Daptomycin-Biomineralized Silver Nanoparticles for Enhanced Photothermal Therapy with Anti-Tumor Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dap-AgNPs
2.3. Preparation of AgNPs
2.4. Determination of Peroxidase-like Property
2.5. Photothermal Performance of Dap-AgNPs
2.6. Cytotoxicity of Dap-AgNPs
2.7. Anti-Tumor Effect In Vitro
3. Results and Discussion
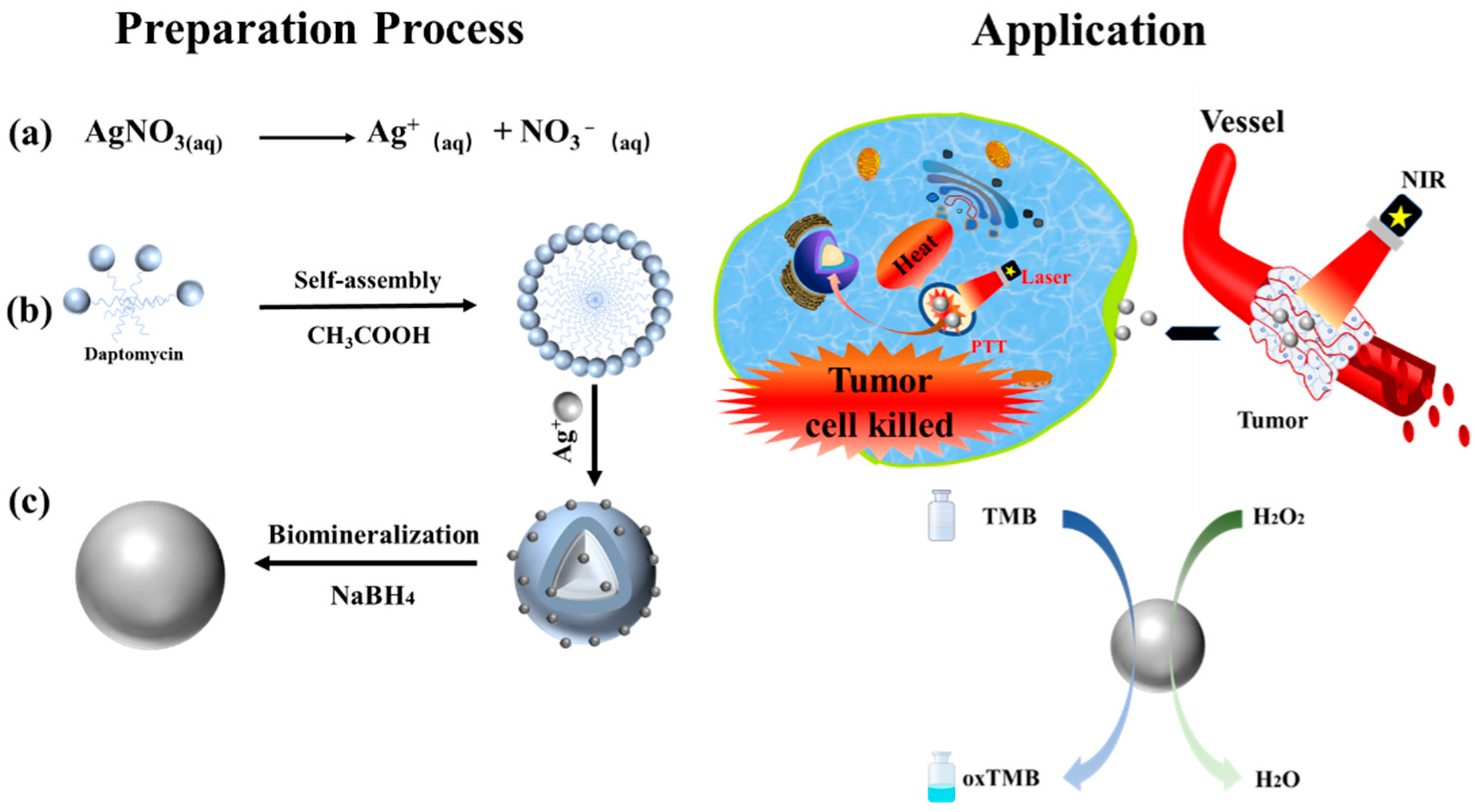
3.1. Preparation of Dap-AgNPs
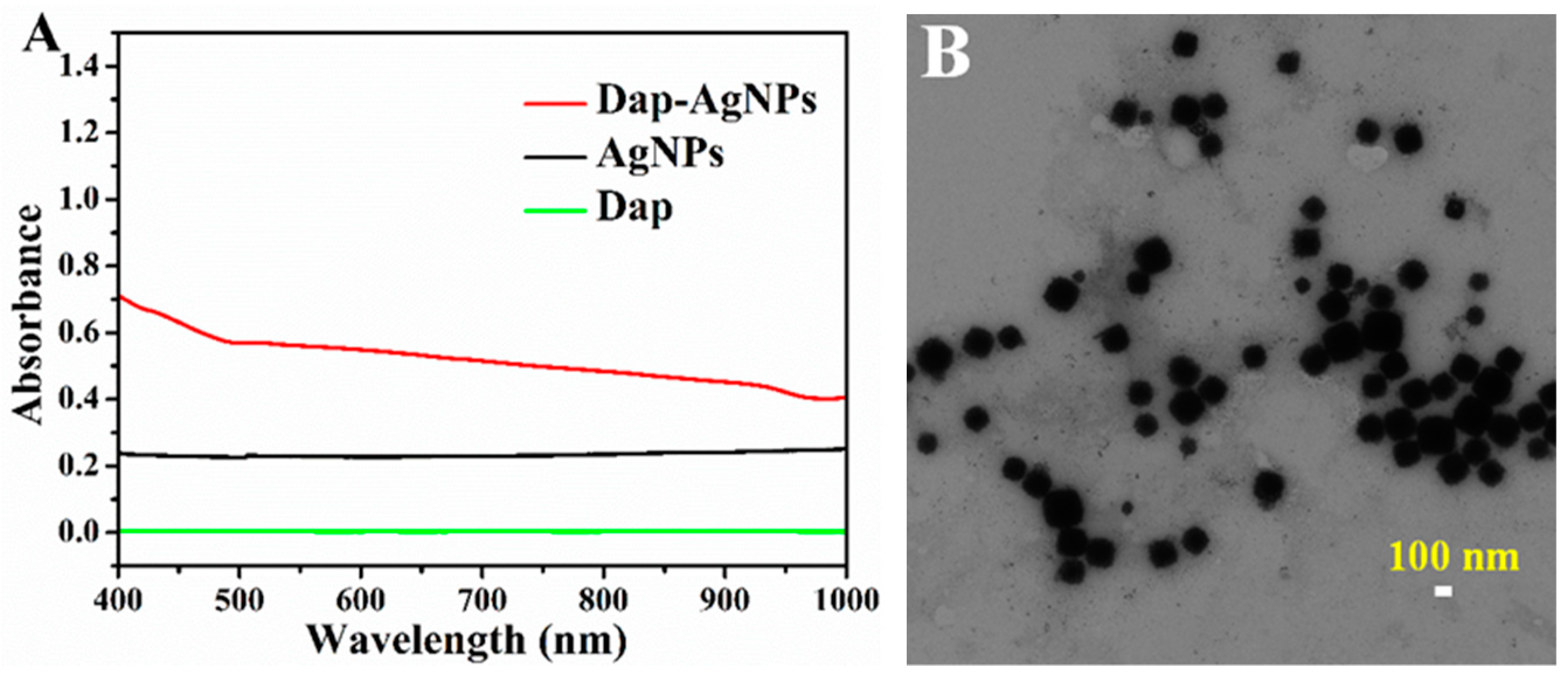
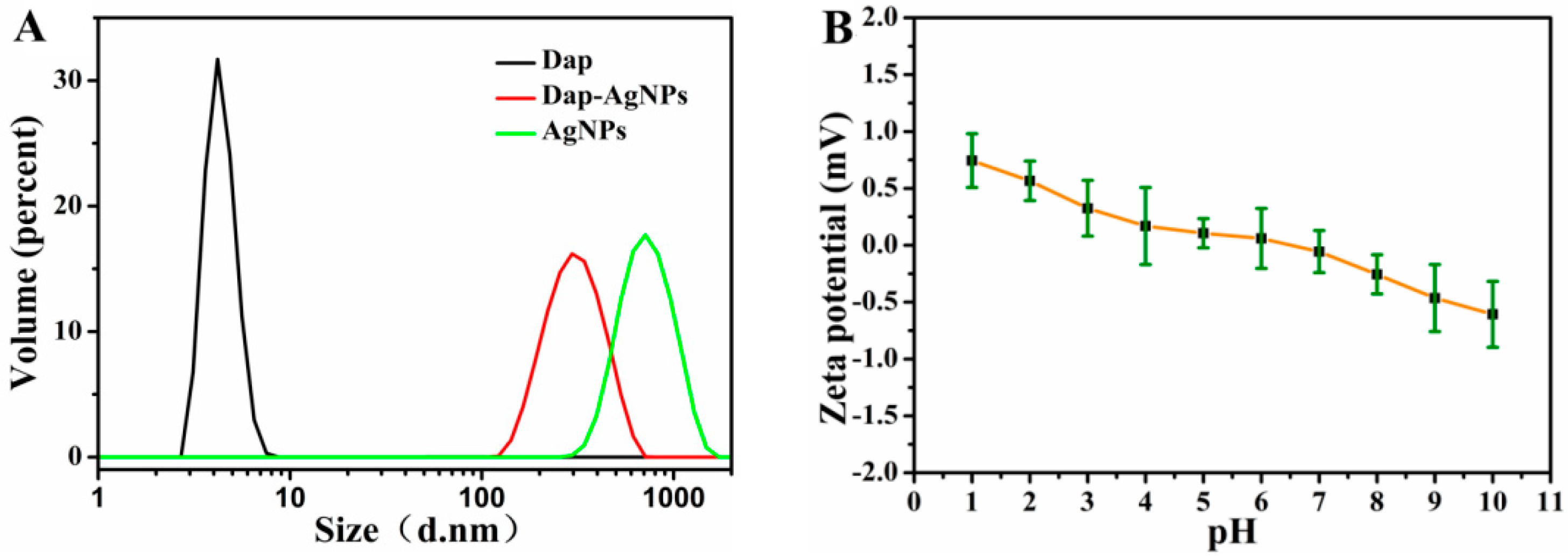
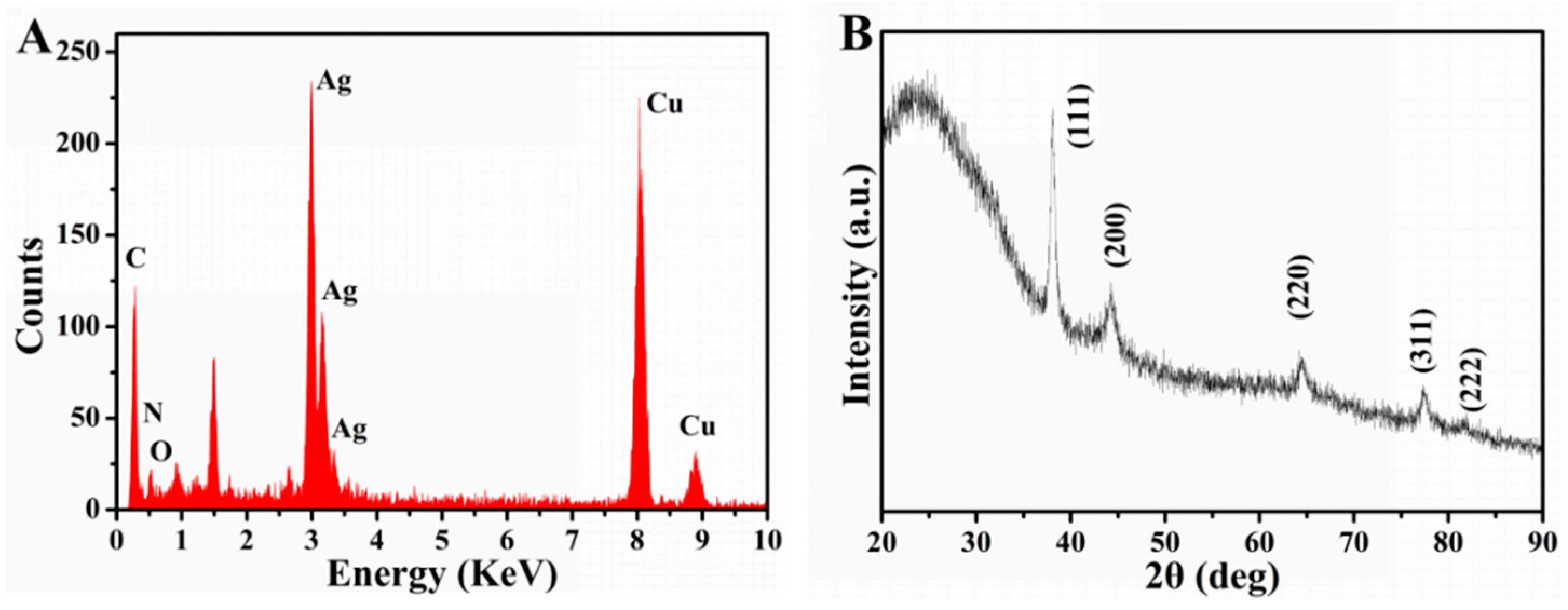
3.2. Characterization of Dap-AgNPs
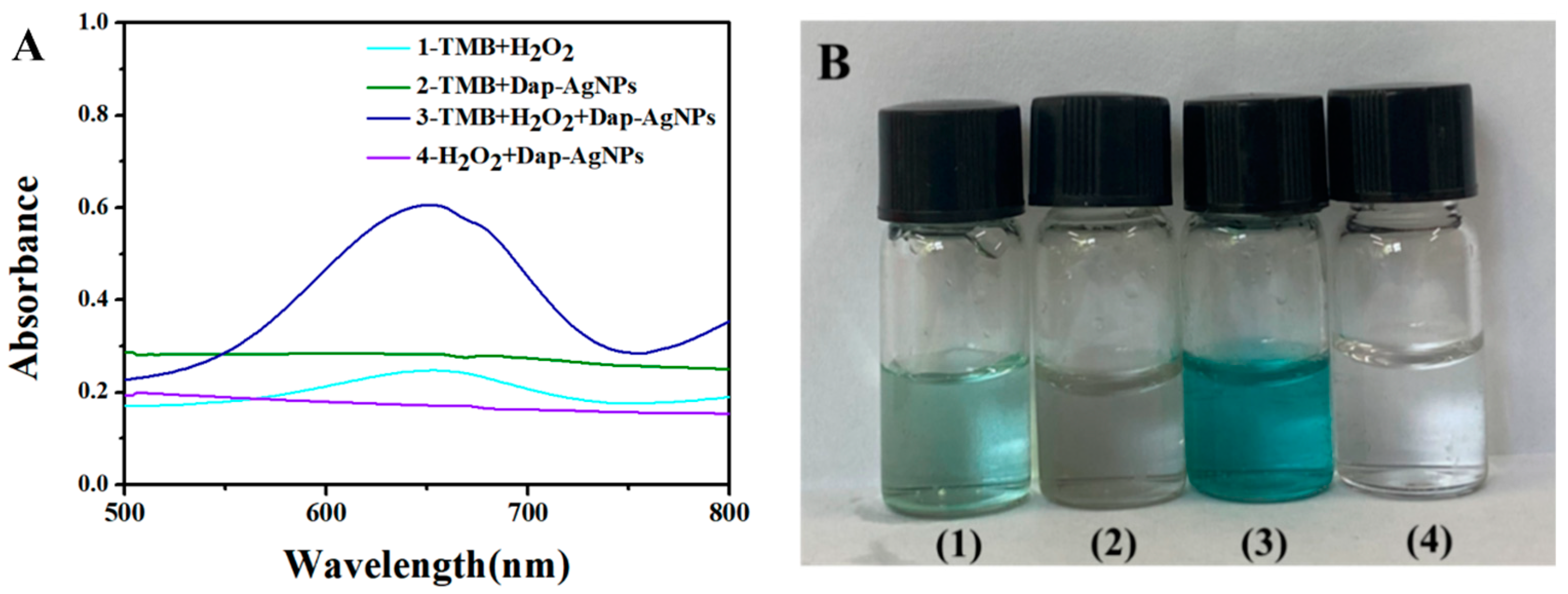
3.3. Peroxidase-like Property of Dap-AgNPs
3.4. Photothermal Performance of Dap-AgNPs
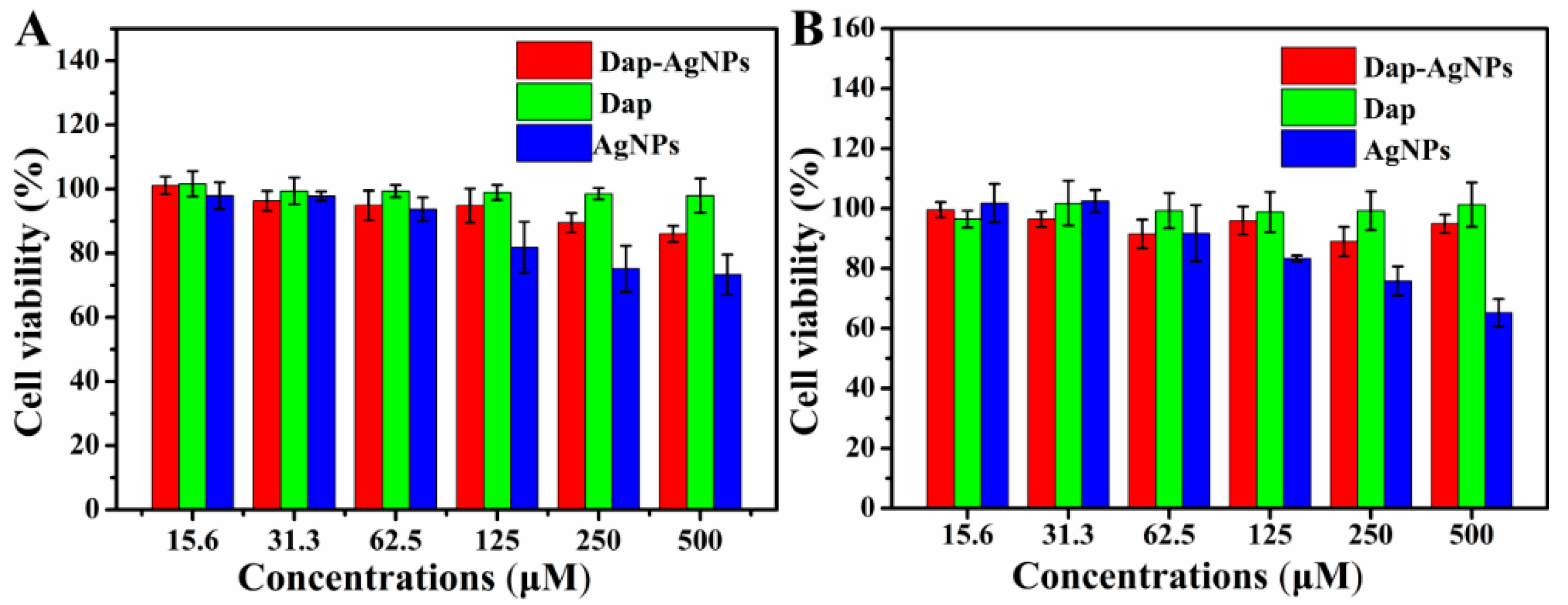
3.5. Cytotoxicity of Dap-AgNPs
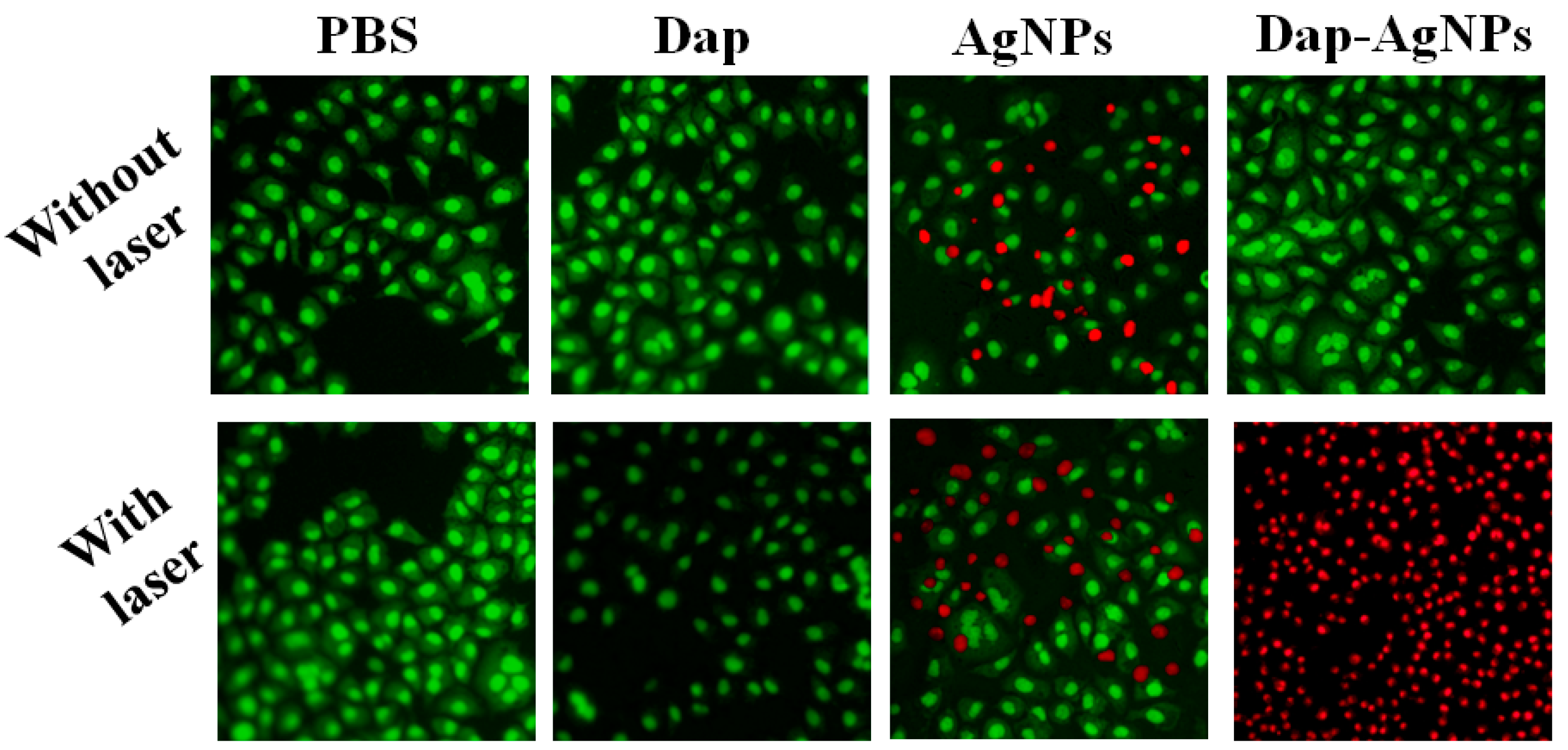
3.6. Anti-Tumor Performance of Dap-AgNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Dutta, T.; Tyagi, A.; Bharti, A.C.; Das, B.; Jain, N.K. Surface-engineered dendrimers for dual drug delivery: A receptor up-regulation and enhanced cancer targeting strategy. J. Drug Target. 