A Molecular Dynamics Approach to the Impacts of Oxidative Aging on the Engineering Characteristics of Asphalt
Abstract
:1. Introduction
2. Material Models and Simulation Method
2.1. Asphalt Bulk Models
2.2. Mineral Substrate
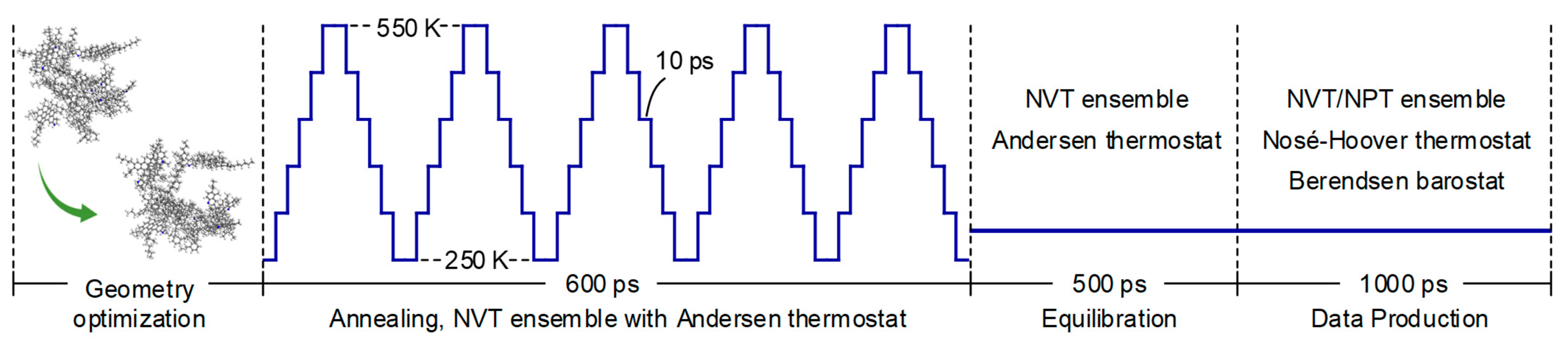
2.3. General Setup for the MD Simulation
- Step 1: geometry optimization to eliminate the unrealistic molecular overlapping and adjust unstable high-energy configurations.
- Step 2: annealing to overcome energy barriers by periodically heating and cooling the system (250 to 550 K) to find the energetically favorable minima using the canonical ensemble NVT (a constant number of atoms N, a constant volume V, and a controlled temperature T).
- Step 3: equilibration to bring the system to equilibration over a simulation time of 500 ps using the NVT ensemble with an Andersen thermostat.
- Step 4: data production run to further equilibrate the system under the NVT or NPT ensemble (a constant number of atoms N, a controlled pressure P, and a controlled temperature T) depending on the model of interest, using the Nosé–Hoover thermostat (for physically sound dynamics) and Berendsen barostat, over a simulation time of 1 ns.
3. Results and Discussion
3.1. Bulk Thermodynamic Properties
3.1.1. Prediction of Density
3.1.2. Prediction of Cohesive Energy Density
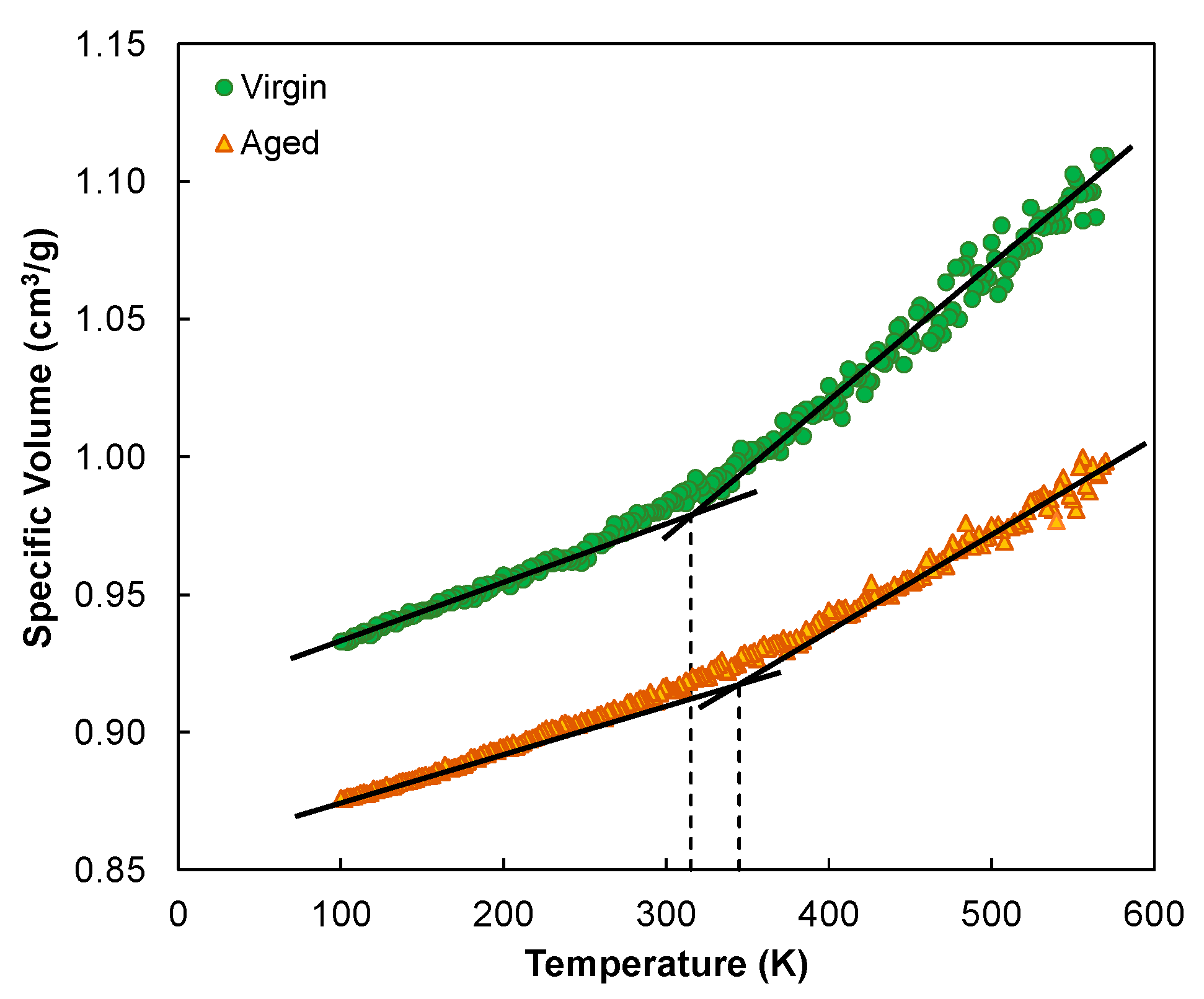
3.1.3. Prediction of Glass Transition Temperature
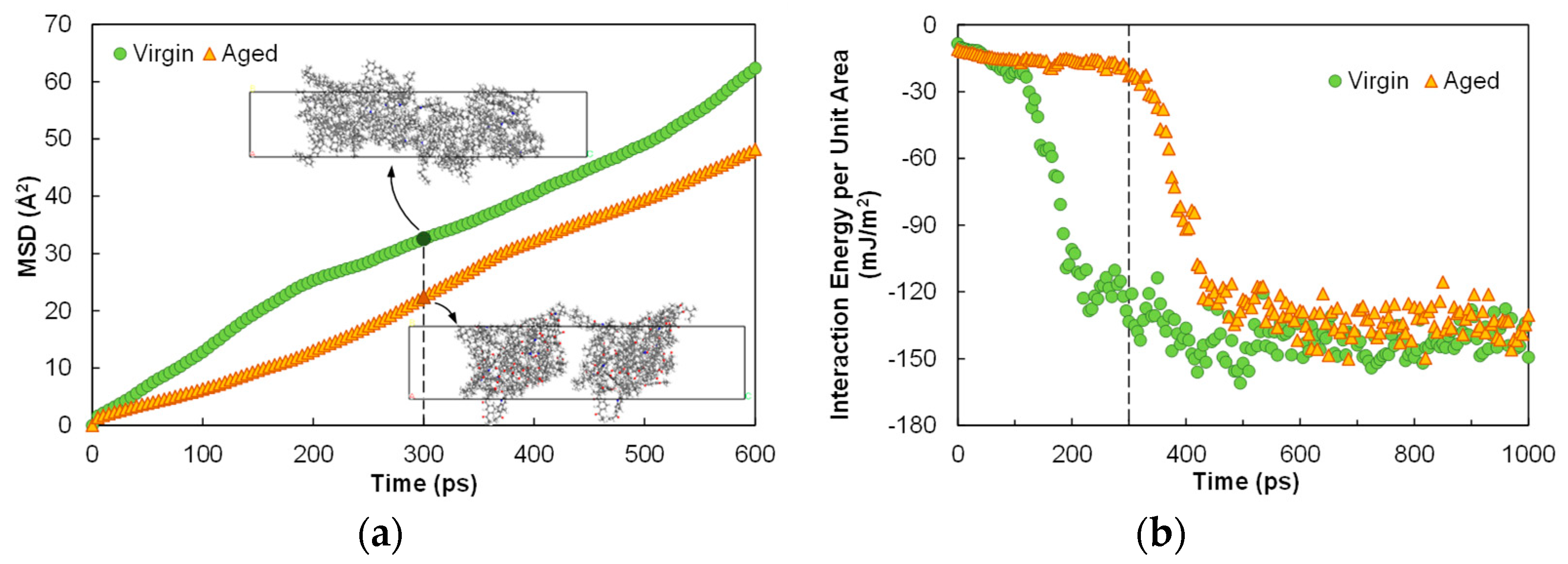
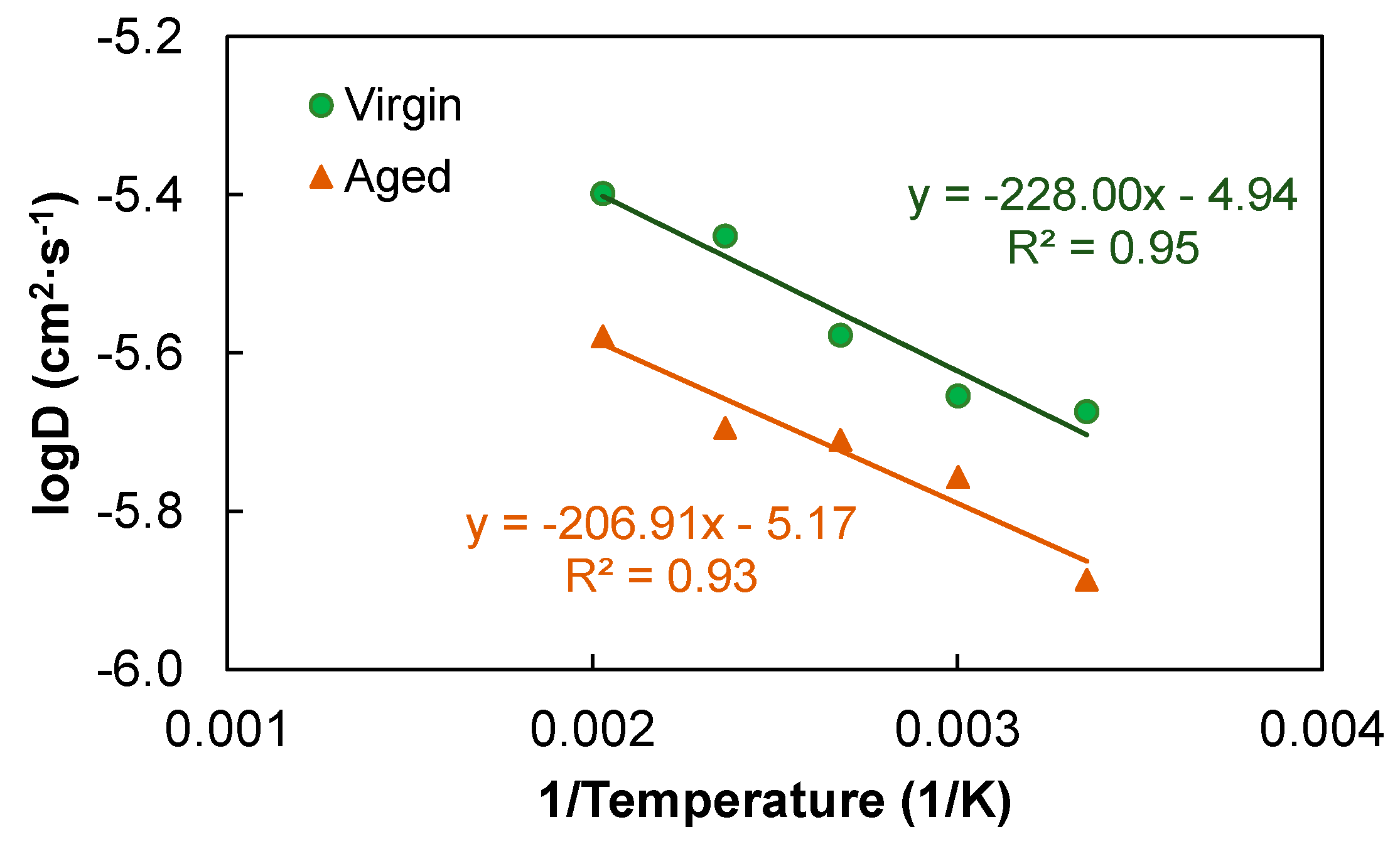
3.2. Asphalt Diffusion Characteristics
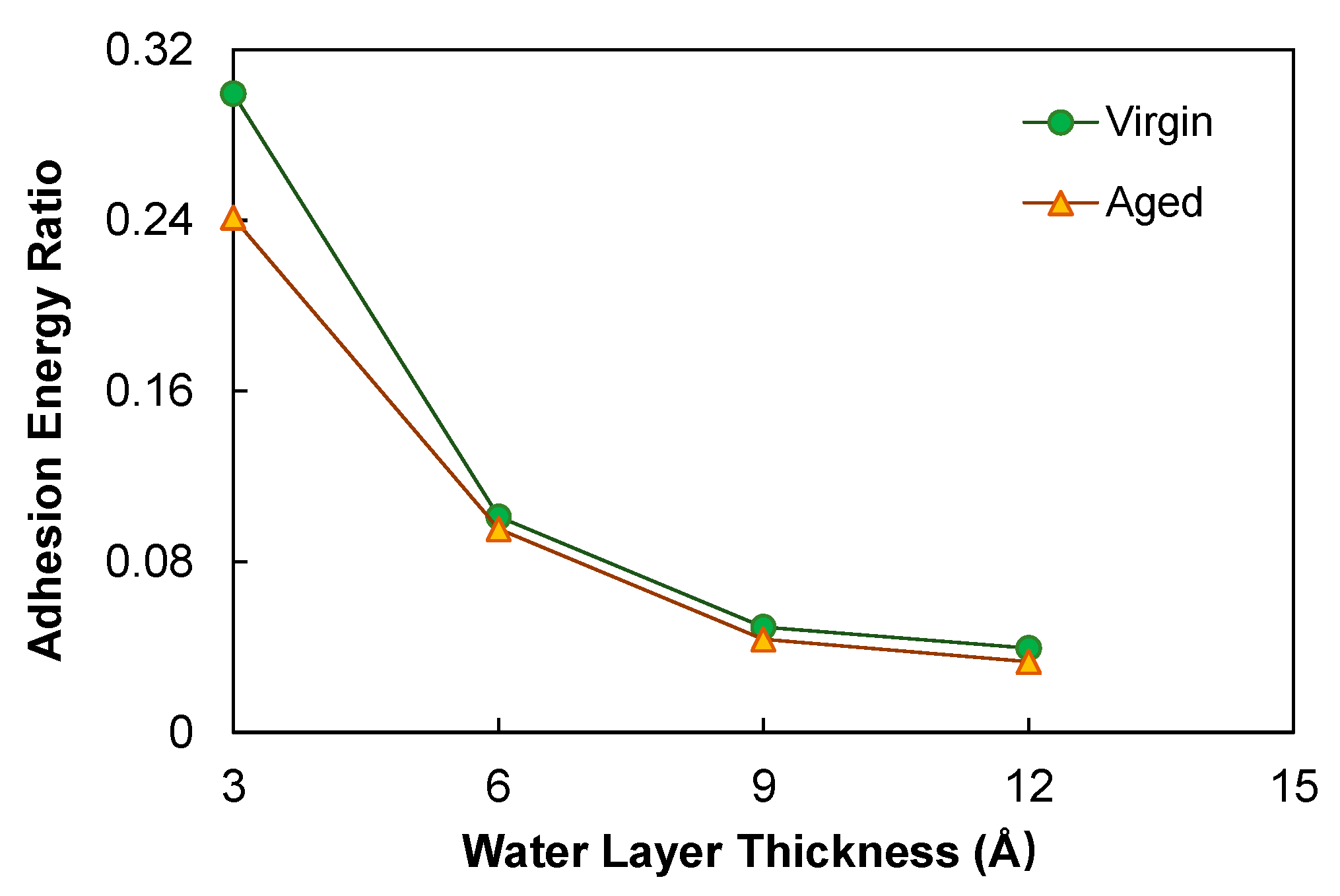
3.3. Asphalt–Aggregate Adhesion Characteristics
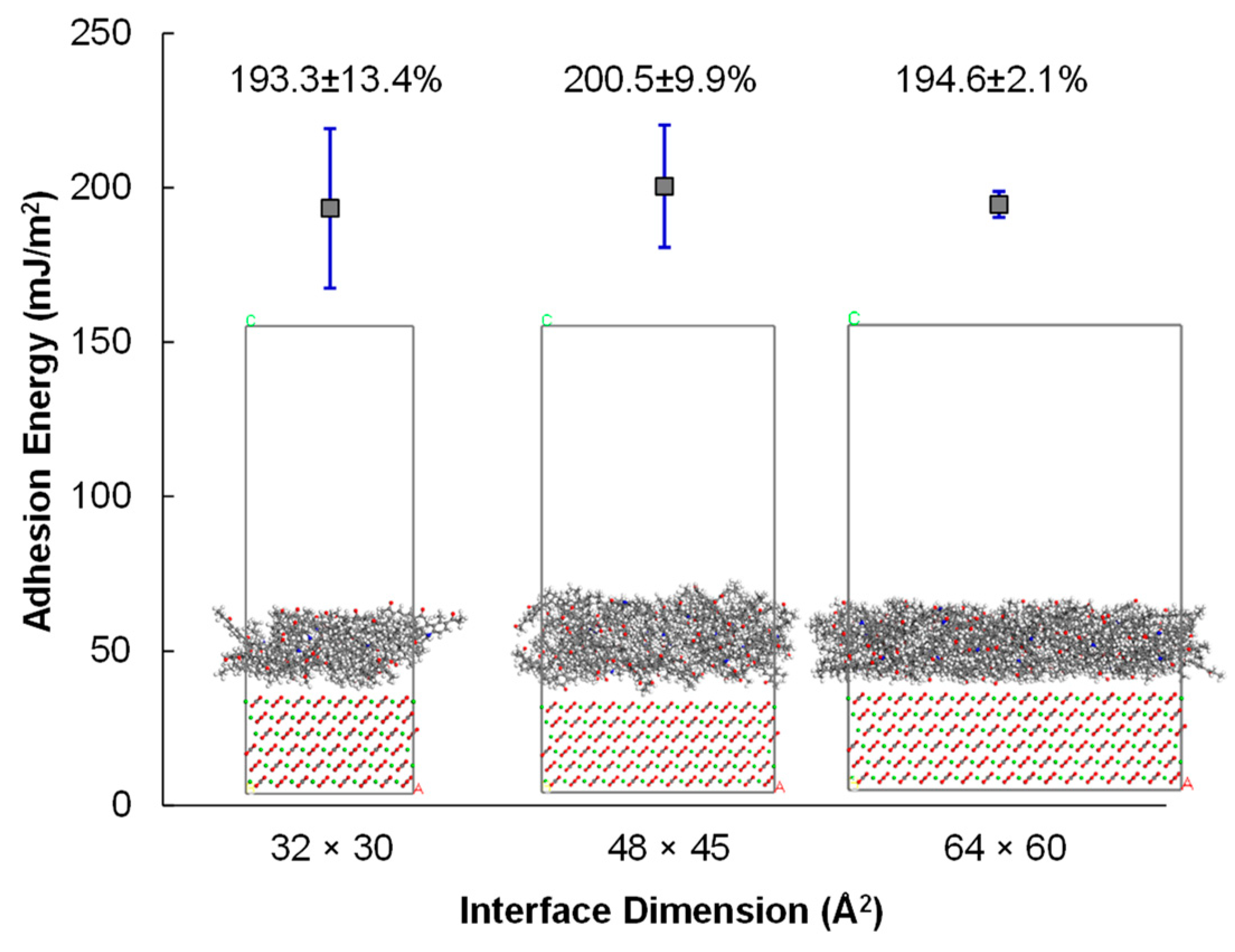
