Novel Magnetic Elastic Phase-Change Nanodroplets as Dual Mode Contrast Agent for Ultrasound and Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Pre-PGS
2.2.2. Synthesis of PEG Coated SPIONS
2.2.3. Preparation of Unloaded and Loaded PCNDs
2.3. Characterizations of Polymeric PCNDs
2.3.1. Chemical Characterization
Fourier Transform Infrared Spectrometry
Contact Angle Measurements
X-Ray Diffractometry
2.3.2. Confirmation of PFP Encapsulation
Encapsulation Efficiency
Optical and Fluorescent Microscopy
2.4. Morphological Analysis
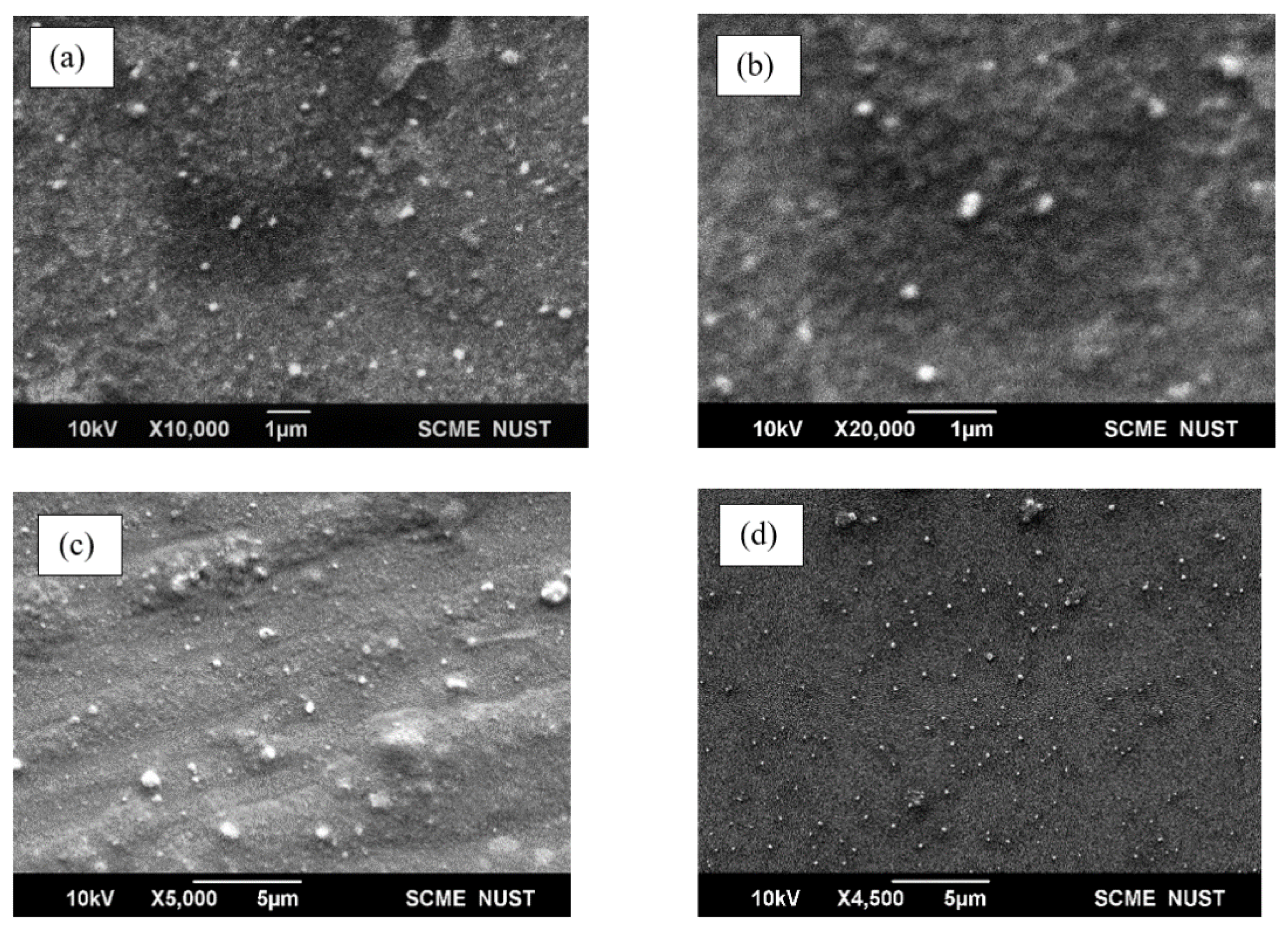
2.4.1. Scanning Electron Microscopy
2.4.2. Dynamic Light Scattering Analysis
2.5. Acoustic and Magnetic Characterization
2.5.1. Vibrating Sample Magnetometry
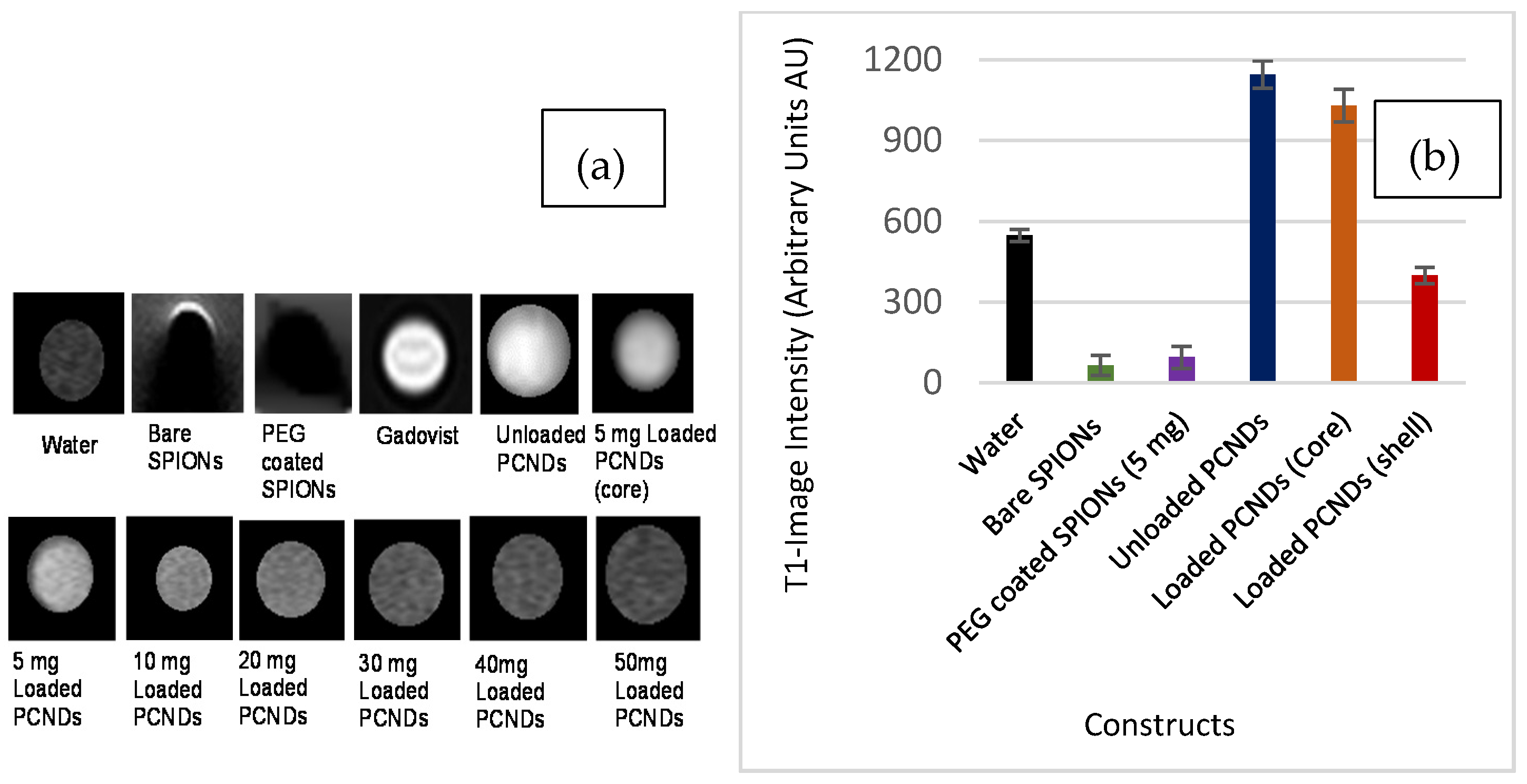
2.5.2. In Vitro Magnetic Resonance Imaging
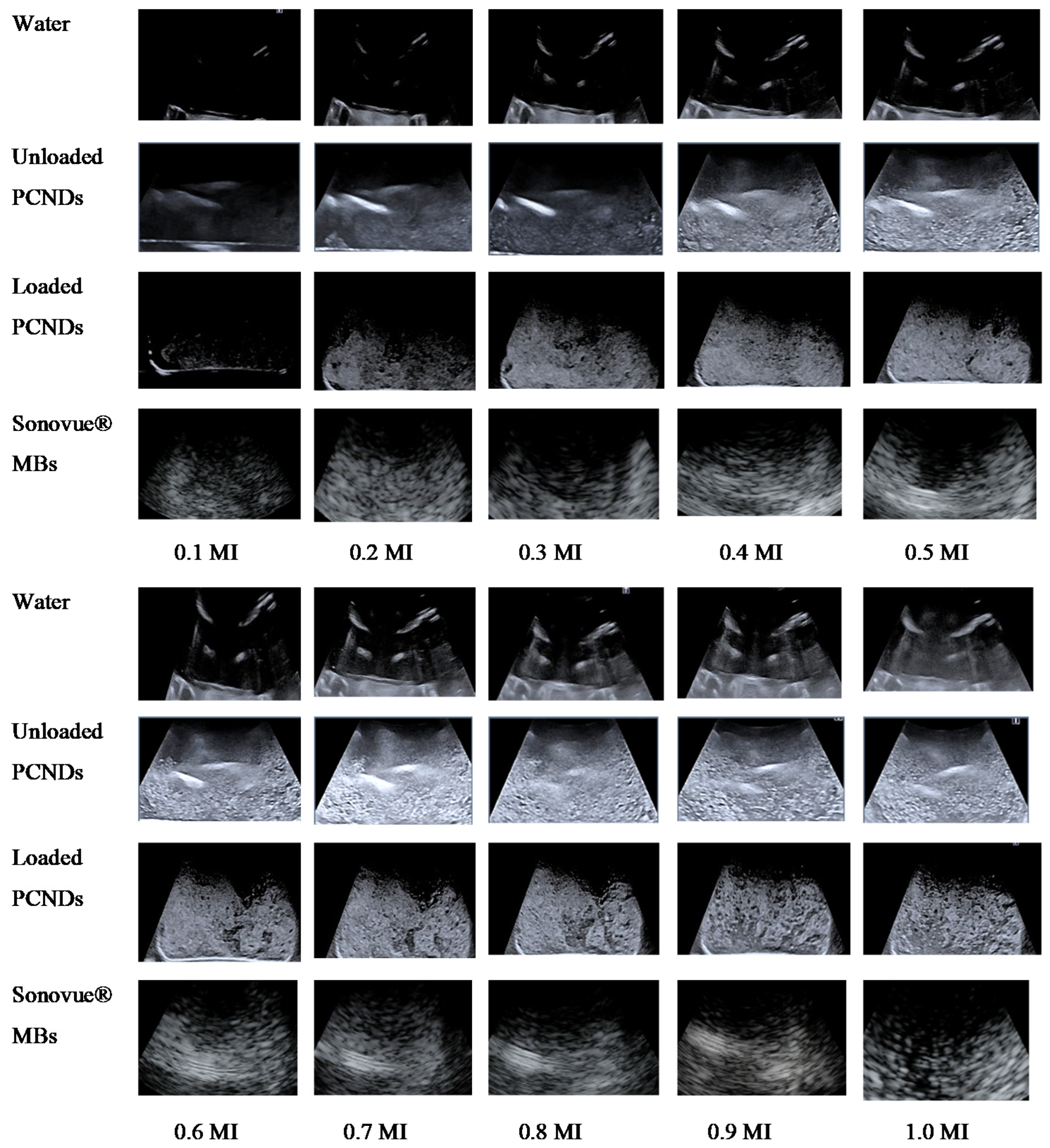
2.5.3. In Vitro Ultrasound Analysis
2.6. Stability Testing
2.7. In Vitro Safety Assays
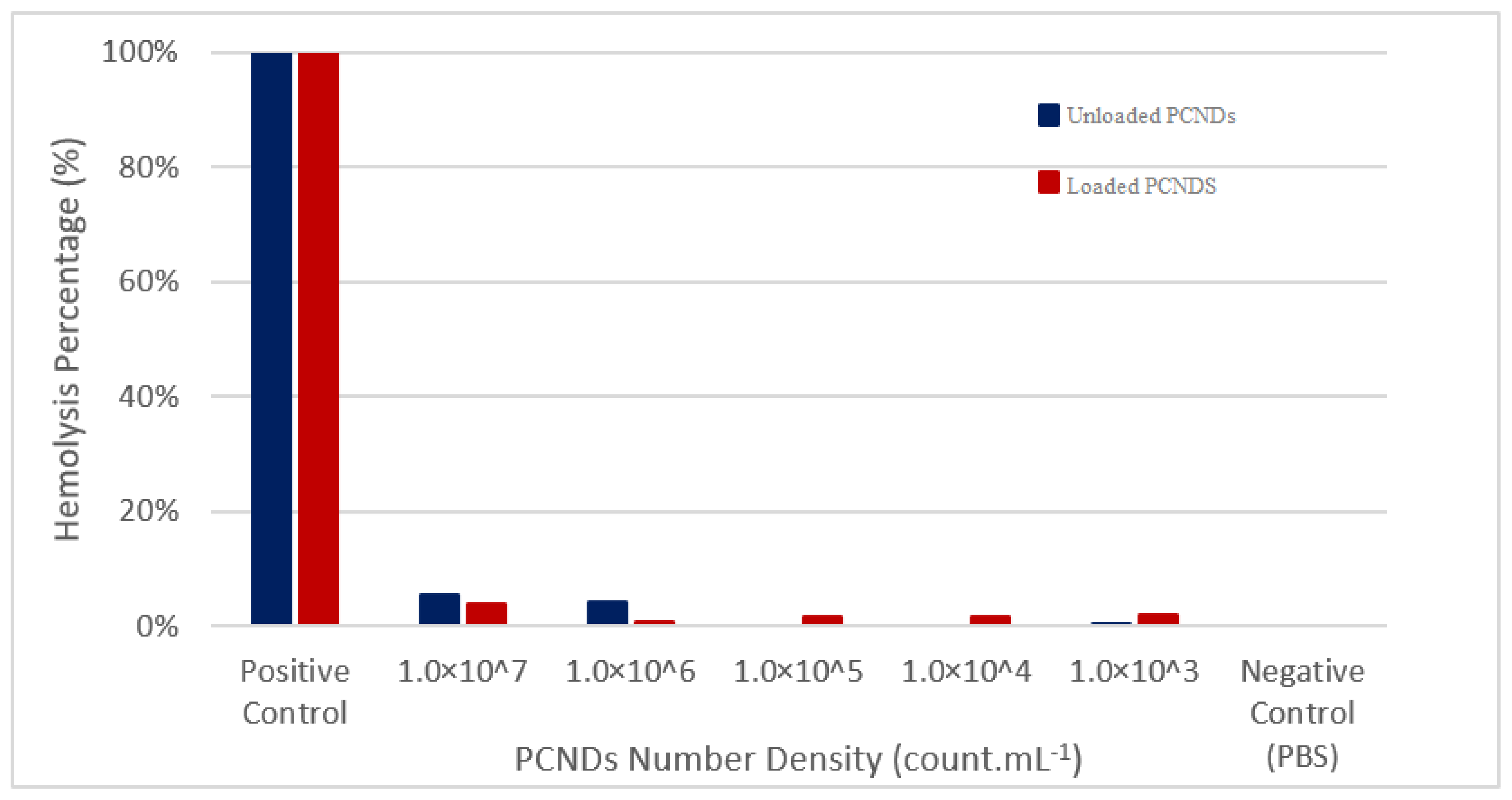
2.7.1. Hemolysis Assay
2.7.2. Thrombin Time
2.7.3. Plasma Recalcification Time
2.7.4. Biocompatibility Assay
2.7.5. Immunogenicity Test
3. Results and Discussion
3.1. Chemical Characterization of Polymeric PCNDs
3.2. Confirmation of PFP Encapsulation
3.3. Morphological Analysis
3.4. Magnetic Characterization of PEG Coated SPIONs
3.5. In Vitro MR Imaging
3.6. In Vitro Ultrasound Imaging
3.7. Stability Testing
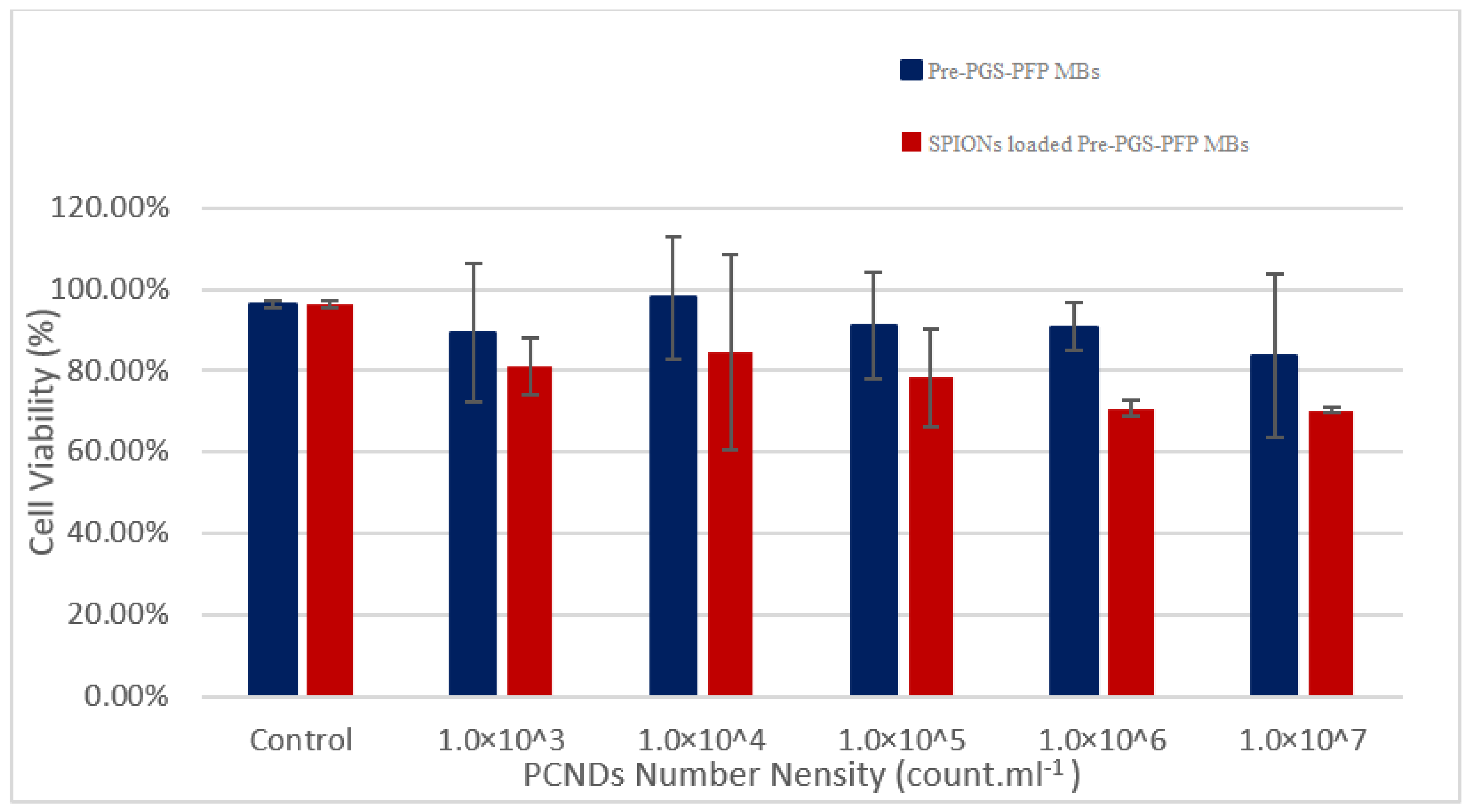
3.8. PCNDs Safety Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.; Perkins, G.; Kirilova, A.; Allen, C.; Jaffray, D.A. Multimodal contrast agent for combined computed tomography and magnetic resonance imaging applications. Investig. Radiol. 2006, 41, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sciallero, C.; Balbi, L.; Paradossi, G.; Trucco, A. Magnetic resonance and ultrasound contrast imaging of polymer-shelled microbubbles loaded with iron oxide nanoparticles. R. Soc. Open Sci. 2016, 3, 160063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, J. Ultrasound fusion imaging. Perspect Med. 2012, 1–12, 80–81. [Google Scholar] [CrossRef] [Green Version]
- Paefgen, V.; Doleschel, D.; Kiessling, F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol. 2015, 6, 197. [Google Scholar] [CrossRef] [Green Version]
- Makov, Y.N. Coated microbubbles: Development of echo-contrast compositions in medical acoustics and dynamic models of such systems with nonlinear elastic shells. Acoust. Phys. 2009, 55, 547–555. [Google Scholar] [CrossRef]
