Starch-Based Hydrogel Nanoparticles Loaded with Polyphenolic Compounds of Moringa Oleifera Leaf Extract Have Hepatoprotective Activity in Bisphenol A-Induced Animal Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Preparation of MOLE
2.3. Fabrication of Encapsulated MOLE
2.4. Extraction and Identification of Phenolic Compound Using HPLC
2.5. Characterization Techniques of Nanoparticles
2.5.1. Measurement of Zeta Potential
2.5.2. Transmission Electron Microscopy (TEM)
2.5.3. Absorbance and Fluorescence Spectrophotometers
2.5.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Animals and Ethical Approval
2.7. Experimental Diet
2.8. Experimental Design and Sampling
2.8.1. Concentration of BPA
2.8.2. Experimental Design
2.9. Homogenization of Liver Tissue
2.10. Biochemical Indices in Liver Tissue Homogenate and Sera
2.10.1. Antioxidant Enzymes
2.10.2. Lipid Profile
2.10.3. Liver Function Biomarkers
2.11. ELISA for Caspase-3 and Bax Detection
2.12. RNA Extraction
2.13. Histopathological Examination
2.14. Biostatistics
3. Results
3.1. HPLC Identification and Quantification
3.2. Characterization
3.3. In Vivo Studies
3.3.1. Liver Functions
3.3.2. Oxidative Stress Markers
3.3.3. Evaluations of Lipid Profile Status
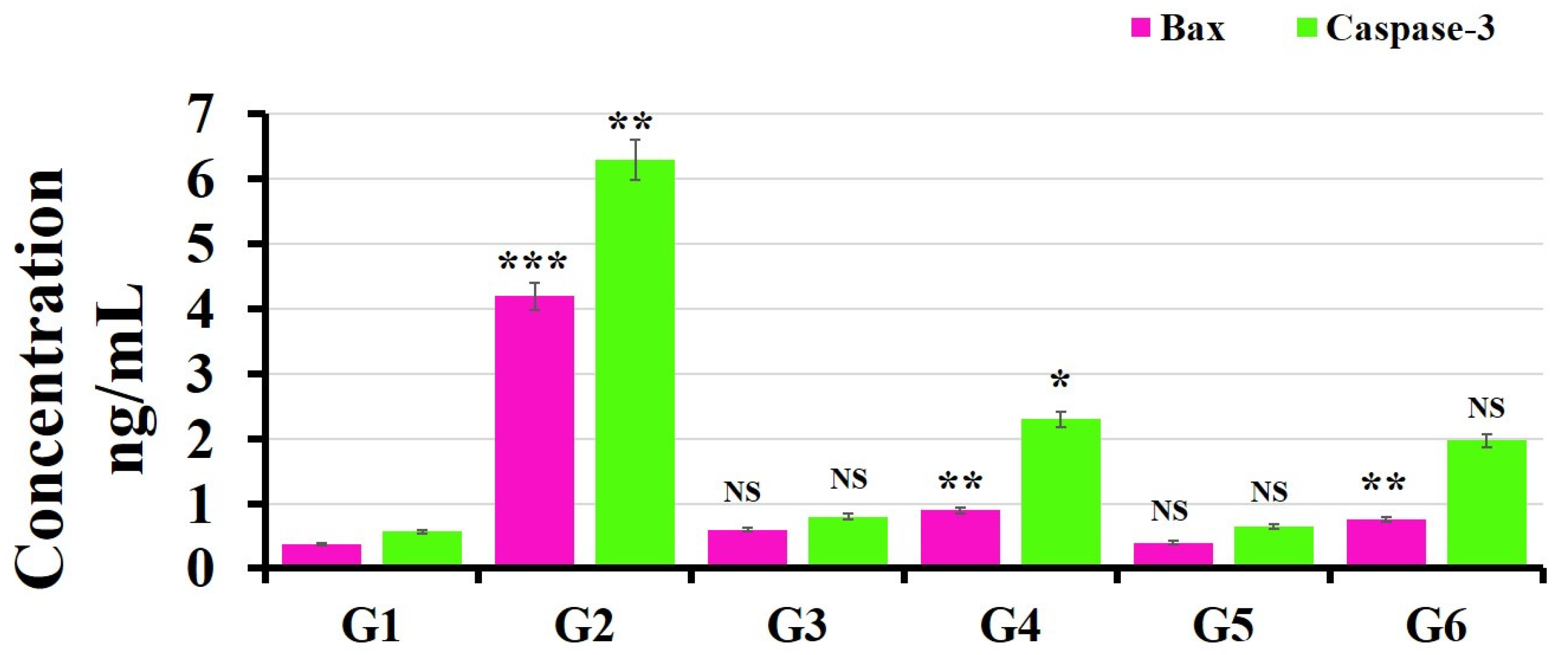
3.4. ELISA Kits for Caspase-3 and Bax Detection
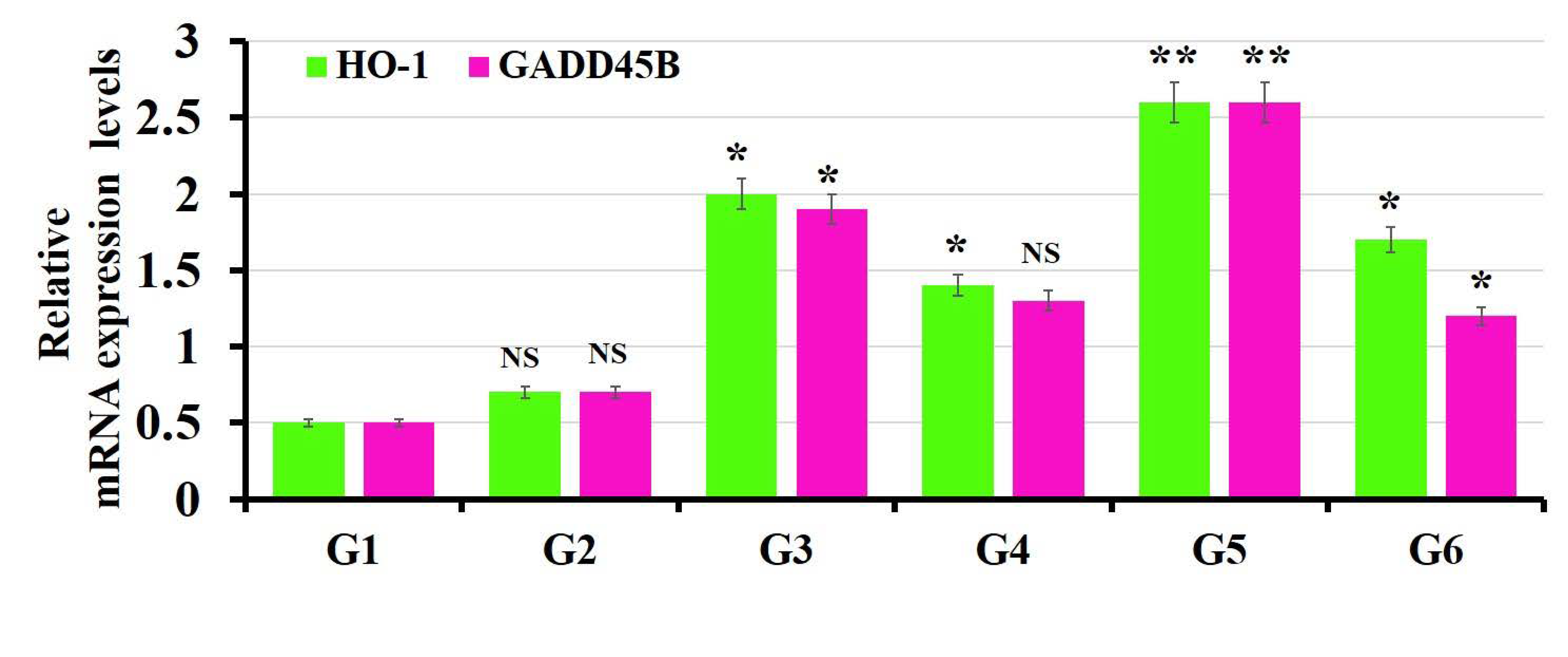
3.5. The Findings of Real-Time PCR
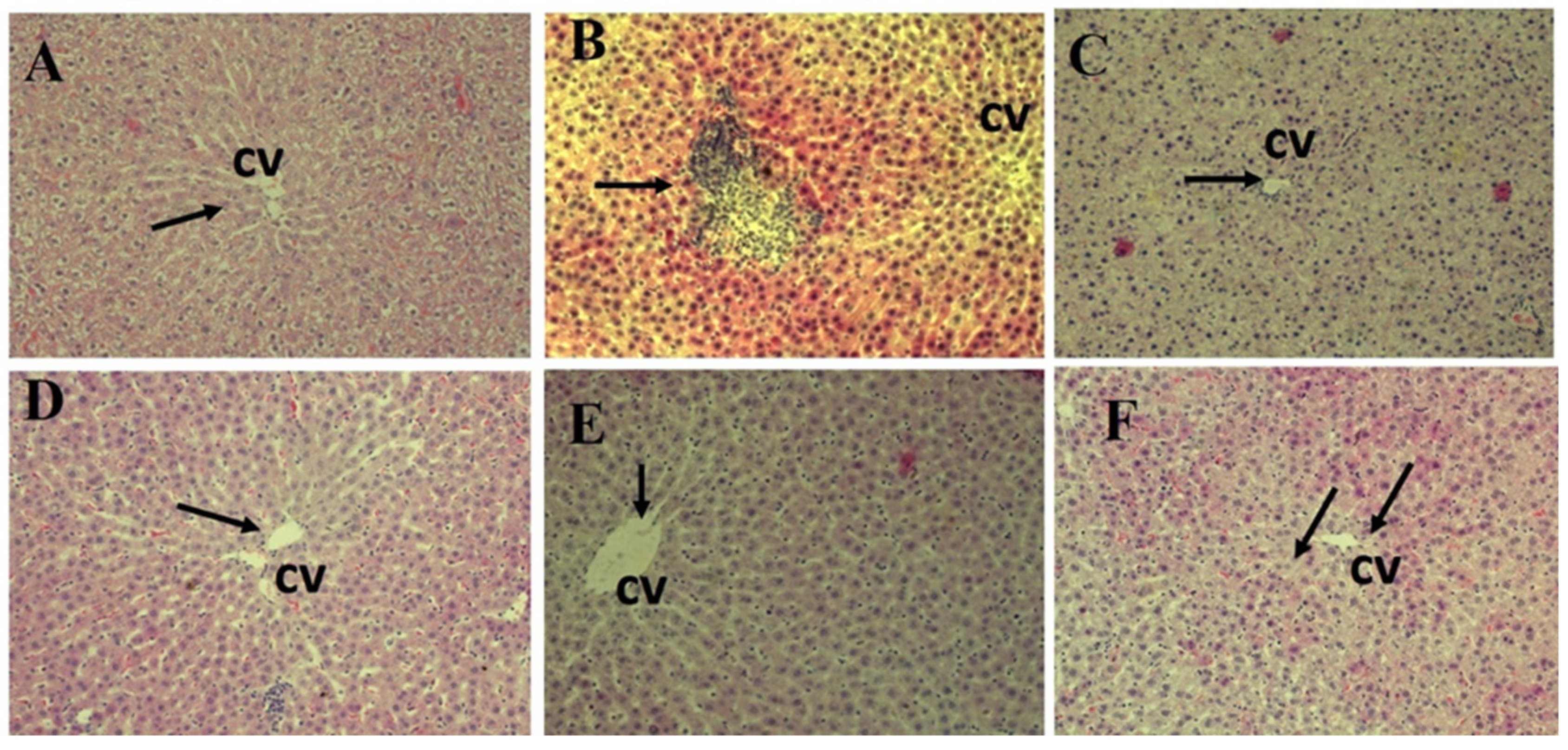
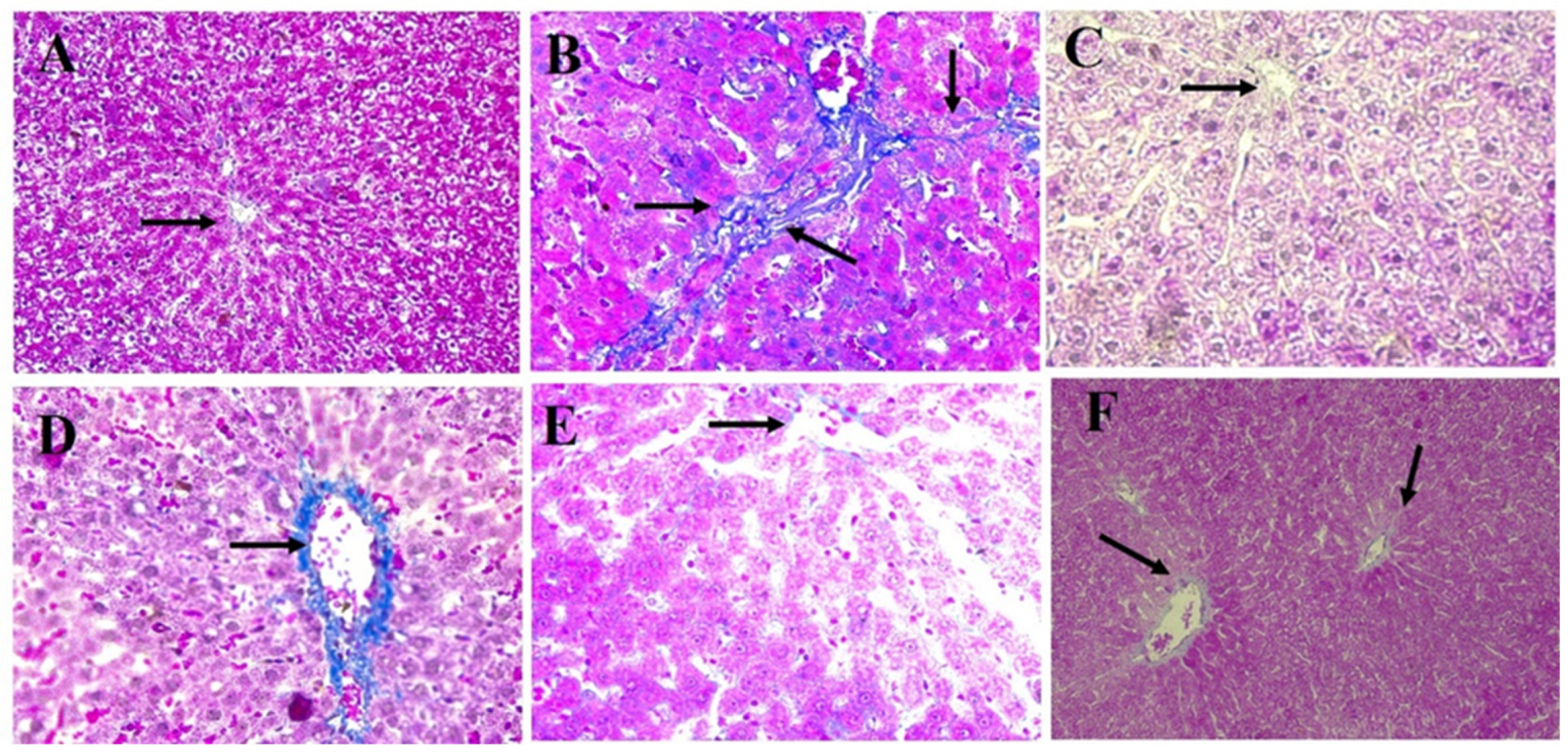
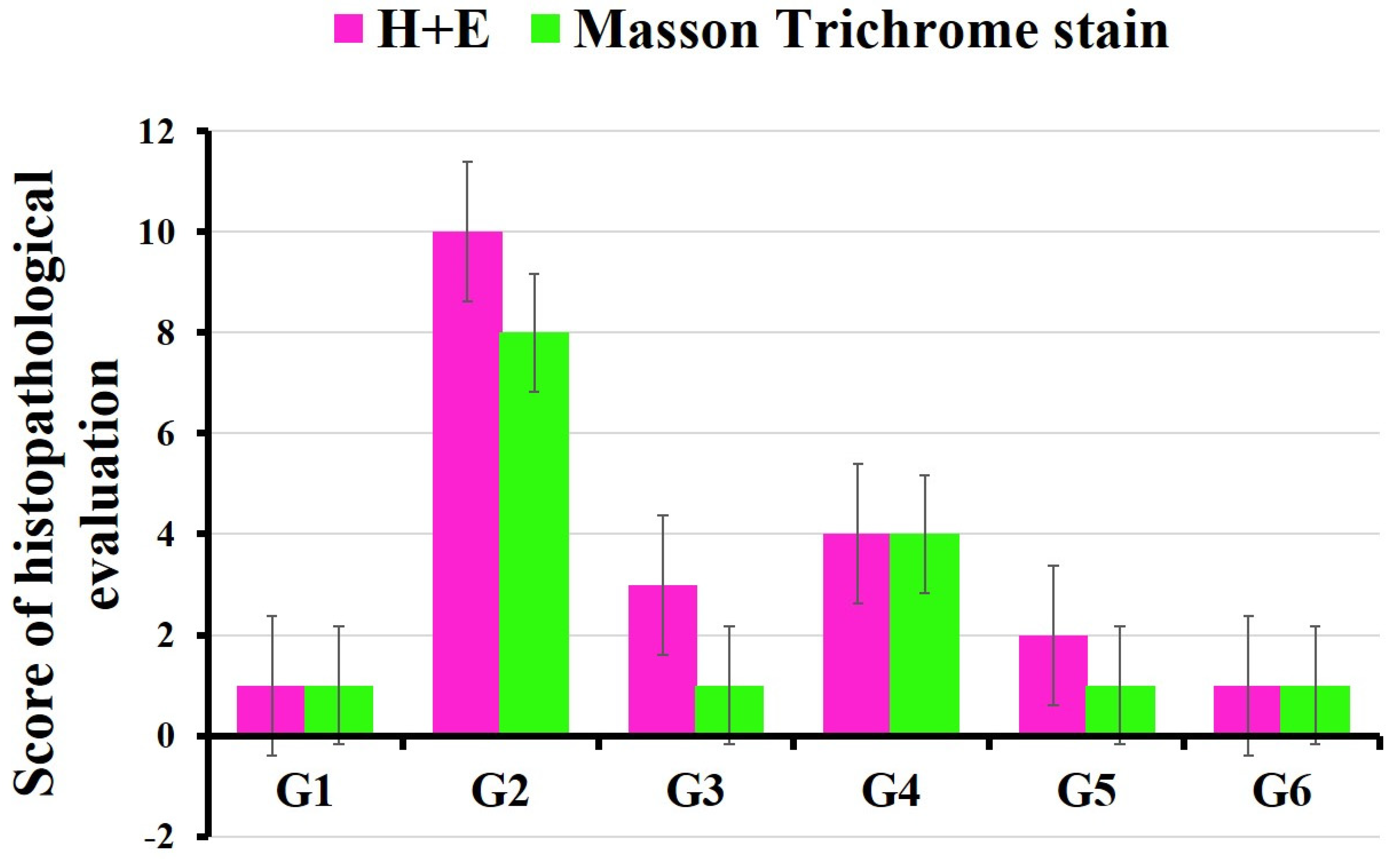
3.6. Histopathology Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Chiang, J. Liver Physiology: Metabolism and Detoxification. Pathobiol. Hum. Dis. 2014, 1770–1782. [Google Scholar] [CrossRef]
- Wahlang, B.; Jin, J.; Beier, J.I.; Hardesty, J.E.; Daly, E.F.; Schnegelberger, R.D.; Falkner, K.C.; Prough, R.A.; A Kirpich, I.; Cave, M.C. Mechanisms of Environmental Contributions to Fatty Liver Disease. Curr. Environ. Health Rep. 2019, 6, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Rytel, L.; Nowicka, N.; Wojtkiewicz, J. The state of bisphenol research in the lesser developed countries of the EU: A mini-review. Toxicol. Res. 2018, 7, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodson, A.; Robin, H.; Summerfield, W.; Cooper, I. Migration of bisphenol A from can coatings—Effects of damage, storage conditions and heating. Food Addit. Contam. 2004, 21, 1015–1026. [Google Scholar] [CrossRef]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [Green Version]
