Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Encapsulating Agents
2.2. Sample Preparation
2.3. Extraction of Polyphenols from the Coproduct
2.4. Microparticle Preparation (MP)
2.5. Encapsulation Efficiency (EE)
2.6. Quantification of Polyphenols by Spectrophotometry
2.6.1. Total Phenolic Content (TPC)
2.6.2. Total Flavonoid Content (TFC)
2.6.3. Tannin Condensate Content (TCC)
2.7. Ultraefficiency Liquid Chromatography (UHPLC-DAD) Analysis
2.8. 2,20-Azinobis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Cation Scavenging Activity
2.9. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radical Scavenging Activity
2.10. Physicochemical Analysis
2.10.1. Infrared Analysis (FTIR)
2.10.2. Thermogravimetry Analysis (TG)
2.10.3. Moisture Content
2.10.4. Water Activity
2.10.5. Microstructure of Particles
2.11. Statistical Analysis
3. Results and Discussion
3.1. Quantification of Polyphenols and Encapsulation Efficiency
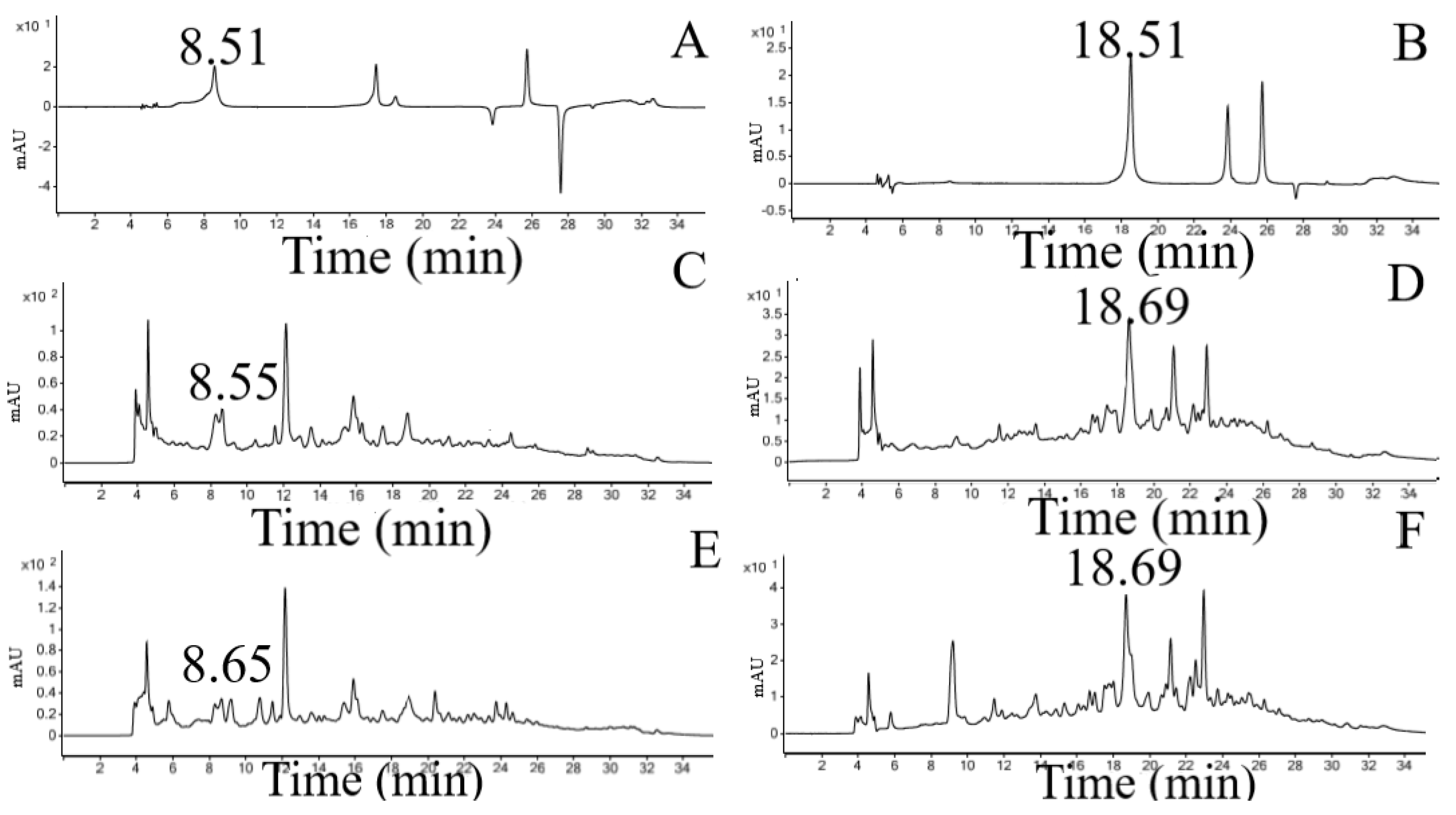
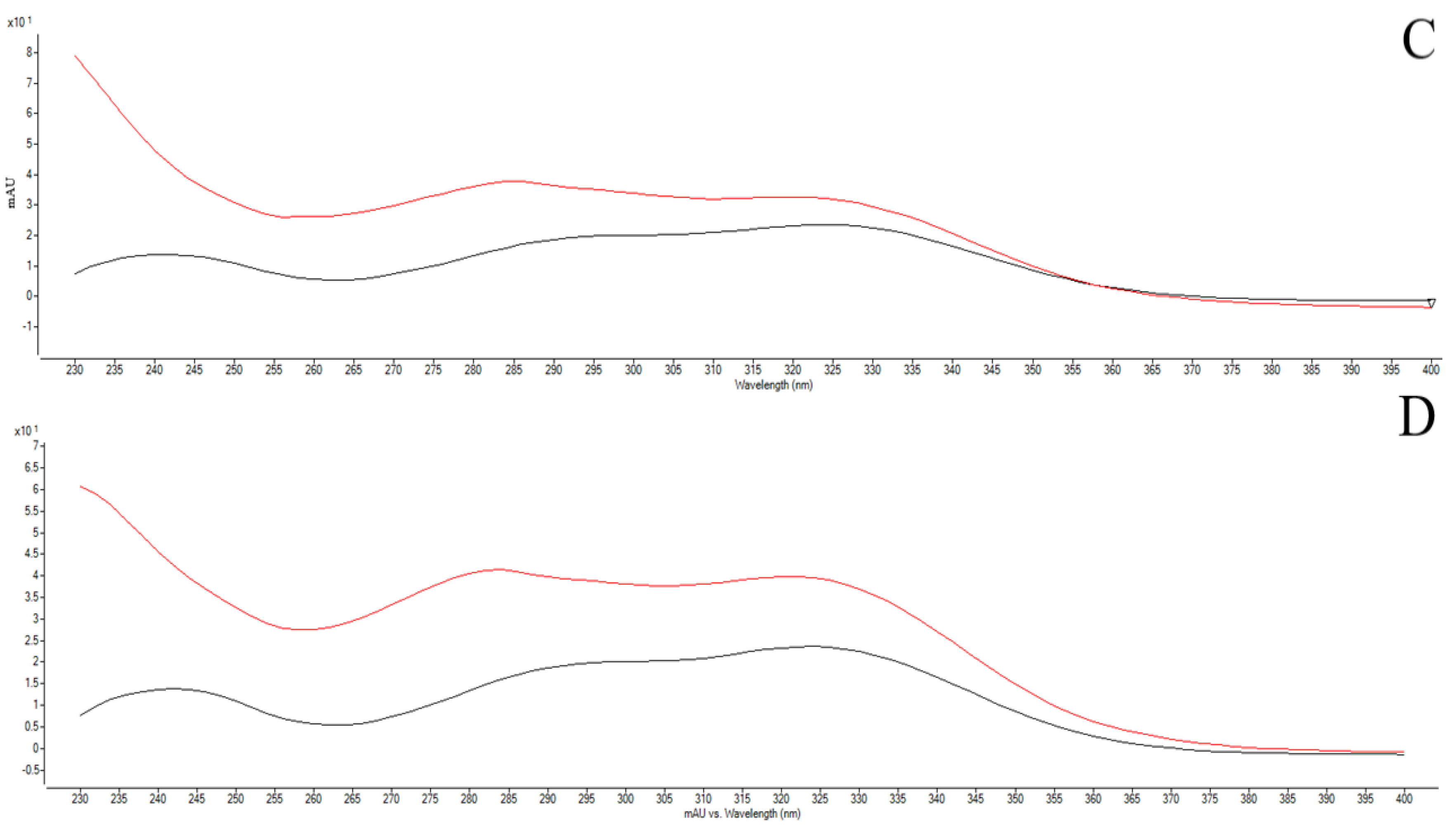
3.2. Ultraefficiency Liquid Chromatography (UHPLC-DAD) Analysis
3.3. Antioxidant Activity
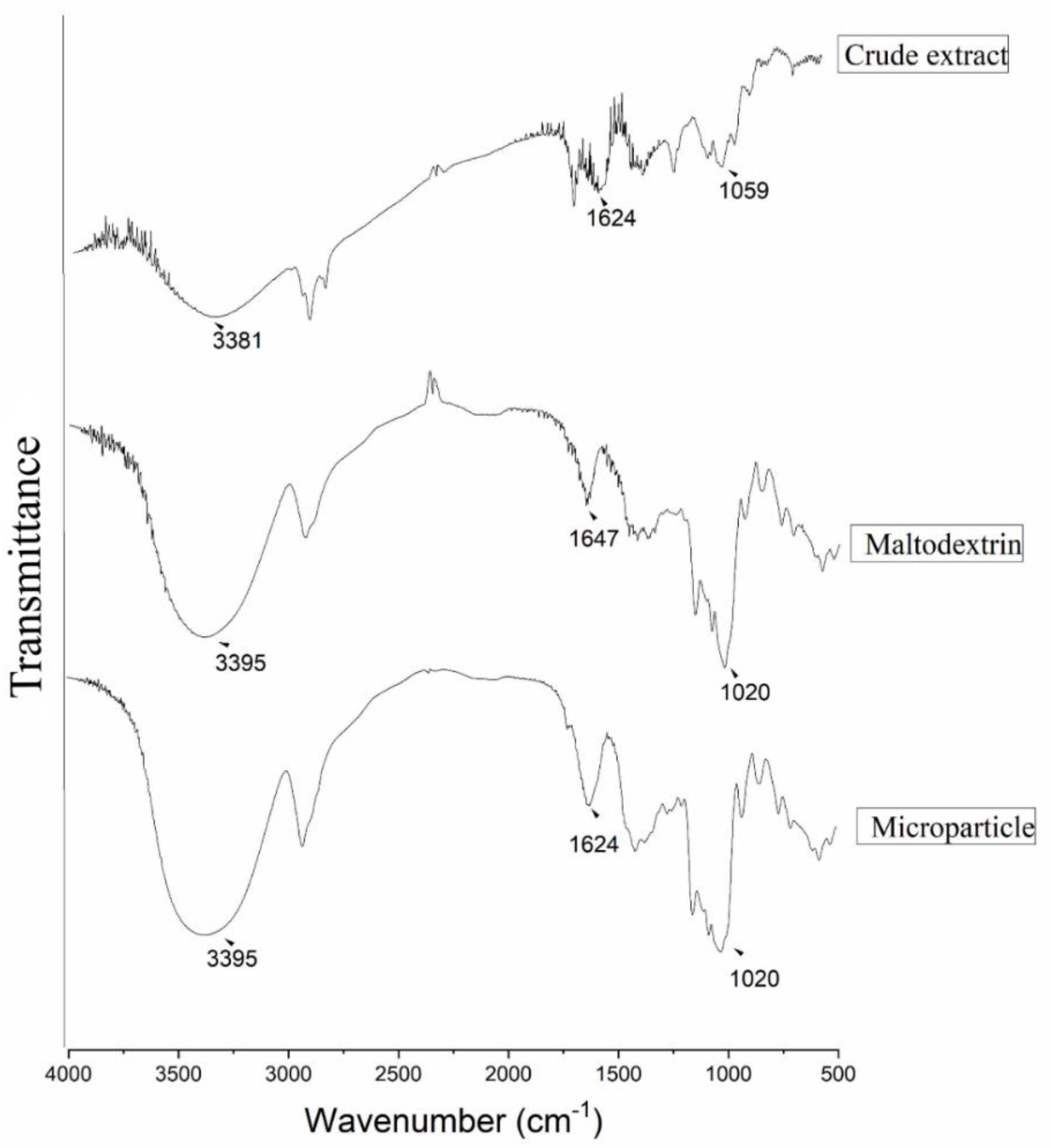
3.4. Infrared Analysis (FTIR)
3.5. Thermogravimetry Analysis (TG)/Derived Thermogravimetry (DTG)
3.6. Moisture Content and Water Activity
3.7. Microstructure of Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferreira, M.J.A.; Mota, M.F.S.; Mariano, R.G.B.; Freitas, S.P. Evaluation of liquid-liquid extraction to reducing the acidity index of the tucuma (Astrocaryum vulgare Mart.) pulp oil. Sep. Purif. Technol. 2021, 257, 117894. [Google Scholar] [CrossRef]
