The Preparation of Hydroxyl-Terminated Deproteinized Natural Rubber Latex by Photochemical Reaction Utilizing Nanometric TiO2 Depositing on Quartz Substrate as a Photocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of TiO2-Depositing Substrate
2.2.2. Preparation of DPNR
2.2.3. Photosensitivity of TiO2 Film by MB
2.2.4. Photosensitivity of TiO2 Film by DPNR
2.3. Characterizations
2.3.1. Crystallinity of TiO2 Film Deposited on the Substrates
2.3.2. Molecular Weight (MW) and Chemical Structure of the Rubber Samples
3. Results and Discussion
3.1. Characterization and Photosensitivity Determination of TiO2 Film
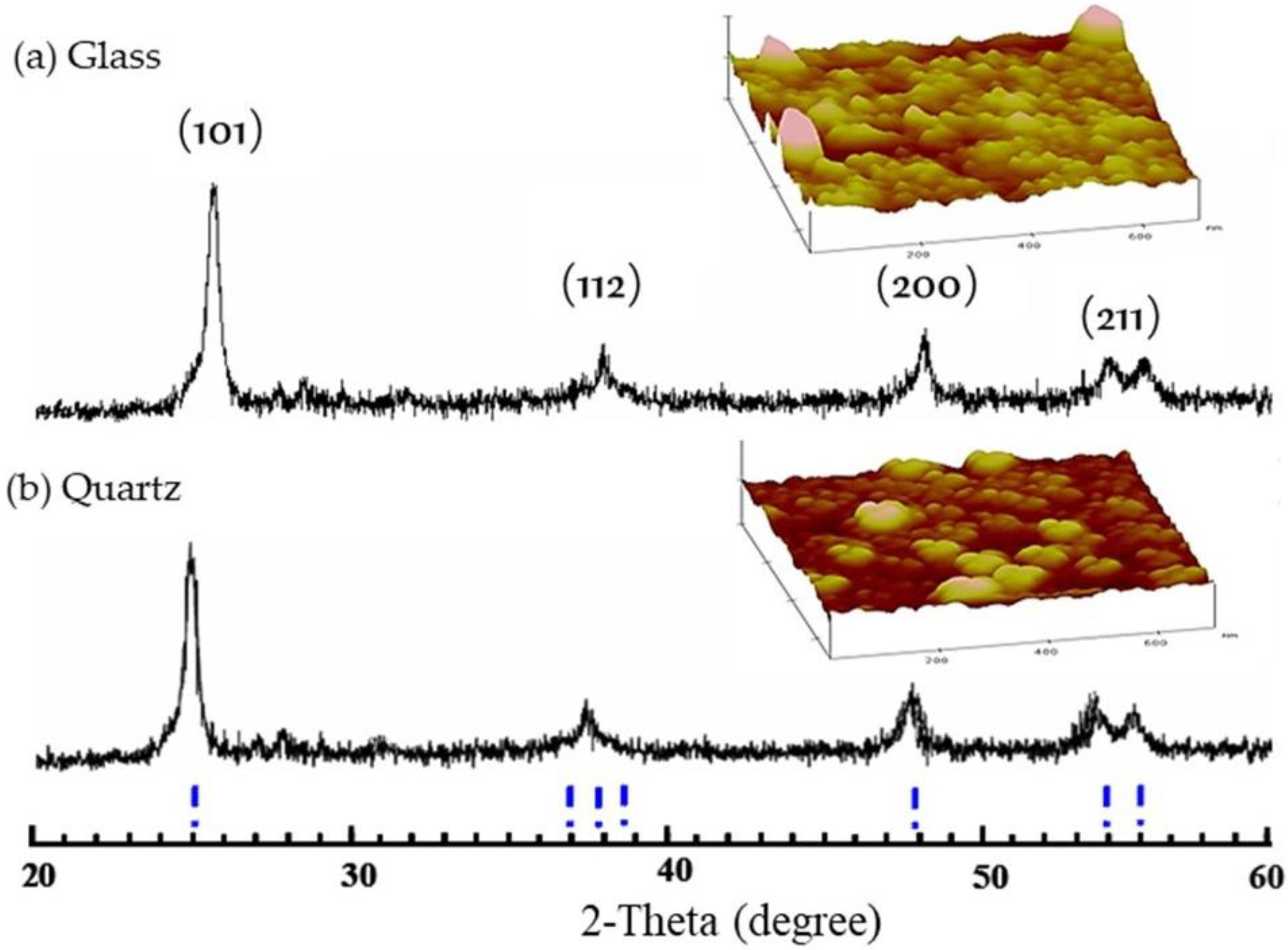
3.1.1. Structure Properties of TiO2 Film
3.1.2. Surface Topology of TiO2 Film
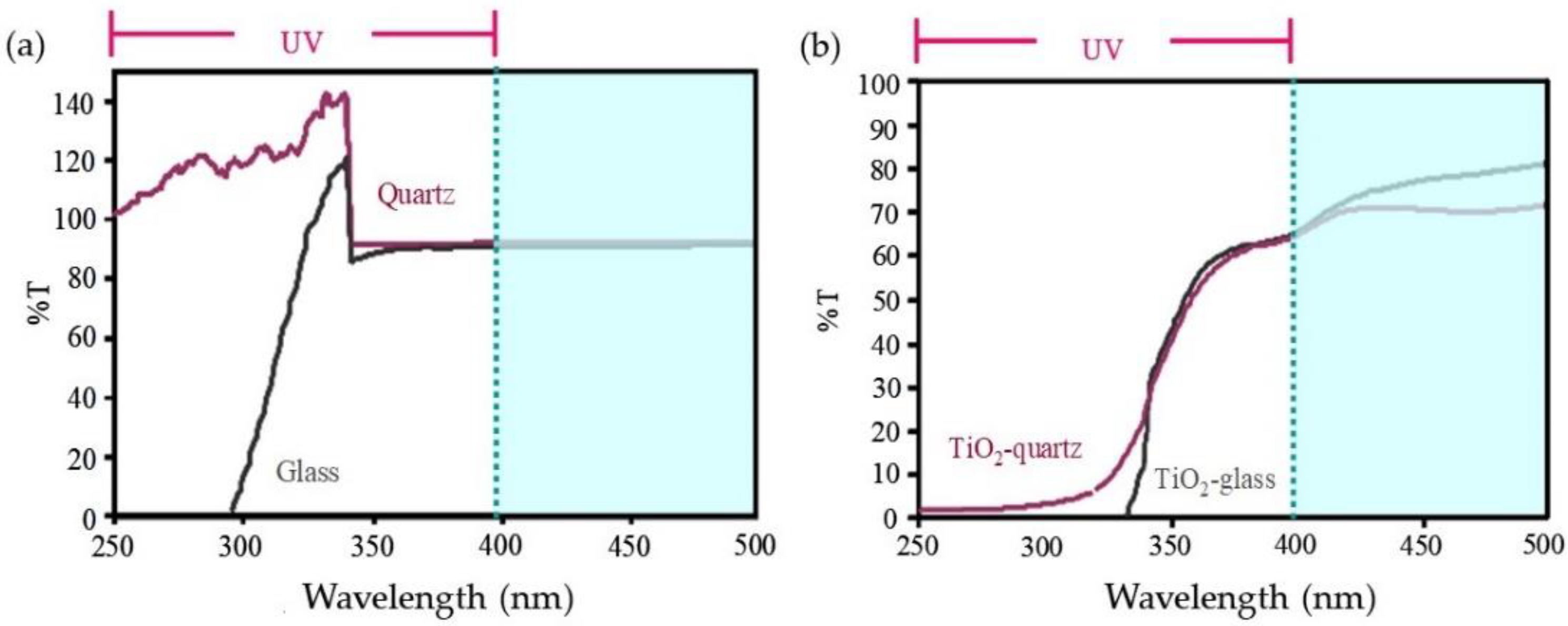
3.1.3. The Transmittance of the Substrate
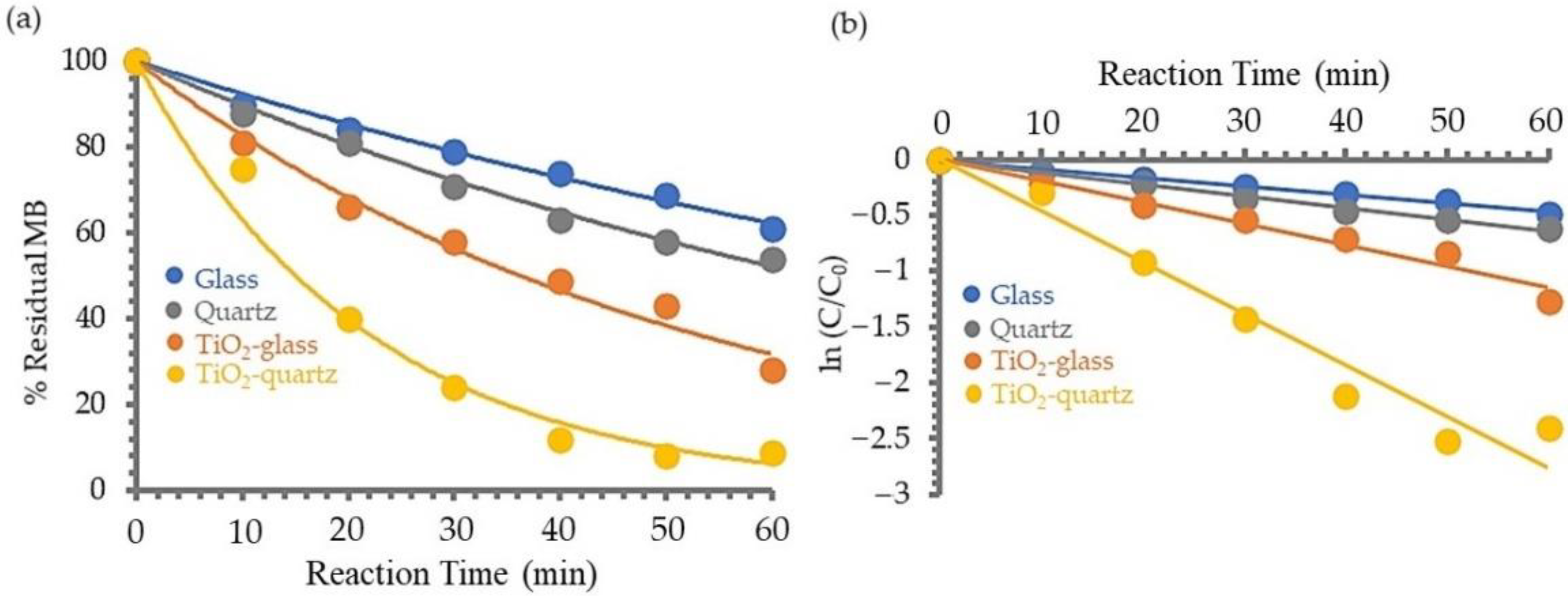
3.1.4. Photosensitivity of TiO2 Film on MB
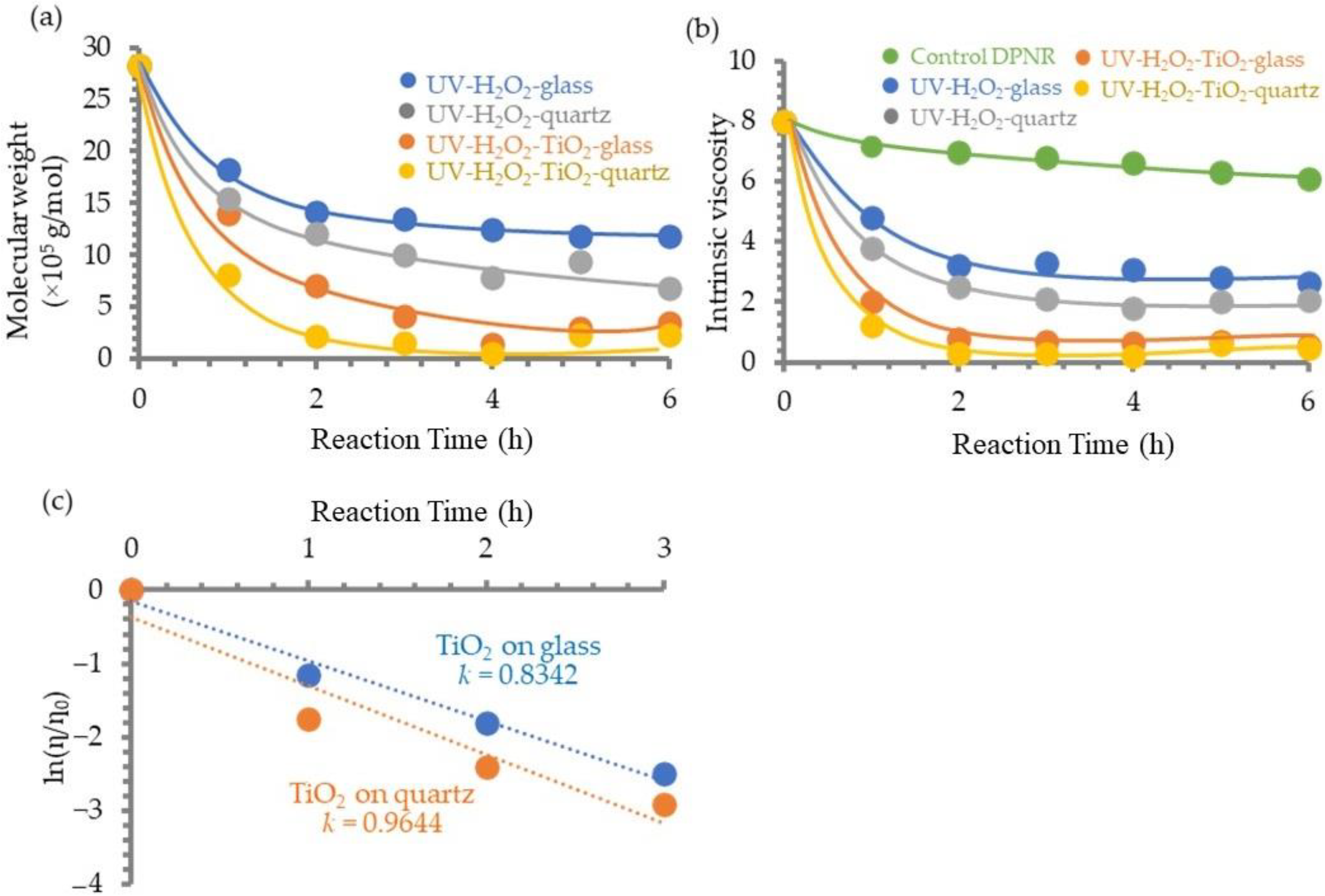
3.1.5. Photosensitivity of TiO2 Film on DPNR
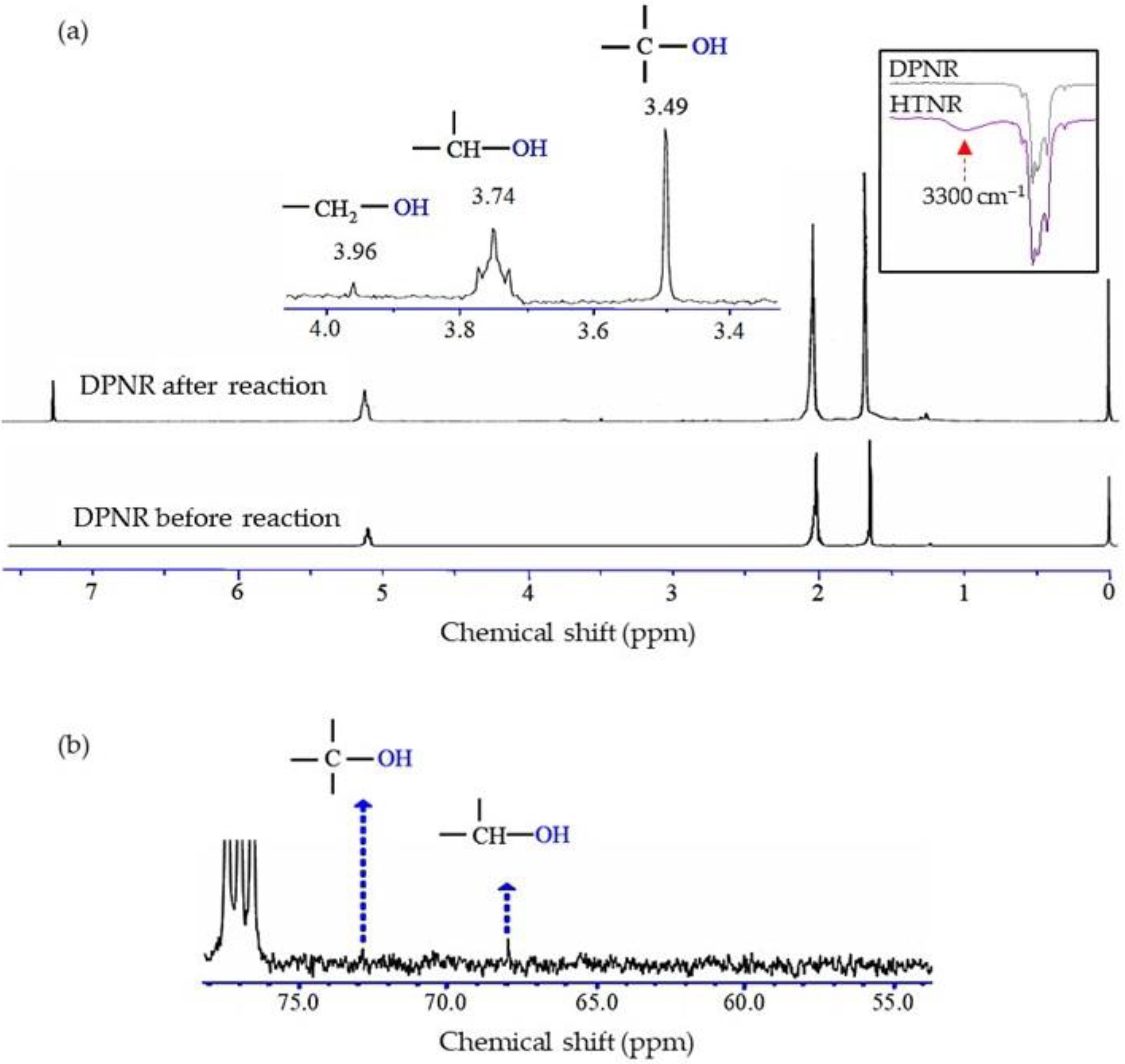
3.2. Structural Characterizations of HTNR
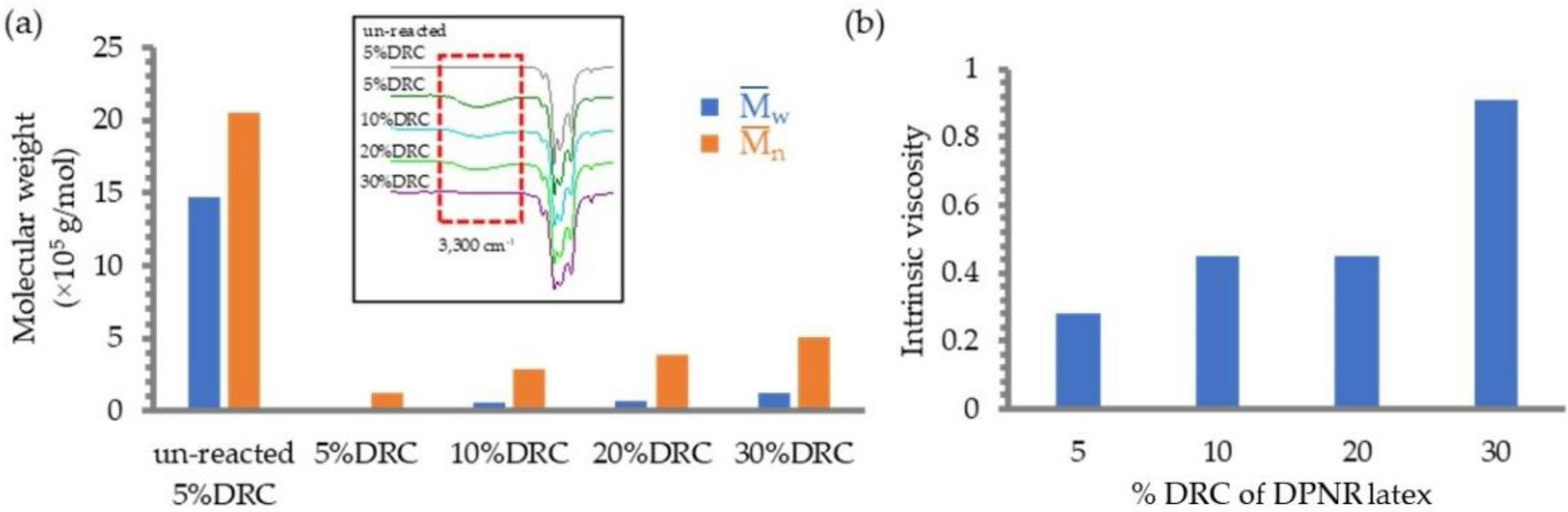
3.3. Effect of Latex Concentration (%DRC)
3.4. Effect of Surfactant