3.3.1. Size Effect
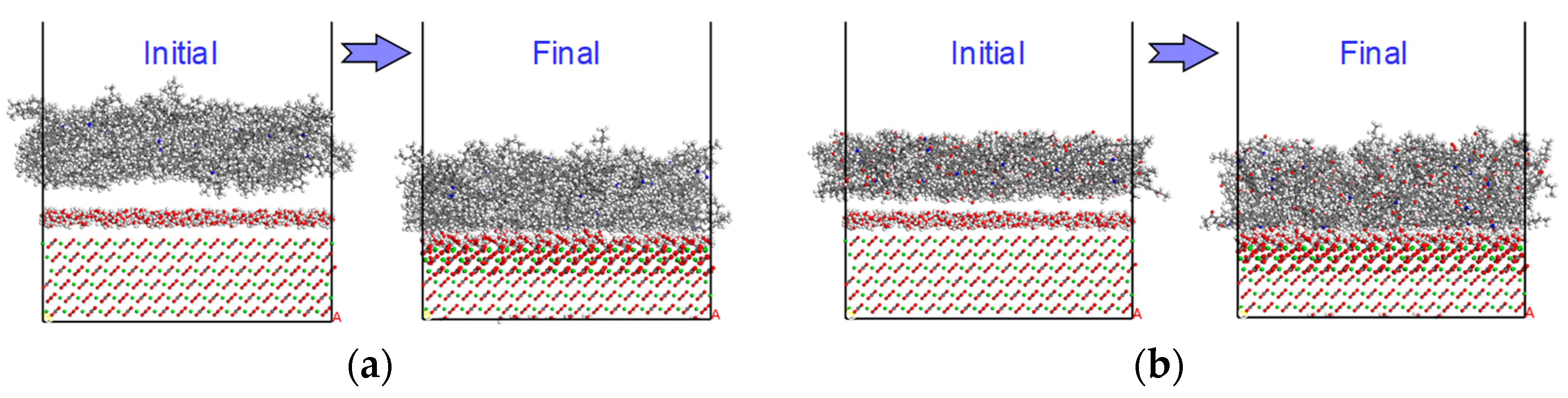
3.3.2. Dry Condition
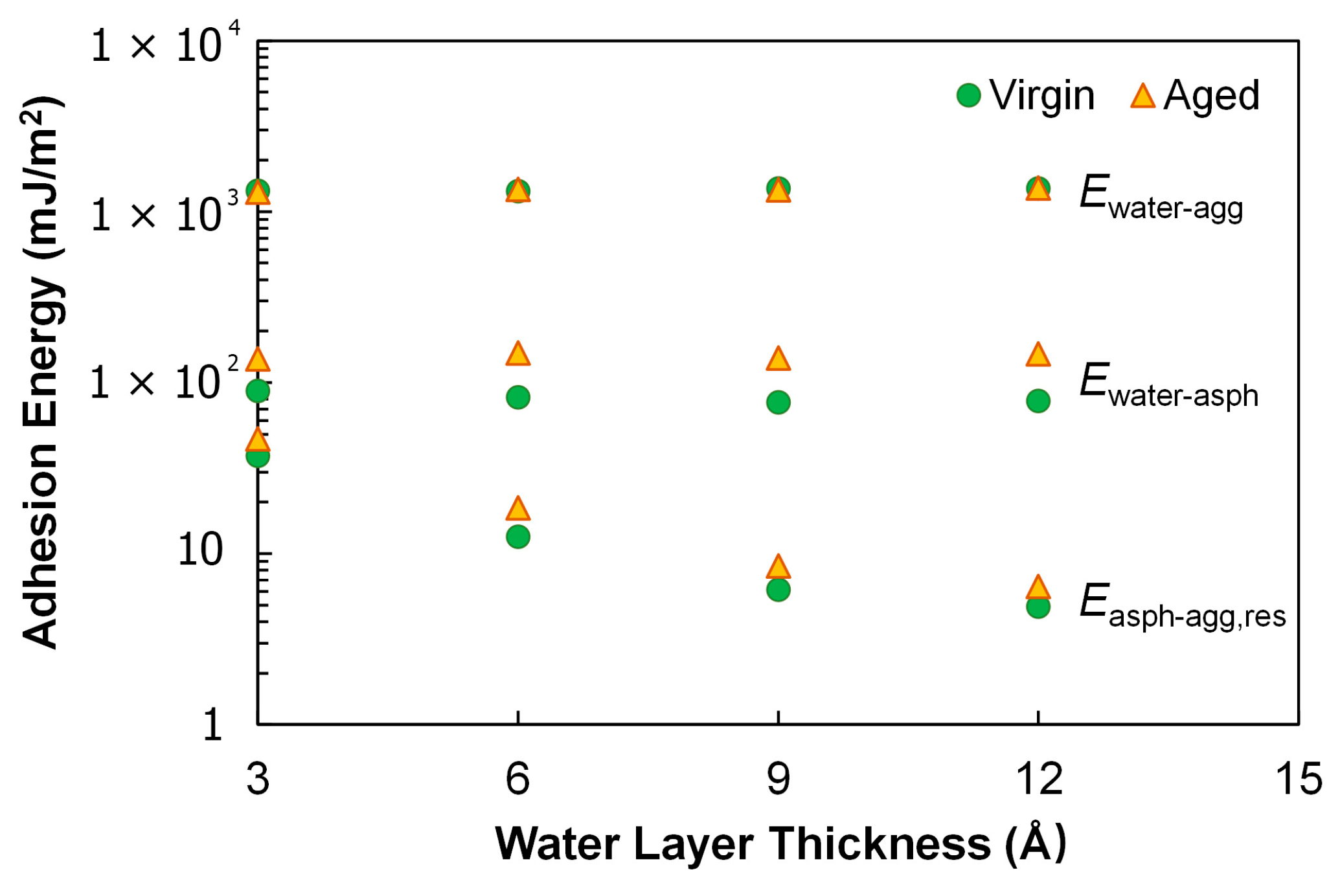
3.3.3. Wet Condition
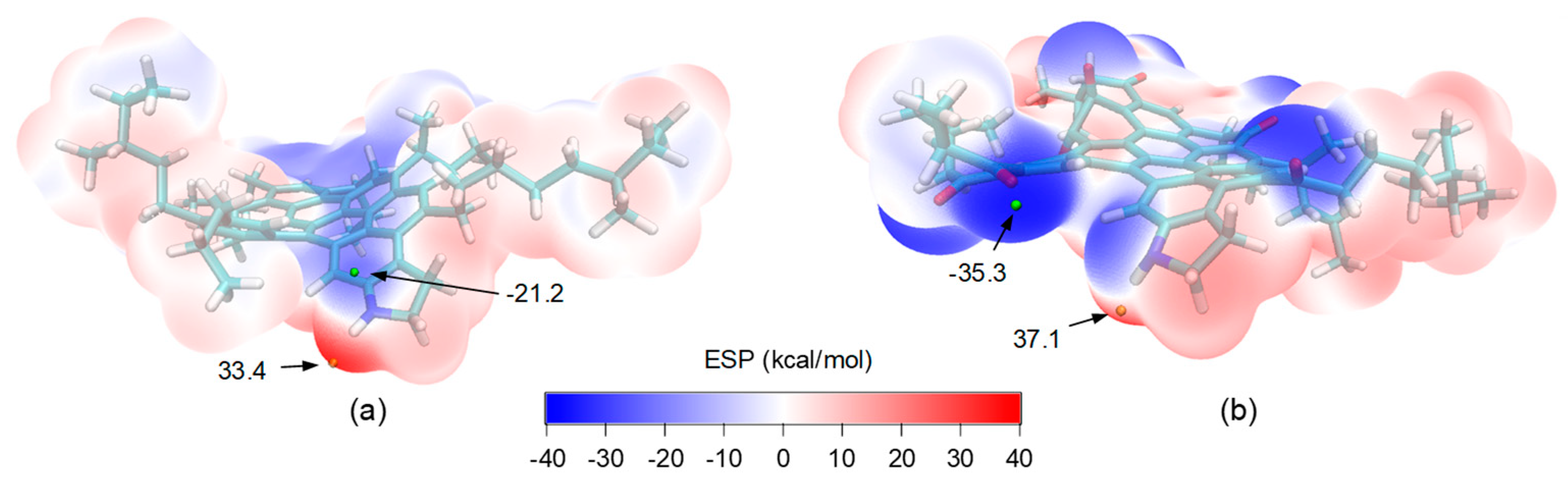
3.4. Further Inspection of Increased Molecular Polarity
4. Conclusions
- The oxidative aging of asphalt raised the density (from 1.008 to 1.081 g/cm3), cohesive energy density (from 3.02 × 108 to 3.49 × 108 J/m3), and glass transition temperature (from 315 to 345 K). The overall molecular polarity was elevated upon aging, as reflected in the increased electrostatic contribution to CED. The increase in Tg was attributed to the stronger intermolecular attractiveness and reduced molecular mobility, which lowers the resistance of asphalt to thermal cracking.
- Aging considerably slowed down the diffusion process (and consequently the self-healing capacity) of asphalt at a given temperature. The diffusion behaviors of both the virgin and aged asphalt systems at different temperatures were well captured by the Arrhenius relationship, in which prefactor D0 was a valid index representing the overall effect of aging on asphalt diffusion. Oxidation substantially decreased the prefactor from 1.1 × 10−5 to 6.8 × 10−6 cm2/s.
- Aging benefited the adhesion between asphalt and calcite substrate in the dry condition, as it substantially improved the electrostatic interactions at the interface due to the increased molecular polarity. In the wet condition, the use of the proposed index, i.e., the ratio of the residual asphalt–aggregate adhesion to that in the dry condition, indicated that aging yielded consistently higher moisture susceptibility. The simulation results