Effect of Tamarind Gum on the Properties of Phase-Separated Poly(vinyl alcohol) Films
Abstract
1. Introduction
2. Results and Discussion
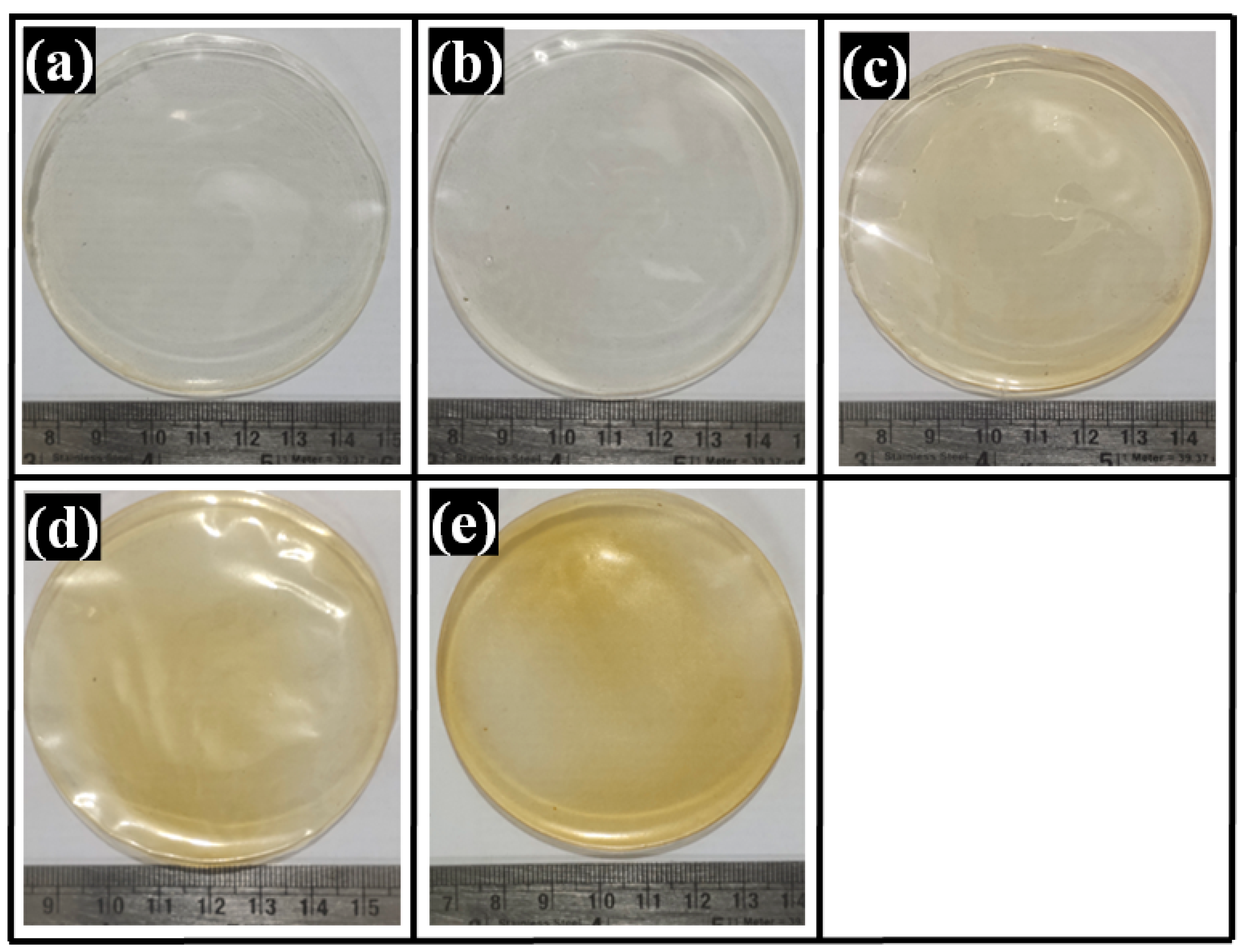
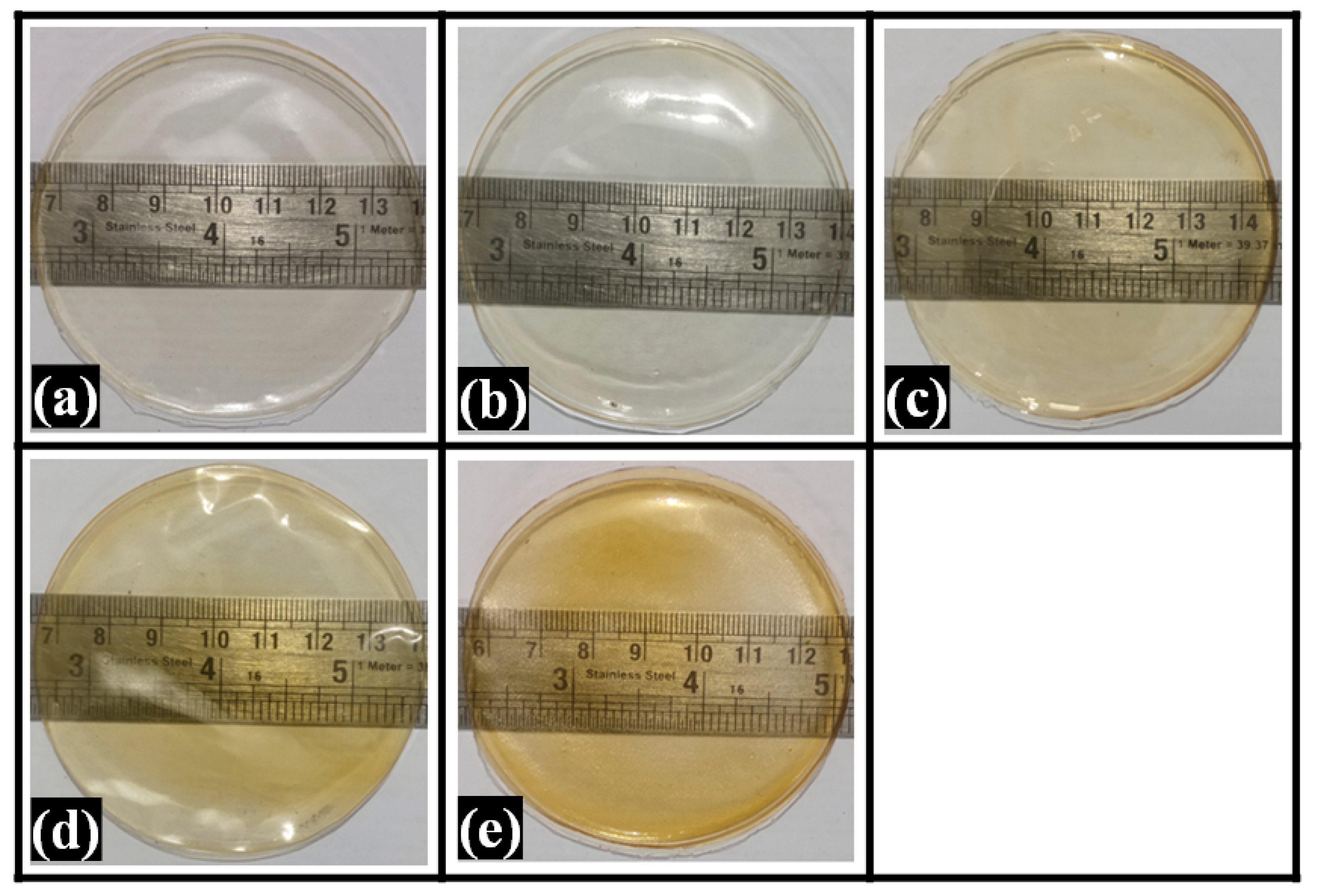
2.1. Visual Appearance of Composite Films
2.2. Microscopic Analysis
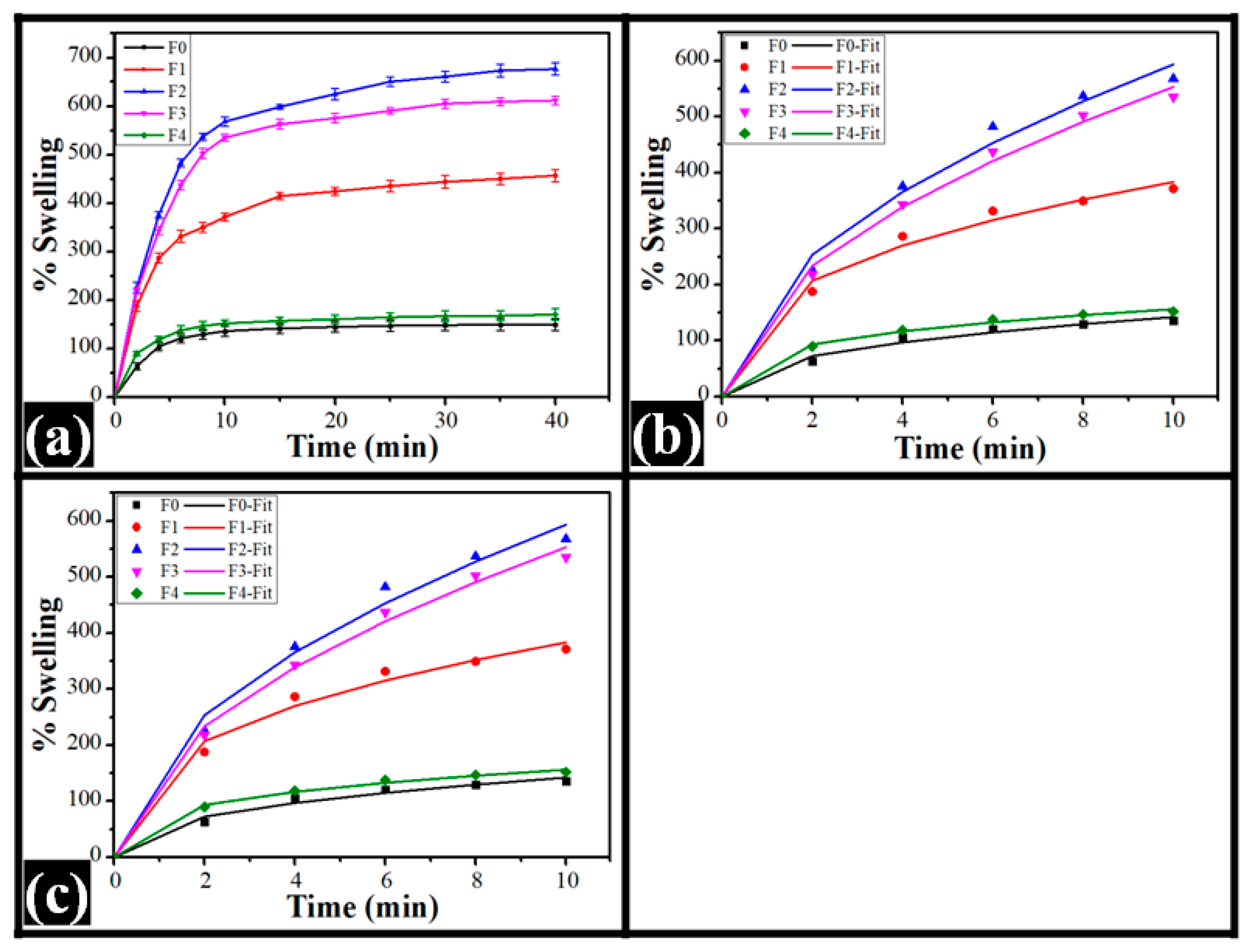
2.3. Swelling Study
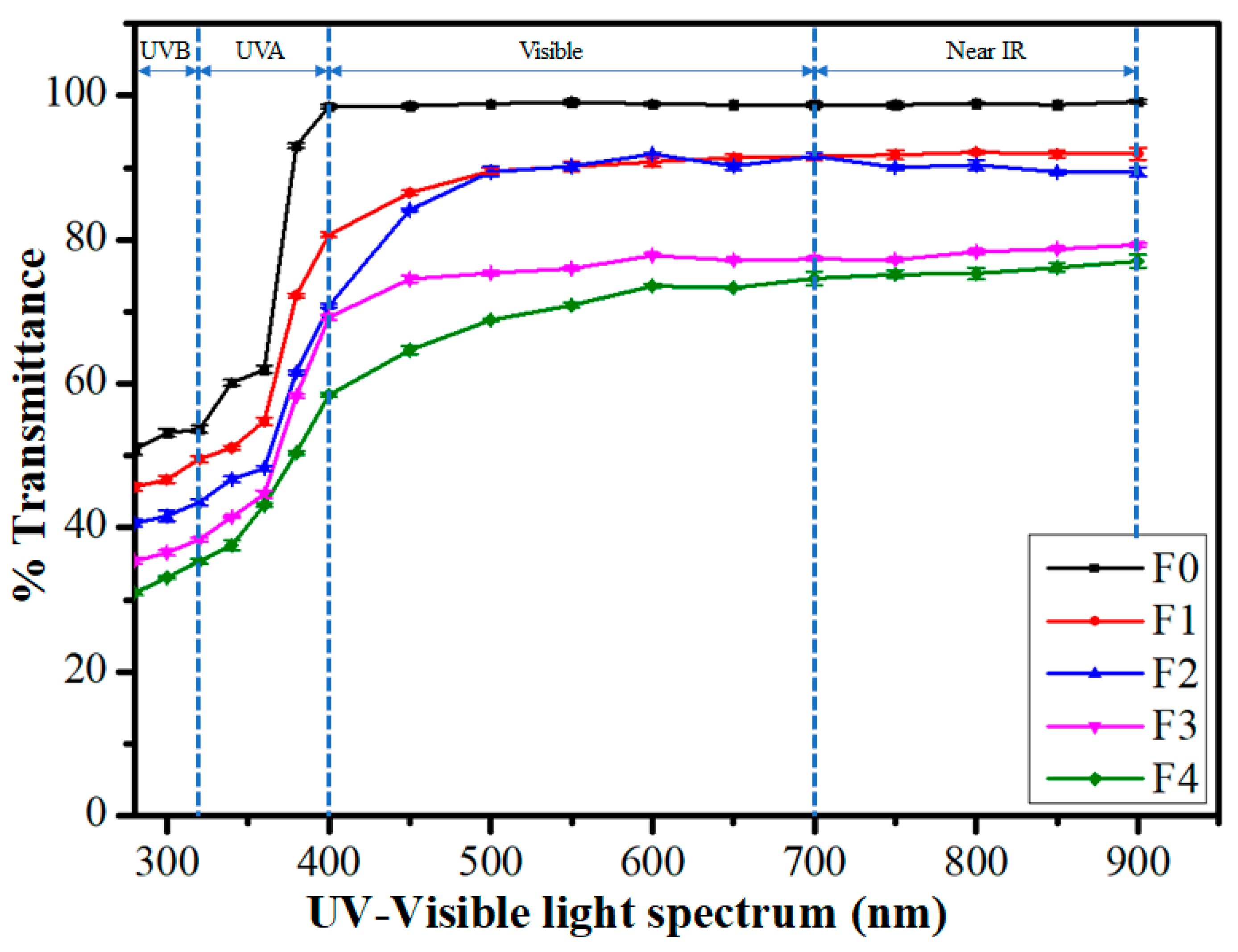
2.4. Transparency Study
2.5. FTIR Analysis
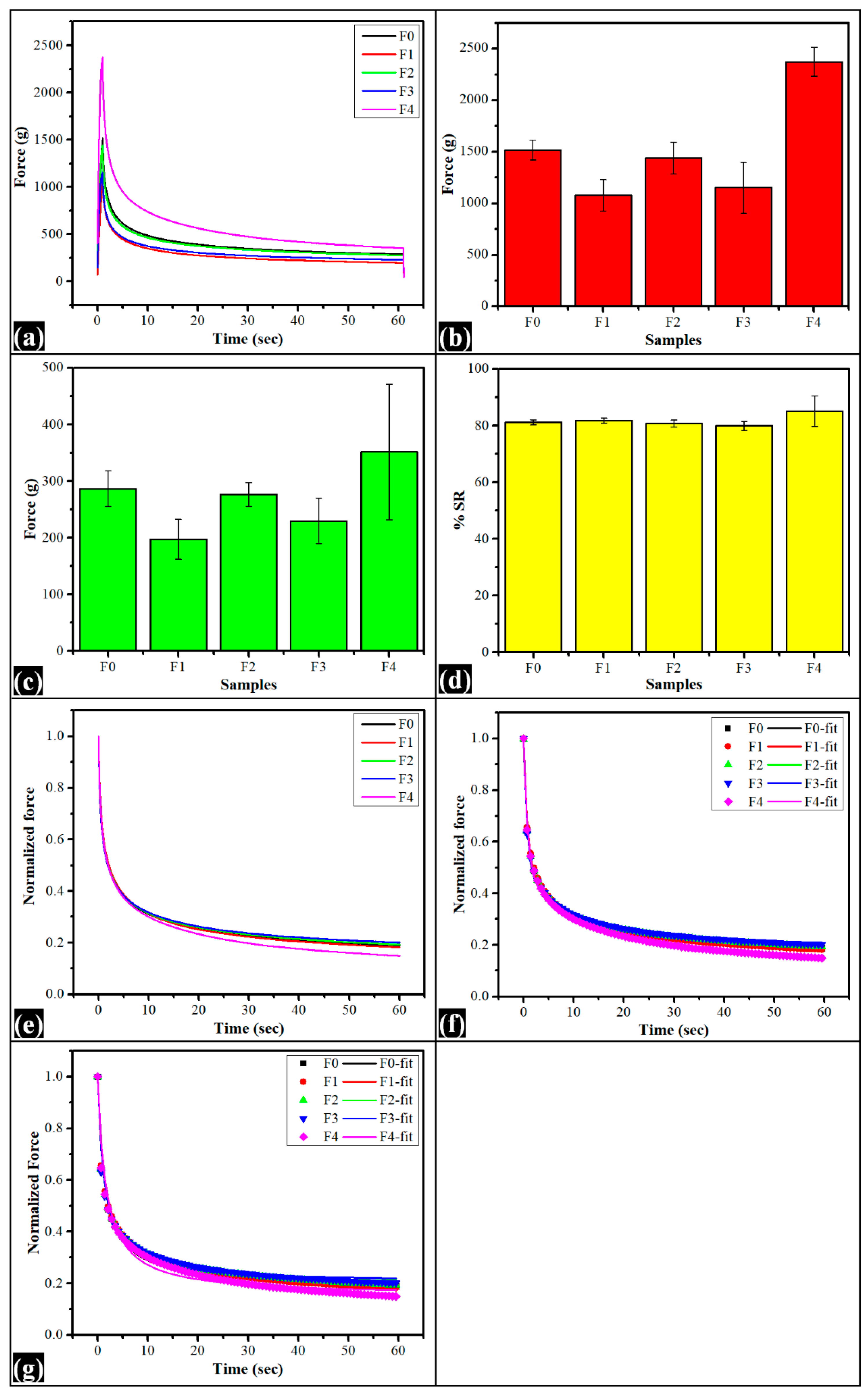
2.6. Stress Relaxation Study
2.7. Impedance Spectroscopy
2.8. In Vitro Drug Release Study
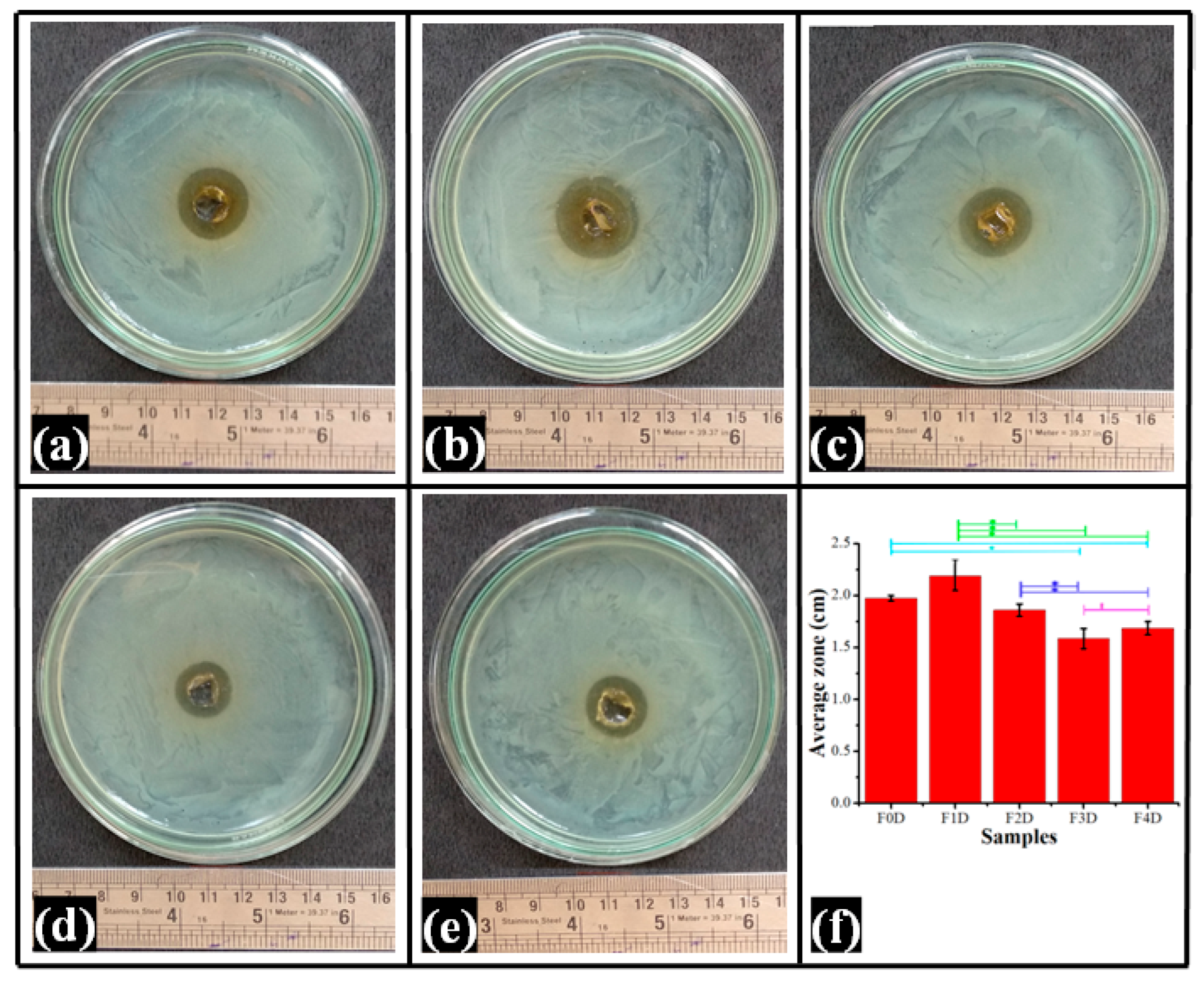
2.9. Antimicrobial Study
3. Materials and Methods
3.1. Preparation of Films
3.2. Microscopy
3.3. Swelling Study
3.4. Transparency Study
3.5. Loss on Drying
3.6. FTIR Analysis
3.7. Impedance Analysis
3.8. Mechanical Studies
3.9. In Vitro Drug Release
3.10. Antimicrobial Study
3.11. Statistical Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamari, Y.; Ghiaci, M. Preparation and characterization of ibuprofen/modified chitosan/TiO2 hybrid composite as a controlled drug-delivery system. Microporous Mesoporous Mater. 2016, 234, 361–369. [Google Scholar] [CrossRef]
- Parhizkar, M.; Reardon, P.J.; Knowles, J.C.; Browning, R.J.; Stride, E.; Barbara, P.R.; Harker, A.H.; Edirisinghe, M. Electrohydrodynamic encapsulation of cisplatin in poly (lactic-co-glycolic acid) nanoparticles for controlled drug delivery. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, J.; Li, X.; Lan, B.; Zhou, S.; Meng, Y.; Cheng, L. Episcleral drug film for better-targeted ocular drug delivery and controlled release using multilayered poly-ε-caprolactone (PCL). Acta Biomater. 2016, 37, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, N. Fabrication of a novel bone ash-reinforced gelatin/alginate/hyaluronic acid composite film for controlled drug delivery. Carbohydr. Polym. 2016, 151, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Sant, S.; Tao, S.L.; Fisher, O.Z.; Xu, Q.; Peppas, N.A.; Khademhosseini, A. Microfabrication technologies for oral drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 496–507. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Manickavasagam, D.; Wehrung, D.; Chamsaz, E.A.; Sanders, M.; Bouhenni, R.; Crish, S.D.; Joy, A.; Oyewumi, M.O. Assessment of alkoxylphenacyl-based polycarbonates as a potential platform for controlled delivery of a model anti-glaucoma drug. Eur. J. Pharm. Biopharm. 2016, 107, 56–66. [Google Scholar] [CrossRef]
- Barbălată-Mândru, M.; Serbezeanu, D.; Butnaru, M.; Rîmbu, C.M.; Enache, A.A.; Aflori, M. Poly (vinyl alcohol)/Plant Extracts Films: Preparation, Surface Characterization and Antibacterial Studies against Gram Positive and Gram Negative Bacteria. Materials 2022, 15, 2493. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly (vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Omata, S.; Sawae, Y.; Murakami, T. GS8-4 Effect of eluted poly (vinyl alcohol)(PVA) molecules in water lubricant on total wear amount of PVA hydrogel (GS8: Artificial Organs and Biomaterials). In Proceedings of the Asian Pacific Conference on Biomechanics: Emerging Science and Technology in Biomechanics, Sapporo, Japan, 16–19 September 2015; p. 201. [Google Scholar]
