Effect of Hydrophilic/Hydrophobic Nanostructured TiO2 on Space Charge and Breakdown Properties of Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
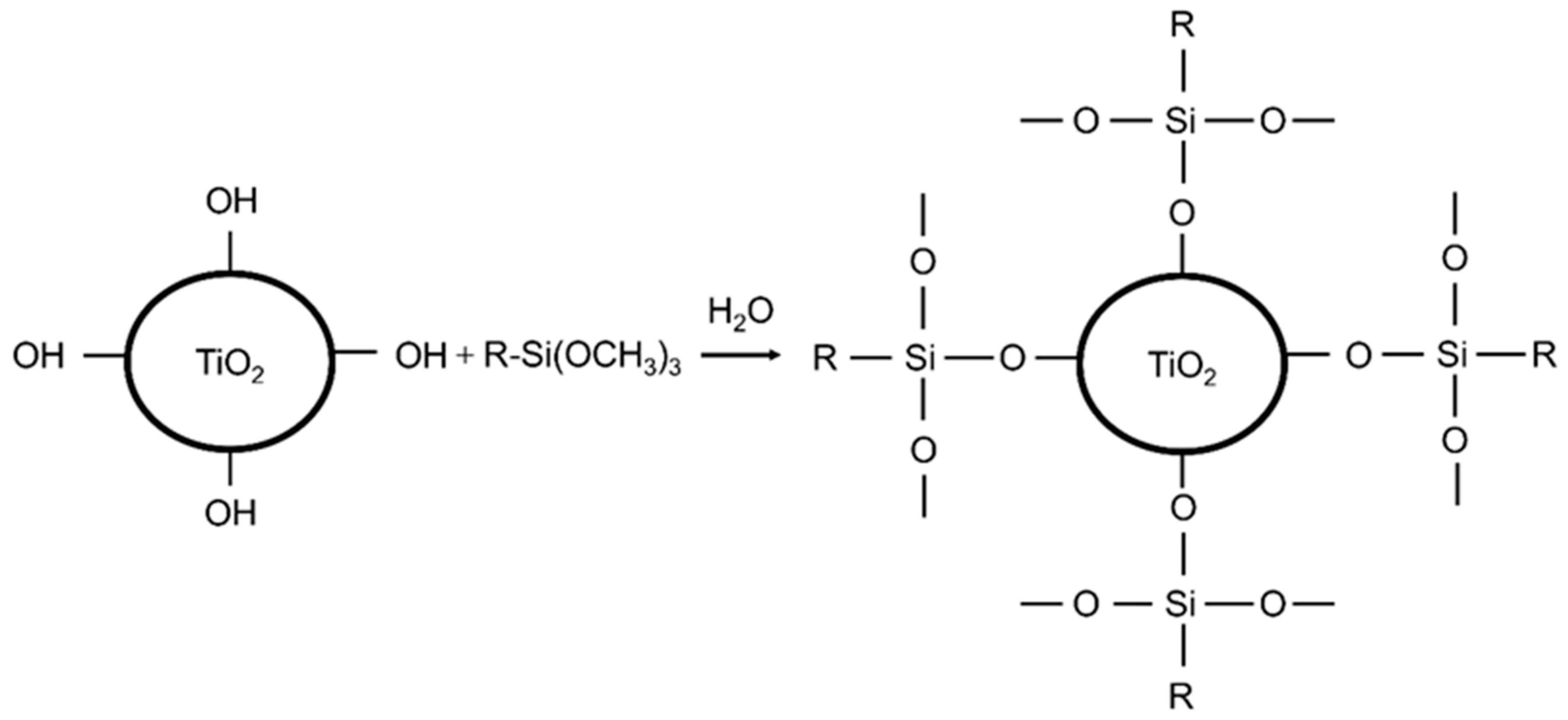
2.2. Sample Preparation
2.3. Experimental Methods
3. Results and Discussion of Simulation
3.1. SEM Analysis
3.2. PLM Morphology
3.3. XRD Analysis
3.4. Space Charge Analysis
- The spherical size of PP is large, and the crystallinity is low from the PLM test results. When the carrier moves in PP, as shown in Figure 7a, there are more amorphous regions and less interfacial regions, only some shallow traps in the amorphous region, and carriers can move easily in the composite material [45,46,47]. Thus, the residual charge density in the samples is larger. When a certain number of nanostructured NKT90 are mixed, the carrier motion is as in Figure 7b, such as 0.5NKT90, 1.0NKT90, and 1.5NKT90. At this time, the spherulite size decreases, and the crystallinity increases, which results in a large number of boundary regions. Deep traps exist in the boundary region between the spherulite and amorphous regions [45,46,47]. The number of deep traps increases, the movement of carriers is hindered, and the charge is captured as soon as it is injected into the sample. At this time, the residual charge in the specimen is less, such as 0.5NKT90, 1.0NKT90, and 1.5NKT90. As shown in Figure 7c, when excessive nanostructured material is mixed, the spherulite size decreases, but the crystallinity decreases. The area of the amorphous region with irregular spherulite distribution increases, and the blocking effect of composite materials on carriers begins to decline. The crystal size of 0.5P90, 1.0P90, and 1.5P90 is between PP and NKT90/PP nanocomposites, so its ability to suppress the movement of carriers is also between them.
