Mechanical Properties, Melting and Crystallization Behaviors, and Morphology of Carbon Nanotubes/Continuous Carbon Fiber Reinforced Polyethylene Terephthalate Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Prepreg Tapes
2.2.1. CNT/PET Composite Pellets
2.2.2. Preparation of Prepreg Tape
2.3. Measurements
2.3.1. Tensile Strength
2.3.2. Differential Scanning Calorimetry
2.3.3. Dynamic Mechanical Analysis
2.3.4. Fractural Analysis
3. Results and Discussion
3.1. Mechanical Property
3.1.1. Tensile Property
3.1.2. Dynamic Mechanical Analysis
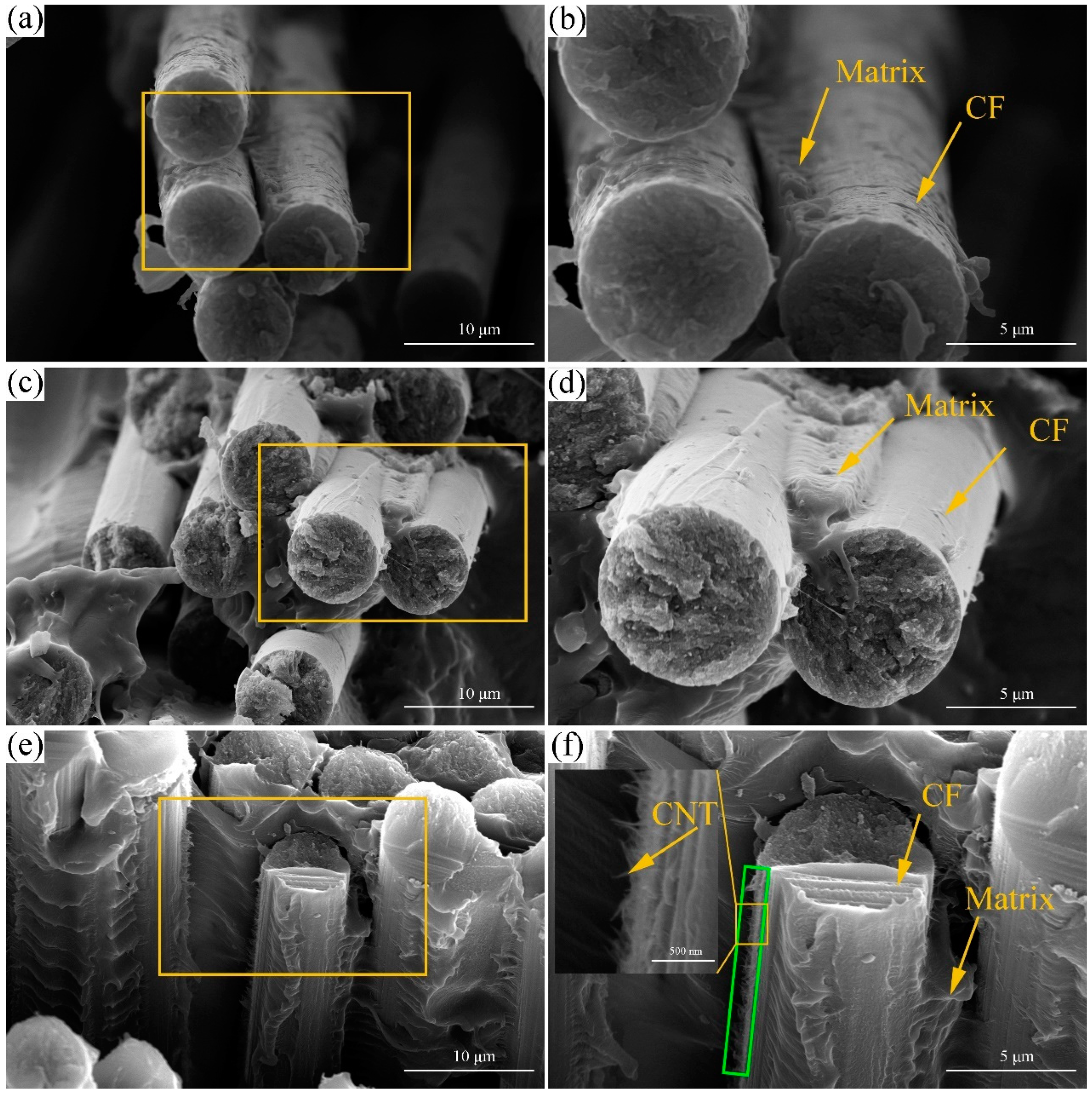
3.2. Morphology Analysis
3.3. Melting and Crystallization Behavior
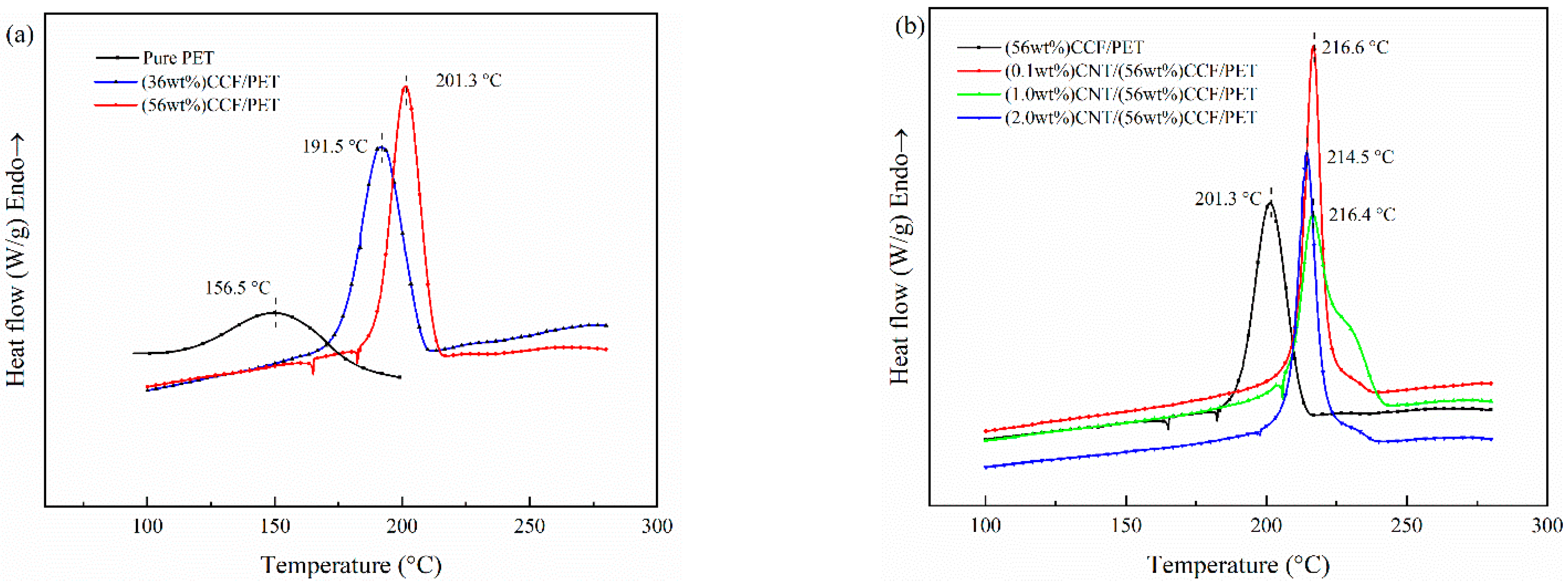
3.3.1. Melting
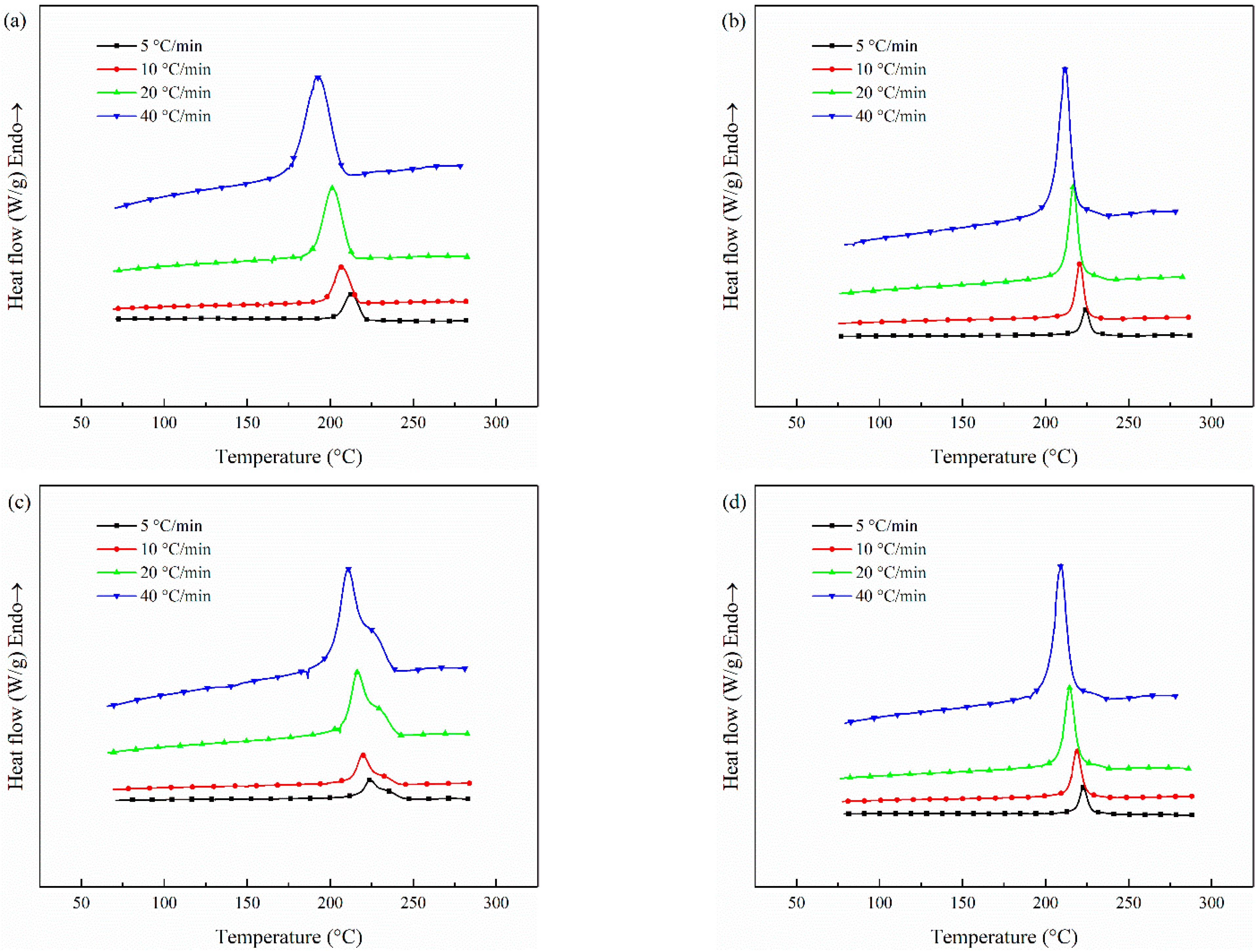
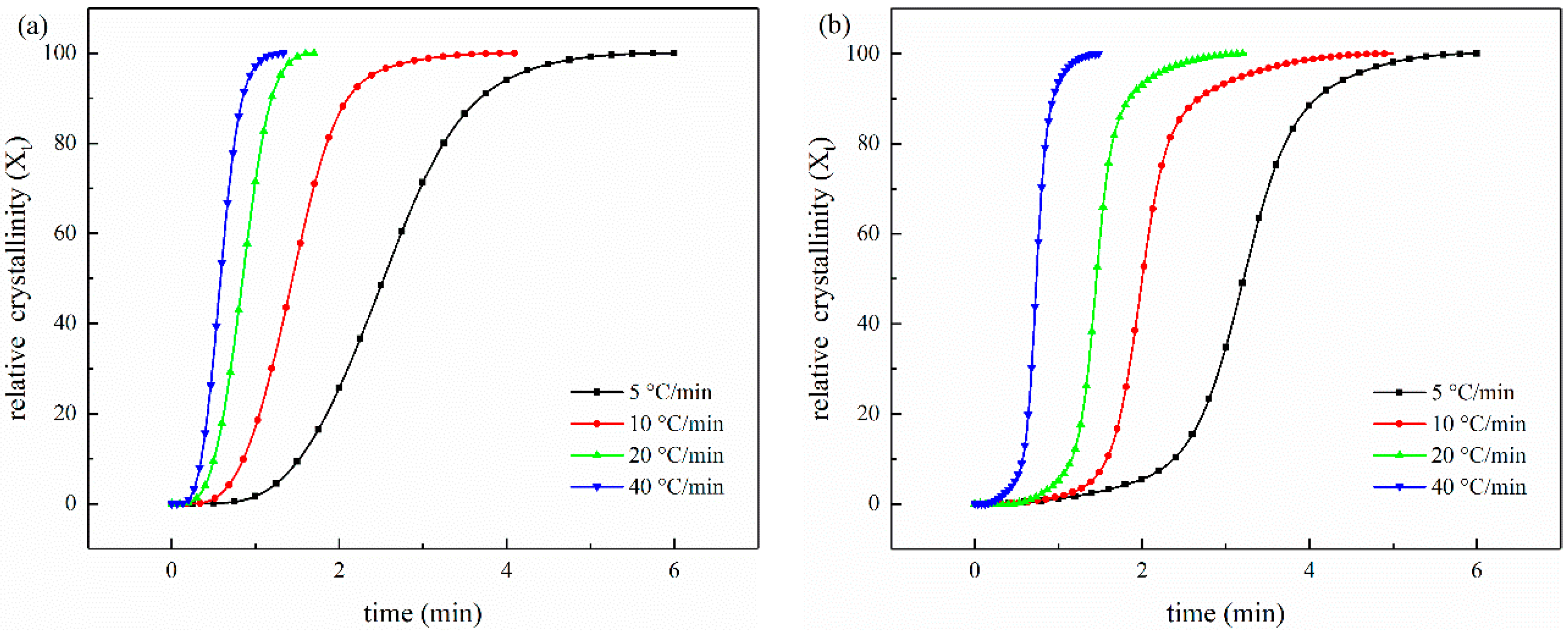
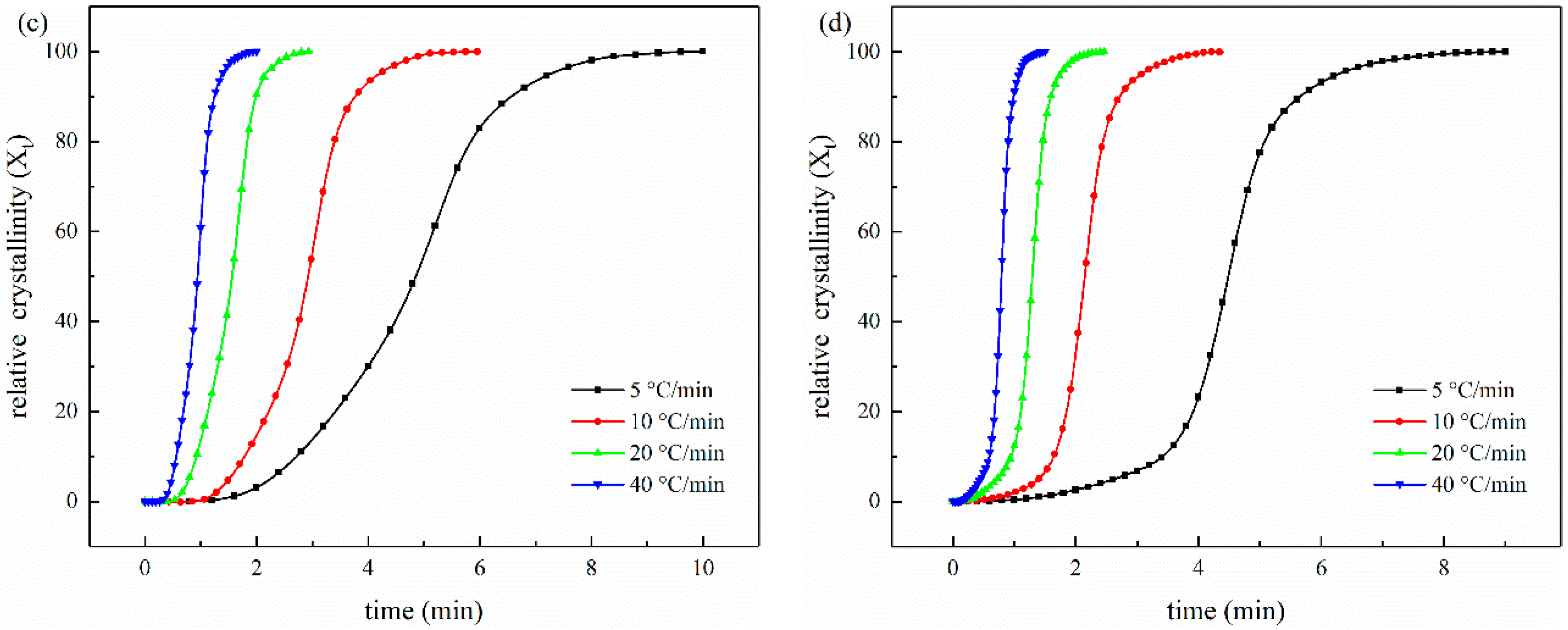
3.3.2. Crystallization
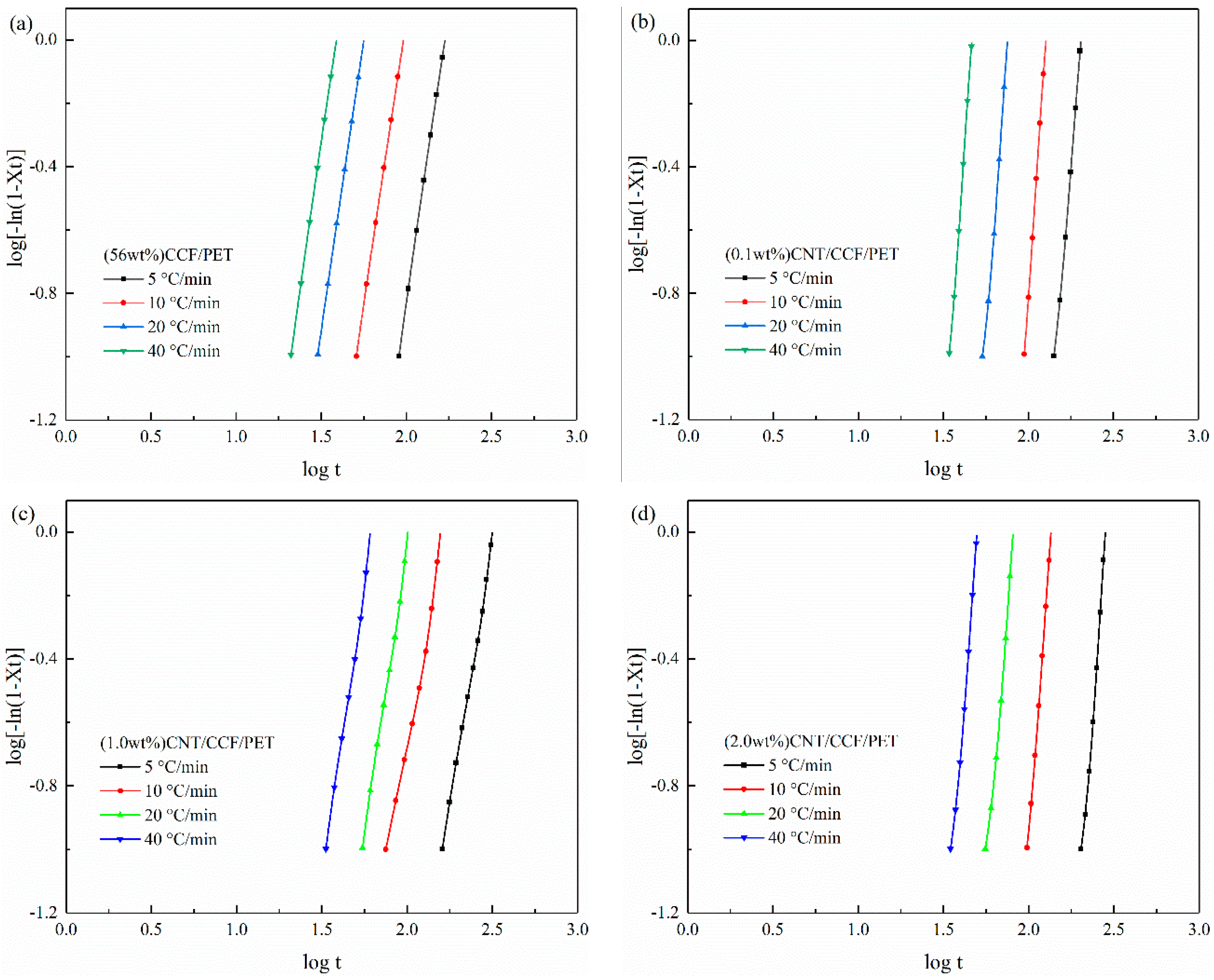
3.4. The Non-Isothermal Melt Crystallization Kinetics
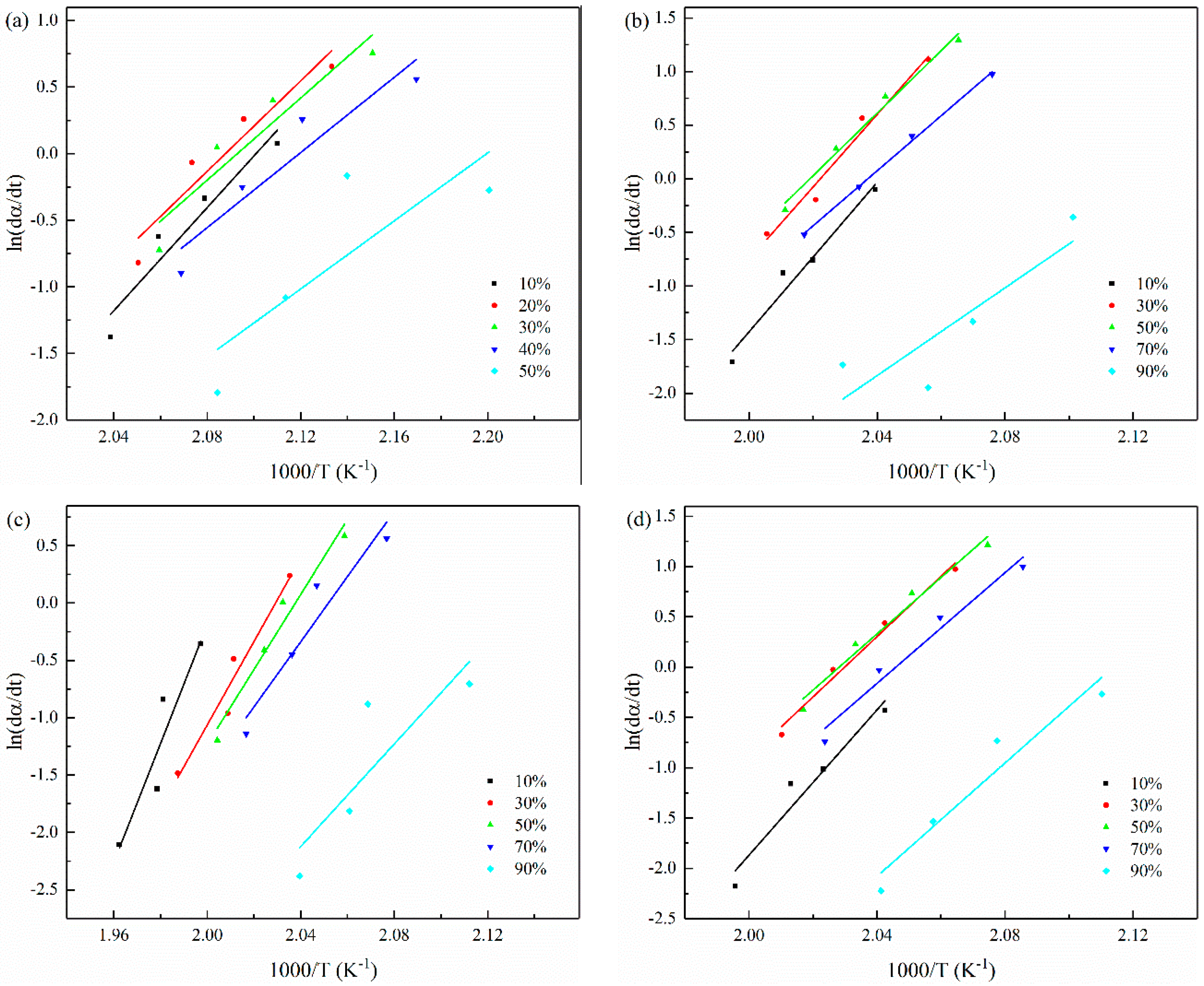
3.5. The Non-Isothermal Melt Crystallization Activation Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goh, G.D.; Toh, W.; Yap, Y.L.; Ng, T.Y.; Yeong, W.Y. Additively manufactured continuous carbon fiber-reinforced thermoplastic for topology optimized unmanned aerial vehicle structures. Compos. Part B 2021, 216, 108840. [Google Scholar] [CrossRef]
- Pearson, A.; Liao, W.; Heydrich, M.; Kakroodi, A.; Hammami, A.; Kazemi, Y.; Naguib, H.E. Role of interfacial adhesion and fiber length on the mechanical performance fiber reinforced thermoplastic elastomers. Compos. Sci. Technol. 2021, 213, 108928. [Google Scholar] [CrossRef]
- Kazano, S.; Osada, T.; Kobayashi, S.; Goto, K. Experimental and analytical investigation on resin impregnation behavior in continuous carbon fiber reinforced thermoplastic polyimide composites. Mech. Adv. Mater. Mod. Process. 2018, 4, 6. [Google Scholar] [CrossRef]
- Wang, B.; Fu, Q.; Sun, L.; Lu, Y.; Liu, Y. Improving the tribological performance of carbon fiber reinforced resin composite by grafting MWCNT and GNPs on fiber surface. Mater. Lett. 2022, 306, 130953. [Google Scholar] [CrossRef]
- Zhang, J. The effect of carbon fibers and carbon nanotubes on the mechanical properties of polyimide composites. Mec. Compos. Mater. 2011, 47, 447. [Google Scholar] [CrossRef]