- Simionescu, B.C.; Olaru, M.; Bordianu, I.; Ursu, C.; Cotofana, C. PVA-based hybrid composites with luminescent properties for drug delivery. High Perform. Polym. 2012, 24, 449–459. [Google Scholar] [CrossRef]
- Chen, W.; Hou, Y.; Tu, Z.; Gao, L.; Haag, R. pH-degradable PVA-based nanogels via photo-crosslinking of thermo-preinduced nanoaggregates for controlled drug delivery. J. Control. Release 2017, 259, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Das, A.; Alzhrani, R.M.; Kang, D.; Bhaduri, S.B.; Boddu, S.H. Comparison of electrospun and solvent cast polylactic acid (PLA)/poly (vinyl alcohol)(PVA) inserts as potential ocular drug delivery vehicles. Mater. Sci. Eng. C 2017, 77, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Muchová, M.; Münster, L.; Capáková, Z.; Mikulcová, V.; Kuřitka, I.; Vícha, J. Design of dialdehyde cellulose crosslinked poly (vinyl alcohol) hydrogels for transdermal drug delivery and wound dressings. Mater. Sci. Eng. C 2020, 116, 111242. [Google Scholar] [CrossRef]
- Sequeira, R.S.; Miguel, S.P.; Cabral, C.S.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Development of a poly (vinyl alcohol)/lysine electrospun membrane-based drug delivery system for improved skin regeneration. Int. J. Pharm. 2019, 570, 118640. [Google Scholar] [CrossRef]
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual cross-linked chitosan/PVA hydrogels containing silver nanoparticles with antimicrobial properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Kurniawan, A.; Muneekaew, S.; Hung, C.-W.; Chou, S.-H.; Wang, M.-J. Modulated transdermal delivery of nonsteroidal anti-inflammatory drug by macroporous poly (vinyl alcohol)-graphene oxide nanocomposite films. Int. J. Pharm. 2019, 566, 708–716. [Google Scholar] [CrossRef]
- Hussein, Y.; Loutfy, S.A.; Kamoun, E.A.; El-Moslamy, S.H.; Radwan, E.M.; Elbehairi, S.E.I. Enhanced anti-cancer activity by localized delivery of curcumin form PVA/CNCs hydrogel membranes: Preparation and in vitro bioevaluation. Int. J. Biol. Macromol. 2021, 170, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Williams, P. Structural characteristics and functional properties of gum Arabic. In Gum Arabic; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 179–187. [Google Scholar]
- Gupta, V.; Puri, R.; Gupta, S.; Jain, S.; Rao, G. Tamarind Kernel Gum: An Upcoming Natural Polysaccharide. Syst. Rev. Pharm. 2010, 1, 50–54. [Google Scholar] [CrossRef][Green Version]