suggest that aging would increase the propensity of asphalt pavement to moisture damage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.W.; Bahia, H.U.; Anderson, D.A. Effects of free volume and intermolecular friction on the viscosity of asphalt binders. J. Assoc. Asph. Paving Technol. 1996, 65, 385–407. [Google Scholar]
- Cao, W. General fractional models for linear viscoelastic characterization of asphalt cements. J. Rheol. 2020, 64, 1439–1453. [Google Scholar] [CrossRef]
- Cao, W.; Barghabany, P.; Mohammad, L.; Cooper, S.B., III; Balamurugan, S. Chemical and rheological evaluation of asphalts incorporating RAP/RAS binders and warm-mix technologies in relation to crack resistance. Constr. Build. Mater. 2019, 198, 256–268. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Cao, W. A chemo-rheological approach to the healing characteristics of asphalt binders under short- and long-term oxidative aging. Constr. Build. Mater. 2019, 221, 553–561. [Google Scholar] [CrossRef]
- Molenaar, A.; Hagos, E.; van de Vev, M. Effects of aging on the mechanical characteristics of bituminous binders in PAC. J. Mater. Civil. Eng. 2010, 22, 779–787. [Google Scholar] [CrossRef]
- Petersen, J.C. A dual, sequential mechanism for the oxidation of petroleum asphalts. Pet. Sci. Technol. 1998, 16, 1023–1051. [Google Scholar] [CrossRef]
- Petersen, J.C.; Harnsberger, P.M. Asphalt aging: A dual oxidation mechanism and its interrelationships with asphalt composition and oxidative age hardening. Transp. Res. Rec. 1998, 1638, 47–55. [Google Scholar] [CrossRef]
- Petersen, J.C.; Glaser, R. Asphalt oxidation mechanisms and the role of oxidation products on age hardening revisited. Road Mater. Pavement Des. 2011, 12, 795–819. [Google Scholar] [CrossRef]