- Cantonwine, D.E.; Hauser, R.; Meeker, J.D. Bisphenol A and human reproductive health. Expert Rev. Obstet. Gynecol. 2013, 8, 329–335. [Google Scholar] [CrossRef]
- Inadera, H. Neurological Effects of Bisphenol A and its Analogues. Int. J. Med Sci. 2015, 12, 926–936. [Google Scholar] [CrossRef] [Green Version]
- Thoene, M.; Rytel, L.; Dzika, E.; Włodarczyk, A.; Kruminis-Kaszkiel, E.; Konrad, P.; Wojtkiewicz, J. Bisphenol A Causes Liver Damage and Selectively Alters the Neurochemical Coding of Intrahepatic Parasympathetic Nerves in Juvenile Porcine Models under Physiological Conditions. Int. J. Mol. Sci. 2017, 18, 2726. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Mbikay, M. Therapeutic Potential of Moringa oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front. Pharmacol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, A.A.A.; Fayed, A.M.; Attia, T.A.; Elbatrna, S.A.; Ismaiel, E.I.; Hassan, A. Protective Effects of Moringa oleifera extract on Isoniazid and Rifampicin Induced Hepatotoxicity in Rats: Involvement of Adiponectin and Tumor Necrosis Factor-α. Egypt. J. Vet. Sci. 2018, 49, 25–34. [Google Scholar]
- Alia, F.; Putri, M.; Anggraeni, N.; Yamsunarno, M.R.A.A. The Potency of Moringa oleifera Lam. as Protective Agent in Cardiac Damage and Vascular Dysfunction. Front. Pharmacol. 2022, 12, 724439. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.S.; Argolo, A.C.C.; Paiva, P.M.G.; Coelho, L.C.B.B. Antioxidant Activity of Moringa oleifera Tissue Extracts. Phytother. Res. 2012, 26, 1366–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakurazi, S.; Sharifudin, S.A.; Arulselvan, P. Moringa oleifera Hydroethanolic Extracts Effectively Alleviate Acetaminophen-Induced Hepatotoxicity in Experimental Rats through Their Antioxidant Nature. Molecules 2012, 17, 8334–8350. [Google Scholar] [CrossRef]
- Osamede Airouyuwa, J.; Kaewmanee, T. Microencapsulation of Moringa oleifera leaf extracts with vegetable protein as wall materials. Food Sci. Technol. Int. 2019, 25, 533–543. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501, Erratum in Front. Microbiol. 2017, 8, 2517. [Google Scholar] [CrossRef] [Green Version]