- The interfacial effect of nanoparticles is also one of the reasons for limiting the movement of carriers. The nanostructured TiO2 will generate an interfacial region with PP, and there are deep traps and shallow traps in the interfacial region [48,49]. When the content of NKT90 is low, the deep traps in the interfacial region can trap mobile charges, thereby inhibiting charge injection. As the nanostructured TiO2 (NKT90 or P90) spacing decreases or agglomerates, the interfacial region will overlap, which is conducive to the generation of low-resistance paths for easier carrier transport and a higher density of residual charge inside the sample, such as 2.0NKT90, 1.5P90, and 2.0P90.
3.5. Electrical Breakdown Strength Analysis
4. Conclusions
- P90 has poor dispersibility in PP and is easy to agglomerate, and its nanocomposite crystals are larger; NKT90 is well dispersed in PP4874, and the crystal size of the corresponding nanocomposites is smaller and more regular. With the increase of its content, the agglomeration of nanostructured gradually increases, and the crystallization becomes more nonsequenced. The addition of TiO2 did not change the crystal form of PP4874, indicating that the electrical properties of PP are changed by the growth rate and crystallinity of the crystal nucleus.
- Nanostructured material can inhibit the space charge accumulation of the PP4874 to a certain extent. With the increase of nanostructured material content, charge accumulation will occur. The hydrophobic TiO2 is more effective in suppressing space charge.
- When the content of NKT90 is 0.5 wt%–1 wt%, its DC breakdown strength is clearly increased, and its AC breakdown strength is also improved but not obvious enough. With the increase of nanostructured material content, the AC and DC breakdown strength gradually decreases. The overall AC/DC breakdown of P90 is lower than that of PP4874 due to agglomeration in PP4874.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic-Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric Nanocomposites with Insulating Properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Hanley, T.L.; Burford, R.P.; Fleming, R.J.; Barber, K.W. A General Review of Polymeric Insulation for Use in HVDC Cables. IEEE Electr. Insul. Mag. 2003, 19, 13–24. [Google Scholar] [CrossRef]
- David, E.; Fréchette, M. Polymer Nanocomposites-Major Conclusions and Achievements Reached So Far. IEEE Electr. Insul. Mag. 2012, 29, 29–36. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Yin, Y. Nanoparticle Surface Modification Induced Space Charge Suppression in Linear Low Density Polyethylene. Appl. Phys. Lett. 2009, 95, 242905. [Google Scholar] [CrossRef]
- Cavallini, A.; Montanari, G.C.; Morshuis, P. HVDC Insulation and Diagnostics. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 4–6. [Google Scholar] [CrossRef]
- Du, B.; Li, Z.; Yang, Z.; Li, J. Application and Research Progress of HVDC XLPE Cables. Gaodianya Jishu/High Volt. Eng. 2017, 43, 344–354. [Google Scholar]
- Thomas, J.; Joseph, B.; Jose, J.P.; Maria, H.J.; Main, P.; Ali Rahman, A.; Francis, B.; Ahmad, Z.; Thomas, S. Recent Advances in Cross-Linked Polyethylene-Based Nanocomposites for High Voltage Engineering Applications: A Critical Review. Ind. Eng. Chem. Res. 2019, 58, 20863–20879. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, S.; Hu, J.; He, J. Polymeric Insulation Materials for HVDC Cables: Development, Challenges and Future Perspective. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1308–1318. [Google Scholar] [CrossRef]
