Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances
Abstract
1. Introduction
| Brand Name | Manufacturer | Composition | Indication | Biodegradation Duration | Refs |
|---|---|---|---|---|---|
| Homopolymer (first generation) | |||||
| Biofix SR-PGA | Bionx Implants (Tampere, Finland) | 100% SR PGA | Midface and mandible fractures and osteotomies | LM: 36 months | [27,28] |
| Biofix SR-PLLA | Bionx Implants (Tampere, Finland) | 100% SR PLLA | Midface and mandible fractures and osteotomies | LM: >54 months | [27,28] |
| FIXORB-MX | Teijin Medical Technologies Co., Ltd. (Osaka, Japan) | 100% PLLA | Midface and mandible fractures and osteotomies | LM: >3 years | [22] |
| GrandFix | Gunze (Kyoto, Japan) | 100% PLLA | Midface and mandible fractures and osteotomies | LM: >3 years | [22,29,30,31] |
| Copolymer (second generation) | |||||
| BioSorb FX | ConMed Linvatec Biomaterials Ltd. (Tampere, Finland) | 70% SR PLLA, 30% SR PDLLA | Midface fractures and osteotomies, and mandibular symphysis factures | SEM with EDX: >4 years | [20,25] |
| Delta | Stryker (Kalamazoo, MI, USA) | 85% PLLA, 10% PGA, 5% PDLA | Midface fractures and osteotomies | Visual inspection: 8–13 months | [18,32] |
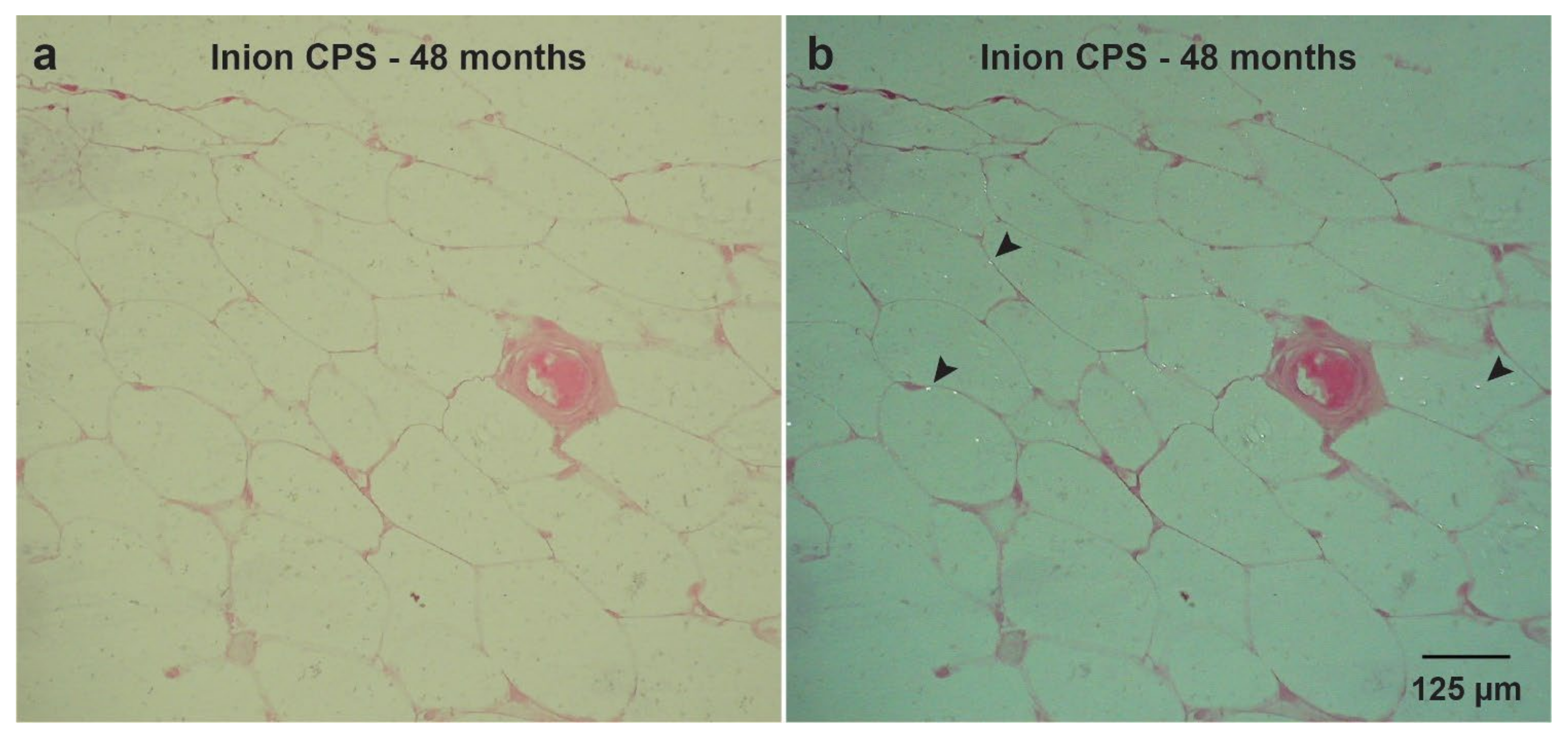
| Inion CPS | Inion Oy (Tampere, Finland) | 70–78.5% PLLA, 16–24% PDLLA, 4% TMC 1 | Midface and mandible fractures and osteotomies | SEM with EDX: >4 years | [20,25] |
| Inion CPS Baby | Inion Oy (Tampere, Finland) | 82% PLLA, 12% PGA, 6% TMC | Cranial reconstructions, including midface and mandibular fracture fixation, in pediatric patients | Ultrasonography: 2–3 years | [33,34] |
| LactoSorb | Biomet Microfixation (Jacksonville, FL, USA) | 82% PLLA, 18% PGA | Midface fractures and osteotomies | SEM with EDX: >4 years | [18,20,25] |
| Macropore | Medtronic, Inc. (Minneapolis, MN, USA) | 70% PLLA, 30% PDLLA | Midface fractures and osteotomies | Unknown | [20] |
| MacroSorb | Medtronic, Inc. (Minneapolis, MN, USA) | 70% PLLA, 30% PDLLA | Midface and mandible fractures and osteotomies | LM: >12 months | [27,35] |
| Polymax | Synthes (Oberdorf, Switzerland) | 70% PLLA, 30% PDLLA | Midface and mandible fractures and osteotomies | LM: >12 months | [20,27,35] |
| Polymax RAPID | Synthes (Oberdorf, Switzerland) | 85% PLLA, 15% PGA | Midface and mandible fractures and osteotomies | Unknown | [27] |
| RapidSorb | DePuy Synthes (West Chester, PA, USA) | 70% PLLA, 30% PDLLA | Midface fractures and osteotomies | In vitro: 12 months | [20,22] |
| Resomer | Evonik Industries (Darmstad, Germany) | 50% PLLA, 50% PDLLA | Midface fractures and osteotomies | Unknown | [27] |
| ResorbX | KLS Martin Group (Gebrüder Martin GmbH & Co., Tuttlingen, Germany) | 100% PDLLA | Midface fractures and osteotomies | LM: 12–30 months | [18,20] |
| SonicWeld Rx | KLS Martin Group (Gebrüder Martin GmbH & Co., Tuttlingen, Germany) | 100% PDLLA | Midface fractures and osteotomies | SEM with EDX: >4 years | [20,25] |
| SonicWeld xG | KLS Martin Group (Gebrüder Martin GmbH & Co., Tuttlingen, Germany) | 85% PLLA, 15% PGA | Midface fractures and osteotomies | LM: 12–14 months | [18,20] |
| Biocomposite (third generation) | |||||
| OsteotransMX | Teijin Medical Technologies Co., Ltd. (Osaka, Japan) | Plate: 60% PLLA, 40% uHA Screw: 70% PLLA, 30% uHA | Midface and mandible fractures and osteotomies | LM: 5.5 years | [20,22,36,37] |
2. Pre-Clinical Evidence
2.1. Biocompatibility
2.1.1. Initial Host Response
2.1.2. Synthetic Biodegradable Polymers
Biodegradation
Late Host Response
| Aspect | Ideal Properties | Method | Potential Solutions | Refs |
|---|---|---|---|---|
| Surgical handling | Easy perioperative adaptation of plates | 3D engineering | Patient specific osteosynthesis systems | [18,26,83] |
| Production process | Plate adaption at room temperature | [20] | ||
| No risk of perioperative screw breakage | Alternative application method | Ultrasound welding of thermoplastic pins instead of using conventional screws | [20] | |
| Elastic modulus of materials | Enough elastic modulus to avoid micromovements, but not stiffer than bone to avoid stress-shielding of the underlying bone | Production process | Create composites to tailor the elastic modulus to the application of interest | [26] |
| Self-reinforcing of polymers to increase the elastic modulus of systems | [20] | |||