2008, 16, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Yang, G.; Yang, P. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, B.; Shen, S.; Tuo, Y.; Luo, Z.; Hu, Y.; Pang, Z.; Jiang, X. Tumor microenvironment modulation by cyclopamine improved photothermal therapy of biomimetic gold nanorods for pancreatic ductal adenocarcinomas. ACS Appl. Mater. Interfaces 2017, 9, 31497–31508. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Lin, H.; Kannan, P. Enhanced antibacterial and food simulant activities of silver nanoparticles/polypropylene nanocomposite films. Langmuir 2018, 34, 14537–14545. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, J.; Liu, S. Self-assembly synthesis of flower-like gold nanoparticles for photothermal treatment of cancer. Colloids Surf. A 2022, 647, 129163. [Google Scholar] [CrossRef]
- Li, X.; Xiao, H.; Xiu, W. Mitochondria-targeting MoS2-based nanoagents for enhanced NIR-II photothermal-chemodynamic synergistic oncotherapy. ACS Appl. Mater. Interfaces 2021, 13, 55928–55938. [Google Scholar] [CrossRef]
- Palmieri, V.; Spirito, M.D.; Papi, M. Graphene-based scaffolds for tissue engineering and photothermal therapy. Nanomedicine 2020, 15, 1411–1417. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, C.; Liu, J.; Huang, F.; Zhang, Y.; Liang, X.J. ICG-conjugated and 125I-Labeled polymeric micelles with high biosafety for multimodality imaging-guided photothermal therapy of tumors. Adv. Healthc. Mater. 2020, 9, 1901616. [Google Scholar] [CrossRef]
- Hua, S.; He, J.; Zhang, F.; Yu, J.; Zhang, W.; Gao, L. Multistage-responsive clustered nanosystem to improve tumor accumulation and penetration for photothermal/enhanced radiation synergistic therapy. Biomaterials 2021, 268, 120590. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Peng, D.; Hao, H.; Hu, J.; Wang, D.; Wang, K. Thermally triggered in situ assembly of gold nanoparticles for cancer multimodal imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 10453–10460. [Google Scholar] [CrossRef]