- Akonda, M.H.; Lawrence, C.A.; Weager, B.M. Recycled Carbon Fibre-Reinforced Polypropylene Thermoplastic Composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 79–86. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Jiang, P.; Tanaka, T. Material progress toward recyclable insulation of power cables part 2: Polypropylene-based thermoplastic materials. IEEE Electr. Insul. Mag. 2020, 36, 8–18. [Google Scholar] [CrossRef]
- Ouyang, Y.; Mauri, M.; Pourrahimi, A.M.; Östergren, I.; Lund, A.; Gkourmpis, T.; Prieto, O.; Xu, X.; Hagstrand, P.O.; Müller, C. Recyclable Polyethylene Insulation via Reactive Compounding with a Maleic Anhydride-Grafted Polypropylene. ACS Appl. Polym. Mater. 2020, 2, 2389–2396. [Google Scholar] [CrossRef]
- Hosier, I.L.; Vaughan, A.S.; Swingler, S.G. An Investigation of the Potential of Polypropylene and Its Blends for Use in Recyclable High Voltage Cable Insulation Systems. J. Mater. Sci. 2011, 46, 4058–4070. [Google Scholar] [CrossRef]
- Huang, X.; Fan, Y.; Zhang, J.; Jiang, P. Polypropylene Based Thermoplastic Polymers for Potential Recyclable HVDC Cable Insulation Applications. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1446–1456. [Google Scholar] [CrossRef]
- Ho, J.; Jow, T.R. High Field Conduction in Biaxially Oriented Polypropylene at Elevated Temperature. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 990–995. [Google Scholar] [CrossRef]
- Awad, S.A.; Khalaf, E.M. Investigation of Improvement of Properties of Polypropylene Modified by Nano Silica Composites. Compos. Commun. 2019, 12, 59–63. [Google Scholar] [CrossRef]
- Nikmatin, S.; Syafiuddin, A.; Hong Kueh, A.B.; Maddu, A. Physical, Thermal, and Mechanical Properties of Polypropylene Composites Filled with Rattan Nanoparticles. J. Appl. Res. Technol. 2017, 15, 386–395. [Google Scholar] [CrossRef]
- Atagur, M.; Seki, Y.; Pasaoglu, Y.; Sever, K.; Seki, Y.; Sarikanat, M.; Altay, L. Mechanical and Thermal Properties of Carpinas Betulus Fiber Filled Polypropylene Composites. Polym. Compos. 2020, 41, 1925–1935. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Awad, S.A. Improvement Chemical, Thermal, and Mechanical Properties of Polypropylene by Using Corn Flour. Usak Univ. J. Mater. Sci. 2017, 6, 15–25. [Google Scholar] [CrossRef]
- Ketsamee, P.; Andritsch, T.; Vaughan, A. Effect of Surface-Modified TiO2 and MgO Nanoparticles on Dielectric Permittivity and Breakdown Strength of PP Nanocomposites. In Proceedings of the Annual Report—Conference on Electrical Insulation and Dielectric Phenomena, CEIDP, Cancun, Mexico, 20–24 October 2002; Institute of Electrical and Electronics Engineers Inc.: Manhattan, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Fuse, N.; Ohki, Y.; Tanaka, T. Comparison of Nano-Structuration Effects in Polypropylene among Four Typical Dielectric Properties. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 671–677. [Google Scholar] [CrossRef]
- Zhou, Y.; Dang, B.; Hu, J.; Chen, X.; Yu, F.; He, J. Effect of Magnesium Oxide Nanoparticles on Tailoring the Properties of Polypropylene. Zhongguo Dianji Gongcheng Xuebao/Proc. Chin. Soc. Electr. Eng. 2016, 36, 6619–6626. [Google Scholar] [CrossRef]
- Xu, H. Modification and Space Charge Investigation of Polypropylene Composites for HVDC Cables. Master’s Thesis, Tianjin University, Tianjin, China, 2017. [Google Scholar]
- Zhou, Y.; Hu, J.; Dang, B.; He, J. Effect of Different Nanoparticles on Tuning Electrical Properties of Polypropylene Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Hamzah, M.S.; Jaafar, M.; Mohd Jamil, M.K. Electrical Insulation Characteristics of Alumina, Titania, and Organoclay Nanoparticles Filled PP/EPDM Nanocomposites. J. Appl. Polym. Sci. 2014, 131, 41184. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Kuttappan, S.K.; Joseph, R. Thermal and Mechanical Properties of Polypropylene/Titanium Dioxide Nanocomposite Fibers. Mater. Des. 2012, 37, 537–542. [Google Scholar] [CrossRef]