3.5. Study the Morphology of DPNR Particles by Scanning Electron Microscopy (SEM)
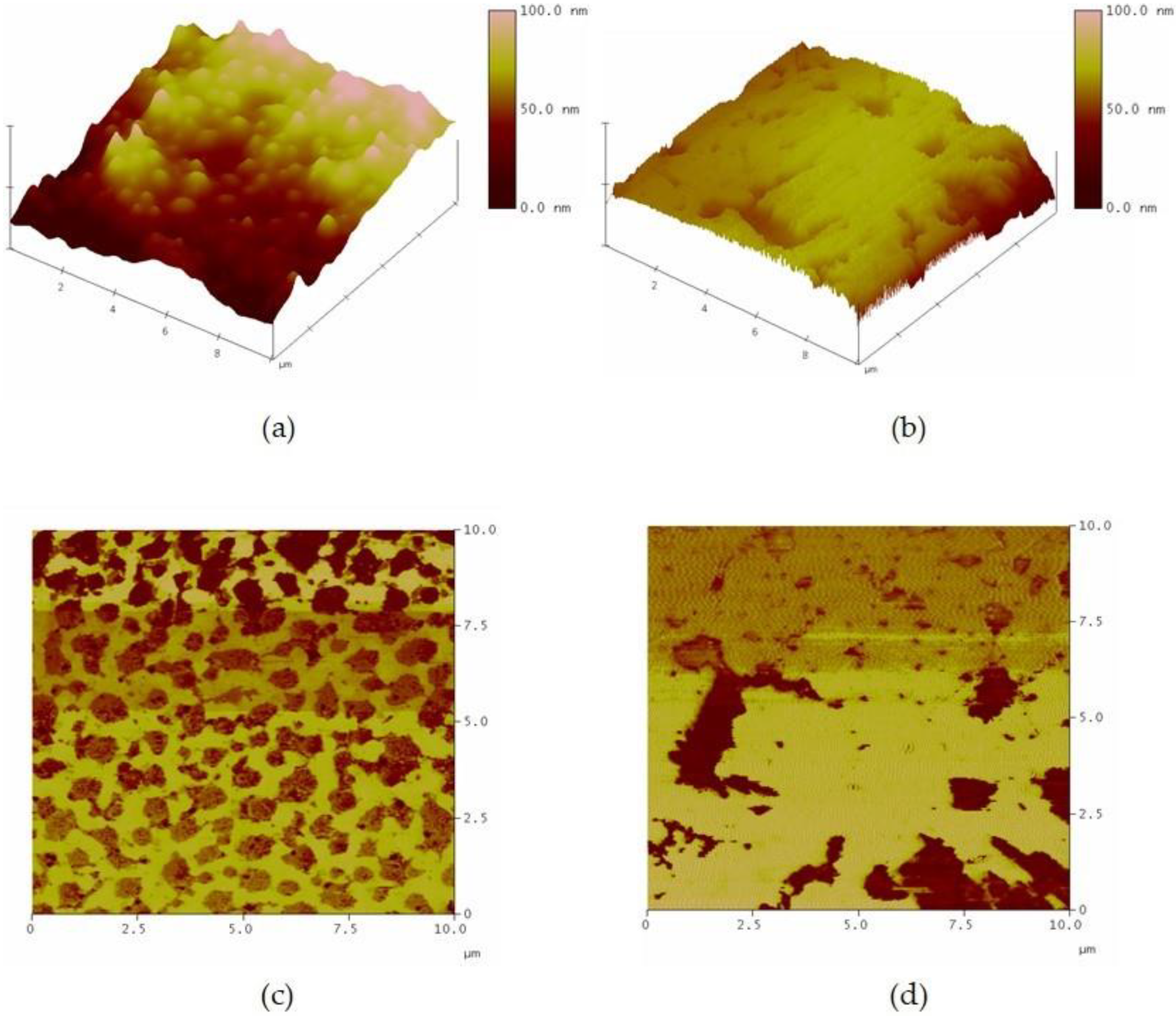
3.6. Characterization of the Morphology of the DPNR Particles by AFM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lake, G.J.; Samsuri, A.; Teo, S.C.; Vaja, J. Time-dependent fracture in vulcanized elastomers. Polymer 1991, 32, 2963–2975. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. Mechanical and thermal properties of natural rubber-modified poly(lactic acid) compatibilized with telechelic liquid natural rubber. Polym. Test. 2016, 54, 196–202. [Google Scholar] [CrossRef]
- Mounir, A.; Darwish, N.; Shehata, A. Effect of maleic anhydride and liquid natural rubber as compatibilizers on the mechanical properties and impact resistance of the NR-NBR blend. Polym. Adv. Technol. 2004, 15, 209–213. [Google Scholar] [CrossRef]
- Wayakron Phetphaisit, C.; Bumee, R.; Namahoot, J.; Ruamcharoen, J.; Ruamcharoen, P. Polyurethane polyester elastomer: Innovative environmental friendly wood adhesive from modified PETs and hydroxyl liquid natural rubber polyols. Int. J. Adhes. Adhes. 2013, 41, 127–131. [Google Scholar] [CrossRef]
