Cadmium-Rich Plant Powder/PAN/PU Foams with Low Thermal Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatment of Heavy Metal Enrichment Biomass
2.3. Preparation of the Composite Foams
2.4. Characterization
2.4.1. Basic Performance Test
2.4.2. Thermal Conductivity Test
2.4.3. Determination of Heavy Metal Stability of Composite Foams
3. Results
3.1. Morphology Characterization
3.2. Specific Surface Area Analysis
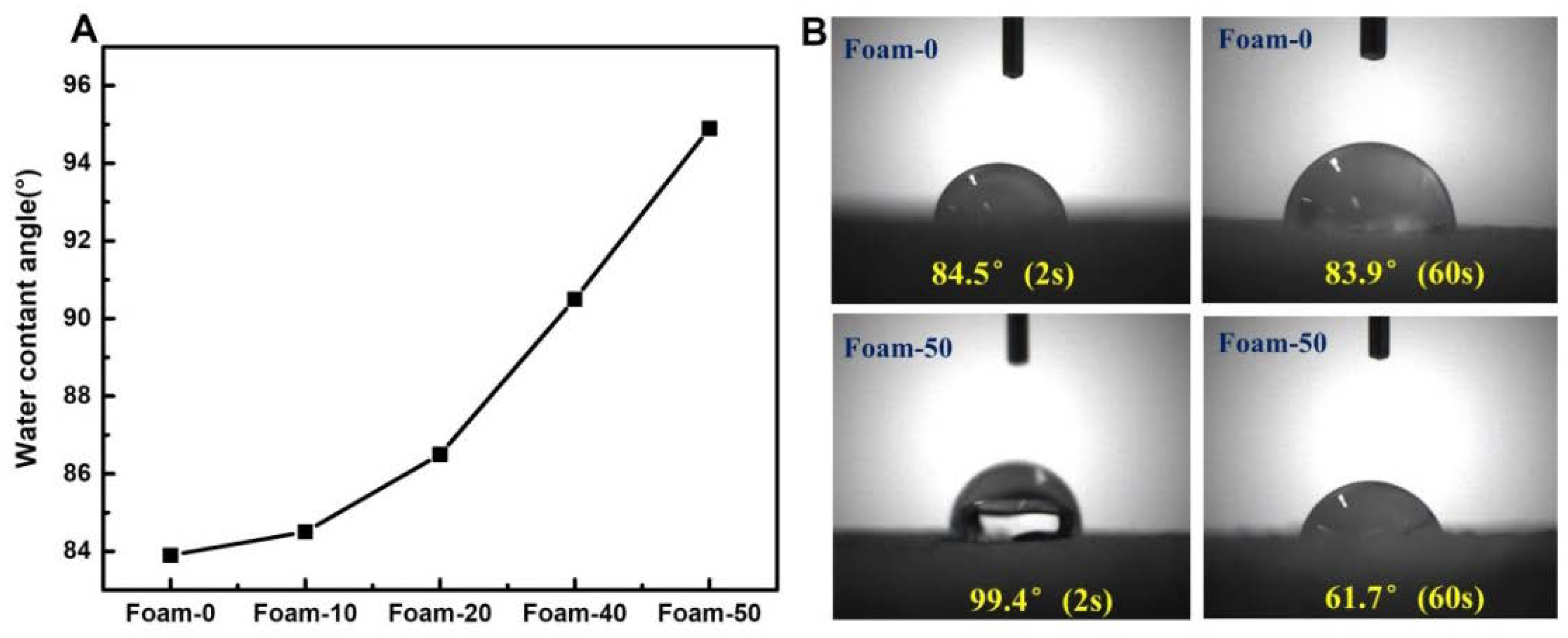
3.3. WCA Test
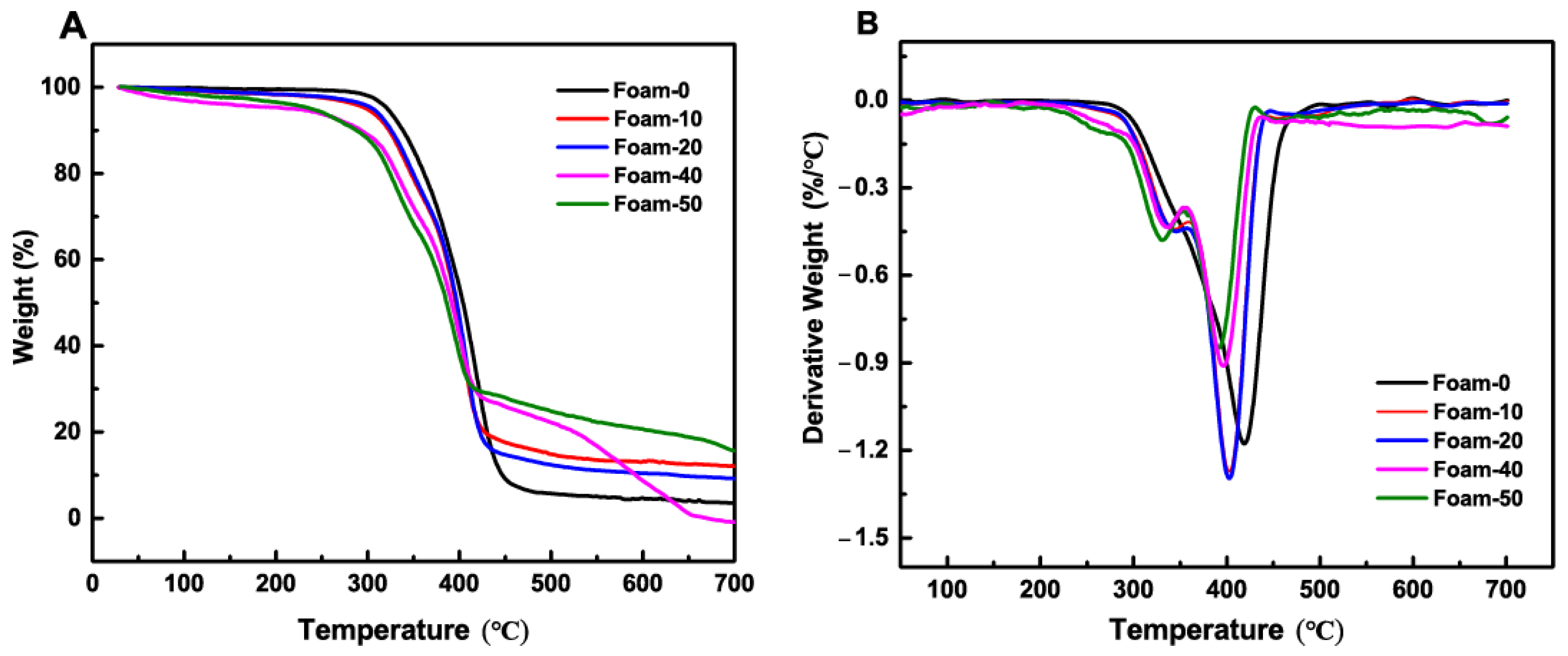
3.4. Thermal Property
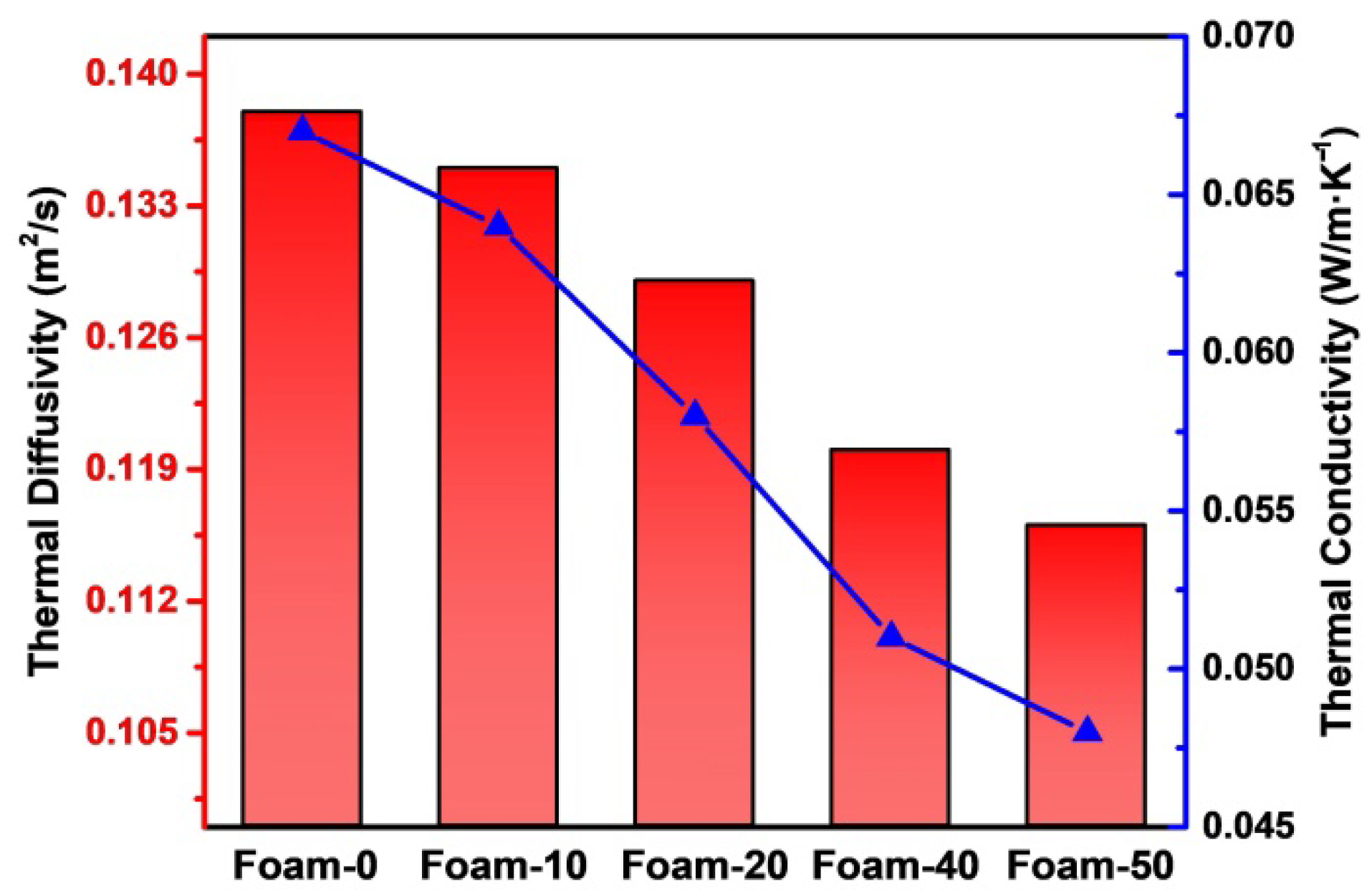
3.5. Thermal Conductivity
3.6. Heavy Metal Stability in Composite Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez, N.; Mclaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; FAO: New York, NY, USA, 2018. [Google Scholar]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M. Phytoremediation of Toxic Metals. Using Plants to Clean up the Environment; Raskin, I., Ensley, B., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; p. 304. ISBN 0-471-19254-6. [Google Scholar]
- Roychowdhury, A.; Datta, R.; Sarkar, D. Green Chemistry: Heavy Metal Pollution and Remediation; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kamath, R.; Rentz, J.A.; Schnoor, J.L.; Alvarez, P. Phytoremediation of hydrocarbon-contaminated soils: Principles and applications. Stud. Surf. Sci. Catal. 2004, 151, 447–478. [Google Scholar]
- Cui, X.; Mao, P.; Sun, S.; Huang, R.; Li, Z. Phytoremediation of cadmium contaminated soils by Amaranthus Hypochondriacus, L.: The effects of soil properties highlighting cation exchange capacity. Chemosphere 2021, 283, 131067. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I.; Schmidt, U.; Londo, M.; Faaij, A. The economic value of the phytoremediation function—Assessed by the example of cadmium remediation by willow (Salix ssp). Agric. Syst. 2006, 89, 68–89. [Google Scholar] [CrossRef] [Green Version]