- Pan, T.; Lu, Y.; Lloyd, S. Quantum-chemistry study of asphalt oxidative aging: An XPS-aided analysis. Ind. Eng. Chem. Res. 2012, 51, 7957–7966. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Viscosity, relaxation time, and dynamics within a model asphalt of larger molecules. J. Chem. Phys. 2014, 140, 034507. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tarefder, R.A. Investigation of asphalt aging behaviour due to oxidation using molecular dynamics simulation. Mol. Simul. 2016, 42, 667–678. [Google Scholar] [CrossRef]
- Ding, H.; Wang, H.; Qu, X.; Varveri, A.; Gao, J. Towards an understanding of diffusion mechanism of bio-rejuvenators in aged asphalt binder through molecular dynamics simulation. J. Clean. Prod. 2021, 299, 126927. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Yang, Y.; Zhang, J.; Gu, F. Molecular dynamics investigation of interfacial adhesion between oxidized bitumen and mineral surfaces. Appl. Surf. Sci. 2019, 479, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Liu, Q.; Guo, M.; Wang, D.; Oeser, M. Study on the effect of aging on physical properties of asphalt binder from a microscale perspective. Constr. Build. Mater. 2018, 187, 718–729. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H. Molecular dynamics study of oxidative aging effect on asphalt binder properties. Fuel 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Bhasin, A.; Little, D.N.; Vasconcelos, K.L.; Masad, E. Surface free energy to identify moisture sensitivity of materials for asphalt mixes. Transp. Res. Rec. 2007, 2001, 37–45. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Ovalles, E.; Caro, S. Assessment of the effect of mineral filler on asphalt-aggregate interfaces based on thermodynamic properties. Constr. Build. Mater. 2012, 28, 599–606. [Google Scholar] [CrossRef]