- Noronha Matos, K.A.; Praia Lima, D.; Pereira Barbosa, A.P.; Zerlotti Mercadante, A.; Campos Chisté, R. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- Ferreira, L.M.M.C.; Pereira, R.R.; De Carvalho, F.B.; Santos, A.S.; Ribeiro-Costa, R.M.; Silva, J. Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Biomolecules 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.A.; Pereira, R.R.; Duarte Junior, A.P.; da Cruz Rodrigues, A.M.; Remédios, C.M.R.; Brasil, D.D.S.B.; Morais, L.R.B.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Tucumã (Astrocaryum vulgare) fat: An Amazonian material as a pharmaceutical input for lipid nanoparticle production. J. Therm. Anal. Calorim. 2020, 147, 355–365. [Google Scholar] [CrossRef]
- Jimenez-Vasquez, V.; Millán, B.; Machahua, M.; Kahn, F.; Ramirez, R.; Pintaud, J.C.; Roncal, J. Dry season characteristics in western Amazonia underlie the divergence of Astrocaryum section Huicungo (Arecaceae) and evaluation of potential anatomical adaptations. Bot. J. Linn. Soc. 2017, 185, 291–306. [Google Scholar] [CrossRef]
- Baurin, N.; Arnoult, E.; Scior, T.; Do, Q.T.; Bernard, P. Preliminary screening of some tropical plants for anti-tyrosinase activity. J. Ethnopharmacol. 2002, 82, 155–158. [Google Scholar] [CrossRef]
- Bergmann, J.C.; Tupinambá, D.D.; Costa, O.Y.A.; Almeida, J.R.M.; Barreto, C.C.; Quirino, B.F. Biodiesel production in Brazil and alternative biomass feedstocks. Renew. Sustain. Energy Rev. 2013, 21, 411–420. [Google Scholar] [CrossRef]