- Kotrba, P.; Najmanova, J.; Macek, T.; Ruml, T.; Mackova, M. Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol. Adv. 2009, 27, 799–810. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Li, N.; Li, Z.; Fu, Q.; Zhuang, P.; Guo, B.; Li, H. Agricultural Technologies for Enhancing the Phytoremediation of Cadmium-Contaminated Soil by Amaranthus hypochondriacus L. Water Air Soil Pollut. 2013, 224, 1–12. [Google Scholar] [CrossRef]
- Gomes, H.I. Phytoremediation for bioenergy: Challenges and opportunities. Environ. Technol. Rev. 2012, 1, 59–66. [Google Scholar] [CrossRef]
- Chami, Z.A.; Amer, N.S.; Smets, K.; Yperman, J.; Carleer, R.; Dumontet, S.; Vangronsveld, J. Evaluation of flash and slow pyrolysis applied on heavy metal contaminated Sorghum bicolor shoots resulting from phytoremediation. Biomass Bioenergy 2014, 63, 268–279. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Arisha, A.; Gabr, A.; El-Badawy, S.; Shwally, S. Using Blends of Construction & Demolition Waste Materials and Recycled Clay Masonry Brick in Pavement. Procedia Eng. 2016, 143, 1317–1324. [Google Scholar]
- Jiao, Q.; Luo, C.; Qiao, C.; Xing, Y. Recycling of the hyperaccumulator Brassica juncea L.: Synthesis of carbon nanotube-Cu/ZnO nanocomposites. J. Mater. Cycles Waste Manag. 2014, 16, 162–166. [Google Scholar]
- Chen, Y.; Dong, L.; Miao, J.; Wang, J.; Liu, J. Hydrothermal liquefaction of corn straw with mixed catalysts for the production of bio-oil and aromatic compounds. Bioresour. Technol. 2019, 294, 122148. [Google Scholar] [CrossRef]
- Kamal, N.; Beddu, S.; Syamsir, A.; Mohammad, D.; Manan, T. Immobilization of Heavy Metals for Building Materials in the Construction Industry—An Overview. Mater. Today: Proc. 2019, 17, 787–791. [Google Scholar] [CrossRef]
- Sas-Nowosielska, A.; Kucharski, R.; Małkowski, E.; Pogrzeba, M.; Kuperberg, J.M.; Kryński, K. Phytoextraction crop disposal—An unsolved problem. Environ. Pollut. 2004, 128, 373–379. [Google Scholar] [CrossRef]