- Kaenhin, L.; Klinpituksa, P.; Rungvichaniwat, A.; Pilard, J.F. Waterborne Polyurethane: Effect of functional groups in aromatic isocyanate and the chain length of hydroxyl terminated natural rubber. Adv. Mat. Res. 2011, 415–417, 2032–2035. [Google Scholar] [CrossRef]
- Ly, P.H. Reinforcement of natural rubber from hydroxyl-terminated liquid natural rubber grafted carbon black. I. Grafting of acyl chloride capped liquid natural rubber onto carbon black. J. Macromol. Sci. A 1996, 33, 1931–1937. [Google Scholar] [CrossRef]
- Duereh, A.; Boonchuay, C.; Buahom, P.; Areerat, S. Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes. Green Process Synth. 2019, 8, 288–296. [Google Scholar] [CrossRef]
- Sukhawipat, N.; Saetung, N.; Pilard, J.-F.; Bistac, S.; Saetung, A. Effects of molecular weight of hydroxyl telechelic natural rubber on novel cationic waterborne polyurethane: A new approach to water-based adhesives for leather applications. Int. J. Adhes. Adhes. 2020, 99, 102593. [Google Scholar] [CrossRef]
- Phetthong, C.; Nakaramontri, Y.; Marthosa, S.; Anancharoenwong, E. Influence of zirconium (IV) in polyurethane based on hydroxyl telechelic natural rubber for coating application. J. Coat. Technol. Res. 2021, 18, 1095–1107. [Google Scholar] [CrossRef]