- John, J.V.; Moon, B.K.; Kim, I. Influence of soft segment content and chain length on the physical properties of poly(ether ester) elastomers and fabrication of honeycomb pattern and electrospun fiber. React. Funct. Polym. 2013, 73, 1213–1222. [Google Scholar] [CrossRef]
- Jiang, F.; Qiu, Z. Crystallization kinetics, mechanical properties, and hydrolytic degradation of novel eco-friendly poly(butylene diglycolate) containing ether linkages. J. Appl. Polym. Sci. 2016, 133, 44186. [Google Scholar] [CrossRef]
- Pilati, F.; Toselli, M.; Messori, M.; Manzoni, C.; Turturro, A.; Gattiglia, E.G. On spesific factors affecting the crystallization of PET: The role of carboxyl terminal groups and residual catalysts on the crystallization rate. Polymer 1997, 38, 4469–4476. [Google Scholar] [CrossRef]
- Yao, S.; Hu, D.; Xi, Z.; Liu, T.; Xu, Z.; Zhao, L. Effect of crystallization on tensile mechanical properties of PET foam: Experiment and model prediction. Polym. Test. 2020, 90, 106649. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Ma, Y.; Agarwal, U.S. Study on mechanical properties, thermal stability and crystallization behavior of PET/MMT nanocomposites. Compos. Part B Eng. 2006, 37, 399–407. [Google Scholar] [CrossRef]
- Antoniadis, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Chrissafis, K. Non-isothermal crystallization kinetic of poly(ethylene terephthalate)/fumed silica (PET/SiO2) prepared by in situ polymerization. Thermochim. Acta 2010, 510, 103–112. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Li, H.; Li, Q.; Chen, J. Nonisothermal melt crystallization kinetics of poly(ethylene terephthalate)/clay nanocomposites. J. Appl. Polym. Sci. 2004, 91, 308–314. [Google Scholar] [CrossRef]
- IIjima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, C.; Zhang, S.; Yang, M.; Wang, Z. Low percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer 2006, 47, 480–488. [Google Scholar] [CrossRef]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M. Predicting effective electrical resistivity and conductivity of carbon nanotube/carbon black-filled polymer matrix hybrid nanocomposites. J. Phys. Chem. Solids 2022, 161, 110444. [Google Scholar] [CrossRef]
- Cruz-Delgado, V.J.; Ávila-Orta, C.A.; Espinoza-Martínez, A.B.; Mata-Padilla, J.M.; Solis-Rosales, S.G.; Jalbout, A.F.; Medellín-Rodríguez, F.J.; Hsiao, B.S. Carbon nanotube surface-induced crystallization of polyethylene terephthalate (PET). Polymer 2014, 55, 642–650. [Google Scholar] [CrossRef]