- Dong, L.; Tianle, Z.; Runwei, L.; Xinghua, L.; Yuan, Z.; Ye, S.; Hongmei, W.; Fan, Z.; Qinglin, Z. Effects of Co-Processing Sewage Sludge in the Cement Kiln on PAHs, Heavy Metals Emissions and the Surrounding Environment. Int. J. Env. Res. Public Health 2018, 15, 698. [Google Scholar]
- Selvaraj, S.; Ravichandran, S.; Prabhu, S.; Prashanth, G.K.; Sathyananda, H.M. Novel Foam Adsorbents in Dyes and Heavy Metals Removal: A Review. Asian J. Chem. 2021, 33, 499–508. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Wang, S. Remediation of a heavy metal-contaminated soil by a rhamnolipid foam. Eng. Geol. 2006, 85, 75–81. [Google Scholar] [CrossRef]
- Hong, H.J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated cellulose nanofibrils (CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef]
- Madadi, M.; Wang, Y.; Xu, C.; Liu, P.; Wang, Y.; Xia, T.; Tu, Y.; Lin, X.; Song, B.; Yang, X. Using Amaranthus green proteins as universal biosurfactant and biosorbent for effective enzymatic degradation of diverse lignocellulose residues and efficient multiple trace metals remediation of farming lands. J. Hazard. Mater. 2021, 406, 124727. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xu, X.; Li, T.; Gong, G.; Jia, Y.; Li, Y.; Deng, L. Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J. Hazard. Mater. 2010, 180, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.T.; Chang, C.Y.; Pan, H.W.; Chiang, T.Y.; Kuo, M.T.; Wang, Y.H. Characterization of polyurethane foam as heat seal coating in medical pouch packaging application. J. Polym. Res. 2012, 19, 9791. [Google Scholar] [CrossRef]