- Hoff, L.; Sontum, P.C.; Hovem, J.M. Oscillations of polymeric microbubbles: Effect of the encapsulating shell. J. Acoust. Soc. Am. 2000, 107, 2272–2280. [Google Scholar] [CrossRef]
- Stride, E.; Pancholi, K.; Edirisinghe, M.J.; Samarasinghe, S. Increasing the nonlinear character of microbubble oscillations at low acoustic pressures. J. R. Soc. Interface 2008, 5, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Domenici, F.; Brasili, F.; Oddo, L.; Cerroni, B.; Bedini, A.; Bordi, F.; Paradossi, G. Long-term physical evolution of an elastomeric ultrasound contrast microbubble. J. Colloid Interface Sci. 2019, 540, 185–196. [Google Scholar] [CrossRef]
- Capece, S.; Domenici, F.; Brasili, F.; Oddo, L.; Cerroni, B.; Bedini, A.; Bordi, F.; Chiessi, E.; Paradossi, G. Complex interfaces in “phase-change” contrast agents. Phys. Chem. Chem. Phys. 2016, 18, 8378–8388. [Google Scholar] [CrossRef]
- Palmieri, D.; Brasili, F.; Capocefalo, A.; Bizien, T.; Angelini, I.; Oddo, L.; Toumia, Y.; Paradossi, G.; Domenici, F. Improved hybrid-shelled perfluorocarbon microdroplets as ultrasound- and laser-activated phase-change platform. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128522. [Google Scholar] [CrossRef]
- Barrefelt, Å.; Paradossi, G.; Asem, H.; Margheritelli, S.; Saghafian, M.; Oddo, L.; Muhammed, M.; Aspelin, P.; Hassan, M.; Brismar, T.B. Dynamic MR imaging, biodistribution and pharmacokinetics of polymer shelled microbubbles containing spion. Nano 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Brismar, T.B.; Grishenkov, D.; Gustafsson, B.; Härmark, J.; Barrefelt, Å.; Kothapalli, S.V.; Margheritelli, S.; Oddo, L.; Caidahl, K.; Hebert, H.; et al. Magnetite nanoparticles can be coupled to microbubbles to support multimodal imaging. Biomacromolecules 2012, 13, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yang, F.; Wu, Y.; Wen, S.; Chen, P.; Zhang, Y.; Gu, N. Microbubbles with surface coated by superparamagnetic iron oxide nanoparticles. Mater. Lett. 2012, 68, 64–67. [Google Scholar] [CrossRef]
- Waqar, H.; Riaz, R.; Ahmed, N.M.; Majeed, A.I.; Abbas, S.R. Monodisperse magnetic lecithin-PFP submicron bubbles as dual imaging contrast agents for ultrasound (US) and MRI. RSC Adv. 2022, 12, 10503–10517. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Guo, Y.; Peng, L.; Fang, H.; Wang, Z.; Zheng, Y.; Ran, H.; Chen, Y. In Vivo Targeted, Responsive, and Synergistic Cancer Nanotheranostics by Magnetic Resonance Imaging-Guided Synergistic High-Intensity Focused Ultrasound Ablation and Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15428–15441. [Google Scholar] [CrossRef]
