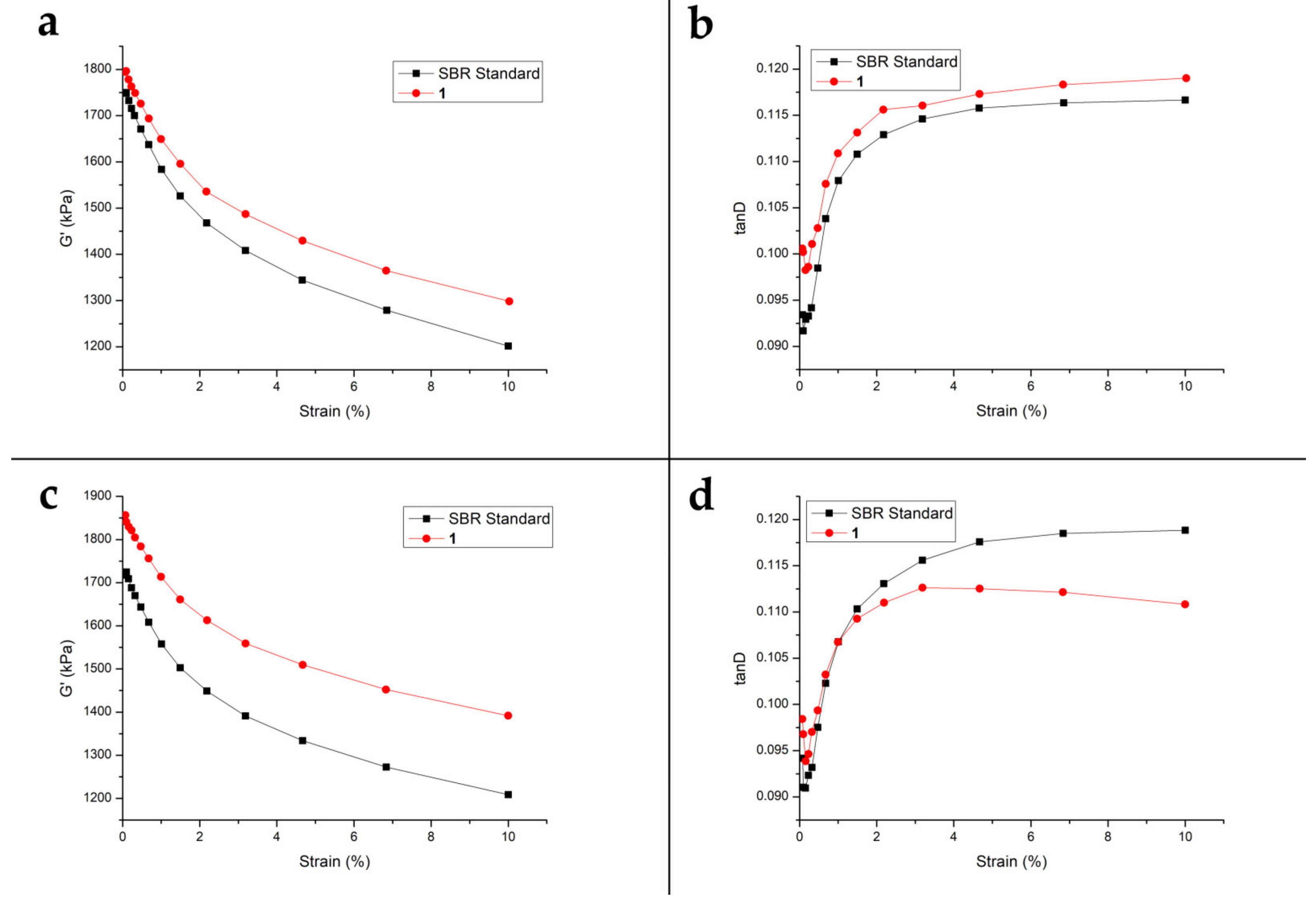
3.4. In Depth Dynamic Mechanical Properties of Elastomeric Compunds Containing 1
3.4.1. Single Temperature Curing
3.4.2. Separated Temperature Curing
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, S.; Stephen, R. Rubber Nanocomposites: Preparation, Properties, and Applications; Thomas, S., Stephen, R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; ISBN 9780470823453. [Google Scholar]
- El-Nemr, K.F. Effect of Different Curing Systems on the Mechanical and Physico-Chemical Properties of Acrylonitrile Butadiene Rubber Vulcanizates. Mater. Des. 2011, 32, 3361–3369. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization. In Science and Technology of Rubber; Elsevier: Amsterdam, The Netherlands, 1994; pp. 339–385. [Google Scholar]
- Xue, L.; Zhang, Y.; Zuo, Y.; Diao, S.; Zhang, J.; Feng, S. Preparation and Characterization of Novel UV-Curing Silicone Rubber via Thiol-Ene Reaction. Mater. Lett. 2013, 106, 425–427. [Google Scholar] [CrossRef]
- Maag, K.; Lenhard, W.; Löffles, H. New UV Curing Systems for Automotive Applications. Prog. Org. Coat. 2000, 40, 93–97. [Google Scholar] [CrossRef]
- Mascia, L.; Clarke, J.; Ng, K.S.; Chua, K.S.; Russo, P. Cure Efficiency of Dodecyl Succinic Anhydride as a Cross-Linking Agent for Elastomer Blends Based on Epoxidized Natural Rubber. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef] [Green Version]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and Peroxide Vulcanisation of Rubber Compounds—Overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of Rubber Compounds with Peroxide Curing Systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Datta, R.N.; Flexsys, B.V. Rubber Curing Systems; Rapra Technology: Shrewsbury, UK, 2001; ISBN 9781859573266. [Google Scholar]
- Rodgers, B. (Ed.) Rubber Compounding: Chemistry and Applications, 2nd ed.; CRC Press: London, UK, 2021; ISBN 9780367783402. [Google Scholar]
- Penumadu, D.; Chin, J.-C.; Young, S.; Ignatz-Hoover, F.; Floyd, T.; Chapman, P. Sulfur Dispersion Quantitative Analysis in Elastomeric Tire Formulations by Using High Resolution X-Ray Computed Tomography. Rubber Chem. Technol. 2021, 94, 626–641. [Google Scholar] [CrossRef]
- Tinker, A.J. Distribution of Crosslinks in Vulcanized Blends. Rubber Chem. Technol. 1995, 68, 461–480. [Google Scholar] [CrossRef]