- Labelle, M.; Ispas-Szabo, P.; Mateescu, M.A. Structure-Functions Relationship of Modified Starches for Pharmaceutical and Biomedical Applications. Starch Stärke 2020, 72, 2000002. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanafy, N.A.N.; Quarta, A.; Di Corato, R.; Dini, L.; Nobile, C.; Tasco, V.; Carallo, S.; Cascione, M.; Malfettone, A.; Soukupova, J.; et al. Hybrid polymeric-protein nano-carriers (HPPNC) for targeted delivery of TGFβ inhibitors to hepatocellular carcinoma cells. J. Mater. Sci. Mater. Med. 2017, 28, 120. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk Zayed, M.M.; Sahyon, H.A.; Hanafy, N.A.N.; El-Kemary, M.A. The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53. Pharmaceutics 2022, 14, 1160. [Google Scholar] [CrossRef] [PubMed]
- Nayak, G.; Honguntikar, S.D.; Kalthur, S.G.; D’Souza, A.S.; Mutalik, S.; Setty, M.M.; Kalyankumar, R.; Krishnamurthy, H.; Kalthur, G.; Adiga, S.K. Ethanolic extract of Moringa oleifera Lam. leaves protect the pre-pubertal spermatogonial cells from cyclophosphamide-induced damage. J. Ethnopharmacol. 2016, 182, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N. Starch based hydrogel NPs loaded by anthocyanins might treat glycogen storage at cardiomyopathy in animal fibrotic model. Int. J. Biol. Macromol. 2021, 183, 171–181. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Extraction of chlorophyll and carotenoids loaded into chitosan as potential targeted therapy and bio imaging agents for breast carcinoma. Int. J. Biol. Macromol. 2021, 182, 1150–1160. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.A. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef]
- Simon, S.; Joseph, J.; George, D. Optimization of extraction parameters of bioactive components from Moringa oleifera leaves using Taguchi method. Biomass Convers. Biorefinery 2022, 1–10. [Google Scholar] [CrossRef]
- El-Hashash, S.A.; El-Sakhawy, M.A.; El-Nahass, E.E.; Abdelaziz, M.A.; Abdelbasset, W.K.; Elwan, M.M. Prevention of Hepatorenal Insufficiency Associated with Lead Exposure by Hibiscus sabdariffa L. Beverages Using In Vivo Assay. BioMed Res. Int. 2022, 2022, 7990129. [Google Scholar] [CrossRef]
- Hass, U.; Christiansen, S.; Boberg, J.; Rasmussen, M.G.; Mandrup, K.; Axelstad, M. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology 2016, 4, 594–607. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Ehrlich, S.; Belcher, S.M.; Ben-Jonathan, N.; Dolinoy, D.C.; Hugo, E.R.; Hunt, P.A.; Newbold, R.R.; Rubin, B.S.; Saili, K.S.; et al. Low dose effects of bisphenol A: An integrated review of in vitro, laboratory animal, and epidemiology studies. Endocr. Disruptors 2013, 1, e26490. [Google Scholar] [CrossRef]
- Ahmed, M.S.W.; Moselhy, A.W.; Nabil, T.M. Bisphenol A Toxicity in Adult Male Rats: Hematological, Biochemical and Histopathological Approach. Glob. Vet. 2015, 14, 228–238. [Google Scholar]