- Khalid, H.R.; Jang, D.; Abbas, N.; Haider, M.S.; Bukhari, S.N.; Mirza, C.R.; Elboughdiri, N.; Ahmad, F. Electrical stability and piezoresistive sensing performance of high strain-range ultra-stretchable CNT-Embedded sensors. Polymers 2022, 14, 1366. [Google Scholar] [CrossRef]
- Jang, D.; Farooq, S.Z.; Yoon, H.N.; Khalid, H.R. Design of a highly flexible and sensitive multi-functional polymeric sensor incorporating CNTs and carbonyl iron powder. Compos. Sci. Technol. 2021, 207, 108725. [Google Scholar] [CrossRef]
- Khalid, H.R.; Choudhry, I.; Jang, D.; Abbas, N.; Haider, M.S.; Lee, H.K. Facile synthesis of sprayed CNTs layer-embedded stretchable sensors with controllable sensitivity. Polymers 2021, 13, 311. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Shi, J.; Bai, H.; Song, B. Functionalized multi-walled carbon nanotubes improve nonisothermal crystallization of poly(ethylene terephthalate). Polym. Test. 2008, 27, 179–188. [Google Scholar] [CrossRef]
- Antoniadis, G.; Paraskevopoulos, K.; Bikiaris, D.; Chrissafis, K. Kinetics study of cold-crystallization of poly(ethylene terephthalate) nanocomposites with multi-walled carbon nanotubes. Thermochim. Acta 2009, 493, 68–75. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Kunc, V.; Velez-Garcia, G.M.; Duty, C.E.; Love, L.J.; Naskar, A.K.; Blue, C.A.; Ozcan, S. Highly oriented carbon fiber–polymer composites via additive manufacturing. Compos. Sci. Technol. 2014, 105, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.K.; Ohdar, R.; Mahapatra, S. Parametric appraisal of mechanical property of fused deposition modelling processed parts. Mater. Des. 2010, 31, 287–295. [Google Scholar] [CrossRef]
- Qi, X.-D.; Yang, J.-H.; Zhang, N.; Huang, T.; Zhou, Z.-W.; Kühnert, I.; Pötschke, P.; Wang, Y. Selective localization of carbon nanotubes and its effect on the structure and properties of polymer blends. Prog. Polym. Sci. 2021, 123, 101471. [Google Scholar] [CrossRef]
- Xia, Z.; Riester, L.; Curtin, W.A.; Li, H.; Sheldon, B.W.; Liang, J.; Xu, J.M. Direct observation of toughening mechanisms in carbon nanotube ceramic matrix composites. Acta. Mater. 2004, 52, 931–944. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, S.; Wang, Q.; Xing, M. A comparison study on mechanical properties of polymer composites reinforced by carbon nanotubes and graphene sheet. Compos. Part B Eng. 2017, 133, 35–41. [Google Scholar] [CrossRef]