| Alternative application method | Ultrasound welding of thermoplastic pins to increase the maximum tensile load and stiffness, and side-bending stiffness | [20] | ||
| Bacterial infection | Preventing bacterial adhesion to implant surface | Coating | Hydrophobic coatings | [26] |
| Eliminating surrounding bacteria without antibiotics | Surface modification | Adjusting the nano-scale surface topography (e.g., pillars on the surface) | [84] | |
| Eliminating surrounding bacteria with local antibiotics | Polymer coating containing stabilized gas bubbles loaded with antibiotics that can be released locally using ultrasound | [85] | ||
| Foreign body response (FBR) | Materials that do not elicit an FBR | Selection of materials | Materials with non-toxic degradation products (e.g., derived from silk) | [18] |
| Production process | Avoid thick materials, especially with points and sharp edges | [26,75,76] | ||
| Tailor the host response so that FBR are avoided | Production process | Avoid particle sizes < 2 µm | [26] | |
| Avoid micromovements (max. 28–150 µm), that can result in fibrous encapsulation of the implant | Selection of materials, production process, and 3D engineering | Osteosynthesis system with material properties that matches with the mechanical properties of the target tissue (e.g., by using ultrasound welding) | [26] | |
| Degradation profile | Predictable degradation, preferably after 3–12 months | 3D engineering | Thinner materials degrade quicker | [17] |
| Production process | Balance the degradation and regeneration equilibrium by, e.g., using L- and D-chirality or by copolymerization | [25,26] |
2.1.3. Biodegradable Metals
Biodegradation
Late Host Response
2.1.4. Silk
Biodegradation
Late Host Response
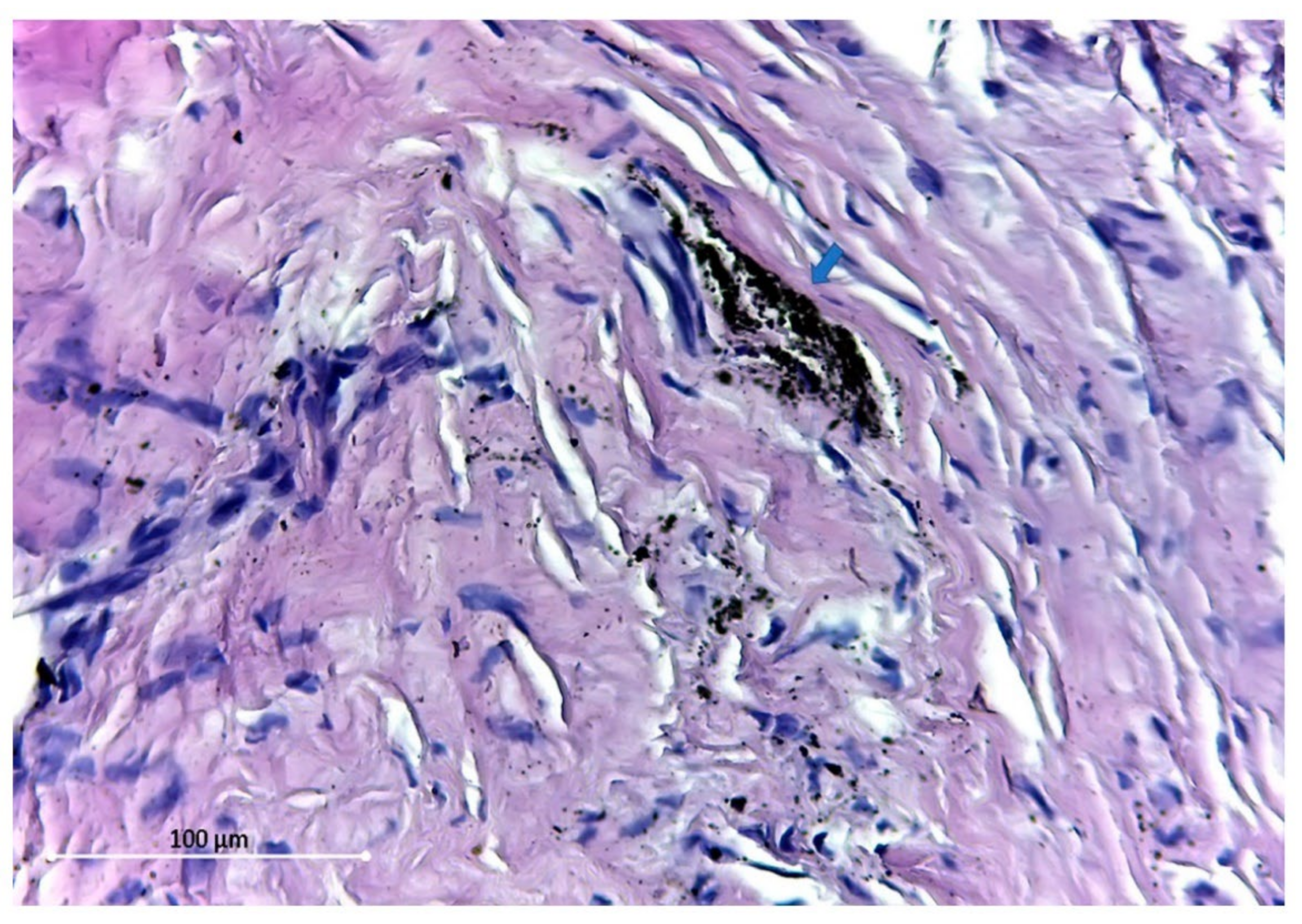
2.1.5. Titanium and Its Alloys
Late Host Response
| Aspect | Ideal Properties | Methods | Potential Solutions | Refs |
|---|---|---|---|---|
| Surgical handling | Easy perioperative adaptation of plates | 3D engineering | Patient specific osteosynthesis systems | [115,116] |
| Production process | Adaption of the production process to alter the mechanical properties of plates (e.g., lower stiffness) | [20,117,118,119,120] | ||
| No risk of perioperative screw breakage | 3D engineering | Adjusting the screw head to improve the grip on the screws | [20] | |
| Elastic modulus | Enough elastic modulus to avoid micromovements, but not stiffer than bone to avoid stress-shielding of the underlying bone | Production process | Adaption of the production process to alter the mechanical properties of plates | [20,117,118,119,120] |
| Bacterial infection | Preventing bacterial adhesion to implant surface | Coating | Hydrophobic coatings | [26] |
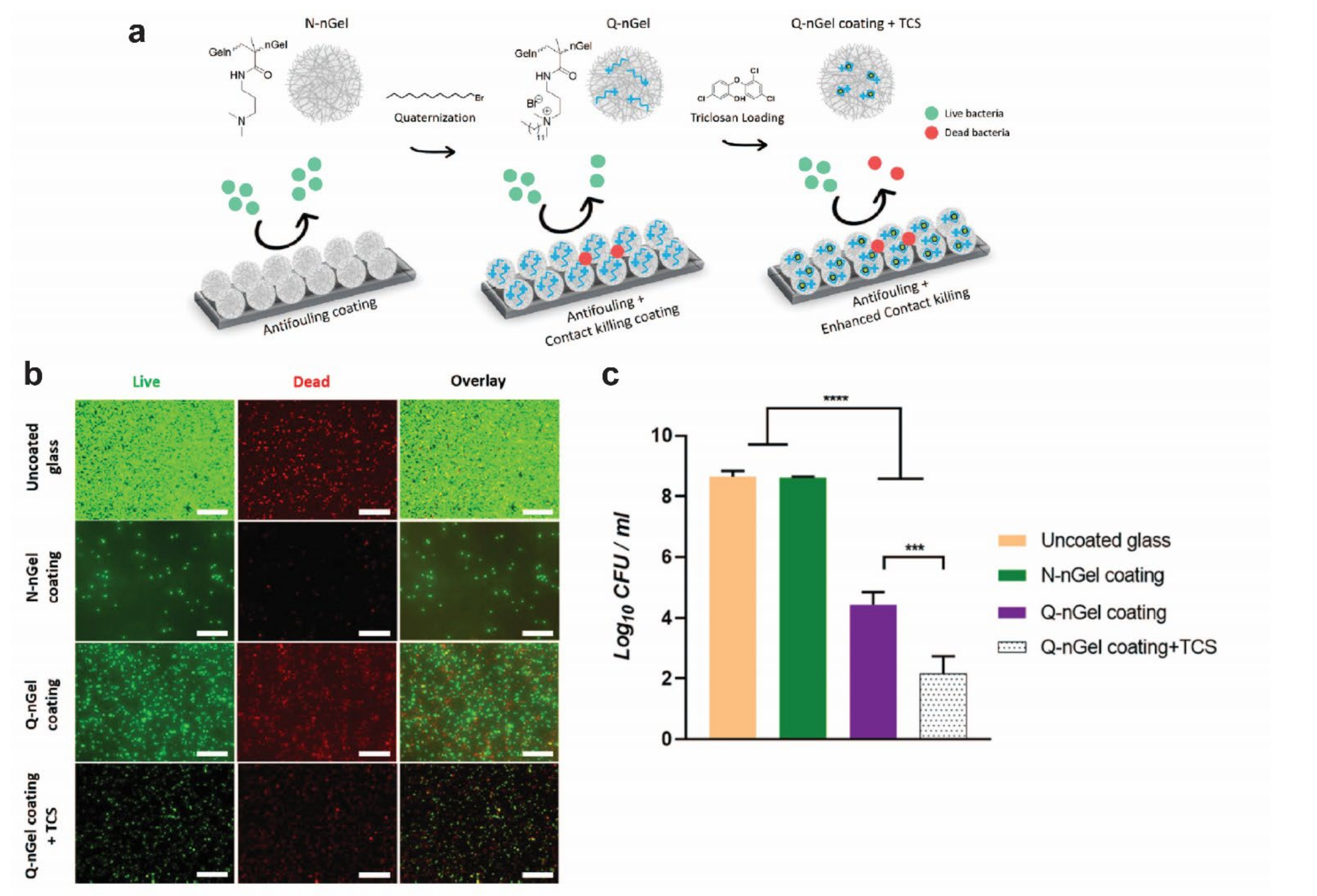
| (Nano)gel coatings | [121,122] | |||
| Surface modification | Plasma immersion ion implantation (surface modification) | [110,123,124] | ||
| Physical vapor deposition | [125,126] | |||
| Increasing surface energy by acid etching | [127] | |||
| Eliminating surrounding bacteria without antibiotics | Coating | Titanium Nitride (TiN) coating | [128,129] | |
| Surface modification | Adjusting the nano-scale surface topography (e.g., pillars on the surface) | [84] | ||
| Plasma immersion ion implantation | [110,130] | |||
| Physical vapor deposition | [131] | |||
| Laser surface modification | [132] | |||
| Anodization | [133,134] | |||
| Micro-Arc oxidation | [135,136] | |||
| Eliminating surrounding bacteria with local antibiotics | Coating | Polymer coating containing stabilized gas bubbles loaded with antibiotics that can be released locally using ultrasound | [85] | |
| (Nano)gel coatings | [122,137] | |||
| Surface modification | Chemical vapor deposition | [138] | ||
| Osteogenesis | Improving bone growth surrounding the implant | Coating | (Nano)gel coatings | [137] |
| Surface modification | Plasma spraying with hydroxyapatite | [139,140,141,142,143] | ||
| Plasma immersion ion implantation | [144,145] | |||
| Physical vapor deposition | [146,147] | |||
| Chemical vapor deposition | [148] | |||
| Increasing surface energy by acid etching | [127] | |||
| Laser surface modification | [132,149,150] | |||
| Anodization | [151] | |||
| Wear resistance | No wearing of titanium (alloy) particles | Coating | Titanium Nitride (TiN) coating | [152,153] |