- Shaikh, S.; Shivsaran, K.; Pawar, R.; Misal, N.; Mene, H.; More, B. Tamarind seed polysaccharide: A versatile pharmaceutical excipient and its modification. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 157–164. [Google Scholar]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Ghorpade, V.S. Delivery of drugs using tamarind gum and modified tamarind gum: A review. Bull. Fac. Pharm. Cairo Univ. 2019, 57, 1–24. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y. Molar mass effect in food and health. Food Hydrocoll. 2021, 112, 106110. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, R.; Palve, Y.P.; Nayak, P. Synthesis and characterization of tamarind-polyvinyl alcohol blended with cloisite 30B nanocomposite for controlled release of the ocular drug timolol maleate. J. Pharm. Biomed. Sci. 2012, 22, 1–7. [Google Scholar]
- Khounvilay, K.; Sittikijyothin, W. Rheological behaviour of tamarind seed gum in aqueous solutions. Food Hydrocoll. 2012, 26, 334–338. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D. Functionalization of tamarind gum for drug delivery. In Functional Biopolymers; Springer: Berlin/Heidelberg, Germany, 2018; pp. 25–56. [Google Scholar]
- Crispín-Isidro, G.; Hernández-Rodríguez, L.; Ramírez-Santiago, C.; Sandoval-Castilla, O.; Lobato-Calleros, C.; Vernon-Carter, E. Influence of purification on physicochemical and emulsifying properties of tamarind (Tamarindus indica L.) seed gum. Food Hydrocoll. 2019, 93, 402–412. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Joshi, A.A.; Patil, C.L.; Amale, P.D.; Patel, H.M.; Surana, S.J.; Belgamwar, V.S.; Chaudhari, K.S.; Pardeshi, C.V. Xyloglucan: A functional biomacromolecule for drug delivery applications. Int. J. Biol. Macromol. 2017, 104, 799–812. [Google Scholar] [CrossRef]
- Fenton, T.; Gholamipour-Shirazi, A.; Daffner, K.; Mills, T.; Pelan, E. Formulation and additive manufacturing of polysaccharide-surfactant hybrid gels as gelatin analogues in food applications. Food Hydrocoll. 2021, 120, 106881. [Google Scholar] [CrossRef]
- Kiniwa, R.; Miyake, M.; Kimura, S.-i.; Itai, S.; Kondo, H.; Iwao, Y. Development of muco-adhesive orally disintegrating tablets containing tamarind gum-coated tea powders for oral care. Int. J. Pharm. X 2019, 1, 100012. [Google Scholar] [CrossRef] [PubMed]
- Doniparthi, J. Novel tamarind seed gum-alginate based multi-particulates for sustained release of dalfampridine using response surface methodology. Int. J. Biol. Macromol. 2020, 144, 725–741. [Google Scholar] [CrossRef]
- Maharana, V.; Gaur, D.; Nayak, S.K.; Singh, V.K.; Chakraborty, S.; Banerjee, I.; Ray, S.S.; Anis, A.; Pal, K. Reinforcing the inner phase of the filled hydrogels with CNTs alters drug release properties and human keratinocyte morphology: A study on the gelatin-tamarind gum filled hydrogels. J. Mech. Behav. Biomed. Mater. 2017, 75, 538–548. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology–a versatile tool for thin film production. In Scattering Methods and the Properties of Polymer Materials; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar]