- Ding, L.; Li, P.; He, L.; Xu, F.; Ding, J.; Luo, B. Lactoferrin-Conjugated Polylactic Acid Nanobubbles Encapsulated Perfluoropentane as a Contrast Agent for Ultrasound/Magnetic Resonance Dual-Modality Imaging. J. Renew. Mater. 2022, 10, 767–780. [Google Scholar] [CrossRef]
- Ginzel, E.; Macneil, R.; Ginzel, R.; Zuber, M.; Sinclar, A.N. Acoustic Properties of the Elastomeric Materials AqualeneTM and ACETM. E-J. Nondestruct. Test. 2015, 20, 1–2. [Google Scholar]
- Sugimoto, T.; Ando, A.; Ono, K.; Morita, Y.; Hosoda, K.; Ishii, D.; Nakamura, K. A lightweight push-pull acoustic transducer composed of a pair of dielectric elastomer films. J. Acoust. Soc. Am. 2013, 134, EL432–EL437. [Google Scholar] [CrossRef] [Green Version]
- Mitsak, A.G.; Dunn, A.M.; Hollister, S.J. Mechanical characterization and non-linear elastic modeling of poly(glycerol sebacate) for soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 3–15. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, W.; Zhou, X.; Nie, W.; Chen, L.; He, C. Synthesis and characterization of poly(glycerol sebacate)-based elastomeric copolyesters for tissue engineering applications. Polym. Chem. 2016, 7, 2553–2564. [Google Scholar] [CrossRef]
- Riaz, R.; Abbas, S.R.; Iqbal, M. Synthesis, rheological characterization, and proposed application of pre-polyglycerol sebacate as ultrasound contrast agent based on theoretical estimation. J. Appl. Polym. Sci. 2022, 139, 51963. [Google Scholar] [CrossRef]
- Louage, B.; Tack, L.; Wang, Y.; De Geest, B.G. Poly(glycerol sebacate) nanoparticles for encapsulation of hydrophobic anti-cancer drugs. Polym. Chem. 2017, 8, 5033–5038. [Google Scholar] [CrossRef]
- Avram, A.M.; Radoi, A.; Schiopu, V.; Avram, M.; Gavrila, H. Synthesis and Characterization of γ-Fe2O3 Nanoparticles for Applications in Magnetic Hyperthermia. Synthesis 2011, 10, 151. [Google Scholar]
- Bravo, I.; Aranda, A.; Hurley, M.D.; Marston, G.; Nutt, D.R.; Shine, K.P. Infrared absorption spectra, radiative efficiencies, and global warming potentials of perfluorocarbons: Comparison between experiment and theory. J. Geophys. Res. Atmos. 2010, 115, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Altarawneh, M.; Almatarneh, M.H.; Dlugogorski, B.Z. Thermal decomposition of perfluorinated carboxylic acids: Kinetic model and theoretical requirements for PFAS incineration. Chemosphere 2022, 286, 131685. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y. Characterization and application of magnetite Particles, synthesized by reverse coprecipitation method in open air from mill scale. J. Magn. Magn. Mater. 2020, 495, 165823. [Google Scholar] [CrossRef]