| Surface modification | Plasma immersion ion implantation | [110] | ||
| Physical vapor deposition | [98] | |||
| Laser surface modification | [150,154] | |||
| Anodization | [134,155,156] |
2.2. Mechanical Properties
2.2.1. Minimally Required Mechanical Properties
2.2.2. Mechanical Properties of Osteosynthesis Systems
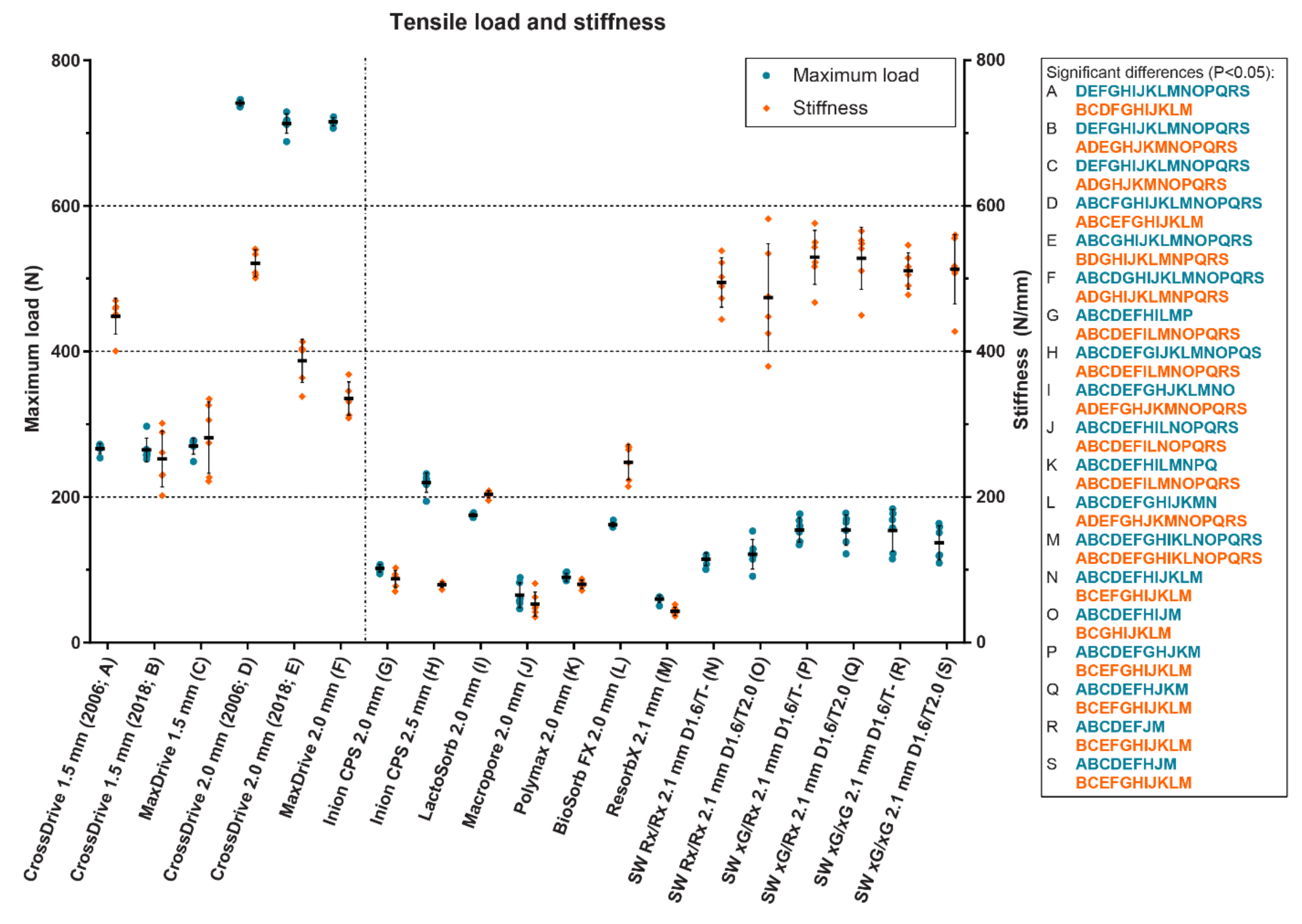
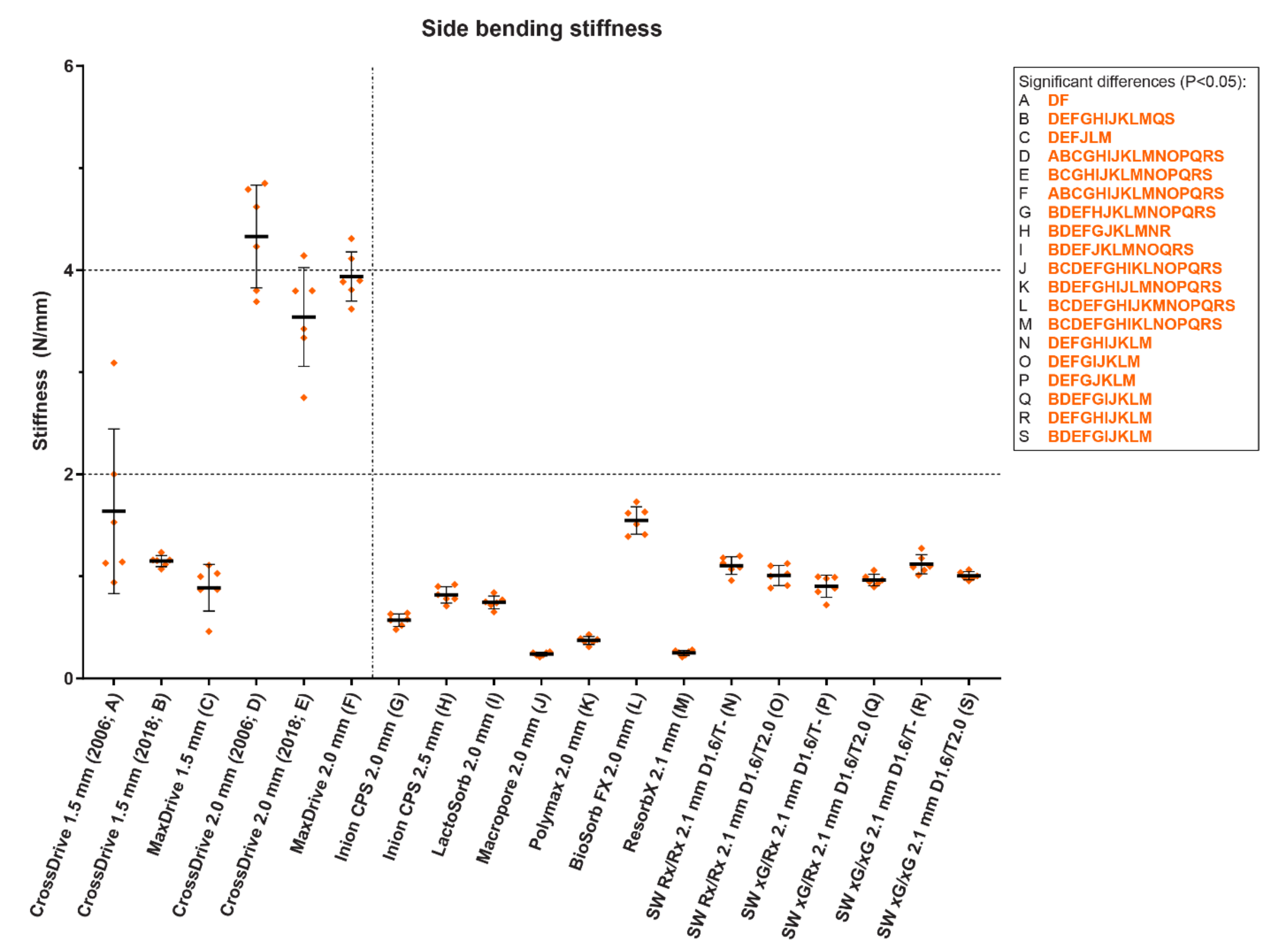
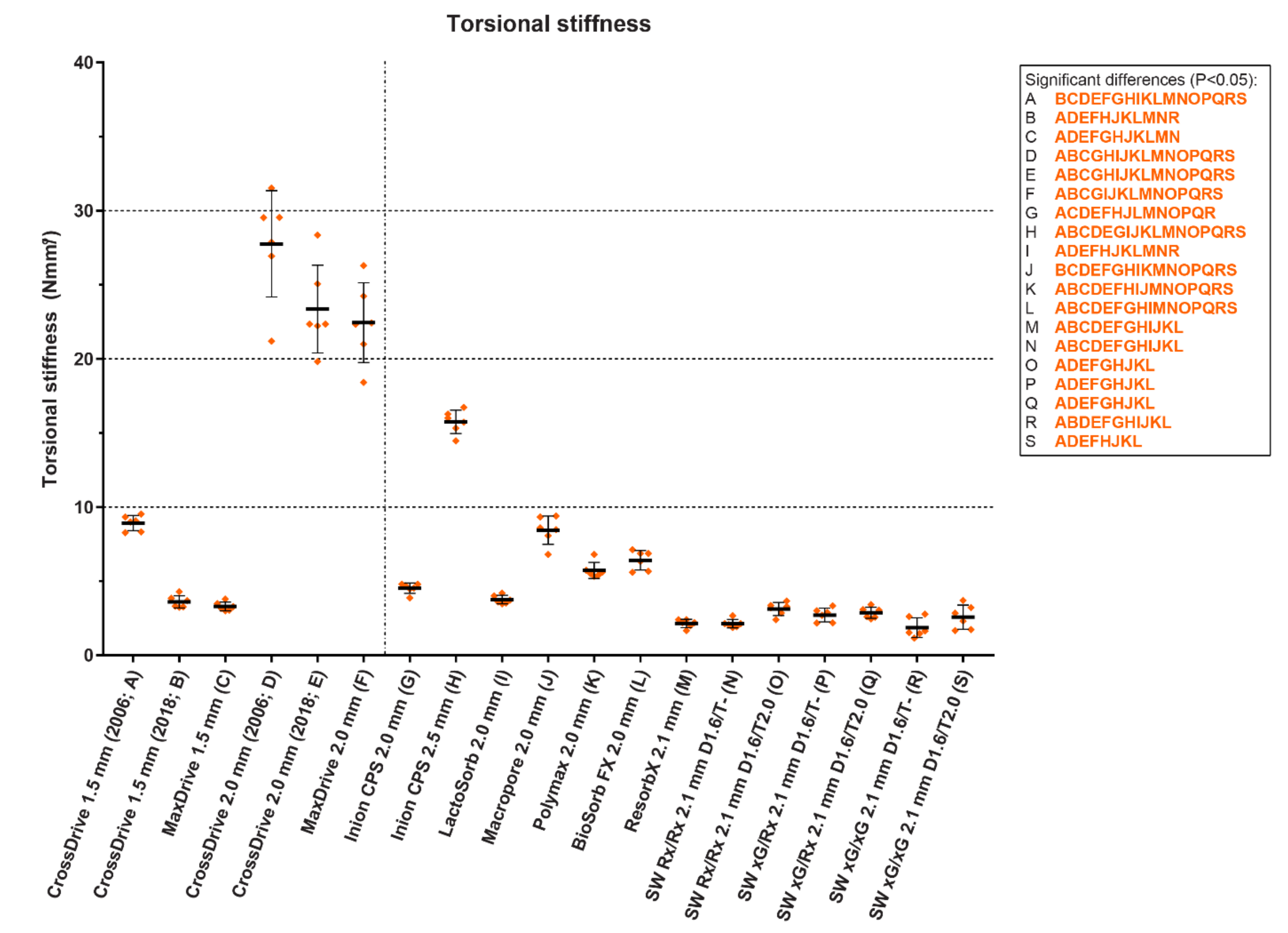
3. Clinical Evidence
3.1. Biodegradable Versus Titanium Osteosyntheses: Efficacy and Symptomatic Removal
3.2. Biodegradable Versus Titanium Osteosyntheses: Secondary Advantages
3.3. Certainty of the Current Evidence
4. Clinical Recommendations: Titanium or Biodegradable Osteosyntheses?
5. Future Perspectives
5.1. Overcoming the Disadvantages of Current Osteosynthesis Systems
5.1.1. Biodegradable (Co)Polymeric Systems
5.1.2. Titanium Systems
5.2. Outlook
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Haerle, F.; Champy, M.; Terry, B.C. Atlas of Craniomaxillofacial Osteosynthesis; Thieme: Stuttgart, Germany, 2009. [Google Scholar]
- Champy, M.; Lodde, J.P.; Wilk, A. A propos des ostèosynthéses fronto-malaires par plaques vissées. Rev. Stomat. 1975, 76, 483–488. [Google Scholar]
- Champy, M.; Lodde, J.P.; Jaeger, J.H.; Wilk, A. Ostéosynthèses mandibulaires selon la technique de Michelet. I Bases biomécaniques. Rev. Stomatol. Chir. Maxillofac. 1976, 77, 569–576. [Google Scholar] [PubMed]
- Champy, M.; Lodde, J.P.; Jaeger, J.H.; Wilk, A.; Gerber, J.C. Ostéosynthèses mandibulaires selon la technique de Michelet. II Présentation d’un nouveau matérial. Résultats. Rev. Stomatol. Chir. Maxillofac. 1976, 77, 252–255. [Google Scholar] [PubMed]
- Champy, M.; Lodde, J.P.; Muster, D.; Wilk, A.; Gastelo, L. Osteosynthesis using miniaturized screws on plates in facial and cranial surgery. Indications and results in 400 cases. Ann. Chir. Plast. 1977, 22, 261–264. [Google Scholar] [PubMed]
- Champy, M.; Lodde, J.P. Étude des contraintes dans la mandibule fracturee chez l’homme: Measures theoriques et verification par jauges extensometriques in situ. Rev. Stomatol. 1977, 78, 545. [Google Scholar]
- Buijs, G.J.; Stegenga, B.; Bos, R.R. Efficacy and safety of biodegradable osteofixation devices in oral and maxillofacial surgery: A systematic review. J. Dent. Res. 2006, 85, 980–989. [Google Scholar] [CrossRef]
- Gareb, B.; Van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; De Visscher, J.G.A.M.; Hoppenreijs, T.J.M.; Bergsma, J.E.; Van Minnen, B.; Stegenga, B.; Bos, R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: A multicenter randomized controlled trial. PLoS ONE 2017, 12, e0177152. [Google Scholar] [CrossRef]
- Yaremchuk, M.J.; Posnick, J.C. Resolving controversies related to plate and screw fixation in the growing craniofacial skeleton. J. Craniofac. Surg. 1995, 6, 525–538. [Google Scholar] [CrossRef]
- Postlethwaite, K.R.; Philips, J.G.; Booth, S.; Shaw, J.; Slater, A. The effects of small plate osteosynthesis on postoperative radiotherapy. Br. J. Oral Maxillofac. Surg. 1989, 27, 375–378. [Google Scholar] [CrossRef]
- Alpert, B.; Seligson, D. Removal of asymptomatic bone plates used for orthognathic surgery and facial fractures. J. Oral Maxillofac. Surg. 1996, 54, 618–621. [Google Scholar] [CrossRef]
- Viljanen, J.; Kinnunen, J.; Bondestam, S.; Majola, A.; Rokkanen, P.; Törmälä, P. Bone changes after experimental osteotomies fixed with absorbable self-reinforced poly-l-lactide screws or metallic screws studied by plain radiographs, quantitative computed tomography and magnetic resonance imaging. Biomaterials 1995, 16, 1353–1358. [Google Scholar] [CrossRef]
- Jorgenson, D.S.; Mayer, M.H.; Ellenbogen, R.G.; Centeno, J.A.; Johnson, F.B.; Mullick, F.G.; Manson, P.N. Detection of titanium in human tissues after craniofacial surgery. Plast. Reconstr. Surg. 1997, 99, 976–981. [Google Scholar] [CrossRef]
- Opris, H.; Armencea, G.; Manea, A.; Mitre, I.; Baciut, M.; Onișor, F.; Imre-Lucaci, F.; Vulpoi, A.; Vacaras, S.; Simion, B.; et al. Titanium Periimplant Tissue Alterations: A Prospective Cohort Plate Retrieval Study. Appl. Sci. 2021, 11, 6315. [Google Scholar] [CrossRef]
- Armencea, G.; Gheban, D.; Onisor, F.; Mitre, I.; Manea, A.; Trombitas, V.; Lazar, M.; Baciut, G.; Baciut, M.; Bran, S. Histological Change in Soft Tissue Surrounding Titanium Plates after Jaw Surgery. Materials 2019, 12, 3205. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Vacaras, S.; Baciut, M.; Lucaciu, O.; Dinu, C.; Baciut, G.; Crisan, L.; Hedesiu, M.; Crisan, B.; Onisor, F.; Armencea, G.; et al. Understanding the basis of medical use of poly-lactide-based resorbable polymers and composites–a review of the clinical and metabolic impact. Drug Metab. Rev. 2019, 51, 570–588. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Gareb, B.; van Bakelen, N.B.; Dijkstra, P.U.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Biodegradable versus titanium osteosyntheses in maxillofacial traumatology: A systematic review with meta-analysis and trial sequential analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 914–931. [Google Scholar] [CrossRef]
- Gareb, B.; Roossien, C.C.; Van Bakelen, N.B.; Verkerke, G.J.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Comparison of the mechanical properties of biodegradable and titanium osteosynthesis systems used in oral and maxillofacial surgery. Sci. Rep. 2020, 10, 18143. [Google Scholar] [CrossRef]
- Bergsma, J.E.; de Bruijn, W.C.; Rozema, F.R.; Bos, R.R.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Low Modulus Titanium Alloys for Inhibiting Bone Atrophy; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Kang, D.H.; Kim, T.W. Mechanical behavior and microstructural evolution of commercially pure titanium in enhanced multi-pass equal channel angular pressing and cold extrusion. Mater. Des. 2010, 31, S54–S60. [Google Scholar] [CrossRef]
- Gareb, B.; van Bakelen, N.B.; Driessen, L.; Buma, P.; Kuipers, J.; Grijpma, D.W.; Vissink, A.; Bos, R.R.; van Minnen, B. Biocompatibility and degradation comparisons of four biodegradable copolymeric osteosynthesis systems used in maxillofacial surgery: A goat model with four years follow-up. Bioact. Mater. 2022, 17, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Dewey, M.J.; Harley, B.A.C. Biomaterial design strategies to address obstacles in craniomaxillofacial bone repair. RSC Adv. 2021, 11, 17809–17827. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: A review on polymers and magnesium-based materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef]
- Bötman, O.M.; Laitinen, O.M.; Tynninen, O.; Salminen, S.T.; Pihlajamäki, H.K. Tissue restoration after resorption of polyglycolide and poly-laevo-lactic acid screws. J. Bone Jt. Surg.—Ser. B 2005, 87, 1575–1580. [Google Scholar] [CrossRef]
- Oiwa, H.; Ishida, R.; Sudo, K. Sternal closure with reabsorbable pin and cord in pediatric less invasive cardiac surgery. Ann. Thorac. Surg. 2004, 78, 358–359. [Google Scholar] [CrossRef]
- Saito, T.; Iguchi, A.; Sakurai, M.; Tabayashi, K. Biomechanical study of a Poly-L-Lactide (PLLA) sternal pin in sternal closure after cardiothoracic surgery. Ann. Thorac. Surg. 2004, 77, 684–687. [Google Scholar] [CrossRef]
- Tatsumi, A.; Kanemitsu, N.; Nakamura, T.; Shimizu, Y. Bioabsorbable poly-L-lactide costal coaptation pins and their clinical application in thoracotomy. Ann. Thorac. Surg. 1999, 67, 765–768. [Google Scholar] [CrossRef]
- Ricalde, P.; Posnick, J.C. Degradation rate of delta (resorbable) internal fixation: Report of 2 cases. J. Oral Maxillofac. Surg. 2004, 62, 250–255. [Google Scholar] [CrossRef]
- Losken, H.W.; van Aalst, J.A.; Mooney, M.P.; Godfrey, V.L.; Burt, T.; Teotia, S.; Dean, S.B.; Moss, J.R.; Rahbar, R. Biodegradation of Inion fast-absorbing biodegradable plates and screws. J. Craniofac. Surg. 2008, 19, 748–756. [Google Scholar] [CrossRef]
- Degala, S.; Shetty, S.; Ramya, S. Fixation of zygomatic and mandibular fractures with biodegradable plates. Ann. Maxillofac. Surg. 2013, 3, 25. [Google Scholar] [CrossRef]
- Landes, C.A.; Kriener, S. Resorbable plate osteosynthesis of sagittal split osteotomies with major bone movement. Plast. Reconstr. Surg. 2003, 111, 1828–1840. [Google Scholar] [CrossRef]