- Zainudin, Z.; Baharulrazi, N.; Che Man, S.H. Natural rubber derivatives for adhesives applications: A review. Chem. Eng. Trans. 2021, 83, 493–498. [Google Scholar]
- Ibrahim, S.; Othman, N.; Sreekantan, S.; Tan, K.S.; Mohd Nor, Z.; Ismail, H. Preparation and characterization of low-molecular-weight natural rubber latex via photodegradation catalyzed by nano TiO2. Polymers 2018, 10, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radabutra, S.; Saengsuwan, S.; Jitchati, R.; Kalapat, M. Preparation and characterization of modified telechelic natural rubber based pressure-sensitive adhesive. J. Adhes. Sci. Technol. 2017, 31, 2682–2696. [Google Scholar] [CrossRef]
- Ibrahim, S.; Othman, N.; Ismail, H. Degradation of natural rubber latex. In Natural Rubber: Properties, Behavior and Applications; Hamilton, J.L., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 105–136. [Google Scholar]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO₂ photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kansal, S.K.; Kundu, P.; Sood, S.; Lamba, R.; Umar, A.; Mehta, S. K, Photocatalytic degradation of the antibiotic levofloxacin using highly crystalline TiO2 nanoparticles. New J. Chem. 2014, 38, 3220–3226. [Google Scholar] [CrossRef]