- Chen, Q.W.; Liu, X.H.; Fan, J.X.; Peng, S.Y.; Wang, J.W.; Wang, X.N. Self-mineralized photothermal bacteria hybridizing with mitochondria-targeted metal–organic frameworks for augmenting photothermal tumor therapy. Adv. Funct. Mater. 2020, 30, 1909806. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B. Fabrication, characterization of chitosan/nanosilver film and its potential antibacterial application. J. Biomater. Sci. Polym. Ed. 2009, 20, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Y.; Zhang, L.; Fan, J.G.; Qiao, L. NAFLD leads to liver cancer: Do we have sufficient evidence? Cancer Lett. 2014, 345, 230–234. [Google Scholar] [CrossRef]
- Chang, S.; Qin, D.; Wang, L.; Zhang, M.; Yan, R.; Zhao, C.J.C. Preparation of novel cinnamaldehyde derivative–BSA nanoparticles with high stability, good cell penetrating ability, and promising anticancer activity. Colloids Surf. A 2021, 624, 126765. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J. Preparation of Au–Ag bimetallic nanoparticles for enhanced solar photothermal conversion. Int. J. Heat Mass Transf. 2017, 114, 1098–1104. [Google Scholar] [CrossRef]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D. ICG-loaded pegylated BSA-silver nanoparticles for effective photothermal cancer therapy. Int. J. Nanomed. 2020, 15, 5459. [Google Scholar] [CrossRef]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-silver nanoparticles: A potential multimodal therapeutics for conventional and photothermal treatment of skin cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef]
- Bian, K.; Zhang, X.; Liu, K.; Tian, Y.; Gao, D. Peptide-directed hierarchical mineralized silver nanocages for anti-tumor photothermal therapy. ACS Sustain. Chem. Eng. 2018, 6, 7574–7588. [Google Scholar] [CrossRef]