- Musa, B.; Hameed, N. Effect of crosslinking agent (glutaraldehyde) on the mechanical properties of (PVA/Starch) blend and (PVA/PEG) binary blend films. J. Phys. Conf. Ser. 2021, 1795, 012064. [Google Scholar] [CrossRef]
- Sirait, B.B.F.; Saepudin, E. Modified Crosslinked Cellulose/PVA with Glutaraldehyde and Reinforced. In Proceedings of the International Conference on Science, Technology, and Environment 2020, Surabaya, Indonesia, 2–3 December 2020. [Google Scholar]
- Cascone, M.G.; Maltinti, S.; Barbani, N.; Laus, M. Effect of chitosan and dextran on the properties of poly (vinyl alcohol) hydrogels. J. Mater. Sci. Mater. Med. 1999, 10, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Chatkitanan, T.; Harnkarnsujarit, N. Effects of nitrite incorporated active films on quality of pork. Meat Sci. 2021, 172, 108367. [Google Scholar] [CrossRef]
- Yadav, I.; Rathnam, V.S.; Yogalakshmi, Y.; Chakraborty, S.; Banerjee, I.; Anis, A.; Pal, K. Synthesis and characterization of polyvinyl alcohol-carboxymethyl tamarind gum based composite films. Carbohydr. Polym. 2017, 165, 159–168. [Google Scholar] [CrossRef]
- Shaw, G.S.; Uvanesh, K.; Gautham, S.; Singh, V.; Pramanik, K.; Banerjee, I.; Kumar, N.; Pal, K. Development and characterization of gelatin-tamarind gum/carboxymethyl tamarind gum based phase-separated hydrogels: A comparative study. Des. Monomers Polym. 2015, 18, 434–450. [Google Scholar] [CrossRef]
- Qureshi, D.; Behera, K.P.; Mohanty, D.; Mahapatra, S.K.; Verma, S.; Sukyai, P.; Banerjee, I.; Pal, S.K.; Mohanty, B.; Kim, D. Synthesis of novel poly (vinyl alcohol)/tamarind gum/bentonite-based composite films for drug delivery applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126043. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Dogaru, B.-I.; Popescu, C.-M. The influence of cellulose nanocrystals content on the water sorption properties of bio-based composite films. Mater. Des. 2017, 132, 170–177. [Google Scholar] [CrossRef]
- Saidi, M.; Dabbaghi, A.; Rahmani, S. Swelling and drug delivery kinetics of click-synthesized hydrogels based on various combinations of PEG and star-shaped PCL: Influence of network parameters on swelling and release behavior. Polym. Bull. 2020, 77, 3989–4010. [Google Scholar] [CrossRef]
- Kim, D. Low temperature deposition of transparent conducting ITO/Au/ITO films by reactive magnetron sputtering. Appl. Surf. Sci. 2010, 256, 1774–1777. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J. Synthesis and characterization of hydrogel films of carboxymethyl tamarind gum using citric acid. Int. J. Biol. Macromol. 2017, 105, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef]
- Pospıšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, H.; Yu, L.; Liu, X.; Chen, L.; Zhang, N. Developing hydroxypropyl methylcellulose/hydroxypropyl starch blends for use as capsule materials. Carbohydr. Polym. 2013, 98, 73–79. [Google Scholar] [CrossRef]
- Peng, X.; Yang, G.; Yue, Q.; Ren, X.; Zhou, Y.; Zhang, M. The Film-Forming Characterization and Structural Analysis of Pectin from Sunflower Heads. Int. J. Polym. Sci. 2021, 2021, 8859108. [Google Scholar] [CrossRef]
- Yadav, I.; Nayak, S.K.; Rathnam, V.S.; Banerjee, I.; Ray, S.S.; Anis, A.; Pal, K. Reinforcing effect of graphene oxide reinforcement on the properties of poly (vinyl alcohol) and carboxymethyl tamarind gum based phase-separated film. J. Mech. Behav. Biomed. Mater. 2018, 81, 61–71. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A. Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res. Int. 2000, 33, 571–578. [Google Scholar] [CrossRef]
- Tomé, L.C.; Silva, N.H.; Soares, H.R.; Coroadinha, A.S.; Sadocco, P.; Marrucho, I.M.; Freire, C.S. Bioactive transparent films based on polysaccharides and cholinium carboxylate ionic liquids. Green Chem. 2015, 17, 4291–4299. [Google Scholar] [CrossRef]
- Hendrawan, H.; Khoerunnisa, F.; Sonjaya, Y.; Putri, A.D. Poly (vinyl alcohol)/glutaraldehyde/Premna oblongifolia merr extract hydrogel for controlled-release and water absorption application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012048. [Google Scholar] [CrossRef]
- Razmjoo, F.; Sadeghi, E.; Rouhi, M.; Mohammadi, R.; Noroozi, R.; Safajoo, S. Polyvinyl alcohol–Zedo gum edible film: Physical, mechanical and thermal properties. J. Appl. Polym. Sci. 2021, 138, 49875. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, H.; Tian, Y.; Song, Z.; Lai, P.F.; Ai, L. Composition and rheological properties of polysaccharide extracted from tamarind (Tamarindus indica L.) seed. Molecules 2019, 24, 1218. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.; Kerry, J.P.; de la Caba, K. FTIR characterization of protein–polysaccharide interactions in extruded blends. Carbohydr. Polym. 2014, 111, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Boroglu, M.; Cavus, S.; Boz, I.; Ata, A. Synthesis and characterization of poly (vinyl alcohol) proton exchange membranes modified with 4, 4-diaminodiphenylether-2, 2-disulfonic acid. Express Polym. Lett. 2011, 5, 470–478. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, S.; Huang, L.; Wang, H.; Wang, W.; Ye, Q. Starch-based hydrogel loading with carbendazim for controlled-release and water absorption. Carbohydr. Polym. 2015, 125, 376–383. [Google Scholar] [CrossRef]
- Parida, U.K.; Nayak, A.K.; Binhani, B.K.; Nayak, P. Synthesis and characterization of chitosan-polyvinyl alcohol blended with cloisite 30B for controlled release of the anticancer drug curcumin. J. Biomater. Nanobiotechnol. 2011, 2, 414. [Google Scholar] [CrossRef]
- Kumar, G.H.; Rao, J.L.; Gopal, N.; Narasimhulu, K.; Chakradhar, R.; Rajulu, A.V. Spectroscopic investigations of Mn2+ ions doped polyvinylalcohol films. Polymer 2004, 45, 5407–5415. [Google Scholar] [CrossRef]