- Nijpanich, S.; Hagio, T.; Murase, K.; Park, J.-H.; Kamimoto, Y.; Sakdapipanich, J.; Terashima, C.; Chanlek, N.; Ichino, R.J. A tri-layer floating photocatalyst/adsorbent for the removal of organic compounds from wastewater: Layer-by-layer deposition of silicalite-1 and titania on hollow glass microspheres. Environ. Technol. Innov. 2022, 26, 102242. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Z.; Luo, D.; Zhao, M.; Zhao, G. A facial approach combining photosensitive sol-gel with self-assembly method to fabricate superhydrophobic TiO2 films with patterned surface structure. Appl. Surf. Sci. 2016, 360, 1030–1035. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Reviews, Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Hattori, A.; Shimoda, K.; Tada, H.; Ito, S. Photoreactivity of sol−gel TiO2 films formed on soda-lime glass substrates: Effect of SiO2 underlayer containing fluorine. Langmuir 1999, 15, 5422–5425. [Google Scholar] [CrossRef]
- Kang, S.; Lu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium dioxide: From engineering to applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Akelah, A.; Moet, A. Functionalized Polymers and Their Applications; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Nijpanich, S.; Nimpaiboon, A.; Sakdapipanich, J. Functionalization of styrene-butadiene rubber and skim latex by photo-catalytic reaction using nanometric TiO2 film as a photocatalyst. Key Eng. Mater. 2015, 659, 409–413. [Google Scholar] [CrossRef]
- Sakdapipanich, J.T.; Suksawad, P.; Insom, K.; Kawahara, S. Preparation of functionalized low molecular weight natural rubber latex using solid nanometric TiO3 film as a photocatalyst. Rubber Chem. Technol. 2005, 78, 597–605. [Google Scholar] [CrossRef]
- Nijpanich, S.; Nimpaiboon, A.; Rojruthai, P.; Sakdapipanich, J. Hydroxyl-terminated saponified natural rubber based on the H2O2/P25-TiO2 powder/UVC-irradiation system. Polymers 2021, 13, 1319. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, X.; Zhao, Q. Effect of film thickness on the grain size and photocatalytic activity of the sol-gel derived nanometer TiO2 thin films. J. Mater. Sci. Lett. 2000, 19, 1015–1017. [Google Scholar] [CrossRef]