- Tong, C.; Li, L.; Xiao, F.; Fan, J.; Zhong, X.; Liu, X. Daptomycin and AgNP co-loaded rGO nanocomposites for specific treatment of Gram-positive bacterial infection in vitro and in vivo. Biomater. Sci. 2019, 7, 5097–5111. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, L.; He, M.; Jiang, X.; Tian, J.; Li, Q. Doxorubicin-loaded natural daptomycin micelles with enhanced targeting and anti-tumor effect in vivo. Eur. J. Med. Chem. 2021, 222, 113582. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, R.; Wang, L.; Lan, X.; Sun, H.; Zhao, Y. Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose. Int. J. Biol. Macromol. 2021, 172, 289–298. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Gao, S.; Cui, Y.; Ji, X.; Zhang, X. Biomineralized synthesis of palladium nanoflowers for photothermal treatment of cancer and wound healing. Int. J. Pharm. 2022, 615, 121489. [Google Scholar] [CrossRef]
- Jung, R.; Kim, Y.; Kim, H.S. Antimicrobial properties of hydrated cellulose membranes with silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2009, 20, 311–324. [Google Scholar] [CrossRef]
- Yu, N.; Cai, T.; Sun, Y.; Jiang, C.; Xiong, H.; Li, Y. A novel antibacterial agent based on AgNPs and Fe3O4 loaded chitin microspheres with peroxidase-like activity for synergistic antibacterial activity and wound-healing. Int. J. Pharm. 2018, 552, 277–287. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Fan, L.; Li, R.; Cui, Y.; Ji, X. Zwitterionic daptomycin stabilized palladium nanoparticles with enhanced peroxidase-like properties for glucose detection. Colloids Surf. A 2022, 633, 127797. [Google Scholar] [CrossRef]
- Shiny, P.J.; Mukherjee, A.; Chandrasekaran, N. DNA damage and mitochondria-mediated apoptosis of A549 lung carcinoma cells induced by biosynthesised silver and platinum nanoparticles. RSC Adv. 2016, 6, 27775–27787. [Google Scholar]
- He, M.; Han, Z.; Liang, Y.; Zhao, H.; Ji, X.; Ma, G.; Cui, Y.; Wang, L. Green synthesis of Ag nanoparticles using elm pod polysaccharide for catalysis and bacteriostasis. Int. J. Biol. Macromol. 2022, 213, 1078–1087. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, J.; Fan, G.; Zhang, B.; Ma, G.; Xiao, H.; Wang, L. Daptomycin-Biomineralized Silver Nanoparticles for Enhanced Photothermal Therapy with Anti-Tumor Effect. Polymers 2022, 14, 2787. https://doi.org/10.3390/polym14142787
Zhang J, Wang J, Fan G, Zhang B, Ma G, Xiao H, Wang L. Daptomycin-Biomineralized Silver Nanoparticles for Enhanced Photothermal Therapy with Anti-Tumor Effect. Polymers. 2022; 14(14):2787. https://doi.org/10.3390/polym14142787
Chicago/Turabian StyleZhang, Jie, Jing Wang, Guixiu Fan, Bingjie Zhang, Guanglong Ma, Haiyan Xiao, and Longgang Wang. 2022. "Daptomycin-Biomineralized Silver Nanoparticles for Enhanced Photothermal Therapy with Anti-Tumor Effect" Polymers 14, no. 14: 2787. https://doi.org/10.3390/polym14142787
APA StyleZhang, J., Wang, J., Fan, G., Zhang, B., Ma, G., Xiao, H., & Wang, L. (2022). Daptomycin-Biomineralized Silver Nanoparticles for Enhanced Photothermal Therapy with Anti-Tumor Effect. Polymers, 14(14), 2787. https://doi.org/10.3390/polym14142787