- Ignatz-Hoover, F.; To, B.H.; Datta, R.N.; De Hoog, A.J.; Huntink, N.M.; Talma, A.G. Chemical Additives Migration in Rubber. Rubber Chem. Technol. 2003, 76, 747–768. [Google Scholar] [CrossRef]
- Huisgen, R.; Sauer, J.; Seidel, M. Ringöffnungen Der Azole, VI. Die Thermolyse 2.5-disubstituierter Tetrazole Zu Nitriliminen. Chem. Ber. 1961, 94, 2503–2509. [Google Scholar] [CrossRef]
- Lesnikovich, A.I.; Levchik, S.V.; Balabanovich, A.I.; Ivashkevich, O.A.; Gaponik, P.N. The Thermal Decomposition of Tetrazoles. Thermochim. Acta 1992, 200, 427–441. [Google Scholar] [CrossRef]
- Meier, H.; Heimgartner, H. Intramolekulare 1,3-Dipolare Cycloadditionen von Diarylnitriliminen Aus 2,5-Diaryltetrazolen. Helv. Chim. Acta 1985, 68, 1283–1300. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Zhao, Z.K. Nucleophilic Trapping Nitrilimine Generated by Photolysis of Diaryltetrazole in Aqueous Phase. Molecules 2013, 19, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Vaghi, L.; Monti, M.; Marelli, M.; Motto, E.; Papagni, A.; Cipolla, L. Photoinduced Porcine Gelatin Cross-Linking by Homobi- and Homotrifunctional Tetrazoles. Gels 2021, 7, 124. [Google Scholar] [CrossRef]
- Clovis, J.S.; Eckell, A.; Huisgen, R.; Sustmann, R.; Wallbillich, G.; Weberndörfer, V. 1.3-Dipolare Cycloadditionen, XXVIII. Diphenylnitrilimin Und Arylkonjugierte Alkene. Chem. Ber. 1967, 100, 1593–1601. [Google Scholar] [CrossRef]
- Huisgen, R.; Knupfer, H.; Sustmann, R.; Wallbillich, G.; Weberndörfer, V. 1.3-Dipolare Cycloadditionen, XXVII. Zur Anlagerung Des Diphenylnitrilimins an Nichtkonjugierte Alkene Und Alkine; Sterischer Ablauf, Orientierung Un Substituenteneinfluß. Chem. Ber. 1967, 100, 1580–1592. [Google Scholar] [CrossRef]
- Eckell, A.; Huisgen, R.; Sustmann, R.; Wallbillich, G.; Grashey, D.; Spindler, E. 1.3-Dipolare Cycloadditionen, XXXI. Dipolarophilen-Aktivitäten Gegenüber Diphenylnitrilimin Und Zahlenmäßige Ermittlung Der Substituenteneinflüsse. Chem. Ber. 1967, 100, 2192–2213. [Google Scholar] [CrossRef]
- Huisgen, R.; Sustmann, R.; Wallbillich, G. 1.3-Dipolare Cycloadditionen, XXIX. Orientierungsphänomene Bei Der Anlagerung von Nitriliminen an α.Β-ungesättigte Carbonester, Vinyläther Und Enamine. Chem. Ber. 1967, 100, 1786–1801. [Google Scholar] [CrossRef]