- Sen Yadav, B.; Singh, R.; Kumar Vishwakarma, A.; Kumar, N. Facile Synthesis of Substantially Magnetic Hollow Nanospheres of Maghemite (γ-Fe2O3) Originated from Magnetite (Fe 3O4) via Solvothermal Method. J. Supercond. Nov. Magn. 2020, 33, 2199–2208. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.N. Papandreou C. Magnetic Nanoparticles in Medical Diagnostic Applications: Synthesis, Characterization and Proteins Conjugation. Curr. Nanosci. 2016, 4, 455–468. [Google Scholar] [CrossRef]
- Perecin, C.J.; Tirich, B.M.; Nagamine, L.C.C.M.; Porto, G.; Rocha, F.V.; Cerize, N.N.P. Aqueous synthesis of magnetite nanoparticles for magnetic hyperthermia: Formation mechanism approach, high water-dispersity and stability. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 627, 127169. [Google Scholar] [CrossRef]
- Bhoopal Reddy, S.; Nagaraja, M.S.; Kadalli, G.G.; Champa, B.V. Fourier Transform Infrared (FTIR) Spectroscopy of Soil Humic and Fulvic Acids Extracted from Paddy Land Use System. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 834–837. [Google Scholar] [CrossRef]
- Moronshing, M.; Seemesh, B.; Sudeshna, M.; Ramamurthy, S.; Subramaniam, C. Surface-enhanced Raman scattering platform operating over wide pH range with minimal chemical enhancement effects: Test case of tyrosine. J. Raman Spectrosc. 2019, 50, 826–836. [Google Scholar]
- Christou, C.; Agapiou, A.; Kokkinofta, R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wardani, R.K.; Dahlan, K.; Wahudi, T. Synthesis and characterization of nanoparticle magnetite for biomedical application. In Proceedings of the AIP Conference Proceedings, Surakarta, Indonesia, 18 December 2019; Volume 2194, p. 020137. [Google Scholar]
- Chen, Q.Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. [Google Scholar]
- Xu, J.; Yang, H.; Fu, W.; Du, K.; Sui, Y.; Chen, J.; Zeng, Y.; Li, M.; Zou, G. Preparation and magnetic properties of magnetite nanoparticles by sol-gel method. J. Magn. Magn. Mater. 2007, 309, 307–311. [Google Scholar]
- Ouyang, Z.W.; Chen, E.C.; Wu, T.M. Thermal stability and magnetic properties of polyvinylidene fluoride/magnetite nanocomposites. Materials 2015, 8, 4553–4564. [Google Scholar] [CrossRef] [PubMed]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar]
