Synthesis and Characterization of High Viscosity Cationic Poly(Proline-Epichlorohydrin) Composite Polymer with Antibacterial Functionalities
Abstract
:1. Introduction
2. Materials and Methods
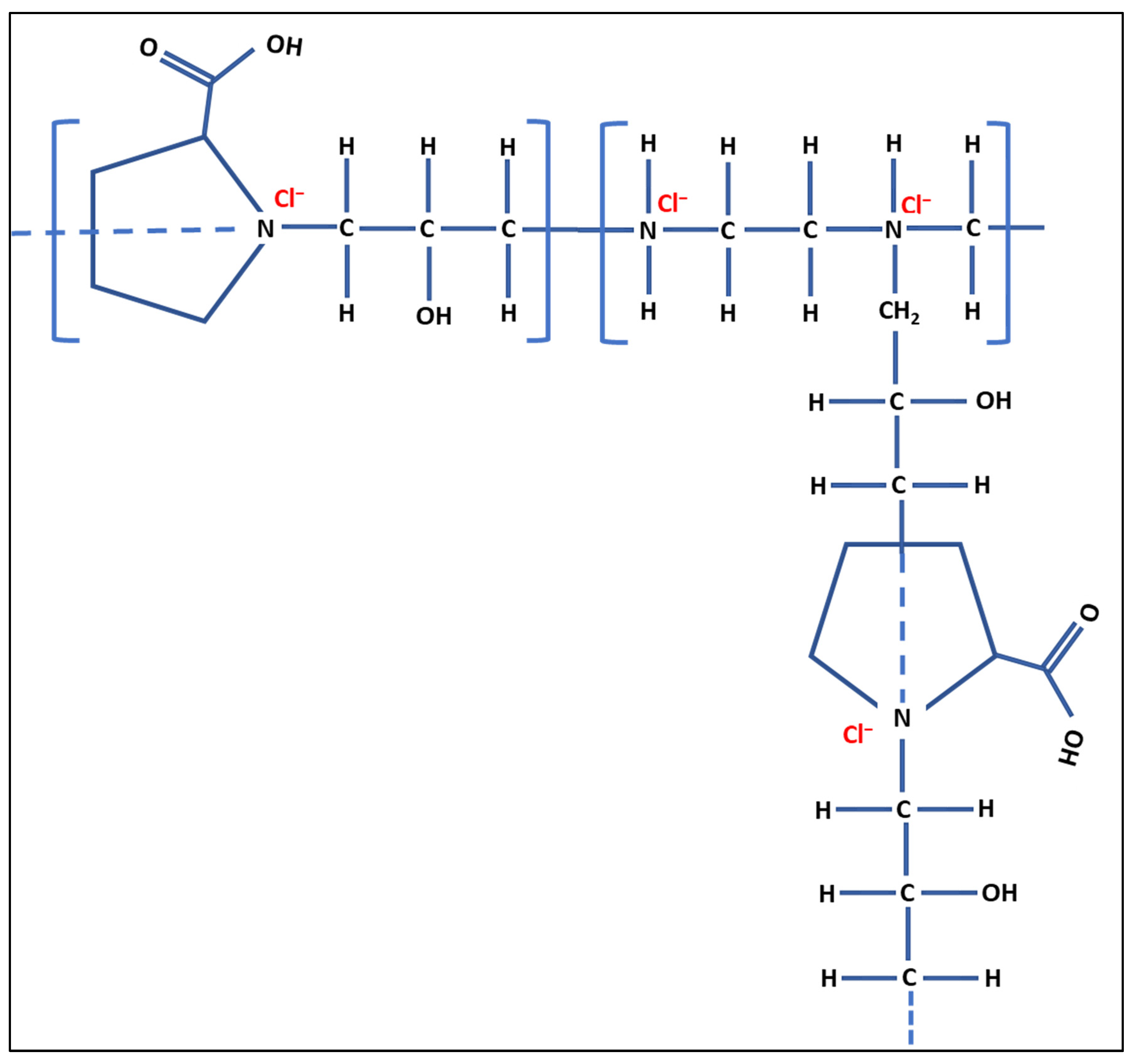
2.1. Preparation of the Cationic Polymer
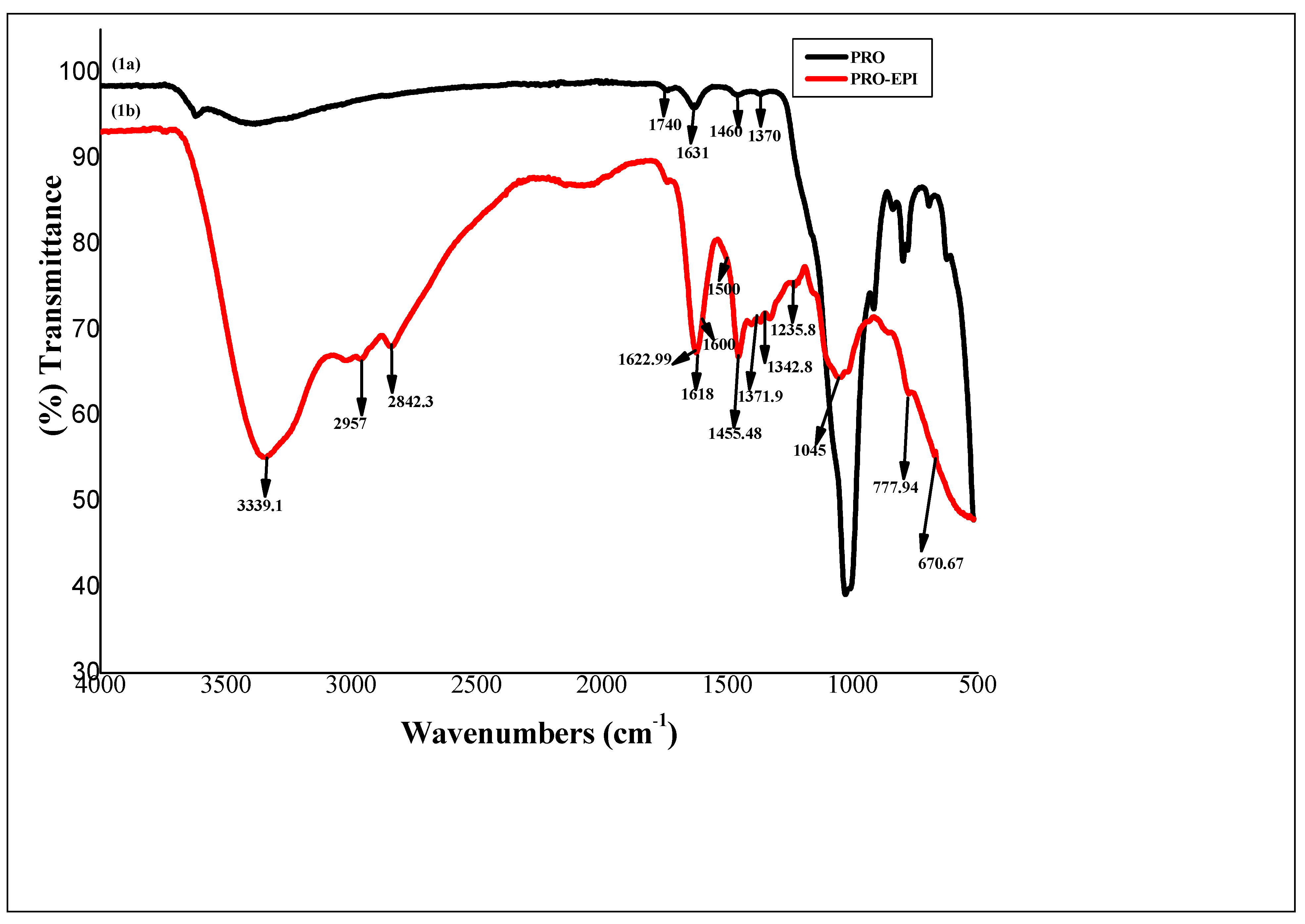
2.2. Characterization Studies
2.3. Antimicrobial Activity
2.3.1. Preparation of Reagent: Microplate Alamar Blue Assay (MABA)
2.3.2. The Preparation of the Nutrient Broth
2.3.3. Minimum Inhibitory Concentrations (MICs)
3. Results and Discussion
3.1. Evaluation of Antimicrobial Activity of PRO-EPI Composite
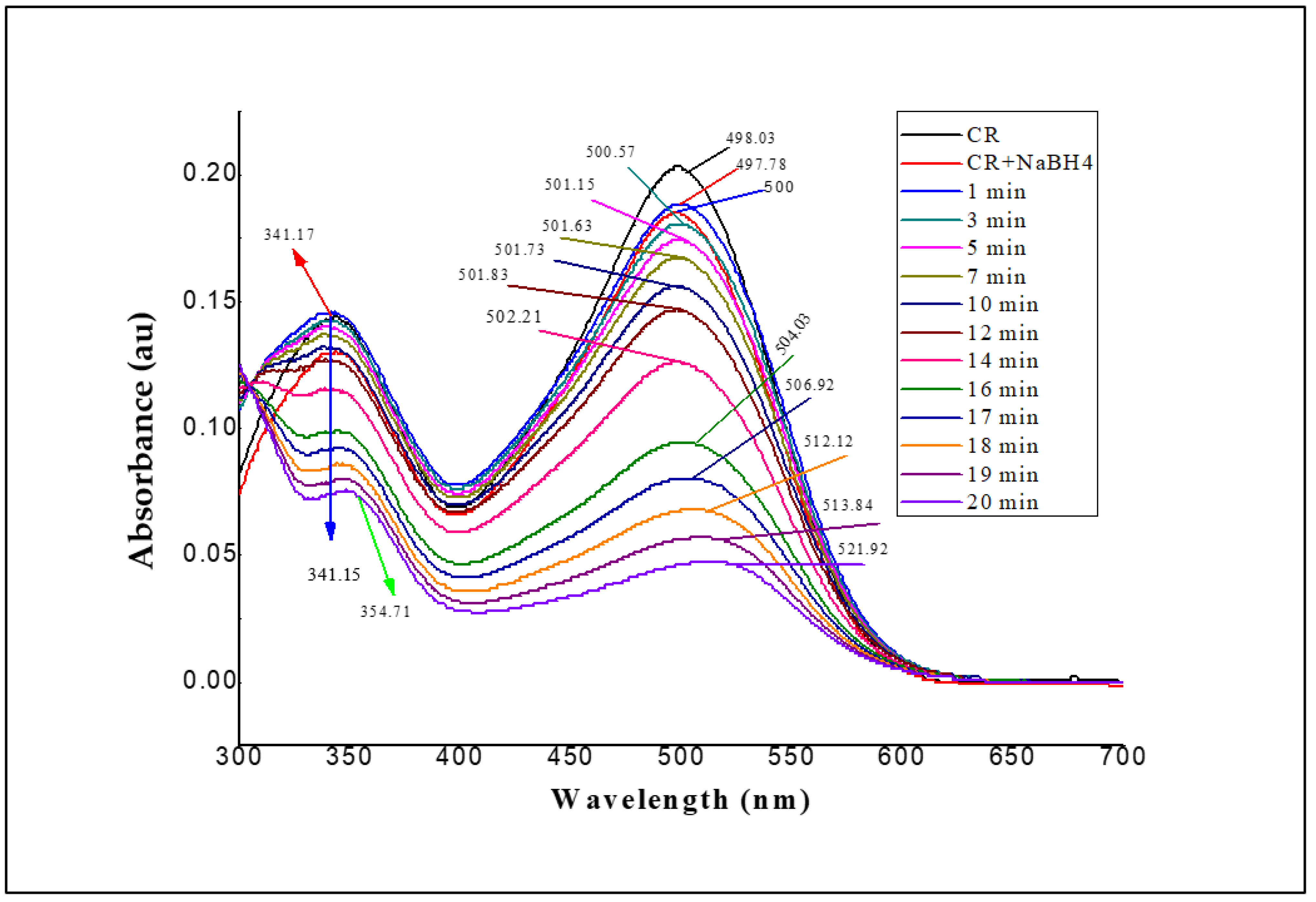
3.2. Adsorption of CR Azo Dye with PRO-EPI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLS | Dynamic Light Scattering |
| DSC | Differential Scanning Calorimetry |
| EPI | Epichlorohydrin |
| FTIR | Fourier Transform Infrared Spectroscopy |
| MIC | Minimum inhibitory concentration |
| NMR | Nuclear Magnetic Resonance |
| PRO | Proline |
| SEM | Scanning Electron Microscope |
| TGA | Thermogravimetric Analyzer |
References
- Lim, S.H.; Hudson, S.M. Application of a Fiber-Reactive Chitosan Derivative to Cotton Fabric as an Antimicrobial Textile Finish. Carbohydr. Polym. 2004, 56, 227–234. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic Antimicrobial Polymers and their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, I.M. A Review of the Role of Antibiotic Policies in the Control of Antibiotic Resistance. J. Antimicrob. Chemother. 1999, 43, 459–465. [Google Scholar] [CrossRef]
- Stone, A. Microbicides: A new Approach to Preventing HIV and other Sexually Transmitted Infections. Nat. Rev. Drug Discov. 2002, 1, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No eskape! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Paino, M.; Muñoz-Bonilla, A.; López-Fabal, F.; Gómez-Garcés, J.L.; Heuts, J.P.A.; Fernández-García, M. Effect of Glycounits on the Antimicrobial Properties and Toxicity Behaviour of Polymers Based on Quaternized DMAEMA. Biomacromolecules 2015, 16, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, A.D.; Tiller, J.C. Contact-Active Antimicrobial Coatings Derived from Aqueous Suspensions. Angew. Chem. Int. Ed. 2006, 45, 6759–6762. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Lenoir, S.; Riga, J.; Jérôme, R.; Detrembleur, C. Grafting of Poly[2-(Tert-Butylamino)Ethyl Methacrylate] onto Polypropylene by Reactive Blending and Antibacterial Activity of the Copolymer. Biomacromolecules 2007, 8, 1171–1177. [Google Scholar] [CrossRef]