- Sahu, C.; Singla, S.; Jena, G. Studies on male gonadal toxicity of bisphenol A in diabetic rats: An example of exacerbation effect. J. Biochem. Mol. Toxicol. 2022, 36, e22996. [Google Scholar] [CrossRef] [PubMed]
- Doshi, T.; D’Souza, C.; Dighe, V.; Vanage, G. Effect of neonatal exposure on male rats to bisphenol a on the expression of DNA methylation machinery in the postimplantation embryo. J. Biochem. Mol. Toxicol. 2012, 26, 337–343. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Richmond, N. Enzymatic colorimetric test for cholesterol determination. Clin. Chem. 1973, 19, 1350–1356. [Google Scholar] [CrossRef]
- Jacobs, N.J.; VanDenmark, P.J. Enzymatic colorimetric determination of triglycerides. Arch. Biochem. Biophys. 1960, 88, 250–255. [Google Scholar] [CrossRef]
- Ray, T.K.; Skipski, V.P.; Barclay, M.; Essner, E.; Archibald, F.M. Lipid Composition of Rat Liver Plasma Membranes. J. Biol. Chem. 1969, 244, 5528–5536. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for determination of oxaloacetic transaminase and serum glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957, 28, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Kind, P.R.N.; King, E.J. Estimation of Plasma Phosphatase by Determination of Hydrolysed Phenol with Amino-antipyrine. J. Clin. Pathol. 1954, 7, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Somade, O.T.; Ajayi, B.O.; Olunaike, O.E.; Jimoh, L.A. Hepatic oxidative stress, up-regulation of pro-inflammatory cytokines, apoptotic and oncogenic markers following 2-methoxyethanol administrations in rats. Biochem. Biophys. Rep. 2020, 24, 100806. [Google Scholar] [CrossRef]
- Kazemi, S.; Mousavi, S.N.; Aghapour, F.; Rezaee, B.; Sadeghi, F.; Moghadamnia, A.A. Induction Effect of Bisphenol A on Gene Expression Involving Hepatic Oxidative Stress in Rat. Oxidative Med. Cell. Longev. 2016, 2016, 6298515. [Google Scholar] [CrossRef] [Green Version]
- Safer, A.M.; Afzal, M.; Hanafy, N.; Sosamma, O.; Mousa, S.A. Curative propensity of green tea extract towards hepatic fibrosis induced by CCl4: A histopathological study Corrigendum in /etm/10/2/835. Exp. Ther. Med. 2012, 3, 781–786. [Google Scholar] [CrossRef] [Green Version]