- Ye, S.Y.; Vijh, A.K.; Dao, L.H. Oxygen reduction on a new electrocatalyst based on highly porous carbonized polyacrylonitrile microcellular foam with very low platinum loading. J. Electroanal. Chem. 1996, 415, 115–121. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Basak, P.; Manorama, S.V. PEO-PU/PAN semi-interpenetrating polymer networks for SPEs: Influence of physical properties on the electrical characteristics. Solid State Ionics 2004, 167, 113–121. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Bio-Renewable Sources for Synthesis of Eco-Friendly Polyurethane Adhesives—Review. Open J. Polym. Chem. 2017, 07, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Mort, R.; Vorst, K.; Curtzwiler, G.; Jiang, S. Biobased foams for thermal insulation: Material selection, processing, modelling, and performance. RSC Adv. 2021, 11, 4375–4394. [Google Scholar] [CrossRef]
- Jiang, X.L.; Liu, T.; Hu, G.H.; Zhao, L.; Zhu, Z.N.; Yuan, W.K. Foaming of polypropylene with supercritical carbon dioxide. J. Supercrit. Fluids 2007, 41, 299–310. [Google Scholar]
- Polaczek, K.; Kurańska, M.; Augucik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell polyurethane foams of very low density modified with various palm oil-based bio-polyols in accordance with cleaner production. J. Clean. Prod. 2021, 290, 125875. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.S.; Li, F.J.; Zhu, N. Preparation and Property Study on Traditional Chinese Medicine Modified Viscose Fiber. Cotton Text. Technol. 2018, 46, 15–17. [Google Scholar]
- Colton, J.S.; Suh, N.P. Nucleation of microcellular foam: Theory and practice. Polym. Eng. Sci. 1987, 27, 500–503. [Google Scholar] [CrossRef]
- Guo, K.Y.; Wu, Q.; Mao, M.; Chen, H.; Zhang, G.D.; Zhao, L.; Gao, J.F.; Song, P.; Tang, L.A. Water-based hybrid coatings toward mechanically flexible, super-hydrophobic and flame-retardant polyurethane foam nanocomposites with high-efficiency and reliable fire alarm response—ScienceDirect. Compos. Part B Eng. 2020, 193, 108017. [Google Scholar] [CrossRef]