- Sheeran, P.S.; Matsuura, N.; Borden, M.A.; Williams, R.; Matsunaga, T.O.; Burns, P.N.; Dayton, P.A. Methods of generating submicrometer phase-shift perfluorocarbon droplets for applications in medical ultrasonography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 252–263. [Google Scholar] [CrossRef]
- Sheeran, P.S.; Dayton, P.A. Phase-Change Contrast Agents for Imaging and Therapy. Curr. Pharm. Des. 2012, 18, 2152–2165. [Google Scholar]
- Ho, T.M.; Razzaghi, A.; Ramachandran, A.; Mikkonen, K.S. Emulsion characterization via microfluidic devices: A review on interfacial tension and stability to coalescence. Adv. Colloid Interface Sci. 2022, 299, 102541. [Google Scholar]
- Jin, J.; Ooi, C.H.; Dao, D.V.; Nguyen, N.T. Coalescence processes of droplets and liquid marbles. Micromachines 2017, 8, 336. [Google Scholar] [CrossRef] [Green Version]
- Joseph, E.; Singhvi, G. Chapter 4—Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu AMBT-N for DD and T, Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. [Google Scholar]
- Anuje, M.; Pawaskar, P.N.; Khot, V.; Sivan, A.; Jadhav, S.; Meshram, J.; Thombare, B. Synthesis, Characterization, and Cytotoxicity Evaluation of Polyethylene Glycol-Coated Iron Oxide Nanoparticles for Radiotherapy Application. J. Med. Phys. 2021, 46, 154–161. [Google Scholar] [PubMed]
- Foong, M.; Chin, T.; Lai, W.; Bee, S.; Hamid, A. Facile Synthesis Polyethylene Glycol Coated Magnetite Nanoparticles for High Colloidal Stability. J. Nanomater. 2016, 2016, 8612505. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zou, P.; Yang, L.; Cao, J.; Sun, Y.; Han, D.; Yang, S.; Wang, Z.; Chen, G.; Wang, B.; et al. A comprehensive study on the synthesis and paramagnetic properties of PEG-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2014, 303, 425–432. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Superparamagnetic iron oxide (Fe3O4) nanoparticles coated with PEG/PEI for biomedical applications: A facile and scalable preparation route based on the cathodic electrochemical deposition method. Adv. Phys. Chem. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Suciu, M.; Mirescu, C.; Crăciunescu, I.; Macavei, S.G.; Leoștean, C.; Ştefan, R.; Olar, L.E.; Tripon, S.C.; Ciorîță, A.; Barbu-Tudoran, L. In vivo distribution of poly(Ethylene glycol) functionalized iron oxide nanoclusters: An ultrastructural study. Nanomaterials 2021, 11, 2184. [Google Scholar] [CrossRef]