- Shikinami, Y.; Matsusue, Y.; Nakamura, T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly l-lactide (F-u-HA/PLLA). Biomaterials 2005, 26, 5542–5551. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Kawai, H.; Shibata, A.; Takahashi, Y.; Nagatsuka, H.; Furuki, Y. Long-Term Bioresorption of Bone Fixation Devices Made from Composites of Unsintered Hydroxyapatite Particles and Poly-L-Lactide. J. Hard Tissue Biol. 2015, 24, 219–224. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In Vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; Al Adham, Z.; Lavoie, S.; Ibourk, M.; et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Bio. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Ravikumar, K.; Basu, B. The Foreign Body Response Demystified. ACS Biomater. Sci. Eng. 2019, 5, 19–44. [Google Scholar] [CrossRef]
- Amini, A.R.; Wallace, J.S.; Nukavarapu, S.P. Short-term and long-term effects of orthopedic biodegradable implants. J. Long. Term. Eff. Med. Implants 2011, 21, 93–122. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, H.H.; Na Shin, Y.; Cho, M.H.; Khang, G.; Lee, H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA-and/or porcine small intestinal submucosa-based scaffolds. Biomaterials 2007, 28, 5137–5143. [Google Scholar] [CrossRef]
- Jones, K.S. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin. Immunol. 2008, 20, 130–136. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: A review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Bostman, O.M.; Pihlajamaki, H.K. Adverse tissue reactions to bioabsorbable fixation devices. Clin. Orthop. Relat. Res. 2000, 371, 216–227. [Google Scholar] [CrossRef]
- Seino, D.; Fukunishi, S.; Yoshiya, S. Late foreign-body reaction to PLLA screws used for fixation of acetabular osteotomy. J. Orthop. Traumatol. 2007, 8, 188–191. [Google Scholar] [CrossRef][Green Version]
- Jeon, H.B.; Kang, D.H.; Gu, J.H.; Oh, S.A. Delayed Foreign Body Reaction Caused by Bioabsorbable Plates Used for Maxillofacial Fractures. Arch. Plast. Surg. 2016, 43, 40–45. [Google Scholar] [CrossRef]
- Van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; de Visscher, J.G.A.M.; Hoppenreijs, T.J.; Bergsma, J.E.; Stegenga, B.; Bos, R.R.M. Comparison of biodegradable and titanium fixation systems in maxillofacial surgery: A two-year multi-center randomized controlled trial. J. Dent. Res. 2013, 92, 1100–1105. [Google Scholar] [CrossRef]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly[m-lactic acid) size- dependence. Biomateriak 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.; Garreau, H. New insights on the degradation of bioresorbable polymeric devices based on lactic and glycolic acids. Clin. Mater. 1992, 10, 3–8. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Gredes, T.; Kunath, F.; Gedrange, T.; Kunert-Keil, C. Bone Regeneration after Treatment with Covering Materials Composed of Flax Fibers and Biodegradable Plastics: A Histological Study in Rats. Bio. Res. Int. 2016, 2016, 5146285. [Google Scholar] [CrossRef]
- Vert, M.; Mauduit, J.; Li, S. Biodegradation of PLA/GA polymers: Increasing complexity. Biomaterials 1994, 15, 1209–1213. [Google Scholar] [CrossRef]
- Laitinen, O.; Pihlajamäki, H.; Sukura, A.; Böstman, O. Transmission electron microscopic visualization of the degradation and phagocytosis of a poly-L-lactide screw in cancellous bone: A long-term experimental study. J. Bio. Mater. Res. 2002, 61, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.; Böstman, O.; Tynninen, O.; Laitinen, O. Long-term tissue response to bioabsorbable poly-l-lactide and metallic screws: An experimental study. Bone 2006, 39, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.R.; de Bruijn, W.C. Foreign body reactions to resorbable poly(L-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Katase, N.; Shibata, A.; Takahashi, Y.; Furuki, Y. Clinical evaluation of an unsintered hydroxyapatite/poly-l-lactide osteoconductive composite device for the internal fixation of maxillofacial fractures. J. Craniofac. Surg. 2016, 27, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Rasse, M.; Moser, D.; Zahl, C.; Gerlach, K.L.; Eckelt, U.; Loukota, R. Resorbable poly(d,l)lactide plates and screws for osteosynthesis of condylar neck fractures in sheep. Br. J. Oral Maxillofac. Surg. 2007, 45, 35–40. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.-A.; Schultze-Mosgau, S.; Schrell, U.; Wénzel, D.; Keler, P. Biodegradable Miniplates (LactoSorb): Long-Term Results in Infant Minipigs and Clinical Results. J. Craniofac. Surg. 2000, 11, 239–243. [Google Scholar] [CrossRef]
- Nieminen, T.; Rantala, I.; Hiidenheimo, I.; Keränen, J.; Kainulainen, H.; Wuolijoki, E.; Kallela, I. Degradative and mechanical properties of a novel resorbable plating system during a 3-year follow-up in vivo and in vitro. J. Mater. Sci. Med. 2008, 19, 1155–1163. [Google Scholar] [CrossRef]
- Buijs, G.J.; van der Houwen, E.B.; Stegenga, B.; Bos, R.R.M.; Verkerke, G.J. Mechanical Strength and Stiffness of Biodegradable and Titanium Osteofixation Systems. J. Oral Maxillofac. Surg. 2007, 65, 2148–2158. [Google Scholar] [CrossRef]
- Xue, A.S.; Koshy, J.C.; Weathers, W.M.; Wolfswinkel, E.M.; Kaufman, Y.; Sharabi, S.E.; Brown, R.H.; Hicks, M.J.; Hollier, L.H., Jr. Local foreign-body reaction to commercial biodegradable implants: An in vivo animal study. Craniomaxillofac. Trauma Reconstr. 2014, 7, 27–34. [Google Scholar] [CrossRef]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef]
- Shi, Q.; Tang, J.; Liu, R.; Wang, L. Toxicity in vitro reveals potential impacts of microplastics and nanoplastics on human health: A review. Crit. Rev. Environ. Sci. Technol. 2021, 195, 1528. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Reports 2020, 10, 7391. [Google Scholar] [CrossRef]
- Vallés, G.; González-Melendi, P.; González-Carrasco, J.L.; Saldaña, L.; Sánchez-Sabaté, E.; Munuera, L.; Vilaboa, N. Differential inflammatory macrophage response to rutile and titanium particles. Biomaterials 2006, 27, 5199–5211. [Google Scholar] [CrossRef]
- Stankevich, K.S.; Gudima, A.; Filimonov, V.D.; Klüter, H.; Mamontova, E.M.; Tverdokhlebov, S.I.; Kzhyshkowska, J. Surface modification of biomaterials based on high-molecular polylactic acid and their effect on inflammatory reactions of primary human monocyte-derived macrophages: Perspective for personalized therapy. Mater. Sci. Eng. C 2015, 51, 117–126. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Deshayes, S.; Kasko, A.M. Polymeric biomaterials with engineered degradation. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3531–3566. [Google Scholar] [CrossRef]
- Matlaga, B.F.; Yasenchak, L.P.; Salthouse, T.N. Tissue response to implanted polymers: The significance of sample shape. J. Bio. Mater. Res. 1976, 10, 391–397. [Google Scholar] [CrossRef]
- Salthouse, T.N. Some aspects of macrophage behavior at the implant interface. J. Bio. Mater. Res. 1984, 18, 395–401. [Google Scholar] [CrossRef]
- Soundararajan, A.; Muralidhar R, J.; Dhandapani, R.; Radhakrishnan, J.; Manigandan, A.; Kalyanasundaram, S.; Sethuraman, S.; Subramanian, A. Surface topography of polylactic acid nanofibrous mats: Influence on blood compatibility. J. Mater. Sci. Mater. Med. 2018, 29, 145. [Google Scholar] [CrossRef]
- Mann, G.S.; Singh, L.P.; Kumar, P.; Singh, S.; Prakash, C. On briefing the surface modifications of polylactic acid: A scope for betterment of biomedical structures. J. Thermoplast. Compos. Mater. 2019, 34, 977–1005. [Google Scholar] [CrossRef]
- Guillaume, O.; Geven, M.A.; Varjas, V.; Varga, P.; Gehweiler, D.; Stadelmann, V.A.; Smidt, T.; Zeiter, S.; Sprecher, C.; Bos, R.R.; et al. Orbital floor repair using patient specific osteoinductive implant made by stereolithography. Biomaterials 2020, 233, 119721. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Zu, G.; Forson, A.M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: A novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Gabryelczak, I.; Bielecki-Kowalski, B. Clinical Evaluation of Magnesium Alloy Osteosynthesis in the Mandibular Head. Materials 2022, 15, 711. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.A.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloys 2020, 8, 352–363. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Seitz, J.M.; Eifler, R.; Bach, F.W.; Maier, H.J. Magnesium degradation products: Effects on tissue and human metabolism. J. Bio. Mater. Res. Part A 2014, 102, 3744–3753. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.S.; et al. The use of silk-based devices for fracture fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef]
- Numata, K.; Cebe, P.; Kaplan, D.L. Mechanism of Enzymatic Degradation of Beta-sheet Crystals. Biomaterials 2010, 31, 2926. [Google Scholar] [CrossRef]
- Riviș, M.; Roi, C.; Roi, A.; Nica, D.; Văleanu, A.; Rusu, L.-C. The Implications of Titanium Alloys Applied in Maxillofacial Osteosynthesis. Appl. Sci. 2020, 10, 3203. [Google Scholar] [CrossRef]