- Selvin, T.P.; Kuruvilla, J.; Sabu, T. Mechanical Properties of Titanium Dioxide-Filled Polystyrene Microcomposites. Mater. Lett. 2004, 58, 281–289. [Google Scholar] [CrossRef]
- Forhad Mina, M.; Seema, S.; Matin, R.; Jellur Rahaman, M.; Bijoy Sarker, R.; Abdul Gafur, M.; Abu Hashan Bhuiyan, M. Improved Performance of Isotactic Polypropylene/Titanium Dioxide Composites: Effect of Processing Conditions and Filler Content. Polym. Degrad. Stab. 2009, 94, 183–188. [Google Scholar] [CrossRef]
- Altan, M.; Yildirim, H. Mechanical and Antibacterial Properties of Injection Molded Polypropylene/TiO2 Nano-Composites: Effects of Surface Modification. J. Mater. Sci. Technol. 2012, 28, 686–692. [Google Scholar] [CrossRef]
- Yang, C.P.; Wu, Y.J.; Liu, C.G. Studies on Crystallizations and Mechanical Properties of Polypropylene/Nano-TiO2 Composites. Adv. Mater. Res. 2013, 690–693, 494–498. [Google Scholar] [CrossRef]
- Chi, X.; Cheng, L.; Liu, W.; Zhang, X.; Li, S. Characterization of Polypropylene Modified by Blending Elastomer and Nano-Silica. Materials 2018, 11, 1321. [Google Scholar] [CrossRef] [Green Version]
- Franciszczak, P.; Wojnowski, J.; Kalniņš, K.; Piesowicz, E. The Influence of Matrix Crystallinity on the Mechanical Performance of Short-Fibre Composites—Based on Homo-Polypropylene and a Random Polypropylene Copolymer Reinforced with Man-Made Cellulose and Glass Fibres. Compos. Part B Eng. 2019, 166, 516–526. [Google Scholar] [CrossRef]
- Hao, M.; Zhou, Y.; Chen, G.; Wilson, G.; Jarman, P. Space Charge Behavior in Oil Gap and Impregnated Pressboard Combined System under HVDC Stresses. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 848–858. [Google Scholar] [CrossRef]
- Dissado, L.A.; Fothergill, J.C. Electrical Degradation and Breakdown in Polymers; Peregrinus, P., Ed.; The Institution of Engineering and Technology: Stevenage, UK, 1992; ISBN 0863411967. [Google Scholar]
- He, X.; Rytoluoto, I.; Anyszka, R.; Mahtabani, A.; Saarimaki, E.; Lahti, K.; Paajanen, M.; Dierkes, W.; Blume, A. Silica Surface-Modification for Tailoring the Charge Trapping Properties of PP/POE Based Dielectric Nanocomposites for HVDC Cable Application. IEEE Access 2020, 8, 87719–87734. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.S. The Role of Interfaces in Polyethylene/Metal-Oxide Nanocomposites for Ultrahigh-Voltage Insulating Materials. Adv. Mater. 2018, 30, 1703624. [Google Scholar] [CrossRef]
- Du, B.X.; Li, J. Interface Charge Behaviors between LDPE and EPDM Filled with Carbon Black Nanoparticles. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3696–3703. [Google Scholar] [CrossRef]
- Zeng-Zeng, X.; Xu, A.N.; Shou-Shan, C.; Huang, J.-Y.; Yi-Fan, J.; Wang, C.; Yang, M.-S. Analysis of Modifi Cation Eff Ect of Peroxide Masterbatch on Homo-Polypropylene from Crystallization Behavior. Synth. Mater. Aging Appl. 2021, 50, 1–4+65. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zha, J.W.; Li, W.K.; Wang, S.J.; Dang, Z.M. A Remarkable Suppression on Space Charge in Isotatic Polypropylene by Inducing the β-Crystal Formation. Appl. Phys. Lett. 2015, 107, 112901. [Google Scholar] [CrossRef]
- Piorkowska, E.; Rutledge, G.C. Handbook of Polymer Crystallization; John Wiley& Son Inc.: Hoboken, NJ, USA, 2013; pp. 38–49. [Google Scholar]