- Kieling, A.C.; Santana, G.P.; dos Santos, M.C.; Jaqtinon, H.D.C.C.; Monteiro, C.C.P. Cadeia do Tucumã comercializado em Manaus-AM. Sci. Amaz. 2019, 8, b1–b9. [Google Scholar]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, F.R.; Rocha, L.C.R.; Santos, T.A.; do Carmo, E.L.; Pereira, L.A.S.; Borges, S.V.; Pereira, R.G.F.A.; Botrel, D.A. Microencapsulation of bioactive compounds from espresso spent coffee by spray drying. LWT 2019, 103, 116–124. [Google Scholar] [CrossRef]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Lagazzo, A.; Botter, R.; Perego, P. Microencapsulation of phenolic compounds from olive pomace using spray drying: A study of operative parameters. LWT–Food Sci. Technol. 2015, 62, 177–186. [Google Scholar] [CrossRef]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Ruggiero, C.; Pastorino, L. Chitosan/dextran multilayer microcapsules for polyphenol co-delivery. Mater. Sci. Eng. C 2015, 46, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of resistant-maltodextrin complemented with sodium caseinate, skim milk, and beta-cyclodextrin on the quality of spray-dried powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.S.; Teixeira, C.B.; Gabbay Alves, T.V.; Ribeiro-Costa, R.M.; Casazza, A.A.; Aliakbarian, B.; Converti, A.; Silva Júnior, J.O.C.; Perego, P. Optimization of spray drying conditions to microencapsulate cupuassu (Theobroma grandiflorum) seed by-product extract. Nat. Prod. Res. 2018, 6419, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gabbay Alves, T.V.; Silva da Costa, R.