- Alanis, A.J. Resistance to antibiotics: Are we in the Post-Antibiotic Era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- Ilker, M.F.; Nüsslein, K.; Tew, G.N.; Coughlin, E.B. Tuning the Hemolytic and Antibacterial Activities of Amphiphilic Polynorbornene Derivatives. J. Am. Chem. Soc. 2004, 126, 15870–15875. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Ye, Y.; Qian, L.; Zhao, G.; He, B.; Xiao, H. Antibacterial Modification of Cellulose Fibers by Grafting β-Cyclodextrin and Inclusion with Ciprofloxacin. Cellulose 2014, 21, 1921–1932. [Google Scholar] [CrossRef]
- Wahab, A.; Hasan, M.C.; Alothman, Z.A.; Hossain, S.A. In-situ Incorporation of Highly Dispersed Silver Nanoparticles in Nanoporous Carbon Nitride for the Enhancement of Antibacterial Activities. J. Hazard. Mater. 2021, 408, 1224919. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harisson, J.J.; Turner, R. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets, and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Luming, L.; Matin, A.; Karim, M.R.; Aijaz, M.O.; Alharbi, H.F.; Abdala, A.; Haque, R. Silver Micro-Nanoparticle -Based Nanoarchitectures: Synthesis Routes. Biomed. Appl. Mech. Act. Polym. 2021, 13, 2870. [Google Scholar]
- Wahab, A.; Luming, L.; Li, H.; Abdala, A. Silver-Nanoparticles-Based Nanocomposites for Combating Infectious Pathogens: Recent Advances and Future Prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef]
- Russell, A.D.; McDonnell, G. Antseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar]
- Majumdar, P.; Lee, E.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Thorson, C.J.; Chisholm, B.J. Synthesis and Antimicrobial Activity of Quaternary Ammonium-Functionalized POSS (Q-POSS) and Polysiloxane Coatings Containing Q-POSS. Polymer 2009, 50, 1124–1133. [Google Scholar] [CrossRef]
- Nayuniagri, M.K.; Kanny, K. Development of Green Dual Polymers for Antibacterial Applications. Polym. Plast. Technol. Eng. 2015, 54, 1715–1722. [Google Scholar] [CrossRef]