- Valentini, L.; Biagiotti, J.; Kenny, J.; Santucci, S. Morphological characterization of single-walled carbon nanotubes-PP composites. Compos. Sci. Technol. 2003, 63, 1149–1153. [Google Scholar] [CrossRef]
- Rezaei, F.; Yunus, R.; Ibrahim, N.A. Effect of fiber length on thermomechanical properties of short carbon fiber reinforced polypropylene composites. Mater. Des. 2009, 30, 260–263. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Iqbal, A.; Frormann, L. Melt mixing of carbon fibers and carbon nanotubes incorporated polyurethanes. J. Appl. Polym. Sci. 2008, 110, 196–202. [Google Scholar] [CrossRef]
- Karsli, N.G.; Aytac, A. Tensile and thermomechanical properties of short carbon fiber reinforced polyamide 6 composites. Compos. Part B Eng. 2013, 51, 270–275. [Google Scholar] [CrossRef]
- Jacob, M.; Francis, B.; Thomas, S.; Varughese, K. Dynamical mechanical analysis of sisal/oil palm hybrid fiber-reinforced natural rubber composites. Polym. Compos. 2006, 27, 671–680. [Google Scholar] [CrossRef]
- Pothan, L.A.; Oommen, Z.; Thomas, S. Dynamic mechanical analysis of banana fiber reinforced polyester composites. Compos. Sci. Technol. 2003, 63, 283–293. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.F. The tensile properties of HNO3 -treated carbon fiber reinforced ABS/PA6 composites. Surf. Interface Anal. 2009, 41, 610–614. [Google Scholar] [CrossRef]
- Li, J.; Cai, C. The carbon fiber surface treatment and addition of PA6 on tensile properties of ABS composites. Curr. Appl. Phys. 2011, 11, 50–54. [Google Scholar] [CrossRef]
- Lee, I.-G.; Kim, D.-H.; Jung, K.-H.; Kim, H.-J.; Kim, H.-S. Effect of the cooling rate on the mechanical properties of glass fiber reinforced thermoplastic composites. Compos. Struct. 2017, 177, 28–37. [Google Scholar] [CrossRef]
- Pötschke, P.; Fornes, T.; Paul, D. Rheological behavior of multiwalled carbon nanotube/polycarbonate composites. Polymer 2002, 43, 3247–3255. [Google Scholar] [CrossRef]
- Coleman, J.N.; Cadek, M.; Blake, R.; Nicolosi, V.; Ryan, K.P.; Belton, C.; Fonseca, A.; Nagy, J.B.; Gun’Ko, Y.K.; Blau, W.J. High performance nanotube-reinforced plastics: Understanding the mechanism of strength increase. Adv. Funct. Mater. 2004, 14, 791–798. [Google Scholar] [CrossRef]
- Assouline, E.; Lustiger, A.; Barber, A.H.; Cooper, C.A.; Klein, E.; Wachtel, E.; Wagner, H.D. Nucleation ability of multiwall carbon nanotubes in polypropylene composites. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 520–527. [Google Scholar] [CrossRef]