- Ansari, A.A.; Gill, S.S.; Gill, R.; Lanza, G.R.; Newman, L. Phytoremediation of Arsenic-Contaminated Soils Using Arsenic Hyperaccumulating Ferns; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Zhang, H.; Fang, W.Z.; Li, Y.M.; Tao, W.Q. Experimental study of the thermal conductivity of polyurethane foams. Appl. Therm. Eng. 2017, 115, 528–538. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Puszka, A.E. New thermoplastic poly(carbonate-urethane)s based on chain extenders with sulfur atoms. Chem. Pap. 2016, 71, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of evening primrose oil-based polyol on the properties of rigid polyurethane-polyisocyanurate foams for thermal insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Berardi, U. The impact of aging and environmental conditions on the effective thermal conductivity of several foam materials. Energy 2019, 182, 777–794. [Google Scholar] [CrossRef]
- Felixhenningsen, P.; Urushadze, T.; Steffens, D.; Kalandadze, B.; Narimanidze, E. Uptake of heavy metals by food crops from highly-polluted Chernozem-like soils in an irrigation district south of Tbilisi, eastern Georgia. Agron. Res. 2010, 8, 781–795. [Google Scholar]
- Xiao, W.; Min, B.G. Comparison of porous poly (vinyl alcohol)/hydroxyapatite composite cryogels and cryogels immobilized on poly (vinyl alcohol) and polyurethane foams for removal of cadmium. J. Hazard. Mater. 2008, 156, 381–386. [Google Scholar]


| Recycling Approach | Advantages | Disadvantages |
|---|---|---|
| Biomass Power Generation | Generate power/heat; reduce greenhouse gas emissions; recover precious metals from fly ash, have high economic value. | Produce secondary pollution (toxic air pollutants); extraction of heavy metals needs chelating agent, requires complex process and high cost. |
| Biofuel Production | Produce bio-oil, industrial ethanol, and other materials; further realize the reduction, harmlessness, and recycling of biomass containing heavy metals. | Require complex operation; the conversion efficiency is low; causes subsequent environmental problems. |