- Copikova, J.; Cerna, M.; Novotna, M.; Kaasova, J.; Synytsya, A. Application of FT-IR spectroscopy in detection of food hydrocolloids confectionery jellies and in food supplements. Czech J. Food Sci. 2001, 19, 51–56. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.; Harnkarnsujarit, N. Morphology and permeability of bio-based poly (butylene adipate-co-terephthalate)(PBAT), poly (butylene succinate)(PBS) and linear low-density polyethylene (LLDPE) blend films control shelf-life of packaged bread. Food Control 2022, 132, 108541. [Google Scholar] [CrossRef]
- Paul, S.R.; Nayak, S.K.; Yogalakshmi, Y.; Singh, V.K.; Rath, A.; Banerjee, I.; Anis, A.; Pal, K. Understanding the effect of tamarind gum proportion on the properties of tamarind gum-based hydroethanolic physical hydrogels. Polym.-Plast. Technol. Eng. 2018, 57, 540–547. [Google Scholar] [CrossRef]
- Satapathy, M.; Quereshi, D.; Hanh Nguyen, T.T.; Pani, D.; Mohanty, B.; Anis, A.; Maji, S.; Kim, D.; Sarkar, P.; Pal, K. Preparation and characterization of cocoa butter and whey protein isolate based emulgels for pharmaceutical and probiotics delivery applications. J. Dispers. Sci. Technol. 2020, 41, 426–440. [Google Scholar] [CrossRef]
- Dhal, S.; Mohanty, A.; Yadav, I.; Uvanesh, K.; Kulanthaivel, S.; Banerjee, I.; Pal, K.; Giri, S. Magnetic nanoparticle incorporated oleogel as iontophoretic drug delivery system. Colloids Surf. B Biointerfaces 2017, 157, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, P.; Cesaro, U.; Moccaldi, N. Noninvasive measurement of transdermal drug delivery by impedance spectroscopy. Sci. Rep. 2017, 7, 44647. [Google Scholar] [CrossRef]
- Sharma, V.; Patnaik, P.; Senthilguru, K.; Nayak, S.K.; Syed, I.; Singh, V.K.; Sarkar, P.; Thakur, G.; Pal, K. Preparation and characterization of novel tamarind gum-based hydrogels for antimicrobial drug delivery applications. Chem. Pap. 2018, 72, 2101–2113. [Google Scholar] [CrossRef]
- Pandey, P.M.; Banerjee, I.; Anis, A.; Pal, K. An Insight on the Swelling, Viscoelastic, Electrical, and Drug Release Properties of Gelatin–Carboxymethyl Chitosan Hydrogels. Polym.-Plast. Technol. Eng. 2018, 57, 404–416. [Google Scholar] [CrossRef]
- Biswal, D.; Anupriya, B.; Uvanesh, K.; Anis, A.; Banerjee, I.; Pal, K. Effect of mechanical and electrical behavior of gelatin hydrogels on drug release and cell proliferation. J. Mech. Behav. Biomed. Mater. 2016, 53, 174–186. [Google Scholar] [CrossRef]
- Rawooth, M.; Qureshi, D.; Hoque, M.; Prasad, M.G.; Mohanty, B.; Alam, M.A.; Anis, A.; Sarkar, P.; Pal, K. Synthesis and characterization of novel tamarind gum and rice bran oil-based emulgels for the ocular delivery of antibiotics. Int. J. Biol. Macromol. 2020, 164, 1608–1620. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Gupta, V.; Sharma, B. Role of phytochemicals in neurotrophins mediated regulation of Alzheimer’s disease. Int. J. Complement. Alt. Med. 2017, 7, 00231. [Google Scholar]