- Madhukar, M.S.; Drzal, L.T. Fiber-matrix adhesion and its effect on composite mechanical properties: II. Longitudinal (0) and transverse (90) tensile and flexure behavior of graphite/epoxy composites. J. Compos. Mater. 1991, 25, 958–991. [Google Scholar] [CrossRef]
- Regis, M.; Zanetti, M.; Pressacco, M.; Bracco, P. Opposite role of different carbon fiber reinforcements on the non-isothermal crystallization behavior of poly(etheretherketone). Mater. Chem. Phys. 2016, 179, 223–231. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Chen, X.; Li, C.; Shao, W. Isothermal crystallization kinetics and melting behaviour of PET/ATO nanocomposites prepared by in situ polymerization. Eur. Polym. J. 2007, 43, 3177–3186. [Google Scholar] [CrossRef]
- Alfonso, G.C.; Pedemonte, E.; Ponzetti, L. Mechanism of densification and crystal perfection of poly(ethylene terephthalate). Polymer 1979, 20, 104–112. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Ashrafi, B.; Naffakh, M.; González-Domínguez, J.M.; Johnston, A.; Simard, B.; Martínez, M.T. Influence of carbon nanotubes on the thermal, electrical and mechanical properties of poly(ether ether ketone)/glass fiber laminates. Carbon 2011, 49, 2817–2833. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Kim, J.-K. Cooling rate influences in carbon fibre/PEEK composites. Part 1. Crystallinity and interface adhesion. Compos. Part A Appl. Sci. Manuf. 2000, 31, 517–530. [Google Scholar] [CrossRef]
- Lu, X.F.; Hay, J.N. Isothermal crystallization kinetics and meeting behaviour of poly(ethylene terephthalate). Polymer 2001, 42, 9423–9431. [Google Scholar] [CrossRef]
- Antoniadis, G.; Paraskevopoulos, K.M.; Vassiliou, A.A.; Papageorgiou, G.Z.; Bikiaris, D.; Chrissafis, K. Nonisothermal melt-crystallization kinetics for in situ prepared poly(ethylene terephthalate)/monmorilonite (PET/OMMT). Thermochim. Acta 2011, 521, 161–169. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Zhou, Q.; Xu, S.; Wu, C. Non-isothermal crystallization kinetics of poly(ethylene terephthalate)/grafted carbon black composite. Polym. Bull. 2007, 59, 685–697. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I. Isoconversional analysis of combined melt and glass crystallization data. Macromol. Chem. Phys. 2007, 207, 20–25. [Google Scholar] [CrossRef]