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Carréra Silva Júnior, J.O.; Ribeiro Costa, R.M.; Converti, A. Microencapsulation of Theobroma cacao L. waste extract: Optimization using response surface methodology. J. Microencapsul. 2017, 34, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers 2022, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Araruna, S.M.; Silva, A.H.; Canuto, K.M.; Silveira, E.R.; Leal, L.K.A.M. Influence of process conditions on the physicochemical characteristics of cumaru (Amburana cearensis) powder produced by spray drying. Braz. J. Pharmacogn. 2013, 23, 132–137. [Google Scholar] [CrossRef]
- Silva da Costa, R.; de Farias Silva, N.; Gabbay Alves, T.V.; Fernandes da Silva, M.; do Socorro Barros Brasil, D.; Ribeiro-Costa, R.M.; Converti, A.; Carréra Silva, J.O. Antioxidant Activity of an Industrial Cupuassu Seed By-product: Molecular Modeling of Phenolic Compounds. Chem. Eng. Technol. 2019, 42, 397–406. [Google Scholar] [CrossRef]
- Gabbay Alves, T.V.; Silva da Costa, R.; Aguiar Gomes, A.T.; Ferreira da Costa, C.E.; Perego, P.; Carréra Silva Júnior, J.O.; Converti, A.; Ribeiro Costa, R.M. Quality control of Amazonian cocoa (Theobroma cacao L.) by-products and microencapsulated extract by thermal analysis. J. Therm. Anal. Calorim. 2018, 134, 993–1000. [Google Scholar] [CrossRef]