- Luo, L.; Chu, L.; Fwa, T.F. Molecular dynamics analysis of oxidative aging effects on thermodynamic and interfacial bonding properties of asphalt mixtures. Constr. Build. Mater. 2021, 269, 121299. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Chemo-physical analysis and molecular dynamics (MD) simulation of moisture susceptibility of nano hydrated lime modified asphalt mixtures. Constr. Build. Mater. 2015, 101, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Aguiar-Moya, J.P.; Salazar-Delgado, J.; Baldi-Sevilla, A.; Leiva-Villacorta, F.; Loria-Salazar, L. Effect of aging on adhesion properties of asphalt mixtures with the use of bitumen bond strength and surface energy measurement tests. Transp. Res. Rec. 2015, 2505, 57–65. [Google Scholar] [CrossRef]
- Dassault Systèmes. BIOVIA Materials Studio; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef]
- King, W.H.; Corbett, L.W. Relative oxygen absorption and volatility properties of submicron films of asphalt using the quartzite crystal microbalance. Anal. Chem. 1969, 41, 580–583. [Google Scholar] [CrossRef]
- Lemarchand, C.A.; Schrøder, T.B.; Dyre, J.C.; Hansen, J.S. Cooee bitumen: Chemical aging. J. Chem. Phys. 2013, 139, 124506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, P.; Bennema, P. The attachment energy as a habit controlling factor: I. Theoretical considerations. J. Cryst. Growth 1980, 49, 145–156. [Google Scholar] [CrossRef]