- Qiao, L.; Zhu, K.; Tan, H.; Yan, X.; Zheng, L.; Dong, S. Effect of carbon nanotubes on the electrical, thermal, mechanical properties and crystallization behavior of continuous carbon fiber reinforced polyether-ether-ketone composites. Mater. Res. Express 2021, 8, 045312. [Google Scholar] [CrossRef]
- Anand, K.A.; Agarwal, U.; Joseph, R. Carbon nanotubes induced crystallization of poly(ethylene terephthalate). Polymer 2006, 47, 3976–3980. [Google Scholar] [CrossRef]
- Valentini, L.; Biagiotti, J.; Lopez-Manchado, M.A.; Santucci, S.; Kenny, J.M. Effects of carbon nanotubes on the crystallization behavior of polypropylene. Polym. Eng. Sci. 2004, 44, 303–311. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change, and microstructure: Kinetics of phase change. II. J. Chem. Phys. 1941, 9, 177. [Google Scholar] [CrossRef]
- Piorkowska, E.; Galeski, A.; Haudin, J.-M. Critical assessment of overall crystallization kinetics theories and predictions. Prog. Polym. Sci. 2006, 31, 549–575. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by DSC. Polymer 1978, 19, 1142. [Google Scholar] [CrossRef]
- Bianchi, O.; Oliveira, R.V.B.; Fiorio, R.; Martins, J.D.N.; Zattera, A.J.; Canto, L.B. Assessment of Avrami, Ozawa and Avrami–Ozawa equations for determination of EVA crosslinking kinetics from DSC measurements. Polym. Test. 2008, 27, 722–729. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Q.; Wang, Y.; Zhang, C.; Mo, Z.; Cao, S. Isothermal and non isothermal crystallization kinetics of nylon-11. J. Appl. Polym. Sci. 1998, 70, 2371–2380. [Google Scholar] [CrossRef]
- Gaonkar, A.A.; Murudkar, V.V.; Deshpande, V.D. Comparison of crystallization kinetics of polyethylene terephthalate (PET) and reorganized PET. Thermochim. Acta 2020, 683, 178472. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Gong, J.; Xu, J. The effect of Pglass state on the non-isothermal cold and melt crystallization processes of PET matrix. Thermochim. Acta 2015, 613, 1–8. [Google Scholar] [CrossRef]
- Yuan, Q.; Awate, S.; Misra, R. Nonisothermal crystallization behavior of polypropylene–clay nanocomposites. Eur. Polym. J. 2006, 42, 1994–2003. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, C.; Fan, X.; Mao, D.; Bian, Z. Nonisothermal crystallization kinetics of poly(butylene terephthalate)/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2006, 99, 3257–3265. [Google Scholar] [CrossRef]