- Kumar, N.M.; Varaprasad, K.; Reddy, G.R.; Reddy, G.S.M.; Sivabharathi, Y.; Reddy, G.V.S.; Naidu, S.V. Biodegradable Water Soluble Copolymer for Antimicrobial Applications. J. Polym. Environ. 2011, 19, 225–229. [Google Scholar] [CrossRef]
- Lienkamp, K.; Madkour, A.E.; Kumar, K.N.; Nusslein, K.; Tew, G.N. Antimicrobial Polymers Prepared by Ring-Opening Metathesis Polymerization: Manipulating Antimicrobial Properties by Organic Counterion and Charge Density Variation. Chem. Eur. J. 2009, 15, 11715–11722. [Google Scholar] [CrossRef] [PubMed]
- Lienkamp, K.; Madkour, A.E.; Musante, A.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Antimicrobial Polymers Prepared by ROMP with Unprecedented Selectivity: A Molecular Construction Kit Approach. J. Am. Chem. Soc. 2008, 130, 9836–9843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madkour, A.E.; Koch, A.H.; Lienkamp, K.; Tew, G.N. End-Functionalized ROMP Polymers for Biomedical Applications. Macromolecules 2012, 43, 4557–4561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutthasupa, S.; Shiotsuki, M.; Sanda, F. Recent Advances in Ring-opening Metathesis Polymerization, and Application to Synthesis of Functional Materials. Polym. J. 2010, 42, 905–915. [Google Scholar] [CrossRef]
- Lienkamp, K.; Madkour, A.E.; Tew, G.N. Antibacterial Peptidomimetics: Polymeric Synthetic Mimics of Antimicrobial Peptides. In Polymer Composites—Polyolefin Fractionation—Polymeric Peptidomimetics—Collagens; Abe, A., Kausch, H.H., Möller, M., Pasch, H., Eds.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2000; Volume 251. [Google Scholar]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Park, S.H.; Wei, S.; Mizaikoff, B.; Taylor, A.E.; Favero, C.; Huang, C.H. DEGRADATION of Amine-Based Water Treatment Polymers During Chloramination as N-Nitrosodimethylamine (NDMA) Precursors. Environ. Sci. Technol. 2009, 43, 1360–1366. [Google Scholar] [CrossRef]
- Yue, Q.Y.; Gao, B.Y.; Wang, Y.; Zhang, H.; Sun, X.; Wang, S.G.; Gu, R.R. Synthesis of Polyamine Flocculants and their Potential use in Treating Dye Wastewater. J. Hazard. Mater. 2008, 152, 221–227. [Google Scholar] [CrossRef]
- Perry, M.R.; Ebrahimi, T.; Morgan, E.; Edwards, P.M.; Hatzikiriakos, S.G.; Schafer, L.L. Catalytic Synthesis of Secondary Amine-Containing Polymers: Variable Hydrogen Bonding for Tunable Rheological Properties. Macromolecules 2016, 49, 4423–4430. [Google Scholar] [CrossRef]