- Rungswang, W.; Jarumaneeroj, C.; Patthamasang, S.; Phiriyawirut, P.; Jirasukho, P.; Soontaranon, S.; Rugmai, S.; Hsiao, B.S. Influences of Tacticity and Molecular Weight on Crystallization Kinetic and Crystal Morphology under Isothermal Crystallization: Evidence of Tapering in Lamellar Width. Polymer 2019, 172, 41–51. [Google Scholar] [CrossRef]
- Mahmood, P.H.; Amiri, O.; Ahmed, S.S.; Hama, J.R. Simple Microwave Synthesis of TiO2/NiS2 Nanocomposite and TiO2/NiS2/Cu Nanocomposite as an Efficient Visible Driven Photocatalyst. Ceram. Int. 2019, 45, 14167–14172. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, J.; Zhang, X.; Guo, N. Composite Micro-Nanoarchitectonics of Mmt-SiO2: Space Charge Characteristics under Tensile State. Polymers 2021, 13, 4354. [Google Scholar] [CrossRef]
- Min, D.; Li, S. Simulation on the Influence of Bipolar Charge Injection and Trapping on Surface Potential Decay of Polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1627–1636. [Google Scholar] [CrossRef]
- Li, X.; Du, Q.; Kang, J.; Tu, D. Influence of Microstructure on Space Charges of Polypropylene. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 365–374. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, B.; Wang, X.; Jia, T. Charge Transport in Low Density Polyethylene Based Micro/Nano-Composite with Improved Thermal Conductivity. J. Phys. D Appl. Phys. 2019, 52, 285302. [Google Scholar] [CrossRef]
- Li, J.; Zhou, F.; Min, D.; Li, S.; Xia, R. The Energy Distribution of Trapped Charges in Polymers Based on Isothermal Surface Potential Decay Model. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1723–1732. [Google Scholar] [CrossRef]
- Seiler, J.; Kindersberger, J. Insight into the Interphase in Polymer Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 537–547. [Google Scholar] [CrossRef]
- Gong, S.; Chen, Q.; Moll, J.F.; Kumar, S.K.; Colby, R.H. Segmental Dynamics of Polymer Melts with Spherical Nanoparticles. ACS Macro Lett. 2014, 3, 773–777. [Google Scholar] [CrossRef]
- Li, S.; Yin, G.; Chen, G.; Li, J.; Bai, S.; Zhong, L.; Zhang, Y.; Lei, Q. Short-Term Breakdown and Long-Term Failure in Nanodielectrics: A Review. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1523–1535. [Google Scholar] [CrossRef] [Green Version]








| Nanocomposites | Abbreviation |
|---|---|
| PP4874 | PP |
| PP + 0.5 wt% P90 | 0.5P90 |
| PP + 1.0 wt% P90 | 1.0P90 |
| PP + 1.5 wt% P90 | 1.5P90 |
| PP + 2.0 wt% P90 | 2.0P90 |
| PP + 0.5 wt% NKT90 | 0.5NKT90 |
| PP + 1.0 wt% NKT90 | 1.0NKT90 |
| PP + 1.5 wt% NKT90 | 1.5NKT90 |
| PP + 2.0 wt% NKT90 | 2.0NKT90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.-G.; Liu, H.-S.; Lee, T.-T.; Schachtely, U.; Kobayashi, H.; Li, L.-L. Effect of Hydrophilic/Hydrophobic Nanostructured TiO2 on Space Charge and Breakdown Properties of Polypropylene. Polymers 2022, 14, 2762. https://doi.org/10.3390/polym14142762
Gao J-G, Liu H-S, Lee T-T, Schachtely U, Kobayashi H, Li L-L. Effect of Hydrophilic/Hydrophobic Nanostructured TiO2 on Space Charge and Breakdown Properties of Polypropylene. Polymers. 2022; 14(14):2762. https://doi.org/10.3390/polym14142762
Chicago/Turabian StyleGao, Jun-Guo, Hong-Shuo Liu, Ting-Tai Lee, Uwe Schachtely, Hitoshi Kobayashi, and Li-Li Li. 2022. "Effect of Hydrophilic/Hydrophobic Nanostructured TiO2 on Space Charge and Breakdown Properties of Polypropylene" Polymers 14, no. 14: 2762. https://doi.org/10.3390/polym14142762
APA StyleGao, J.-G., Liu, H.-S., Lee, T.-T., Schachtely, U., Kobayashi, H., & Li, L.-L. (2022). Effect of Hydrophilic/Hydrophobic Nanostructured TiO2 on Space Charge and Breakdown Properties of Polypropylene. Polymers, 14(14), 2762. https://doi.org/10.3390/polym14142762