- Tang, S.; Xin, Z. Structural effects of ionomers on the morphology, isothermal crystallization kinetics and melting behaviors of PET/ionomers. Polymer 2009, 50, 1054–1061. [Google Scholar] [CrossRef]
- Ke, Y.C.; Wu, T.B.; Xia, Y.F. The nucleation, crystallization and dispersion behavior of PET–monodisperse SiO2 composites. Polymer 2007, 48, 3324–3336. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Achilias, D.; Bikiaris, D.; Karayannidis, G. Isothermal and non-isothermal crystallization kinetics of branched and partially crosslinked PET: DSC study. J. Therm. Anal. Calorim. 2006, 4, 85–89. [Google Scholar] [CrossRef]
- Yu, F.; Xiao, L. Non-isothermal crystallization kinetics of poly(ether sulfone) functionalized graphene reinforced poly(ether ether ketone) composites. Polym. Test. 2021, 97, 107150. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional analysis of the nonisothermal crystallization of a polymer melt. Macromol. Rapid Common. 2002, 23, 766–770. [Google Scholar] [CrossRef]
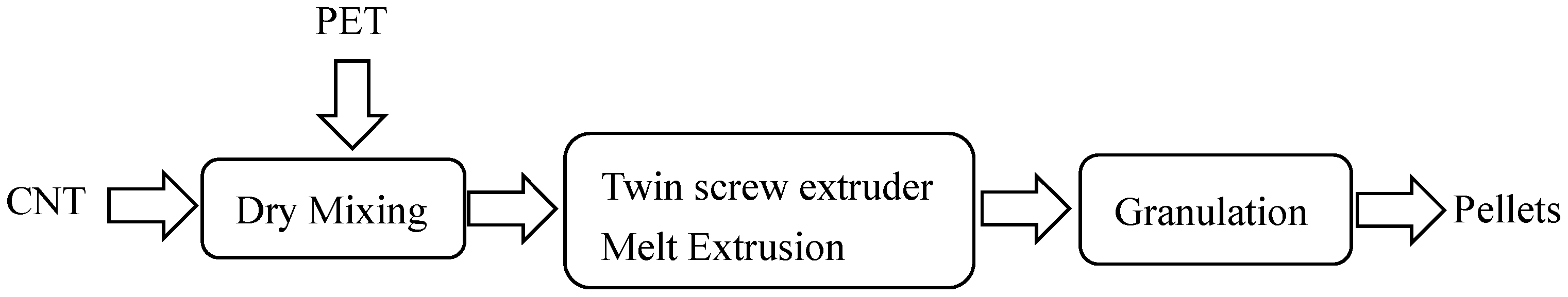





| Samples | Matrix (PET) (wt%) | Antioxidant (wt %) | Carbon Nanotube (wt%) | |
|---|---|---|---|---|
| 1010 | 168 | |||
| PET | 99.6 | 0.2 | 0.2 | — |
| CPET 1# | 99.5 | 0.2 | 0.2 | 0.1 |
| CPET 2# | 99.0 | 0.2 | 0.2 | 0.5 |
| CPET 3# | 98.6 | 0.2 | 0.2 | 1.0 |
| CPET 4# | 97.6 | 0.2 | 0.2 | 2.0 |
| Samples | Fiber Content (wt%) | CNT Content (wt%) |
|---|---|---|
| CCPET 1# | 36 | 0 |
| CCPET 2# | 56 | 0 |
| CCPET 3# | 56 | 0.1 |
| CCPET 4# | 56 | 1.0 |
| CCPET 5# | 56 | 2.0 |
| Samples | Tensile Strength (MPa) | Tensile Modulus (GPa) | Failure Strain (%) |
|---|---|---|---|
| CCPET 1# | 998.6 ± 29.5 | 11.8 ± 0.31 | 1.010 |
| CCPET 2# | 1567.1 ± 83.6 | 19.8 ± 0.27 | 0.850 |
| CCPET 3# | 1668.6 ± 56.8 | 20.9 ± 0.36 | 0.895 |
| CCPET 4# | 1728.7 ± 53.5 | 25.1 ± 0.22 | 0.721 |
| CCPET 5# | 1580.5 ± 72.4 | 21.9 ± 0.39 | 0.731 |
| Samples | Tm (°C) | ∆Hm (J/g) | Xc (%) |
|---|---|---|---|
| Pure PET | 242.6 | — | — |
| CCPET 1# | 248.7 | 24.79 | 17.7 |
| CCPET 2# | 249.1 | 23.14 | 16.5 |
| CCPET 3# | 248.6 | 26.32 | 18.8 |
| CCPET 4# | 248.5 | 28.14 | 20.1 |
| CCPET 5# | 248.8 | 21.15 | 15.1 |
| Samples | To (°C) | Tc (°C) |
|---|---|---|
| Pure PET | 242.6 | — |
| CCPET 1# | 248.1 | 24.79 |
| CCPET 2# | 249.1 | 23.14 |
| CCPET 3# | 247.6 | 24.92 |
| CCPET 4# | 248.5 | 25.19 |
| CCPET 5# | 248.8 | 21.15 |