- Lian, R.; Cao, J.; Jiang, X.; Rogachev, A.V. Prolonged release of ciprofloxacin hydrochloride from chitosan/gelatin/poly (vinyl alcohol) composite films. Mater. Today Commun. 2021, 27, 102219. [Google Scholar] [CrossRef]
- David, J.; Mahanty, B. Optimized ciprofloxacin release from citric acid crosslinked starch-PVA hydrogel film: Modelling with mixture design. J. Polym. Res. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Functional Biopolymers; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]






| Study | Model | Parameter | Formulations | ||||
|---|---|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F4 | |||
| % swelling | Korsmeyer -Peppas | k | 54.64 ± 8.05 a | 158.99 ± 10.18 bd | 176.03 ± 6.43 bc | 161.59 ± 4.95 d | 75.02 ± 4.60 e |
| n | 0.42 ± 0.07 abcd | 0.38 ± 0.02 a | 0.53 ± 0.01 b | 0.54 ± 0.01 bc | 0.32 ± 0.02 d | ||
| R2 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | ||
| Peppas -Shalin | kd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| kr | 54.64 ± 8.05 a | 158.99 ± 10.18 bd | 176.03 ± 6.43 bc | 161.59 ± 4.95 d | 75.02 ± 4.60 e | ||
| m | 0.21 ± 0.04 abcd | 0.19 ± 0.01 a | 0.26 ± 0.01 b | 0.27 ± 0.00 bc | 0.16 ± 0.01 d | ||
| kd/kr | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| R2 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | ||
| Frequency Range (cm−1) | Functional Group | References |
|---|---|---|
| 3298 | Stretching vibration (-OH) | [56] |
| 2933.1 | Stretching of the alkyl (C-H) | [57] |
| 1721.1 | Stretching vibration carbonyl (C=O) | [58,59] |
| 1655.1 | stretching vibration (C=C) | [60] |
| 1564.4,1424.3 | Bending vibration (-CH2) | [60] |
| 1370.7, 1327.4 | Bending vibration of -CH3) | [61,62,63] |
| 1249.1 | C-O-C | [60] |
| 1086.2, 1032.6 | Stretching(C-O) | [64] |
| 958.4 | Bending vibration of Alkyl (C-H) | [64] |
| 836.8 | Rocking vibration of Alkyl (C-H) | [64] |
| Model | Parameter | F0 | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|---|
| ƒ max | 1517.04 ± 95.38 | 1078.27 ± 152.33 | 1439.90 ± 155.14 | 1152.66 ± 247.09 | 2373.57 ± 140.98 | |
| ƒ min | 286.71 ± 31.61 | 197.07 ± 34.72 | 276.38 ± 20.92 | 230.00 ± 40.35 | 351.46 ± 80.35 | |
| %SR | 81.14 ± 0.93 | 81.76 ± 0.82 | 80.72 ± 1.21 | 79.91 ± 1.21 | 85.12 ± 5.39 | |
| Weichert model | P0 | 0.19 ± 0.01 a | 0.19 ± 0.01 b | 0.20 ± 0.01 c | 0.21 ± 0.02 d | 0.15± 0.05 e |
| P1 | 0.52 ± 0.01 a | 0.51 ± 0.01 b | 0.52± 0.01 c | 0.52 ± 0.01 d | 0.53 ± 0.03 e | |
| τ1 | 0.82 ± 0.04 a | 0.83 ± 0.01 b | 0.79 ± 0.03 c | 0.72 ± 0.02 abcd | 0.83± 0.03 d | |
| P2 | 0.27± 0.01 a | 0.29 ± 0.01 b | 0.27 ± 0.00 c | 0.268 ± 0.00 d | 0.312 ± 0.03 e | |
| τ2 | 12.76± 0.66 a | 12.64 ± 0.35 b | 12.93 ± 2.11 c | 12.33 ± 0.73 d | 14.60 ± 0.50 ab | |