| Building Materials | Replace part of cement; reduce the cost of building materials; reduce CO2 and NOx emissions under the condition of coordinated high-temperature disposal in cement kiln [19]. | Heavy metals used in building materials need to be fixed; heavy metals may leach. |
| Samples | Specific Surface Area/m2·g |
|---|---|
| Foam-0 | 0.1381 |
| Foam-10 | 0.3158 |
| Foam-20 | 0.3179 |
| Foam-40 | 0.3268 |
| Foam-50 | 0.4392 |
| Samples | d (mm) | a (mm2/s) | ρ (g/cm3) | Cp (J/g·K) |
|---|---|---|---|---|
| Foam-0 | 2.400 ± 0.043 | 0.138 | 0.253 | 1.861 |
| Foam-10 | 2.500 ± 0.012 | 0.135 | 0.260 | 1.823 |
| Foam-20 | 2.500 ± 0.068 | 0.129 | 0.266 | 1.690 |
| Foam-40 | 2.800 ± 0.018 | 0.120 | 0.273 | 1.506 |
| Foam-50 | 2.900 ± 0.021 | 0.116 | 0.300 | 1.386 |
| pH of the Solution | Mass of Foam (g) | Volume of Leachate (mL) | Cadmium Concentration in Leachate (μg/L) | Cadmium Leaching Rate (%) | Cadmium Immobilization Rate (%) |
|---|---|---|---|---|---|
| 3 | 0.2 | 10 | 1.779 | 0.14 | 99.86 |
| 5 | 0.2 | 10 | 1.529 | 0.12 | 99.88 |
| 7 | 0.2 | 10 | 0.645 | 0.05 | 99.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Sun, J.; Tang, J.; Chen, Z.; Shi, Y.; Zhao, R.; Jiang, Y.; Tan, L. Cadmium-Rich Plant Powder/PAN/PU Foams with Low Thermal Conductivity. Polymers 2022, 14, 2893. https://doi.org/10.3390/polym14142893
Tang W, Sun J, Tang J, Chen Z, Shi Y, Zhao R, Jiang Y, Tan L. Cadmium-Rich Plant Powder/PAN/PU Foams with Low Thermal Conductivity. Polymers. 2022; 14(14):2893. https://doi.org/10.3390/polym14142893
Chicago/Turabian StyleTang, Wenying, Jin Sun, Jie Tang, Zheng Chen, Yidong Shi, Ruifang Zhao, Yuanzhang Jiang, and Lin Tan. 2022. "Cadmium-Rich Plant Powder/PAN/PU Foams with Low Thermal Conductivity" Polymers 14, no. 14: 2893. https://doi.org/10.3390/polym14142893