- Donnay, J.D.H.; Harker, D. A new law of crystal morphology extending the law of Bravais. Am. Mineral. 1937, 22, 446–467. [Google Scholar]
- Stipp, S.L.S.; Eggleston, C.M.; Nielsen, B.S. Calcite surface structure observed at microtopographic and molecular scales with atomic force microscopy (AFM). Geochim. Cosmochim. Acta 1994, 58, 3023–3033. [Google Scholar] [CrossRef]
- Herman, A.; Addadi, L.; Weiner, S. Interactions of sea-urchin skeleton macromolecules with growing calcite crystals—A study of intracrystalline proteins. Nature 1988, 331, 546–548. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Z.; Tan, Y.; Liu, Z. Investigating the interactions of the saturate, aromatic, resin, and asphaltene four fractions in asphalt binders by molecular simulations. Energy Fuels 2015, 29, 112–121. [Google Scholar] [CrossRef]
- Tabatabaee, H.A.; Velasquez, R.; Bahia, H.U. Modeling thermal stress in asphalt mixtures undergoing glass transition and physical hardening. Transp. Res. Rec. 2012, 2296, 106–114. [Google Scholar] [CrossRef]
- Anderson, D.A.; Marasteanu, M.O. Physical hardening of asphalt binders relative to their glass transition temperatures. Transp. Res. Rec. 1999, 1661, 27–34. [Google Scholar] [CrossRef]
- Tabatabaee, H.A.; Velasquez, R.; Bahia, H.U. Predicting low temperature physical hardening in asphalt binders. Constr. Build. Mater. 2012, 34, 162–169. [Google Scholar] [CrossRef]
- Zhang, L.; Greenfield, M.L. Analyzing properties of model asphalts using molecular simulation. Energy Fuels 2007, 21, 1712–1716. [Google Scholar] [CrossRef]
- Soldera, A.; Metatla, N. Glass transition of polymers: Atomistic simulation versus experiments. Phys. Rev. E 2006, 74, 061803. [Google Scholar] [CrossRef]