- Liu, Z.; Lammers, T.; Ehling, J.; Fokong, S.; Bornemann, J.; Kiessling, F.; Gätjens, J. Iron oxide nanoparticle-containing microbubble composites as contrast agents for MR and ultrasound dual-modality imaging. Biomaterials 2011, 32, 6155–6163. [Google Scholar] [CrossRef]
- Faria, M.R.; Cruz, M.M.; Gonçalves, M.C.; Carvalho, A.; Feio, G.; Martins, M.B.F. Synthesis and characterization of magnetoliposomes for MRI contrast enhancement. Int. J. Pharm. 2013, 446, 183–190. [Google Scholar] [CrossRef]
- Martina, M.S.; Fortin, J.P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef]
- Kostevšek, N.; Cheung, C.C.L.; Serša, I.; Kreft, M.E.; Monaco, I.; Franchini, M.C.; Vidmar, J.; Al-Jamal, W.T. Magneto-liposomes as MRI contrast agents: A systematic study of different liposomal formulations. Nanomaterials 2020, 10, 889. [Google Scholar] [CrossRef]
- Martínez-González, R.; Estelrich, J.; Busquets, M.A. Liposomes loaded with hydrophobic iron oxide nanoparticles: Suitable T2 contrast agents for MRI. Int. J. Mol. Sci. 2016, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Sun, Y.; Liu, W.; Guan, W.; Liu, H.; Duan, Y.; Chen, Y. Magnetic polymeric nanobubbles with optimized core size for MRI/ultrasound bimodal molecular imaging of prostate cancer. Nanomedicine 2020, 15, 2901–2916. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, F.; Zhou, X.; Zhuang, Y.; Liu, B.; Mu, Y.; Wang, X.; Shen, H.; Zhi, G.; Wu, D. Uniform PEGylated PLGA microcapsules with embedded Fe3O4 nanoparticles for US/MR dual-modality imaging. ACS Appl. Mater. Interfaces 2015, 7, 20460–20468. [Google Scholar] [CrossRef] [PubMed]
- Perlman, O.; Weitz, I.S.; Azhari, H. Copper oxide nanoparticles as contrast agents for MRI and ultrasound dual-modality imaging. Phys. Med. Biol. 2015, 60, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- Kuźnik, N.; Wyskocka, M.; Jarosz, M.; Oczek, L.; Goraus, S.; Komor, R.; Krawczyk, T.; Kempka, M. Amino-phenol complexes of Fe(III) as promising T1 accelerators. Arab. J. Chem. 2019, 12, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.I.; Ha, S.W.; Lee, H.J. An ultrasound-responsive dual-modal US/T1-MRI contrast agent for potential diagnosis of prostate cancer. J. Magn. Reson. Imaging 2018, 48, 1610–1616. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, M.; Zu, G.; Kuang, Y.; Tong, X.; Xiong, D.; Pei, R. Hyperbranched poly(glycerol) as a T1 contrast agent for tumor-targeted magnetic resonance imaging in vivo. Polym. Chem. 2017, 8, 1104–1113. [Google Scholar] [CrossRef]