- Gupta, K.; Laubscher, R.F. Sustainable machining of titanium alloys: A critical review. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2017, 231, 2543–2560. [Google Scholar] [CrossRef]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’H, M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Jt. Surg.—Ser. A 2000, 82, 457–476. [Google Scholar] [CrossRef]
- Meningaud, J.-P.; Poupon, J.; Bertrand, J.-C.; Chenevier, C.; Galliot-Guilley, M.; Guilbert, F. Dynamic study about metal release from titanium miniplates in maxillofacial surgery. Int. J. Oral Maxillofac. Surg. 2001, 30, 185–188. [Google Scholar] [CrossRef]
- Acero, J.; Calderon, J.; Salmeron, J.I.; Verdaguer, J.J.; Concejo, C.; Somacarrera, M.L. The behaviour of titanium as a biomaterial: Microscopy study of plates and surrounding tissues in facial osteosynthesis. J. Cranio-Maxillofac. Surg. 1999, 27, 117–123. [Google Scholar] [CrossRef]
- Lalor, P.A.; Revell, P.A. T-lymphocytes and titanium aluminium vanadium (TiAlV) alloy: Evidence for immunological events associated with debris deposition. Clin. Mater. 1993, 12, 57–62. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Consolo, U. Element release from titanium devices used in oral and maxillofacial surgery. Biomaterials 2003, 24, 1093–1099. [Google Scholar] [CrossRef]
- Coen, N.; Kadhim, M.A.; Wright, E.G.; Case, C.P.; Mothersill, C.E. Particulate debris from a titanium metal prosthesis induces genomic instability in primary human fibroblast cells. Br. J. Cancer 2003, 88, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxid. Med. Cell. Longev. 2018, 2018, 3714725. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Krȩtowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jabłońska, E.; Załęski, P.; Waszkiel, D.; Ładny, J.R.; Żukowski, P.; et al. The redox balance in erythrocytes, plasma, and periosteum of patients with titanium fixation of the jaw. Front. Physiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimer’s Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef]
- Schifman, R.B.; Luevano, D.R. Aluminum Toxicity: Evaluation of 16-Year Trend Among 14 919 Patients and 45 480 Results. Arch. Pathol. Lab. Med. 2018, 142, 742–746. [Google Scholar] [CrossRef]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface modification techniques of titanium and its alloys to functionally optimize their biomedical properties: Thematic review. Front. Bioeng. Biotechnol. 2020, 8, 1261. [Google Scholar] [CrossRef]
- Do Nascimento, R.M.; de Carvalho, V.R.; Govone, J.S.; Hernandes, A.C.; da Cruz, N.C. Effects of negatively and positively charged Ti metal surfaces on ceramic coating adhesion and cell response. J. Mater. Sci. Mater. Med. 2017, 28, 33. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-Based Implant Infections in Orthopaedics. Adv. Exp. Med. Biol. 2015, 830, 29–46. [Google Scholar]
- Katsikogianni, M.; Missirlis, Y.F.; Harris, L.; Douglas, J. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Kraeima, J.; Glas, H.H.; Witjes, M.J.H.; Schepman, K.P. Patient-specific pre-contouring of osteosynthesis plates for mandibular reconstruction: Using a three-dimensional key printed solution. J. Cranio-Maxillofac. Surg. 2018, 46, 1037–1040. [Google Scholar] [CrossRef]
- Kraeima, J.; Schepers, R.; Spijkervet, F.; Maal, T.; Baan, F.; Witjes, M.; Jansma, J. Splintless surgery using patient-specific osteosynthesis in Le Fort I osteotomies: A randomized controlled multi-centre trial. Int. J. Oral Maxillofac. Surg. 2020, 49, 454–460. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kumar, V.A.; Mathew, C.; Rao, G.S. Strain hardening of Titanium alloy Ti6Al4V sheets with prior heat treatment and cold working. Mater. Sci. Eng. A 2016, 662, 537–550. [Google Scholar] [CrossRef]
- Koike, M.; Greer, P.; Owen, K.; Lilly, G.; Murr, L.E.; Gaytan, S.M.; Martinez, E.; Okabe, T.H. Evaluation of Titanium Alloys Fabricated Using Rapid Prototyping Technologies—Electron Beam Melting and Laser Beam Melting. Materials 2011, 4, 1776–1792. [Google Scholar] [CrossRef]
- Venkatesh, B.D.; Chen, D.L.; Bhole, S.D. Effect of heat treatment on mechanical properties of Ti-6Al-4V ELI alloy. Mater. Sci. Eng. A 2009, 506, 117–124. [Google Scholar] [CrossRef]
- Yang, F.; Pi, Z.Q.; Zhao, Q.Y.; Raynova, S.; Liu, Q.; Sharp, K.; Brandt, M.; Bolzoni, L.; Qian, M. Strong and Ductile Ti-6Al-4V Alloy Produced by Hot Pressing of Ti-6Al-4V Swarf. JOM 2019, 71, 1056–1061. [Google Scholar] [CrossRef]
- Guo, L.; Feng, W.; Liu, X.; Lin, C.; Li, B.; Qiang, Y. Sol-gel synthesis of antibacterial hybrid coatings on titanium. Mater. Lett. 2015, 160, 448–451. [Google Scholar] [CrossRef]
- Keskin, D.; Tromp, L.; Mergel, O.; Zu, G.; Warszawik, E.; van der Mei, H.C.; van Rijn, P. Highly Efficient Antimicrobial and Antifouling Surface Coatings with Triclosan-Loaded Nanogels. ACS Appl. Mater. Interfaces 2020, 12, 57721–57731. [Google Scholar] [CrossRef]
- Yu, L.; Tian, Y.; Qiao, Y.; Liu, X. Mn-containing titanium surface with favorable osteogenic and antimicrobial functions synthesized by PIII&D. Colloids Surf. B Biointerfaces 2017, 152, 376–384. [Google Scholar] [PubMed]
- Shanaghi, A.; Chu, P.K. Investigation of corrosion mechanism of NiTi modified by carbon plasma immersion ion implantation (C-PIII) by electrochemical impedance spectroscopy. J. Alloys Compd. 2019, 790, 1067–1075. [Google Scholar] [CrossRef]
- Fabritius, M.; Al-Munajjed, A.A.; Freytag, C.; Jülke, H.; Zehe, M.; LeMarchand, T.; Arts, J.J.; Schumann, D.; Alt, V.; Sternberg, K. Antimicrobial silver multilayer coating for prevention of bacterial colonization of orthopedic implants. Materials 2020, 13, 1415. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, C.; Zaatreh, S.; Wegner, K.; Arndt, K.; Podbielski, A.; Bader, R.; Prinz, C.; Lembke, U.; Nebe, J.B. Copper as an alternative antimicrobial coating for implants—An in vitro study. World J. Transplant. 2017, 7, 193–202. [Google Scholar] [CrossRef]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Vrespa, G.; Caputi, S.; Piattelli, A. Bacterial adhesion on titanium nitride-coated and uncoated implants: An in vivo human study. J. Oral Implantol. 2003, 29, 80–85. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, X.M.; Tang, H.Q.; Liu, T.; Gu, H.Q.; Cui, R.Z. Bactericidal and biocompatible properties of TiN/Ag multilayered films by ion beam assisted deposition. J. Mater. Sci. Mater. Med. 2008, 20, 101–105. [Google Scholar] [CrossRef]
- Soares, T.P.; Garcia, C.S.; Roesch-Ely, M.; da Costa, M.E.M.; Giovanela, M.; Aguzzoli, C. Cytotoxicity and antibacterial efficacy of silver deposited onto titanium plates by low-energy ion implantation. J. Mater. Res. 2018, 33, 2545–2553. [Google Scholar] [CrossRef]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Bio. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef]
- Chan, C.W.; Carson, L.; Smith, G.C.; Morelli, A.; Lee, S. Enhancing the antibacterial performance of orthopaedic implant materials by fibre laser surface engineering. Appl. Surf. Sci. 2017, 404, 67–81. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Shirai, T.; Nishida, H.; Murakami, H.; Kabata, T.; Yamamoto, N.; Watanabe, K.; Nakase, J. Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci. 2012, 17, 595–604. [Google Scholar] [CrossRef]
- Feng, W.; Geng, Z.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Wang, R.; Yang, X. Controlled release behaviour and antibacterial effects of antibiotic-loaded titania nanotubes. Mater. Sci. Eng. C 2016, 62, 105–112. [Google Scholar] [CrossRef]
- Sedelnikova, M.; Komarova, E.; Sharkeev, Y.; Tolkacheva, T.; Khlusov, I.; Litvinova, L.; Yurova, K.; Shupletsova, V. Comparative investigations of structure and properties of micro-arc wollastonite-calcium phosphate coatings on titanium and zirconium-niobium alloy. Bioact. Mater. 2017, 2, 177–184. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, H.; Lei, J.; Zhang, J.; Zhou, H.; Qi, M. Formation and in vitro/in vivo performance of “cortex-like” micro/nano-structured TiO2 coatings on titanium by micro-arc oxidation. Mater. Sci. Eng. C 2018, 87, 90–103. [Google Scholar] [CrossRef]
- Yu, S.; Li, Z.; Han, L.; Zhao, Y.; Fu, T. Biocompatible MgO Film on Titanium Substrate Prepared by Sol-gel Method. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2018, 47, 2663–2667. [Google Scholar]
- Martin, T.P.; Kooi, S.E.; Chang, S.H.; Sedransk, K.L.; Gleason, K.K. Initiated chemical vapor deposition of antimicrobial polymer coatings. Biomaterials 2006, 28, 909–915. [Google Scholar] [CrossRef]
- Shahali, H.; Jaggessar, A.; Yarlagadda, P.K.D.V. Recent Advances in Manufacturing and Surface Modification of Titanium Orthopaedic Applications. Procedia Eng. 2017, 174, 1067–1076. [Google Scholar] [CrossRef]