- Safer, A.; Afzal, M.; Hanafy, N.; Mousa, S. Green tea extract therapy diminishes hepatic fibrosis mediated by dual exposure to carbon tetrachloride and ethanol: A histopathological study Corrigendum in /etm/10/3/1239. Exp. Ther. Med. 2015, 9, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Olasehinde, T.A.; Oyeleye, S.I.; Boligon, A.A.; Athayde, M.L. Phenolic Extract from Moringa oleifera Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Oxidative Stress in Rats’ Penile Tissues. Biochem. Res. Int. 2015, 2015, 175950. [Google Scholar] [CrossRef] [Green Version]
- Fattah, M.E.A.; Sobhy, H.M.; Reda, A.; Abdelrazek, H.M.A. Hepatoprotective effect of Moringa oleifera leaves aquatic extract against lead acetate–induced liver injury in male Wistar rats. Environ. Sci. Pollut. Res. 2020, 27, 43028–43043. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinozzi, F.; Ferrero, C.; Perez, S. The architecture of starch blocklets follows phyllotaxic rules. Sci. Rep. 2020, 10, 20093. [Google Scholar] [CrossRef] [PubMed]
- Sit, N.; Deka, S.C.; Misra, S. Optimization of starch isolation from taro using combination of enzymes and comparison of properties of starches isolated by enzymatic and conventional methods. J. Food Sci. Technol. 2014, 52, 4324–4332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniz, P.H.G.D.; Barbosa, M.F.; De Melo Milanez, K.D.T.; Pistonesi, M.F.; de Araújo, M.C.U. Using UV–Vis spectroscopy for simultaneous geographical and varietal classification of tea infusions simulating a home-made tea cup. Food Chem. 2016, 192, 374–379 . [Google Scholar] [CrossRef] [PubMed]
- Bronze-Uhle, E.; Costa, B.C.; Ximenes, V.F.; Lisboa-Filho, P.N. Synthetic nanoparticles of bovine serum albumin with entrapped salicylic acid. Nanotechnol. Sci. Appl. 2016, 10, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani, H.; Behzad, T.; Bagheri, R. Synergistic effect of graphene oxide nanoplatelets and cellulose nanofibers on mechanical, thermal, and barrier properties of thermoplastic starch. Polym. Adv. Technol. 2020, 31, 553–565. [Google Scholar] [CrossRef]
- El-Houssiny, A.S.; Fouad, E.A.; Hegazi, A.G. A Comparative Antimicrobial Activity Study of Moringa oleifera Extracts Encapsulated within ALg Nanoparticles. Nanosci. Nanotechnol.-Asia 2021, 11, 144–152. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, A.B. Preparation and characterization of BSA as a model protein loaded chitosan nanoparticles for the development of protein-/peptide-based drug delivery system. Future J. Pharm. Sci. 2021, 7, 200. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elobeid, M.A.; Virk, P.; Omer, S.A.; ElAmin, M.; Daghestani, M.H.; AlOlayan, E.M. Bisphenol A Induces Hepatotoxicity through Oxidative Stress in Rat Model. Oxidative Med. Cell. Longev. 2012, 2012, 194829. [Google Scholar] [CrossRef] [Green Version]