- Teraphongphom, N.; Chhour, P.; Eisenbrey, J.R.; Naha, P.C.; Witschey, W.R.T.; Opasanont, B.; Jablonowski, L.; Cormode, D.P.; Wheatley, M.A. Nanoparticle Loaded Polymeric Microbubbles as Contrast Agents for Multimodal Imaging. Langmuir 2015, 31, 11858–11867. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lai, X.; Zhu, Y.; Guo, F.; Su, L.; Arkin, G.; He, T.; Xu, J.; Ran, H. Contrast-enhanced ultrasound imaging using long-circulating cationic magnetic microbubbles in vitro and in vivo validations. Int. J. Pharm. 2022, 616, 121299. [Google Scholar] [CrossRef]
- Niu, C.; Xu, Y.; An, S.; Zhang, M.; Hu, Y.; Wang, L.; Peng, Q. Near-infrared induced phase-shifted ICG/Fe3O4 loaded PLGA nanoparticles for photothermal tumor ablation. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- US-FDA. Use of International Standard ISO 10993-1, “Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process” guidance for industry and Food and Drug Administration staff. US Dep. Health Hum. Serv. Food Drug Adm. 2020, 301, 1–68. [Google Scholar]
- ISO 10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009; Volume 104. [Google Scholar]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, C.; Wang, L.; Cheng, W.P.; Cuschieri, A. Dilemmas in the reliable estimation of the in-vitro cell viability in magnetic nanoparticle engineering: Which tests and what protocols? Nanoscale Res. Lett. 2012, 7, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef] [Green Version]
- Díaz, B.; Sánchez-Espinel, C.; Arruebo, M.; Faro, J.; De Miguel, E.; Magadán, S.; Yaguee, C.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaria, J.; et al. Assessing methods for blood cell cytotoxic responses to inorganic nanoparticles and nanoparticle aggregates. Small 2008, 4, 2025–2034. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Bremer-Hoffmann, S. Main trends of immune effects triggered by nanomedicines in preclinical studies. Int. J. Nanomed. 2018, 13, 5419–5431. [Google Scholar] [CrossRef] [Green Version]
- Szebeni, J.; Simberg, D.; González-Fernández, Á.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef]
- Muskula, P.R.; Main, M.L. Safety with Echocardiographic Contrast Agents. Circ. Cardiovasc. Imaging 2017, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Neun, B.W.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of Complement Activation by Nanoparticles BT. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Springer: New York, NY, USA, 2018; pp. 149–160. [Google Scholar]
- Bayly-Jones, C.; Bubeck, D.; Dunstone, M.A. The mystery behind membrane insertion: A review of the complement membrane attack complex. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160221. [Google Scholar] [CrossRef] [Green Version]