- Sun, L. Thermal Spray Coatings on Orthopedic Devices: When and How the FDA Reviews Your Coatings. J. Therm. Spray Technol. 2018, 27, 1280–1290. [Google Scholar] [CrossRef]
- Fox, K.E.; Tran, N.L.; Nguyen, T.A.; Nguyen, T.T.; Tran, P.A. Surface modification of medical devices at nanoscale—Recent development and translational perspectives. Biomater. Transl. Med. A Biomater. Approach 2019, 163–189. [Google Scholar] [CrossRef]
- Guimond-Lischer, S.; Ren, Q.; Braissant, O.; Gruner, P.; Wampfler, B.; Maniura-Weber, K. Vacuum plasma sprayed coatings using ionic silver doped hydroxyapatite powder to prevent bacterial infection of bone implants. Biointerphases 2016, 11, 011012. [Google Scholar] [CrossRef]
- Ullah, I.; Siddiqui, M.A.; Liu, H.; Kolawole, S.K.; Zhang, J.; Zhang, S.; Ren, L.; Yang, K. Mechanical, Biological, and Antibacterial Characteristics of Plasma-Sprayed (Sr,Zn) Substituted Hydroxyapatite Coating. ACS Biomater. Sci. Eng. 2020, 6, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Qiao, S.; Cao, H.; Zhao, X.; Lo, H.; Zhuang, L.; Gu, Y.; Shi, J.-Y.; Liu, X. Ag-plasma modification enhances bone apposition around titanium dental implants: An animal study in Labrador dogs. Int. J. Nanomed. 2015, 10, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Kamiura, Y.; Yamamoto, S.; Kohgo, T.; Amemiya, A.; Ukai, H.; Murakami, K.; Asaoka, K. Early bone formation around calcium-ion-implanted titanium inserted into rat tibia. J. Bio. Mater. Res. 1997, 36, 131–136. [Google Scholar] [CrossRef]
- Hong, Z.; Mello, A.; Yoshida, T.; Luan, L.; Stern, P.H.; Rossi, A.; Ellis, D.E.; Ketterson, J.B. Osteoblast proliferation on hydroxyapatite coated substrates prepared by right angle magnetron sputtering. J. Bio. Mater. Res. Part A 2010, 93, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, G.; Hardes, J.; Gosheger, G.; Stoeppeler, S.; Ahrens, H.; Blaske, F.; Wehe, C.; Karst, U.; Höll, S. Evaluation of osseous integration of PVD-silver-coated hip prostheses in a canine model. Bio. Res. Int. 2015, 2015, 292406. [Google Scholar] [CrossRef]
- Giavaresi, G.; Ambrosio, L.; Battiston, G.A.; Casellato, U.; Gerbasi, R.; Finia, M.; Aldini, N.N.; Martini, L.; Rimondini, L.; Giardino, R. Histomorphometric, ultrastructural and microhardness evaluation of the osseointegration of a nanostructured titanium oxide coating by metal-organic chemical vapour deposition: An in vivo study. Biomaterials 2004, 25, 5583–5591. [Google Scholar] [CrossRef]
- Chien, C.S.; Liao, T.Y.; Hong, T.F.; Kuo, T.Y.; Chang, C.H.; Yeh, M.L.; Lee, T.M. Surface microstructure and bioactivity of hydroxyapatite and fluorapatite coatings deposited on Ti-6Al-4V substrates using Nd-YAG laser. J. Med. Biol. Eng. 2014, 34, 109–115. [Google Scholar] [CrossRef]
- Chen, J.; Bly, R.; Saad, M.; AlKhodary, M.; El-Backly, R.; Cohen, D.; Kattamis, N.; Fatta, M.; Moore, W.; Arnold, C.; et al. In-vivo study of adhesion and bone growth around implanted laser groove/RGD-functionalized Ti-6Al-4V pins in rabbit femurs. Mater. Sci. Eng. C 2011, 31, 826–832. [Google Scholar] [CrossRef]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Van Hove, R.P.; Sierevelt, I.N.; Van Royen, B.J.; Nolte, P.A. Titanium-Nitride Coating of Orthopaedic Implants: A Review of the Literature. Bio. Res. Int. 2015, 2015, 485975. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dhara, S.; Saha, P. Enhancing the biocompatibility of Ti6Al4V implants by laser surface microtexturing: An in vitro study. Int. J. Adv. Manuf. Technol. 2015, 76, 5–15. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem.—Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Kim, K.; Lee, B.A.; Piao, X.H.; Chung, H.J.; Kim, Y.J. Surface characteristics and bioactivity of an anodized titanium surface. J. Periodontal Implant Sci. 2013, 43, 198–205. [Google Scholar] [CrossRef]
- Gerlach, K. Elektrische Messverfahren zur Bestimmung der Belastbarkeit des Unterkiefers bei Patienten mit vollbezahntem Gebiss. Dtsch. Zahnarztl. Z. 1986, 39, 146–149. [Google Scholar]
- Tams, J.; van Loon, J.-P.; Otten, E.; Rozema, F.R.; Bos, R.R.M. A three-dimensional study of bending and torsion moments for different fracture sites in the mandible: An in vitro study. Int. J. Oral Maxillofac. Surg. 1997, 26, 383–388. [Google Scholar] [CrossRef]
- Tams, J.; Van Loon, J.P.; Rozema, F.R.; Otten, E.; Bos, R.R.M. A three-dimensional study of loads across the fracture for different fracture sites of the mandible. Br. J. Oral Maxillofac. Surg. 1996, 34, 400–405. [Google Scholar] [CrossRef]
- Pistner, H.; Kukiz, P. Chewing forces after orthodontic therapy, osteotomies and fractures of the mandible. Dtsch. Zahnarztl. Z. 1998, 53, 528–531. [Google Scholar]
- Tate, G.S.; Ellis, E.; Throckmorton, G. Bite forces in patients treated for mandibular angle fractures: Implications for fixation recommendations. J. Oral Maxillofac. Surg. 1994, 52, 734–736. [Google Scholar] [CrossRef]
- Feller, K.U.; Richter, G.; Schneider, M.; Eckelt, U. Combination of microplate and miniplate for osteosynthesis of mandibular fractures: An experimental study. Int. J. Oral Maxillofac. Surg. 2002, 31, 78–83. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, J.; Xu, C.-T.; Zhang, J.; Jin, Y.-J.; Sun, G.-L. Biomechanical evaluation of maxillary Lefort Ι fracture with bioabsorbable osteosynthesis internal fixation. Dent. Traumatol. 2014, 30, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Maurer, P.; Holweg, S.; Knoll, W.D.; Schubert, J. Study by finite element method of the mechanical stress of selected biodegradable osteosynthesis screws in sagittal ramus osteotomy. Br. J. Oral Maxillofac. Surg. 2002, 40, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Maurer, P.; Holweg, S.; Schubert, J. Finite-element-analysis of different screw-diameters in the sagittal split osteotomy of the mandible. J. Cranio-Maxillofac. Surg. 1999, 27, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C.; Sellenschloh, K.; Polster, V.; Heyland, M.; Vollmer, M.; Morlock, M.M.; Heiland, M.; Huber, G.; Rendenbach, C. Biomechanical comparison of polylactide-based versus titanium miniplates in mandible reconstruction in vitro. J. Stomatol. Oral Maxillofac. Surg. 2019, 121, 377–382. [Google Scholar] [CrossRef]
- Huang, S.-F.; Lo, L.-J.; Lin, C.-L. Biomechanical interactions of different mini-plate fixations and maxilla advancements in the Le Fort I Osteotomy: A finite element analysis. Comput. Methods Biomech. Bio. Eng. 2016, 19, 1704–1713. [Google Scholar] [CrossRef]
- Proffit, W.R.; Turvey, T.A.; Fields, H.W.; Phillips, C. The effect of orthognathic surgery on occlusal force. J. Oral Maxillofac. Surg. 1989, 47, 457–463. [Google Scholar] [CrossRef]
- Tams, J.; Otten, B.; Van Loon, J.P.; Bos, R.R.M. A computer study of fracture mobility and strain on biodegradable plates used for fixation of mandibular fractures. J. Oral Maxillofac. Surg. 1999, 57, 973–981. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Manson, P.N.; Prein, J. Principles of Internal Fixation of the Craniomaxillofacial Skeleton: Trauma and Orthognathic Surgery; Thieme: Stuttgart, Germany, 2012. [Google Scholar]
- Buijs, G.; van Bakelen, N.; Jansma, J.; de Visscher, J.; Hoppenreijs, T.; Bergsma, J.; Stegenga, B.; Bos, R. A randomized clinical trial of biodegradable and titanium fixation systems in maxillofacial surgery. J. Dent. Res. 2012, 91, 299–304. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, H.S.; Kim, Y.C.; Lee, J.Y.; Lee, B.K. Stability of biodegradable metal (Mg–Ca–Zn alloy) screws compared with absorbable polymer and titanium screws for sagittal split ramus osteotomy of the mandible using the finite element analysis model. J. Cranio-Maxillofac. Surg. 2017, 45, 1639–1646. [Google Scholar] [CrossRef]
- Chang, L.-R.; Chen, C.-C.; Jeng, S.F.; Chen, Y.-R.; Hwang, L.-C.; Lin, T.-S. Investigation of a Modified Novel Technique in Bilateral Sagittal Splitting Osteotomy Fixation: Finite Element Analysis and in Vitro Biomechanical Test. Bio. Res. Int. 2020, 2020, 8707389. [Google Scholar] [CrossRef]
- Mutombo, K.; Rossouw, P.; Govender, G. Mechanical Properties of Mill-Annealed Ti6Al4V Investment Cast. Mater. Sci. Forum 2011, 690, 69–72. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Peltoniemi, H.; Waris, E.; Suuronen, R.; Serlo, W.; Kellomäki, M.; Törmälä, P.; Waris, T. Developments in Craniomaxillofacial Surgery: Use of Self-Reinforced Bioabsorbable Osteofixation Devices. Plast. Reconstr. Surg. 2001, 108, 167–180. [Google Scholar] [CrossRef]