- Habibi, M.H.; Mikhak, M. Titania/zinc oxide nanocomposite coatings on glass or quartz substrate for photocatalytic degradation of direct blue 71. Appl. Surf. Sci. 2012, 258, 6745–6752. [Google Scholar] [CrossRef]
- Damkale, S.R.; Arbuj, S.S.; Umarji, G.G.; Rane, S.B.; Kale, B.B. Highly crystalline anatase TiO2 nanocuboids as an efficient photocatalyst for hydrogen generation. RSC Adv. 2021, 11, 7587–7599. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, D.; Jia, G.; Wang, B.; Yang, Z.; Yang, Z.; Qiao, H.; Zhang, Y.; Zheng, Z. Hydrofluoric acid controlled TiO2 phase transformation from rutile to anatase at room temperature and their photocatalytic performance. J. Nanosci. Nanotechnol. 2015, 15, 6636–6641. [Google Scholar] [CrossRef]
- Kandil, U.F. Novel Functional Ethylene/Propylene Elastomers: Synthesis and Characterization. Ph.D. Thesis, Pennsylvania State University, University Park, PA, USA, 2005. [Google Scholar]
- Herrmann, J.M.; Duchamp, C.; Karkmaz, M.; Hoai, B.T.; Lachheb, H.; Puzenat, E.; Guillard, C. Environmental green chemistry as defined by photocatalysis. J. Hazard. Mater. 2007, 146, 624–629. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillapasuwan, A.; Saekhow, P.; Rojruthai, P.; Sakdapipanich, J. The Preparation of Hydroxyl-Terminated Deproteinized Natural Rubber Latex by Photochemical Reaction Utilizing Nanometric TiO2 Depositing on Quartz Substrate as a Photocatalyst. Polymers 2022, 14, 2877. https://doi.org/10.3390/polym14142877
Sillapasuwan A, Saekhow P, Rojruthai P, Sakdapipanich J. The Preparation of Hydroxyl-Terminated Deproteinized Natural Rubber Latex by Photochemical Reaction Utilizing Nanometric TiO2 Depositing on Quartz Substrate as a Photocatalyst. Polymers. 2022; 14(14):2877. https://doi.org/10.3390/polym14142877
Chicago/Turabian StyleSillapasuwan, Apisara, Phattharawadi Saekhow, Porntip Rojruthai, and Jitladda Sakdapipanich. 2022. "The Preparation of Hydroxyl-Terminated Deproteinized Natural Rubber Latex by Photochemical Reaction Utilizing Nanometric TiO2 Depositing on Quartz Substrate as a Photocatalyst" Polymers 14, no. 14: 2877. https://doi.org/10.3390/polym14142877
APA StyleSillapasuwan, A., Saekhow, P., Rojruthai, P., & Sakdapipanich, J. (2022). The Preparation of Hydroxyl-Terminated Deproteinized Natural Rubber Latex by Photochemical Reaction Utilizing Nanometric TiO2 Depositing on Quartz Substrate as a Photocatalyst. Polymers, 14(14), 2877. https://doi.org/10.3390/polym14142877