- Olukole, S.G.; Ola-Davies, E.O.; Lanipekun, D.O.; Oke, B.O. Chronic exposure of adult male Wistar rats to bisphenol A causes testicular oxidative stress: Role of gallic acid. Endocr. Regul. 2020, 54, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Fakurazi, S.; Hairuszah, I.; Nanthini, U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem. Toxicol. 2008, 46, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Methods 2015, 25, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, S.; Zhao, Z.; Chen, Y.; Xu, Y.; Li, M.; Xu, M.; Wang, W.; Ning, G.; Bi, Y.; et al. Bisphenol A exposure in relation to altered lipid profile and dyslipidemia among Chinese adults: A repeated measures study. Environ. Res. 2020, 184, 109382. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Balaraman, R.; Amin, A.H.; Bafna, P.A.; Gulati, O.D. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef]
- Mallat, Z.; Tedgui, A. Apoptosis in the vasculature: Mechanisms and functional importance. Br. J. Pharmacol. 2000, 130, 947–962. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Jiang, Y.; Li, Y.; Wan, Y.; Liu, J.; Ma, Y.; Mao, Z.; Chang, H.; Li, G.; Xu, B.; et al. Early-Life Exposure to Bisphenol A Induces Liver Injury in Rats Involvement of Mitochondria-Mediated Apoptosis. PLoS ONE 2014, 9, e90443. [Google Scholar] [CrossRef] [Green Version]
- Akter, T.; Rahman, M.A.; Moni, A.; Apu, M.A.I.; Fariha, A.; Hannan, M.A.; Uddin, M.J. Prospects for Protective Potential of Moringa oleifera against Kidney Diseases. Plants 2021, 10, 2818. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumeci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-κB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Qian, J.-M. Cytoprotective role of heme oxygenase-1 in liver ischemia reperfusion injury. Int. J. Clin. Exp. Med. 2015, 8, 19867–19873. [Google Scholar] [PubMed]
- Rodríguez-Jiménez, P.; Fernández-Messina, L.; Ovejero-Benito, M.C.; Chicharro, P.; Vera-Tomé, P.; Vara, A.; Cibrian, D.; Martínez-Fleta, P.; Jiménez-Fernández, M.; Sánchez-García, I.; et al. Growth arrest and DNA damage-inducible proteins (GADD45) in psoriasis. Sci. Rep. 2021, 11, 14579. [Google Scholar] [CrossRef]