- Stille, J.K.; Chen, A.T. Synthesis and Copolymerization of Styryl-Substituted Tetrazoles. Thermal Cross-Linking of Copolymers Containing Dipolarophiles and the Tetrazoles as Nitrile Imine Dipole Precursors. Macromolecules 1972, 5, 377–384. [Google Scholar] [CrossRef]
- Dürr, C.J.; Lederhose, P.; Hlalele, L.; Abt, D.; Kaiser, A.; Brandau, S.; Barner-Kowollik, C. Photo-Induced Ligation of Acrylonitrile-Butadiene Rubber: Selective Tetrazole–Ene Coupling of Chain-End-Functionalized Copolymers of 1,3-Butadiene. Macromolecules 2013, 46, 5915–5923. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. Preparation of 5-Substituted 1H-Tetrazoles from Nitriles in Water. J. Org. Chem. 2001, 66, 7945–7950. [Google Scholar] [CrossRef] [PubMed]




| Compound | Activation Temperature (Ta) |
|---|---|
| 1 | 185 °C |
| 2 | 185 °C |
| 3 | 170 °C |
| 4 | 190 °C |
| 5 | 230 °C |
| Elastomer | SSBR 1 | Silica 1 | Tetrazole 1 | TESPD 1 |
|---|---|---|---|---|
| SBR standard | 137 | 60 | - | 4.8 |
| SBR + 1 | 137 | 60 | 4.2 | 4.8 |
| SBR + 2 | 137 | 60 | 5.1 | 4.8 |
| SBR + 3 | 137 | 60 | 4.3 | 4.8 |
| SBR + 4 | 137 | 60 | 5.2 | 4.8 |
| Elastomer | SSBR 1 | Silica 1 | Tetrazole 1 | 6PPD 1 | TESPD 1 | Stearic Acid 1 | ZnO 1 | CBS 1 | Sulfur 1 |
|---|---|---|---|---|---|---|---|---|---|
| SBR standard | 137 | 60 | - | 2.5 | 4.8 | 1 | 2 | 3 | 1 |
| SBR + 1 | 137 | 60 | 4.2 | 2.5 | 4.8 | 1 | 2 | 3 | 1 |
| SBR + 2 | 137 | 60 | 5.1 | 2.5 | 4.8 | 1 | 2 | 3 | 1 |
| SBR + 3 | 137 | 60 | 4.3 | 2.5 | 4.8 | 1 | 2 | 3 | 1 |
| SBR + 4 | 137 | 60 | 5.2 | 2.5 | 4.8 | 1 | 2 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti, M.; Giannini, L.; Tadiello, L.; Guerra, S.; Papagni, A.; Vaghi, L. Thermally Activable Bistetrazoles for Elastomers Crosslinking. Polymers 2022, 14, 2919. https://doi.org/10.3390/polym14142919
Monti M, Giannini L, Tadiello L, Guerra S, Papagni A, Vaghi L. Thermally Activable Bistetrazoles for Elastomers Crosslinking. Polymers. 2022; 14(14):2919. https://doi.org/10.3390/polym14142919
Chicago/Turabian StyleMonti, Mauro, Luca Giannini, Luciano Tadiello, Silvia Guerra, Antonio Papagni, and Luca Vaghi. 2022. "Thermally Activable Bistetrazoles for Elastomers Crosslinking" Polymers 14, no. 14: 2919. https://doi.org/10.3390/polym14142919
APA StyleMonti, M., Giannini, L., Tadiello, L., Guerra, S., Papagni, A., & Vaghi, L. (2022). Thermally Activable Bistetrazoles for Elastomers Crosslinking. Polymers, 14(14), 2919. https://doi.org/10.3390/polym14142919