| R2 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Parameters | Formulations | ||||
|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F4 | |
| R (Q) | 17.87 | 19.51 | 19.07 | 61.19 | 518.06 |
| Q (µF) | 355.20 | 334.56 | 372.78 | 154.34 | 20.09 |
| Q1 (µF) | 2.67 | 2.67 | 2.67 | 2.67 | 2.67 |
| n | 0.84 | 0.81 | 0.82 | 0.85 | 0.99 |
| n1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| R2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Study | Model | Parameter | Formulations | ||||
|---|---|---|---|---|---|---|---|
| F0D | F1D | F2D | F3D | F4D | |||
| Diffusion | Korsemeyer –Peppas | k | 1.37 ± 0.29 | 1.67 ± 0.05 | 2.29 ± 0.43 | 0.80 ± 0.13 | 0.67 ± 0.11 |
| n | 0.62 ± 0.06 | 0.58 ± 0.01 | 0.51 ± 0.04 | 0.75 ± 0.03 | 0.77 ± 0.04 | ||
| R2 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | ||
| Peppas –Shalin | kd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| kr | 1.36 ± 0.29 | 1.67 ± 0.05 | 2.01 ± 0.21 | 0.80 ± 0.13 | 0.67 ± 0.11 | ||
| m | 0.31 ± 0.03 | 0.29 ± 0.00 | 0.27 ± 0.01 | 0.37 ± 0.01 | 0.39 ± 0.02 | ||
| kd/kr | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| R2 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | ||
| Composition | Formulations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F4 | F0D | F1D | F2D | F3D | F4D | |
| PVA solution (g) | 20 | 18 | 18 | 18 | 18 | 20 | 18 | 18 | 18 | 18 |
| Tamarind gum suspension (g) | -- | 2 (1% w/v) | 2 (2.5% w/v) | 5 (5% w/v) | 2 (10% w/v) | -- | 2 (1% w/v) | 2 (2.5% w/v) | 5 (5% w/v) | 2 (10% w/v) |
| Ciprofloxacin HCl (g) | -- | -- | -- | -- | -- | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Crosslinking solutions (mL) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Distilled water (g) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawooth, M.; Habibullah, S.; Qureshi, D.; Bharti, D.; Pal, A.; Mohanty, B.; Jarzębski, M.; Smułek, W.; Pal, K. Effect of Tamarind Gum on the Properties of Phase-Separated Poly(vinyl alcohol) Films. Polymers 2022, 14, 2793. https://doi.org/10.3390/polym14142793
Rawooth M, Habibullah S, Qureshi D, Bharti D, Pal A, Mohanty B, Jarzębski M, Smułek W, Pal K. Effect of Tamarind Gum on the Properties of Phase-Separated Poly(vinyl alcohol) Films. Polymers. 2022; 14(14):2793. https://doi.org/10.3390/polym14142793
Chicago/Turabian StyleRawooth, Madhusmita, SK Habibullah, Dilshad Qureshi, Deepti Bharti, Ankit Pal, Biswaranjan Mohanty, Maciej Jarzębski, Wojciech Smułek, and Kunal Pal. 2022. "Effect of Tamarind Gum on the Properties of Phase-Separated Poly(vinyl alcohol) Films" Polymers 14, no. 14: 2793. https://doi.org/10.3390/polym14142793
APA StyleRawooth, M., Habibullah, S., Qureshi, D., Bharti, D., Pal, A., Mohanty, B., Jarzębski, M., Smułek, W., & Pal, K. (2022). Effect of Tamarind Gum on the Properties of Phase-Separated Poly(vinyl alcohol) Films. Polymers, 14(14), 2793. https://doi.org/10.3390/polym14142793