- Verzella, D.; Bennett, J.; Fischietti, M.; Thotakura, A.K.; Recordati, C.; Pasqualini, F.; Capece, D.; Vecchiotti, D.; D’Andrea, D.; Di Francesco, B.; et al. GADD45β Loss Ablates Innate Immunosuppression in Cancer. Cancer Res. 2018, 78, 1275–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-S.; Liang, S.; Li, D.-Y.; Wen, J.-H.; Tang, J.-X.; Liu, H.-F. Cell Cycle Dysregulation and Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 714320. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Jeong, C.H.; Seo, H.G.; Han, S.G. Moringa Extract Attenuates Inflammatory Responses and Increases Gene Expression of Casein in Bovine Mammary Epithelial Cells. Animals 2019, 9, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, A.H.; Foaud, M.A.; Moussa, H.M. The adverse effects of bisphenol A on male albino rats. J. Basic Appl. Zool. 2018, 79, 6. [Google Scholar] [CrossRef] [Green Version]
- Poormoosavi, S.M.; Najafzadehvarzi, H.; Behmanesh, M.A.; Amirgholami, R. Protective effects of Asparagus officinalis extract against Bisphenol A-induced toxicity in Wistar rats. Toxicol. Rep. 2018, 5, 427–433. [Google Scholar] [CrossRef]
- Gasmalbari, E.; El-Kamali, H.H.; Abbadi, O.S. Biochemical and Haematological Effects and Histopathological Changes caused by Moringa oleifera on Albino Rats. Chin. J. Med. Res. 2020, 3, 84–88. [Google Scholar] [CrossRef]
- Elswefy, S.E.-S.; Abdallah, F.R.; Atteia, H.H.; Wahba, A.S.; Hasan, R.A. Inflammation, oxidative stress and apoptosis cascade implications in bisphenol A-induced liver fibrosis in male rats. Int. J. Exp. Pathol. 2016, 97, 369–379. [Google Scholar] [CrossRef]
- Wilujeng, L.K.; Safitri, F.N.; Supriono, S.; Kalim, H.; Poeranto, S. The effect of Moringa oleifera (Lam) leaves ethanol extracts as anti-inflammatory and anti-fibrotic through TNF-α and p38-MAPK expression: In Vivo model of liver fibrosis approach. In Proceedings of the International Conference on Life Sciences and Technology (ICoLiST 2020), Java, Indonesia, 29 September 2020; Volume 2353, p. 030046. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Paliwal, R.; Janmeda, P.; Sharma, S. Chemopreventive efficacy of Moringa oleifera pods against 7,12-dimethylbenz[a]anthracene induced hepatic carcinogenesis in mice. Asian Pac. J. Cancer Prev. 2012, 13, 2563–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrahim, T.; Binobead, M.A. Roles of Moringa oleifera Leaf Extract in Improving the Impact of High Dietary Intake of Monosodium Glutamate-Induced Liver Toxicity, Oxidative Stress, Genotoxicity, DNA Damage, and PCNA Alterations in Male Rats. Oxidative Med. Cell. Longev. 2018, 2018, 4501097. [Google Scholar] [CrossRef] [Green Version]