- Engel, B.; Baccar, N.D.M.; Marquardt, L.; De Oliveira, M.S.R.; Rohlfes, A.L.B. Tecnologias de atomização e desidratação: Alternativas para a produção de farinhas a partir de vegetais. Rev. Jovens Pesqui. 2016, 6, 31–44. [Google Scholar] [CrossRef][Green Version]
- Silva, R.M.F.; Gomes, T.C.B.L.; Albuquerque, M.M.; Silva Junior, J.O.C.; Barbosa, W.L.R.; Rolim Neto, P.J. Abordagem sobre os diferentes processos de secagem empregados na obtenção de extratos secos de plantas medicinais. Rev. Bras. Plantas Med. 2012, 14, 103–109. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Taip, F.S.; Aziz, N.A.; Talib, R.A. Physical Properties of Spray-dried Pink Guava (Psidium guajava) Powder. Agric. Agric. Sci. Procedia 2014, 2, 74–81. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Effect of encapsulated beet extracts (Beta vulgaris) added to yogurt on the physicochemical characteristics and antioxidant activity. Molecules 2021, 26, 4768. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Santos, P.D.D.F.; Rubio, F.T.V.; Balieiro, J.C.D.C.; Thomazini, M.; Favaro-Trindade, C.S. Application of spray drying for production of microparticles containing the carotenoid-rich tucumã oil (Astrocaryum vulgare Mart.). LWT 2021, 143, 111106. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Marques, G.R.; Borges, S.V.; de Mendonça, K.S.; de Barros Fernandes, R.V.; Menezes, E.G.T. Application of maltodextrin in green corn extract powder production. Powder Technol. 2014, 263, 89–95. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA). Farmacopeia Brasileira; ANVISA: Brasília, Brazil, 2010; Volume 2, ISBN 9788588233416.