- Frier, M. Derivatives of 4-Amino-Quinaldinium and 8-Hydroxyquinolone. In Inhibition and Destruction of the Microbial Cell; Hugo, W.B., Ed.; Academic Press, Ltd.: London, UK, 1971; pp. 107–120. [Google Scholar]
- Merianos, J.J. Quaternary Ammonium Antimicrobial Compounds. In Disinfection, Sterilization, and Preservation, 4th ed.; Block, S.S., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1991; pp. 225–255. [Google Scholar]
- Hugo, W.B.; Frier, M. Mode of Action of Antibacterial Compound Dequalinium Acetate. Appl. Microbiol. 1969, 17, 118–127. [Google Scholar] [CrossRef]
- Hugo, W.B. Disinfection Mechanisms. In Principles and Practice of Disinfection, Preservation and Sterilization, 3rd ed.; Russell, A.D., Hugo, W.B., Ayliffe, G.A.J., Eds.; Blackwell Scientific Publications: Oxford, UK, 1999; pp. 258–283. [Google Scholar]
- Salton, M.R.J. Lytic Agents, cell Permeability and Monolayer Penetrability. J. Gen. Physiol. 1968, 52, 227S–252S. [Google Scholar] [CrossRef]
- Denyer, S.P. Mechanisms of Action of Antibacterial Biocides. Int. Biodeterior. Biodegrad. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Vieira, D.B.; Lincopan, N. Cationic Lipids and Surfactants as Anti-Infective Agents. Anti-Infect. Agents Med. Chem. 2006, 5, 33–54. [Google Scholar] [CrossRef]
- Nishihara, T.; Okamoto, T.; Nishiyama, N. Biodegradation of Didecyldimethylammonium Chloride by Pseudomonas fluorescens TN4 Isolated from Activated Sludge. J. Appl. Microbiol. 2000, 88, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, R.; Xiao, L.; Chang, Y.; Guan, Y.; Li, X.; Zeng, G. Photocatalytic Decolorization and Degradation of Congo Red on Innovative Crosslinked Chitosan/Nano-CdS Composite Catalyst under Visible Light Irradiation. J. Hazard. Mater. 2009, 169, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Choi, Y.H.; Park, J. A Self-Destroying Polycationic Polymer: Biodegradable Poly(4-Hydroxy-l-Proline Ester). J. Am. Chem. Soc. 1999, 121, 5633–5639. [Google Scholar] [CrossRef]