- Daly, W.H.; Negulescu, I.; Glover, I. A Comparative Analysis of Modified Binders: Original Asphalts and Materials Extracted from Existing Pavements; Report No. FHWA/LA.10/462; Louisiana Transportation Research Center: Baton Rouge, LA, USA, 2010. [Google Scholar]
- Sun, D.; Sun, G.; Zhu, X.; Pang, Q.; Yu, F.; Lin, T. Identification of wetting and molecular diffusion stages during self-healing process of asphalt binder via fluorescence microscope. Constr. Build. Mater. 2017, 132, 230–239. [Google Scholar] [CrossRef]
- Sun, D.; Sun, G.; Zhu, X.; Ye, F.; Xu, J. Intrinsic temperature sensitive self-healing character of asphalt binders based on molecular dynamics simulations. Fuel 2018, 211, 609–620. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, B.; Shu, X.; Zhang, Y.; Woods, M.E. Use of molecular dynamics to investigate diffusion between virgin and aged asphalt binders. Fuel 2016, 174, 267–273. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, C.; Wan, M.; Zhou, X.; Wang, Y.; Wu, S. Study of the diffusion of rejuvenators and its effect on aged bitumen binder. Appl. Sci. 2017, 7, 397. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Guo, M. Using surface free energy method to study the cohesion and adhesion of asphalt mastic. Constr. Build. Mater. 2013, 47, 254–260. [Google Scholar] [CrossRef]
- Chu, L.; Luo, L.; Fwa, T.F. Effects of aggregate mineral surface anisotropy on asphalt-aggregate interfacial bonding using molecular dynamics (MD) simulation. Constr. Build. Mater. 2019, 225, 1–12. [Google Scholar] [CrossRef]