- De Cássia Almeida Sampaio, R.; Da Costa, R.S.; De Souza, C.R.F.; Duarte Júnior, A.P.; Ribeiro-Costa, R.M.; Da Costa, C.E.F.; De Oliveira, W.P.; Converti, A.; Silva Júnior, J.O.C. Thermal characterization of Arrabidaea chica (Humb. & Bonpl.) B. Verl. dry extracts obtained by spray dryer. J. Therm. Anal. Calorim. 2016, 123, 2469–2475. [Google Scholar] [CrossRef]
- Norulfairuz, D.; Zaidel, A.; Aqilah, N.; Mohd, Y.M. Efficiency and Thermal Stability of Encapsulated Anthocyanins from Red Dragon Fruit (Hylocereus polyrhizus (Weber) Britton & Rose) using Microwave-assisted Technique. Chem. Eng. Trans. 2015, 43, 127–132. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, V.T.; Minh, L.V.; Trieu, L.H.; Cang, M.H.; Bui, L.B.; Le, X.T.; Danh, V.T. Determination of the phytochemical screening, total polyphenols, flavonoids content, and antioxidant activity of soursop leaves (Annona muricata Linn.). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062011. [Google Scholar] [CrossRef]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2021, 337, 127970. [Google Scholar] [CrossRef]
- Nicácio, A.E.; Rodrigues, C.A.; Jardim, I.C.S.F.; Visentainer, J.V.; Maldaner, L. Modified QuEChERS method for phenolic compounds determination in mustard greens (Brassica juncea) using UHPLC-MS/MS. Arab. J. Chem. 2019, 13, 4681–4690. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Laghari, A.H.; Ali Memon, A.; Memon, S.; Nelofar, A.; Khan, K.M.; Yasmin, A. Determination of free phenolic acids and antioxidant capacity of methanolic extracts obtained from leaves and flowers of camel thorn (Alhagi maurorum). Nat. Prod. Res. 2012, 26, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.R.; Gomes, A.T.A.; Testi, M.; Bianchera, A.; Ribeiro-Costa, R.M.; Padula, C.; Silva Júnior, J.O.C.; Sonvico, F. Ucuùba Fat Characterization and Use to Obtain Lipid Nanoparticles by High-Pressure Homogenization with Full Factorial Design. Chem. Eng. Technol. 2021, 44, 1009–1016. [Google Scholar] [CrossRef]
- Pereira, R.R.; Testi, M.; Rossi, F.; Silva Junior, J.O.C.; Ribeiro-Costa, R.M.; Bettini, R.; Santi, P.; Padula, C.; Sonvico, F. Ucuùba (Virola surinamensis) fat-based nanostructured lipid carriers for nail drug delivery of ketoconazole: Development and optimization using box-behnken design. Pharmaceutics 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Longo, E.; Mattoso, L.H.C. Investigação do processo de absorção de água de hidrogéis de polissacarídeo: Efeito da carga iônica, presença de sais, concentrações de monômero e polissacarídeo. Polímeros 2012, 22, 311–317. [Google Scholar] [CrossRef][Green Version]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef]
- Sagrillo, M.R.; Garcia, L.F.M.; De Souza Filho, O.C.; Duarte, M.M.M.F.; Ribeiro, E.E.; Cadoná, F.C.; Da Cruz, I.B.M. Tucumã fruit extracts (Astrocaryum aculeatum Meyer) decrease cytotoxic effects of hydrogen peroxide on human lymphocytes. Food Chem. 2015, 173, 741–748. [Google Scholar] [CrossRef]
- De Souza Schmidt Gonçalves, A.E.; Lajolo, F.M.; Genovese, M.I. Chemical composition and antioxidant/antidiabetic potential of brazilian native fruits and commercial frozen pulps. J. Agric. Food Chem. 2010, 58, 4666–4674. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, M.D. Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. Int. J. Food Sci. Technol. 2009, 44, 1950–1958. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Blagojević, B.; Četojević-Simin, D.; Djurić, S.; Lazzara, G.; Milioto, S.; Agić, D.; Vasile, B.S.; Popović, B.M. Anthocyanins and phenolic acids from Prunus spinosa L. encapsulation in halloysite and maltodextrin based carriers. Appl. Clay Sci. 2022, 222, 106489. [Google Scholar] [CrossRef]
- Slavov, A.; Denev, P.; Panchev, I.; Shikov, V.; Nenov, N.; Yantcheva, N.; Vasileva, I. Combined recovery of polysaccharides and polyphenols from Rosa damascena wastes. Ind. Crops Prod. 2017, 100, 85–94. [Google Scholar] [CrossRef]