- Raghunath, S.; Anand, K.; Gengan, R.M.; Nayunigari, M.K.; Maity, A. Sorption Isotherms, Kinetic and Optimization Process of Amino Acid Proline Based Polymer Nanocomposite for the Removal of Selected Textile Dyes from Industrial Wastewater. J. Photochem. Photobiol. B 2016, 165, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols, 1st ed.; CRC Press: New York, NY, USA, 2007; pp. 75–79. [Google Scholar]
- To, W.K.; Fothergill, A.W.; Rinaldi, M.G. Comparative Evaluation of Macrodilution and Alamar Colorimetric Microdilution broth Methods for Antifungal Susceptibility Testing of Yeast Isolates. J. Clin. Microbiol. 1995, 33, 2660–2664. [Google Scholar] [CrossRef] [Green Version]
- Kobra, A.; Akbar, H. A simple, green, one-pot synthesis of magnetic-nanoparticle-supported proline without any source of supplemental linkers and application as a highly efficient base catalyst. RSC Adv. 2014, 4, 6508–6512. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Noeline, B.F.; Manohar, D.M. Phosphate Removal from Wastewaters using a Weak Anion Exchanger Prepared from a Lignocellulosic Residue. Environ. Sci. Technol. 2006, 40, 2740–2745. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xiao, H.; Ziaee, Z.; Wang, H.; Guan, Y.; Zheng, A. Novel Comb-like Ionenes with Aliphatic Side Chains: Synthesis and Antimicrobial Properties. J. Mater. Sci. 2013, 48, 1162–1171. [Google Scholar] [CrossRef]
- Kang, J.J.; Li, W.Y.; Lin, Y.; Li, X.P.; Xiao, X.R.; Fang, S.B. Synthesis and Ionic Conductivity of a Polysiloxane Containing Quaternary Ammonium Groups. Polym. Adv. Technol. 2004, 15, 61–64. [Google Scholar] [CrossRef]
- Guiqian, L.; Dingcai, W.; Ruowen, F. Studies on the Synthesis and Antibacterial Activities of Polymeric Quaternary Ammonium Salts from Dimethylaminoethyl Methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Som, A.; Vemparala, S.; Ivanov, I.; Tew, G.N. Synthetic Mimics of Antimicrobial Peptides. Biopolymers 2008, 90, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Wiradharma, N.; Khoe, U.; Hauser, C.A.E.; Seow, S.V.; Zhang, S.G.; Yang, Y.Y. Synthetic Cationic Amphiphilic Alpha-Helical Peptides as Antimicrobial Agents. Biomaterials 2011, 32, 2204–2212. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, L.; Yu, H.; Chen, Y. Quan Shi, Study on Antibacterial Behaviour of Insoluble Quaternaryammonium. J. Appl. Polym. Sci. 2006, 99, 2389–2394. [Google Scholar] [CrossRef]