- Cucalon, L.G.; Kassem, E.; Little, D.N.; Masad, E. Fundamental evaluation of moisture damage in warm-mix asphalts. Road Mater. Pavement Des. 2017, 18, 258–283. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. ωB97M-V: A combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J. Chem. Phys. 2016, 144, 214110. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
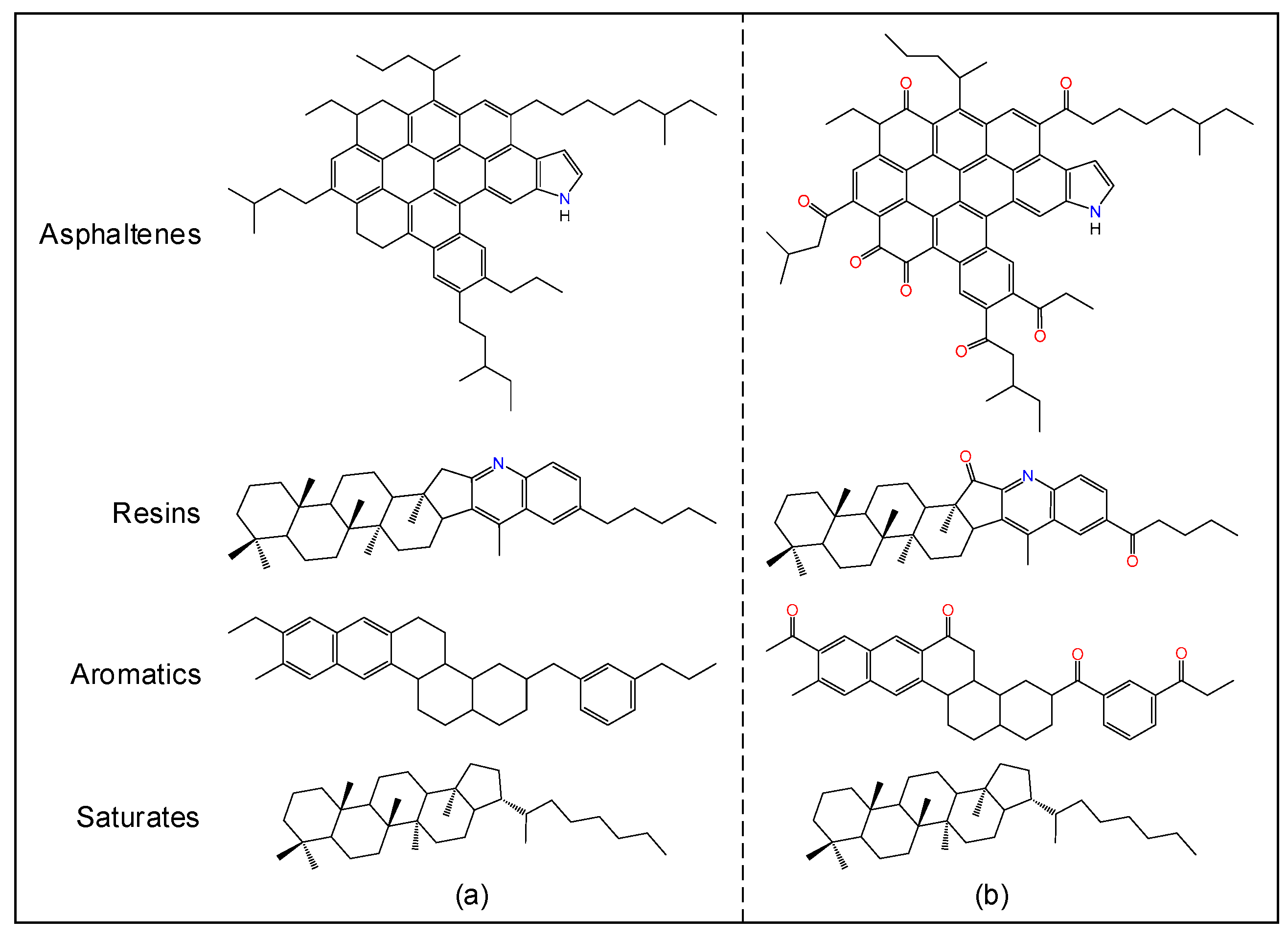



| Components | Virgin | Aged | ||||
|---|---|---|---|---|---|---|
| Formula | No. Ratio | Mass Ratio | Formula | No. Ratio | Mass Ratio | |
| Asphaltene–pyrrole (asphaltenes) | C66H81N | 2 | 18.2% | C66H67NO7 | 3 | 25.4% |
| Quinolinohopane (resins) | C40H59N | 6 | 34.0% | C40H55NO2 | 7 | 35.0% |
| PHPN (aromatics) | C35H44 | 8 | 38.0% | C35H36O4 | 7 | 31.3% |
| Hopane (saturates) | C35H62 | 2 | 9.9% | C35H62 | 2 | 8.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Fini, E. A Molecular Dynamics Approach to the Impacts of Oxidative Aging on the Engineering Characteristics of Asphalt. Polymers 2022, 14, 2916. https://doi.org/10.3390/polym14142916
Cao W, Fini E. A Molecular Dynamics Approach to the Impacts of Oxidative Aging on the Engineering Characteristics of Asphalt. Polymers. 2022; 14(14):2916. https://doi.org/10.3390/polym14142916
Chicago/Turabian StyleCao, Wei, and Elham Fini. 2022. "A Molecular Dynamics Approach to the Impacts of Oxidative Aging on the Engineering Characteristics of Asphalt" Polymers 14, no. 14: 2916. https://doi.org/10.3390/polym14142916
APA StyleCao, W., & Fini, E. (2022). A Molecular Dynamics Approach to the Impacts of Oxidative Aging on the Engineering Characteristics of Asphalt. Polymers, 14(14), 2916. https://doi.org/10.3390/polym14142916