- Botrel, D.A.; de Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Da Silva Port’s, P.; Chisté, R.C.; Godoy, H.T.; Prado, M.A. The phenolic compounds and the antioxidant potential of infusion of herbs from the Brazilian Amazonian region. Food Res. Int. 2013, 53, 875–881. [Google Scholar] [CrossRef]
- Siddhuraju, P. Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. LWT–Food Sci. Technol. 2007, 40, 982–990. [Google Scholar] [CrossRef]
- Savalekar, K.; Ahammed Shabeer, T.P.; Khan, Z.; Oulkar, D.; Jain, P.; Patil, C.; Banerjee, K. Targeted phenolic profiling of Sauvignon blanc and Shiraz grapes grown in two regions of India by liquid chromatography-tandem mass spectrometry. J. Food Sci. Technol. 2019, 56, 3300–3312. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chemie–Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Melo, E.D.A.; Maciel, M.I.S.; Lima, V.L.A.G.; Araújo, C.R. Teor de fenólicos totais e capacidade antioxidante de polpas congeladas de frutas. Aliment. Nutr. Araraquara 2008, 19, 67–72. [Google Scholar]
- Rabelo, D.S.; Paula, J.R.; Bara, M.T.F. Quantificação de fenóis totais presentes nas cascas de Trichillia catigua A. Juss (Meliaceae). Rev. Bras. Plantas Med. 2013, 15, 230–236. [Google Scholar] [CrossRef][Green Version]
- Cataneo, C.B.; Caliari, V.; Gonzaga, L.V.; Kuskoski, E.M.; Fett, R. Atividade antioxidante e conteúdo fenólico do resíduo agroindustrial da produção de vinho. Semin. Agrar. 2008, 29, 93–102. [Google Scholar] [CrossRef]
- Soares, M.; Welter, L.; Gonzaga, L.; Lima, A.; Mancini-Filho, J.; Fett, R. Avaliação da atividade antioxidante e identificação dos ácidos fenólicos presentes no bagaço de maçã cv. Gala (Evaluation of antioxidant activity and identification of phenolic acids present in the pomace of Gala variety apples). Ciênc. Tecnol. Aliment 2008, 28, 727–732. [Google Scholar] [CrossRef]
- Raimundo, T.R.F.; Salvador, M.J.; Corrêa, W.R. Avaliação da Atividade Antioxidante de Pfaffia glabrata. Rev. Univap 2017, 22, 67. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Çam, M.; Içyer, N.C.; Erdoǧan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT–Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Pinto, A.C.; Veiga, V.F., Jr.; Grynberg, N.F.; Echevarria, A. Plantas medicinais: A necessidade de estudos multidisciplinares. Quim. Nova 2002, 25, 429–438. [Google Scholar] [CrossRef]
- Da Costa, R.S.; Dos Santos, O.V.; Rodrigues, A.M.D.C.; Ribeiro-Costa, R.M.; Converti, A.; Silva Júnior, J.O.C. Functional product enriched with the microencapsulated extract of cupuassu (Theobroma grandiflorum schum.) seed by-product. Food Sci. Technol. 2020, 40, 543–550. [Google Scholar] [CrossRef]
- Da Costa, R.S.; Negrão, C.A.B.; Camelo, S.R.P.; Ribeiro-Costa, R.M.; Barbosa, W.L.R.; Da Costa, C.E.F.; Silva Júnior, J.O.C. Investigation of thermal behavior of Heliotropium indicum L. lyophilized extract by TG and DSC. J. Therm. Anal. Calorim. 2013, 111, 1959–1964. [Google Scholar] [CrossRef]
- Fiorda, F.A.; Soares Júnior, M.S.; da Silva, F.A.; Fontinelle Souto, L.R.; Eiras Grossmann, M.V. Farinha de bagaço de mandioca: Aproveitamento de subproduto e comparação com fécula de mandioca. Pesqui. Agropecu. Trop. 2013, 43, 408–416. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Sandulachi, E. Water Activity Concept and Its Role in Food Preservation; IRTUM: Chișinău, Moldova, 2010; pp. 40–48. [Google Scholar]