- Duranti, G.; Maldini, M.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Ceci, R. Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells. Molecules 2021, 26, 5041. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Han, S.; Song, Y.O. Sesame Oil Attenuates Renal Oxidative Stress Induced by a High Fat Diet. Prev. Nutr. Food Sci. 2019, 24, 114–120. [Google Scholar] [CrossRef] [PubMed]








| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | CTACATGGCCTCCAAGGAGTAAG | TGGAATTGTGAGGGAGATGCTC |
| GADD45B | GAAGATGCAGGCGGTGACTG | CCTCCTCTTCTTCGTCTATGGC |
| HO-1 | ACAGCATGTCCCAGGATTTGTC | GGAGGCCATCACCAGCTTAAAG |
| Polyphenol Compounds | MOLE (µg/mL) | Encap. MOLE (µg/mL) |
|---|---|---|
| Gallic acid | 2.5 | 1.44 |
| Chlorogenic acid | 9.55 | 5.54 |
| Methyl gallat | 0.5 | 0.25 |
| Syringic acid | 0.33 | 0.59 |
| Pyro catechol | 4.83 | 1.07 |
| Rutin | 3.3 | 2.76 |
| Ellagic acid | 26 | 4.09 |
| Coumaric acid | 6.87 | 1.08 |
| Ferulic acid | 2.97 | 1.49 |
| Naringenin | 4.82 | 3.08 |
| Taxifolin | 0.69 | 0.26 |
| Cinnamic acid | 0.03 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou El-Naga, H.M.H.; El-Hashash, S.A.; Yasen, E.M.; Leporatti, S.; Hanafy, N.A.N. Starch-Based Hydrogel Nanoparticles Loaded with Polyphenolic Compounds of Moringa Oleifera Leaf Extract Have Hepatoprotective Activity in Bisphenol A-Induced Animal Models. Polymers 2022, 14, 2846. https://doi.org/10.3390/polym14142846
Abou El-Naga HMH, El-Hashash SA, Yasen EM, Leporatti S, Hanafy NAN. Starch-Based Hydrogel Nanoparticles Loaded with Polyphenolic Compounds of Moringa Oleifera Leaf Extract Have Hepatoprotective Activity in Bisphenol A-Induced Animal Models. Polymers. 2022; 14(14):2846. https://doi.org/10.3390/polym14142846
Chicago/Turabian StyleAbou El-Naga, Hend Mohamed Hasanin, Samah A. El-Hashash, Ensaf Mokhtar Yasen, Stefano Leporatti, and Nemany A. N. Hanafy. 2022. "Starch-Based Hydrogel Nanoparticles Loaded with Polyphenolic Compounds of Moringa Oleifera Leaf Extract Have Hepatoprotective Activity in Bisphenol A-Induced Animal Models" Polymers 14, no. 14: 2846. https://doi.org/10.3390/polym14142846
APA StyleAbou El-Naga, H. M. H., El-Hashash, S. A., Yasen, E. M., Leporatti, S., & Hanafy, N. A. N. (2022). Starch-Based Hydrogel Nanoparticles Loaded with Polyphenolic Compounds of Moringa Oleifera Leaf Extract Have Hepatoprotective Activity in Bisphenol A-Induced Animal Models. Polymers, 14(14), 2846. https://doi.org/10.3390/polym14142846