- Ziaee, Z.; Qian, L.; Guan, Y.; Fatehi, P.; Xiao, H. Antimicrobial/Antimold Polymer-Grafted Starches for Recycled Cellulose Fibers. J. Biomater. Sci. Polym. 2010, 21, 1359–1370. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Hirayama, H.; Suzuki, K.; Yamaguchi, H.; Tazuke, S. Polymeric Pyridinium salts with Well-Defined Main Chain Structure. Makromol. Chem. 1986, 187, 333–340. [Google Scholar] [CrossRef]
- Dizman, B.; Elasri, M.O.; Mathias, L.J. Synthesis and Antimicrobial Activities of New Water-Soluble Bis-Quaternary Ammonium Methacrylate Polymers. J. Appl. Polym. Sci. 2004, 94, 635–642. [Google Scholar] [CrossRef]
- Vigh, J.E.; Lo, P.; Dziabo, A.J.; Wong, M.P. Opthalmic Compositions and Methods for Preserving and Using Same. U.S. Patent 5,277,901,1994, 1 July 1998. [Google Scholar]
- Eren, T.; Som, A.; Rennie, J.R.; Nelson, C.F.; Urgina, Y.; Nüsslein, K.; Coughlin, E.B.; Tew, G.N. Antibacterial and Hemolytic Activities of Quaternary Pyridinium Functionalized Polynorbornenes. Macromol. Chem. Phys. 2008, 209, 516–524. [Google Scholar] [CrossRef]
- Ikeda, T.; Tazuke, S.; Suzuki, Y. Biologically Active Polycations, 4. † Synthesis and Antimicrobial Activity of Poly(Trialkylvinylbenzylammonium Chloride)s. Macromol. Chem. Phys. 1984, 185, 869–876. [Google Scholar] [CrossRef]
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. New polymeric biocides: Synthesis and Antibacterial Activities of Polycations with Pendant Biguanide Groups. Antimicrob. Agents. Chemother. 1984, 26, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, O.M. (Ed.) Handbook of Maleic Anhydride Based Materials, Syntheses, Properties and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Kumar, A.B.V.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of Chito-Oligosaccharides Prepared by Chitosanolysis with the Aid of Papain and Pronase, and their Bactericidal Action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic Biocides with Pendant Active Groups—Molecular-Weight Dependence of Antibacterial Activity. Antimicrob. Agents Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlinson, L.A.B.; O’Gara, J.P.; Jones, D.S.; Brayden, D.J. Resistance of Staphylococcus aureus to the Cationic Antimicrobial Agent Poly(2-(Dimethylamino Ethyl)Methacrylate) (pDMAEMA) is Influenced by Cell-Surface Charge and Hydrophobicity. J. Med. Microbiol. 