| Functional Group | Wavenumber (cm−1) | References |
|---|---|---|
| C-F stretch | 1100–1300 | [24,25] |
| Fe-O Stretching | 630, 795, 880, 2920 | [26,27,28,29] |
| C=O stretch | 1735–1760 | [30,31] |
| C-H stretch | 2800–2900 | [32] |
| C-H bending | 600–900 | [32] |
| Constructs | 2θ Peaks Observed (°) | Reference |
|---|---|---|
| Magnetite | 31.35, 35.35, 45.1, 57.15, 63.07 and 74.5 | [33,35,36] |
| Pre-PGS | 25° and 35° | [22,34] |
| Suspensions | SEM Avg Size (nm) | DLS Avg Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| PEG-coated SPIONs | 173 ± 29 | 190.1 ± 0.0 | +32 ± 7 |
| Unloaded PCNDs (non-converted) | 132 ± 58 | 210 ± 61 | −21 ± 4 |
| PEG-coated SPION-loaded PCNDs (non-converted) | 156 ± 95 | 164 ± 26 | −12 ± 5 |
| Unloaded PCNDs (phase-converted) | 1076 ± 288 5359 ± 342 | −21 ± 4 | |
| PEG-coated SPION-loaded PCNDs (phase-converted) | 927 ± 167 | −12 ± 5 |
| Concentrations | Average Thrombin Time (s) | Plasma Recalcification Time (s) | ||
|---|---|---|---|---|
| Unloaded PCNDs | 10 mg SPIONs Loaded PCNDs | Unloaded PCNDs | 10 mg SPIONs Loaded PCNDs | |
| Plasma | 8 | 8 | 240 | 240 |
| Plasma with PBS | 10 | 10 | 220 | 220 |
| PCNDs (1 × 107) | 13 | 9 | 210 | 240 |
| PCNDs (1 × 105) | 10 | 6 | 210 | 245 |
| PCNDs (1 × 103) | 10 | 6 | 210 | 250 |
| Aspirin | 35 | 35 | 410 | 410 |
| Normal Time | <21 | <21 | 220–350 | 220–350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, R.; Waqar, H.; Ahmad, N.M.; Abbas, S.R. Novel Magnetic Elastic Phase-Change Nanodroplets as Dual Mode Contrast Agent for Ultrasound and Magnetic Resonance Imaging. Polymers 2022, 14, 2915. https://doi.org/10.3390/polym14142915
Riaz R, Waqar H, Ahmad NM, Abbas SR. Novel Magnetic Elastic Phase-Change Nanodroplets as Dual Mode Contrast Agent for Ultrasound and Magnetic Resonance Imaging. Polymers. 2022; 14(14):2915. https://doi.org/10.3390/polym14142915
Chicago/Turabian StyleRiaz, Ramish, Hira Waqar, Nasir M. Ahmad, and Shah Rukh Abbas. 2022. "Novel Magnetic Elastic Phase-Change Nanodroplets as Dual Mode Contrast Agent for Ultrasound and Magnetic Resonance Imaging" Polymers 14, no. 14: 2915. https://doi.org/10.3390/polym14142915
APA StyleRiaz, R., Waqar, H., Ahmad, N. M., & Abbas, S. R. (2022). Novel Magnetic Elastic Phase-Change Nanodroplets as Dual Mode Contrast Agent for Ultrasound and Magnetic Resonance Imaging. Polymers, 14(14), 2915. https://doi.org/10.3390/polym14142915