- Barros, S.L.; Santos, N.C.; Almeida, R.L.J.; Silva, S.N.; Nascimento, A.P.S.; Almeida, R.D.; Ribeiro, V.H.D.A.; Silva, W.P.; Gomes, J.P.; Silva, V.M.D.A.; et al. Influence of Pulp, Sugar and Maltodextrin Addiction in the Formulation of Kiwi Jellies with Lemon Grass Tea. J. Agric. Sci. 2019, 11, 125–134. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Soria, A.C.; Villamiel, M.; Montilla, A. Quality parameters in convective dehydrated carrots blanched by ultrasound and conventional treatment. Food Chem. 2013, 141, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujita, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of spray drying conditions on the physical properties of Cagaita (Eugenia dysenterica DC.) fruit extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; Andrade, G.R.S.; da Cunha Nascimento, C.; Barbosa, P.F.; Jesus, M.S.; Narain, N. Development and characterization of microencapsules containing spray dried powder obtained from Brazilian brown, green and red propolis. Food Res. Int. 2018, 109, 278–287. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Freitas, E.D.F.M.; Lopes, L.D.L.; Alves, S.M.D.F.; de Campos, A.J. Efeito da maltodextrina no sumo da polpa de abacaxi Pérola atomizado. Rev. Ciências Agrárias 2019, 42, 271–280. [Google Scholar] [CrossRef]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of ginger (Zingiber officinale) extract by spray drying technology. LWT–Food Sci. Technol. 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Klein, T.; Longhini, R.; Bruschi, M.L.; De Mello, J.C.P. Microparticles containing Guaraná extract obtained by spray-drying technique: Development and characterization. Braz. J. Pharmacogn. 2015, 25, 292–300. [Google Scholar] [CrossRef]
- Koç, B.; Sakin-Yılmazer, M.; Kaymak-Ertekin, F.; Balkır, P. Physical properties of yoghurt powder produced by spray drying. J. Food Sci. Technol. 2014, 51, 1377–1383. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]





| Sample | Total Phenolic Content (mg GAE/100 g) | Total Flavonoid Content (mg QE/100 g) | Tannin Condensate Content (mg CA/100 g) |
|---|---|---|---|
| Crude extract | 135.1 ± 0.078 a | 32.73 ± 0.009 a | 76.29 ± 0.001 a |
| Microparticle | 130.5 ± 0.024 b | 27.17 ± 0.002 b | 62.07 ± 0.002 b |
| Encapsulation Efficiency (%) | |
|---|---|
| Total Phenolic Content | 96.60 ± 0.10 |
| Total Flavonoid Content | 83.01 ± 0.01 |
| Tannin Condensate Content | 81.36 ± 0.01 |
| CE RT (min) Area | MP 5% RT (min) Area | Standards RT (min) Area | ||||
|---|---|---|---|---|---|---|
| Gallic acid (280 nm) | 8.55 | 909.86 | 8.65 | 423.70 | 8.51 | 647.74 |
| Caffeic acid (325 nm) | 18.69 | 537.95 | 18.69 | 822.01 | 18.51 | 462.07 |
| Sample | DPPH | ABTS+ (µM Trolox) | ||
|---|---|---|---|---|
| Conc Inhibition (%) | IC50 (µg/mL) | |||
| 10 | 9.5 ± 0.52 a | 60.22 ± 0.86 a | 1094.01 ± 7.33 a | |
| Crude extract | 25 | 40.14 ± 0.31 a | 38.29 ± 0.24 a | 1101.33 ± 3.66 a |
| 40 | 81.99 ± 0.50 a | 8.29 ± 0.80 a | 1247.88 ± 3.60 a | |
| Microparticle | 10 | 54.28 ± 0.97 b | 24.12 ± 0.61 b | 938.91 ± 4.23 b |
| 25 | 61.87 ± 1.25 b | 19.37 ± 0.79 b | 941.35 ± 6.23 b | |
| 40 | 83.19 ± 1.21 b | 5.91 ± 0.76 b | 956.01 ± 7.63 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.M.d.M.C.; Pereira, R.R.; Carvalho-Guimarães, F.B.d.; Remígio, M.S.d.N.; Barbosa, W.L.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Polymers 2022, 14, 2905. https://doi.org/10.3390/polym14142905
Ferreira LMdMC, Pereira RR, Carvalho-Guimarães FBd, Remígio MSdN, Barbosa WLR, Ribeiro-Costa RM, Silva-Júnior JOC. Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Polymers. 2022; 14(14):2905. https://doi.org/10.3390/polym14142905
Chicago/Turabian StyleFerreira, Lindalva Maria de Meneses Costa, Rayanne Rocha Pereira, Fernanda Brito de Carvalho-Guimarães, Myrth Soares do Nascimento Remígio, Wagner Luiz Ramos Barbosa, Roseane Maria Ribeiro-Costa, and José Otávio Carréra Silva-Júnior. 2022. "Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds" Polymers 14, no. 14: 2905. https://doi.org/10.3390/polym14142905
APA StyleFerreira, L. M. d. M. C., Pereira, R. R., Carvalho-Guimarães, F. B. d., Remígio, M. S. d. N., Barbosa, W. L. R., Ribeiro-Costa, R. M., & Silva-Júnior, J. O. C. (2022). Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Polymers, 14(14), 2905. https://doi.org/10.3390/polym14142905