- Buijs, G.J.; van der Houwen, E.B.; Stegenga, B.; Verkerke, G.J.; Bos, R.R.M. Mechanical Strength and Stiffness of the Biodegradable SonicWeld Rx Osteofixation System. J. Oral Maxillofac. Surg. 2009, 67, 782–787. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym. Degrad. Stab. 2003, 79, 465–474. [Google Scholar] [CrossRef]
- Grabow, N.; Schlun, M.; Sternberg, K.; Hakansson, N.; Kramer, S.; Schmitz, K.-P. Mechanical properties of laser cut poly(L-lactide) micro-specimens: Implications for stent design, manufacture, and sterilization. J. Biomech. Eng. 2005, 127, 25–31. [Google Scholar] [CrossRef]
- Gogolewski, S.; Mainil-Varlet, P. Effect of thermal treatment on sterility, molecular and mechanical properties of various polylactides. 2. Poly(L/D-lactide) and poly(L/DL-lactide). Biomaterials 1997, 18, 251–255. [Google Scholar] [CrossRef]
- Engroff, S.L.; Blanchaert, R.H.; Von Fraunhofer, J.A. Mandibulotomy Fixation: A Laboratory Analysis. J. Oral Maxillofac. Surg. 2003, 61, 1297–1301. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef]
- Park, B.; Jung, B.T.; Kim, W.H.; Lee, J.-H.; Kim, B.; Lee, J.-H. The Stability of Hydroxyapatite/Poly-L-Lactide Fixation for Unilateral Angle Fracture of the Mandible Assessed Using a Finite Element Analysis Model. Materials 2020, 13, 228. [Google Scholar] [CrossRef]
- Song, I.S.; Choi, J.; Kim, S.R.; Lee, J.H. Stability of bioresorbable plates following reduction of mandibular body fracture: Three-dimensional analysis. J. Cranio-Maxillofac. Surg. 2019, 47, 1752–1757. [Google Scholar] [CrossRef]
- Gareb, B.; van Bakelen, N.B.; Dijkstra, P.U.; Vissink, A.; Bos, R.R.M.; van Minnen, B. Efficacy and morbidity of biodegradable versus titanium osteosyntheses in orthognathic surgery: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Oral Sci. 2021, 29, e12800. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Muramatsu, H.; Sato, M.; Tomizuka, Y.; Inoue, M.; Yoshimoto, S. Surgical treatment of facial fracture by using unsintered hydroxyapatite particles/poly l-lactide composite device (OSTEOTRANS MX®): A clinical study on 17 cases. J. Cranio-Maxillofac. Surg. 2013, 41, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Landes, C.; Ballon, A.; Ghanaati, S.; Tran, A.; Sader, R. Treatment of Malar and Midfacial Fractures With Osteoconductive Forged Unsintered Hydroxyapatite and Poly-L-Lactide Composite Internal Fixation Devices. J. Oral Maxillofac. Surg. 2014, 72, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Karino, M.; Yoshino, A.; Koike, T.; Ide, T.; Tatsumi, H.; Tsunematsu, K.; Yoshimatsu, H.; Sekine, J. Feasibility of Single Folded Unsintered Hydroxyapatite Particles/Poly-L-Lactide Composite Sheet in Combined Orbital Floor and Medial Wall Fracture Reconstruction. J. Hard Tissue Biol. 2017, 26, 237–244. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Jin, X.; Xu, J.; Lu, J.; Zhang, C.; Tian, T.; Teng, L. Complications of absorbable fixation in maxillofacial surgery: A meta-analysis. PLoS ONE 2013, 8, e67449. [Google Scholar] [CrossRef]
- Turvey, T.A.; Bell, R.B.; Tejera, T.J.; Proffit, W.R. The use of self-reinforced biodegradable bone plates and screws in orthognathic surgery. J. Oral Maxillofac. Surg. 2002, 60, 59–65. [Google Scholar] [CrossRef]
- Shand, J.M.; Heggie, A.A.C. Use of a resorbable fixation system in orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2000, 38, 335–337. [Google Scholar] [CrossRef]
- Park, Y.-W. Bioabsorbable osteofixation for orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 6. [Google Scholar] [CrossRef]
- Moure, C.; Qassemyar, Q.; Dunaud, O.; Neiva, C.; Testelin, S.; Devauchelle, B. Skeletal stability and morbidity with self-reinforced P (l/dL) LA resorbable osteosynthesis in bimaxillary orthognathic surgery. J. Cranio-Maxillofac. Surg. 2012, 40, 55–60. [Google Scholar] [CrossRef]
- Haers, P.E.; Sailer, H.F. Biodegradable self-reinforced poly-L/DL-lactide plates and screws in bimaxillary orthognathic surgery: Short term skeletal stability and material related failures. J. Cranio-Maxillofac. Surg. 1998, 26, 363–372. [Google Scholar] [CrossRef]
- Fedorowicz, Z.; Nasser, M.; Newton, J.; Oliver, R. Resorbable versus titanium plates for orthognathic surgery. Cochrane Database Syst. Rev. 2007, 4, 20. [Google Scholar]
- Sukegawa, S.; Kanno, T.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Furuki, Y. Complications of a poly-l-lactic acid and polyglycolic acid osteosynthesis device for internal fixation in maxillofacial surgery. Odontology 2018, 106, 360–368. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Ellis, E. Biodegradable and titanium osteosynthesis provide similar stability for orthognathic surgery. J. Oral Maxillofac. Surg. 2015, 73, 1795–1808. [Google Scholar] [CrossRef]
- Mosbah, M.R.; Oloyede, D.; Koppel, D.A.; Moos, K.F.; Stenhouse, D. Miniplate removal in trauma and orthognathic surgery—A retrospective study. Int. J. Oral Maxillofac. Surg. 2003, 32, 148–151. [Google Scholar] [CrossRef]
- O’Connell, J.; Murphy, C.; Ikeagwuani, O.; Adley, C.; Kearns, G. The fate of titanium miniplates and screws used in maxillofacial surgery: A 10 year retrospective study. Int. J. Oral Maxillofac. Surg. 2009, 38, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barrachina, R.; Montiel-Company, J.M.; García-Sanz, V.; Almerich-Silla, J.M.; Paredes-Gallardo, V.; Bellot-Arcís, C. Titanium plate removal in orthognathic surgery: Prevalence, causes and risk factors. A systematic literature review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 49, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.P.; Houppermans, P.N.W.J.; Gooris, P.; Mensink, G.; van Merkesteyn, J.P.R. Risk factors for common complications associated with bilateral sagittal split osteotomy: A literature review and meta-analysis. J. Cranio-Maxillofac. Surg. 2016, 44, 1170–1180. [Google Scholar] [CrossRef]
- Falter, B.; Schepers, S.; Vrielinck, L.; Lambrichts, I.; Politis, C. Plate removal following orthognathic surgery. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Turvey, T.A.; Proffit, W.P.; Phillips, C. Biodegradable fixation for craniomaxillofacial surgery: A 10-year experience involving 761 operations and 745 patients. Int. J. Oral Maxillofac. Surg. 2011, 40, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.N.; Lane, J.M. Newest factors in fracture healing. Clin. Orthop. Relat. Res. 1992, 297–311. [Google Scholar] [CrossRef]
- Daniels, J.S.; Albakry, I.; Braimah, R.O.; Samara, M.I. Is the Routine Removal of Titanium Plates and Screws Following Miniplate Osteosynthesis of Maxillofacial Bone Fractures Justified? A Fifteen-Year Experience in a Maxillofacial Centre, Saudi Arabia. Craniomaxillofacial Trauma Reconstr. Open 2021, 6, 247275122110652. [Google Scholar] [CrossRef]
- Nail, L.N.; Zhang, D.; Reinhard, J.L.; Grunlan, M.A. Fabrication of a Bioactive, PCL-based ‘Self-fitting’ Shape Memory Polymer Scaffold. J. Vis. Exp. 2015, 23, e52981. [Google Scholar] [CrossRef]
- Dewey, M.J.; Johnson, E.M.; Weisgerber, D.W.; Wheeler, M.B.; Harley, B.A.C. Shape-fitting collagen-PLA composite promotes osteogenic differentiation of porcine adipose stem cells. J. Mech. Behav. Biomed. Mater. 2019, 95, 21–33. [Google Scholar] [CrossRef]
- Zhang, D.; George, O.J.; Petersen, K.M.; Jimenez-Vergara, A.C.; Hahn, M.S.; Grunlan, M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10, 4597–4605. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef]
- Leonhardt, H.; Ziegler, A.; Lauer, G.; Franke, A. Osteosynthesis of the Mandibular Condyle With Magnesium-Based Biodegradable Headless Compression Screws Show Good Clinical Results During a 1-Year Follow-Up Period. J. Oral Maxillofac. Surg. 2021, 79, 637–643. [Google Scholar] [CrossRef]
- Naujokat, H.; Seitz, J.-M.; Açil, Y.; Damm, T.; Möller, I.; Gülses, A.; Wiltfang, J. Osteosynthesis of a cranio-osteoplasty with a biodegradable magnesium plate system in miniature pigs. Acta Biomater. 2017, 62, 434–445. [Google Scholar] [CrossRef]
- Leonhardt, H.; Franke, A.; McLeod, N.M.H.; Lauer, G.; Nowak, A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625. [Google Scholar] [CrossRef]
- Rendenbach, C.; Fischer, H.; Kopp, A.; Schmidt-Bleek, K.; Kreiker, H.; Stumpp, S.; Thiele, M.; Duda, G.; Hanken, H.; Beck-Broichsitter, B.; et al. Improved in vivo osseointegration and degradation behavior of PEO surface-modified WE43 magnesium plates and screws after 6 and 12 months. Mater. Sci. Eng. C 2021, 129, 112380. [Google Scholar] [CrossRef]
- Yerit, K.C.; Hainich, S.; Turhani, D.; Klug, C.; Wittwer, G.; Ockher, M.; Ploder, O.; Undt, G.; Baumann, A.; Ewers, R. Stability of biodegradable implants in treatment of mandibular fractures. Plast. Reconstr. Surg. 2005, 115, 1863–1870. [Google Scholar] [CrossRef]