2011, 60, 968–976. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The “Accidental” Pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Pablo, H.; Moise, P.L.; Luis, M.L.; Joachim, D.; Yan, L.; Matthias, B. Catalysis by Metallic Nanoparticles in Aqueous Solution: Model Reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Erdemoğlu, S.; Aksu, S.K.; Sayılkan, F.; İzgi, B.; Asiltürk, M.; Sayılkan, H.; Frimmel, F.; Güçer, Ş. Photocatalytic Degradation of Congo Red by Hydrothermally Synthesized Nanocrystalline TiO2 and Identification of Degradation Products by LC–MS. J. Hazard. Mater. 2008, 155, 469–476. [Google Scholar] [CrossRef]
- Huang, M.; Xu, C.; Wu, Z.; Huang, Y.; Lin, J.; Wu, J. Photocatalytic Decolorization of Methyl Orange Solution by Pt Modified TiO2 Loaded on Natural Zeolite. Dyes Pigment. 2008, 77, 327–334. [Google Scholar] [CrossRef]
- Weir, R.J.; Fisher, R.S. Toxicologic Studies on Borax and Boric Acid. Toxicol. Appl. Pharmacol. 1972, 23, 351–364. [Google Scholar] [CrossRef]
| Polymer (PRO-EPI) Code | Staphylococcus aureus (µg/mL) | Pseudomonas aeruginosa (µg/mL) | Escherichia coli (µg/mL) |
|---|---|---|---|
| M5 | - | 128 ± 3.5 | 128 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayunigari, M.K.; Suri, R.; Andaluri, G. Synthesis and Characterization of High Viscosity Cationic Poly(Proline-Epichlorohydrin) Composite Polymer with Antibacterial Functionalities. Polymers 2022, 14, 2797. https://doi.org/10.3390/polym14142797
Nayunigari MK, Suri R, Andaluri G. Synthesis and Characterization of High Viscosity Cationic Poly(Proline-Epichlorohydrin) Composite Polymer with Antibacterial Functionalities. Polymers. 2022; 14(14):2797. https://doi.org/10.3390/polym14142797
Chicago/Turabian StyleNayunigari, Mithil Kumar, Rominder Suri, and Gangadhar Andaluri. 2022. "Synthesis and Characterization of High Viscosity Cationic Poly(Proline-Epichlorohydrin) Composite Polymer with Antibacterial Functionalities" Polymers 14, no. 14: 2797. https://doi.org/10.3390/polym14142797
APA StyleNayunigari, M. K., Suri, R., & Andaluri, G. (2022). Synthesis and Characterization of High Viscosity Cationic Poly(Proline-Epichlorohydrin) Composite Polymer with Antibacterial Functionalities. Polymers, 14(14), 2797. https://doi.org/10.3390/polym14142797