| Samples | Φ/°C min−1 | n | Zc | t1/2/s |
|---|---|---|---|---|
| (56 wt%) CCF/PET | 5 | 3.67 | 0.002 | 153.28 |
| 10 | 3.59 | 0.194 | 86.43 | |
| 20 | 3.66 | 0.478 | 50.89 | |
| 40 | 3.71 | 0.712 | 35.26 | |
| (0.1 wt%) CNT/CCF/ PET | 5 | 6.50 | 0.001 | 193.55 |
| 10 | 6.87 | 0.021 | 112.48 | |
| 20 | 7.02 | 0.218 | 72.05 | |
| 40 | 7.72 | 0.476 | 44.38 | |
| (1.0 wt%) CNT/CCF/ PET | 5 | 6.59 | 0.001 | 276.33 |
| 10 | 6.89 | 0.033 | 130.38 | |
| 20 | 7.09 | 0.209 | 78.07 | |
| 40 | 7.36 | 0486 | 47.53 | |
| (2.0 wt%) CNT/CCF/ PET | 5 | 3.24 | 0.023 | 291.75 |
| 10 | 3.39 | 0.164 | 182.57 | |
| 20 | 3.53 | 0.439 | 93.68 | |
| 40 | 3.62 | 0.686 | 56.79 |
| α(%) | 10 | 30 | 50 | 70 | 90 | |
|---|---|---|---|---|---|---|
| Samples | Effective Activation Energy (kJ/mol) | |||||
| 56 wt% CCF/PET | −161.20 | −141.17 | −128.20 | −117.22 | −106.08 | |
| 0.1 wt% CNT/CCF/PET | −289.57 | −281.01 | −242.35 | −213.08 | −170.10 | |
| 1.0 wt% CNT/CCF/PET | −431.08 | −302.71 | −271.95 | −235.95 | −185.56 | |
| 2.0 wt% CNT/CCF/PET | −299.80 | −249.00 | −232.37 | −228.61 | −233.87 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, L.; Yan, X.; Tan, H.; Dong, S.; Ju, G.; Shen, H.; Ren, Z. Mechanical Properties, Melting and Crystallization Behaviors, and Morphology of Carbon Nanotubes/Continuous Carbon Fiber Reinforced Polyethylene Terephthalate Composites. Polymers 2022, 14, 2892. https://doi.org/10.3390/polym14142892
Qiao L, Yan X, Tan H, Dong S, Ju G, Shen H, Ren Z. Mechanical Properties, Melting and Crystallization Behaviors, and Morphology of Carbon Nanotubes/Continuous Carbon Fiber Reinforced Polyethylene Terephthalate Composites. Polymers. 2022; 14(14):2892. https://doi.org/10.3390/polym14142892
Chicago/Turabian StyleQiao, Liang, Xu Yan, Hongsheng Tan, Shuhua Dong, Guannan Ju, Hongwang Shen, and Zhaoying Ren. 2022. "Mechanical Properties, Melting and Crystallization Behaviors, and Morphology of Carbon Nanotubes/Continuous Carbon Fiber Reinforced Polyethylene Terephthalate Composites" Polymers 14, no. 14: 2892. https://doi.org/10.3390/polym14142892
APA StyleQiao, L., Yan, X., Tan, H., Dong, S., Ju, G., Shen, H., & Ren, Z. (2022). Mechanical Properties, Melting and Crystallization Behaviors, and Morphology of Carbon Nanotubes/Continuous Carbon Fiber Reinforced Polyethylene Terephthalate Composites. Polymers, 14(14), 2892. https://doi.org/10.3390/polym14142892