- Ghali, G.E.; Sinn, D.P.; Tantipasawasin, S. Management of nonsyndromic craniosynostosis. Atlas Oral Maxillofac. Surg. Clin. North Am. 2002, 10, 1–41. [Google Scholar] [CrossRef]
- Liu, J.N.; Ong, H.S.; Wang, M.Y.; Wan, K.; Qu, X.Z.; Zhang, C.P. Randomized control trial comparing the titanium osteosynthesis and the biodegradable osteosynthesis in mandibulotomy access. Head Neck 2019, 41, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-C.; Kim, S.-G.; Oh, J.-S.; You, J.-S.; Kim, W.-G. Mini-plate removal in maxillofacial trauma patients during a five-year retrospective study. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, O.; Biermann, L.; Kolk, A.; Wolff, K.D.; Götz, C. Osteosynthesis plate removal: Patient benefits and burdens. Appl. Sci. 2020, 10, 5296. [Google Scholar] [CrossRef]
- Bakathir, A.A.; Margasahayam, M.V.; Al-Ismaily, M.I. Removal of bone plates in patients with maxillofacial trauma: A retrospective study. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, e32–e37. [Google Scholar] [CrossRef]
- Landes, C.; Hoefer, S.; Richards, T.; Walcher, F.; Sader, R. Perspectives of patients about bioabsorbable internal fixation for maxillofacial fractures. Ann. Maxillofac. Surg. 2015, 5, 185. [Google Scholar] [CrossRef]
- Ballon, A.; Laudemann, K.; Sader, R.; Landes, C.A. Patients’ preoperative expectations and postoperative satisfaction of dysgnathic patients operated on with resorbable osteosyntheses. J. Craniofac. Surg. 2011, 22, 730–734. [Google Scholar] [CrossRef]
- Sukegawa, S.; Masui, M.; Sukegawa-Takahashi, Y.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H.; Furuki, Y. Maxillofacial Trauma Surgery Patients With Titanium Osteosynthesis Miniplates: Remove or Not? J. Craniofac. Surg. 2020, 31, 1338–1342. [Google Scholar] [CrossRef]
- Berryhill, W.E.; Rimell, F.L.; Ness, J.; Marentette, L.; Haines, S.J. Fate of rigid fixation in pediatric craniofacial surgery. Otolaryngol. Head. Neck Surg. 1999, 121, 269–273. [Google Scholar] [CrossRef]
- Bos, R.R.M. Treatment of pediatric facial fractures: The case for metallic fixation. J. Oral Maxillofac. Surg. 2005, 63, 382–384. [Google Scholar] [CrossRef]
- Wheeler, J.; Phillips, J. Pediatric Facial Fractures and Potential Long-Term Growth Disturbances. Craniomaxillofac. Trauma Reconstr. 2011, 4, 43. [Google Scholar] [CrossRef]
- Baumeister, S.; Peek, A.; Friedman, A.; Levin, L.S.; Marcus, J.R. Management of postneurosurgical bone flap loss caused by infection. Plast. Reconstr. Surg. 2008, 122, 195e–208e. [Google Scholar] [CrossRef]
- Pontell, M.E.; Niklinska, E.B.; Braun, S.A.; Jaeger, N.; Kelly, K.J.; Golinko, M.S. Resorbable Versus Titanium Hardware for Rigid Fixation of Pediatric Upper and Midfacial Fractures: Which Carries a Lower Risk Profile? J. Oral Maxillofac. Surg. 2021, 79, 2103–2114. [Google Scholar] [CrossRef]
- Stanton, D.C.; Liu, F.; Yu, J.W.; Mistretta, M.C. Use of bioresorbable plating systems in paediatric mandible fractures. J. Cranio-Maxillofac. Surg. 2014, 42, 1305–1309. [Google Scholar] [CrossRef]
- An, J.; Jia, P.; Zhang, Y.; Gong, X.; Han, X.; He, Y. Application of biodegradable plates for treating pediatric mandibular fractures. J. Cranio-Maxillofac. Surg. 2015, 43, 515–520. [Google Scholar] [CrossRef]
- Aldana, P.R.; Roy, S.; Postlethwait, R.A.; James, H.E. Ultrasound-aided fixation of a biodegradable cranial fixation system: Uses in pediatric neurosurgery: Technical note. J. Neurosurg. Pediatr. 2009, 3, 420–424. [Google Scholar] [CrossRef]
- Aldana, P.R.; Wieder, K.; Postlethwait, R.A.; James, H.E.; Steinberg, B. Ultrasound-Aided Fixation of Biodegradable Implants in Pediatric Craniofacial Surgery. Pediatr. Neurosurg. 2011, 47, 349–353. [Google Scholar] [CrossRef]
- Siy, R.W.; Brown, R.H.; Koshy, J.C.; Stal, S.; Hollier, L.H. General management considerations in pediatric facial fractures. J. Craniofac. Surg. 2011, 22, 1190–1195. [Google Scholar] [CrossRef]
- Uckan, S.; Bayram, B.; Kecik, D.; Araz, K. Effects of titanium plate fixation on mandibular growth in a rabbit model. J. Oral Maxillofac. Surg. 2009, 67, 318–322. [Google Scholar] [CrossRef]
- Suuronen, R.; Pohjonen, T.; Hietanen, J.; Lindqvist, C. A 5-year in vitro and in vivo study of the biodegradation of polylactide plates. J. Oral Maxillofac. Surg. 1998, 56, 604–605. [Google Scholar] [CrossRef]
- Cordewener, F.W.; Schmitz, J.P. The Future of Biodegradable Osteosyntheses. Tissue Eng. 2004, 6, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Chhabra, P.; Dover, M.S. Removal of miniplates in maxillofacial surgery: A follow-up study. J. Oral Maxillofac. Surg. 2005, 63, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Barbateskovic, M.; Koster, T.M.; Eck, R.J.; Maagaard, M.; Afshari, A.; Blokzijl, F.; Cronhjort, M.; Dieperink, W.; Fabritius, M.L.; Feinberg, J.; et al. A new tool to assess Clinical Diversity In Meta-analyses (CDIM) of interventions. J. Clin. Epidemiol. 2021, 135, 29–41. [Google Scholar] [CrossRef]
- Bayat, M.; Garajei, A.; Ghorbani, K.; Motamedi, M.H.K. Treatment of Mandibular Angle Fractures Using a Single Bioresorbable Miniplate. J. Oral Maxillofac. Surg. 2010, 68, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- van Bakelen, N.; Buijs, G.; Jansma, J.; de Visscher, J.; Hoppenreijs, T.; Bergsma, J.; Stegenga, B.; Bos, R. Decision-making considerations in application of biodegradable fixation systems in maxillofacial surgery—A retrospective cohort study. J. Cranio-Maxillofac. Surg. 2014, 42, 417–422. [Google Scholar] [CrossRef]
- Buijs, G.J.; van der Houwen, E.B.; Stegenga, B.; Bos, R.R.M.; Verkerke, G.J. Torsion Strength of Biodegradable and Titanium Screws: A Comparison. J. Oral Maxillofac. Surg. 2007, 65, 2142–2147. [Google Scholar] [CrossRef]
- Shetty, V.; Caputo, A.A.; Kelso, I. Torsion-axial force characteristics of SR-PLLA screws. J. Cranio-Maxillofac. Surg. 1997, 25, 19–23. [Google Scholar] [CrossRef]
- Eckelt, U.; Nitsche, M.; Müller, A.; Pilling, E.; Pinzer, T.; Roesner, D. Ultrasound aided pin fixation of biodegradable osteosynthetic materials in cranioplasty for infants with craniosynostosis. J. Cranio-Maxillofac. Surg. 2007, 35, 218–221. [Google Scholar] [CrossRef]
- Park, S.M.; Park, S.; Park, J.; Choi, M.; Kim, L.; Noh, G. Design process of patient-specific osteosynthesis plates using topology optimization. J. Comput. Des. Eng. 2021, 8, 1257–1266. [Google Scholar] [CrossRef]
- Merema, B.B.J.; Kraeima, J.; Visscher, S.A.H.J.; Minnen, B.; Spijkervet, F.K.L.; Schepman, K.; Witjes, M.J.H. Novel finite element-based plate design for bridging mandibular defects: Reducing mechanical failure. Oral Dis. 2020, 26, 1265–1274. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Shao, X.; Wang, X.; Xu, F.; Dai, T.; Zhou, J.G.; Liu, J.; Song, K.; Tian, L.; Liu, B.; Liu, Y. In vivo biocompatibility and degradability of a Zn–Mg–Fe alloy osteosynthesis system. Bioact. Mater. 2022, 7, 154–166. [Google Scholar] [CrossRef]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.-Y.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [Google Scholar] [CrossRef]
- Ge, L.; Yang, L.; Bron, R.; Burgess, J.K.; Van Rijn, P. Topography-Mediated Fibroblast Cell Migration Is Influenced by Direction, Wavelength, and Amplitude. ACS Appl. Bio Mater. 2020, 3, 2104–2116. [Google Scholar] [CrossRef]
- Kou, P.M.; Pallassana, N.; Bowden, R.; Cunningham, B.; Joy, A.; Kohn, J.; Babensee, J.E. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials 2012, 33, 1699. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.-J.; Kaiser, C.; et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2016, 387, 31–39. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Essig, H.; Lindhorst, D.; Gander, T.; Schumann, P.; Könü, D.; Altermatt, S.; Rücker, M. Patient-specific biodegradable implant in pediatric craniofacial surgery. J. Cranio-Maxillofac. Surg. 2017, 45, 216–222. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782. https://doi.org/10.3390/polym14142782
Gareb B, Van Bakelen NB, Vissink A, Bos RRM, Van Minnen B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers. 2022; 14(14):2782. https://doi.org/10.3390/polym14142782
Chicago/Turabian StyleGareb, Barzi, Nico B. Van Bakelen, Arjan Vissink, Ruud R. M. Bos, and Baucke Van Minnen. 2022. "Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances" Polymers 14, no. 14: 2782. https://doi.org/10.3390/polym14142782
APA StyleGareb, B., Van Bakelen, N. B., Vissink, A., Bos, R. R. M., & Van Minnen, B. (2022). Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers, 14(14), 2782. https://doi.org/10.3390/polym14142782








