Chiral Porous Carbon Surfaces for Enantiospecific Synthesis
Abstract
:1. Introduction
2. Materials and Methods
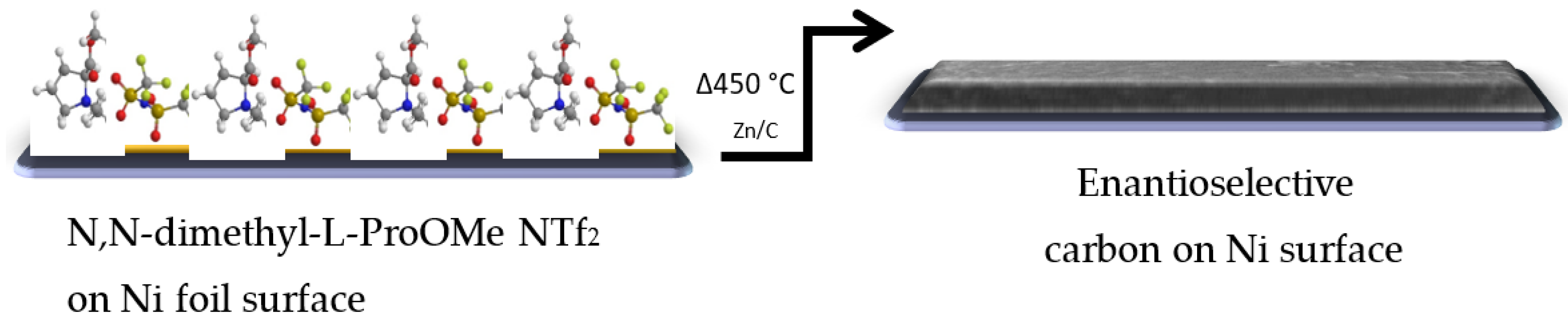
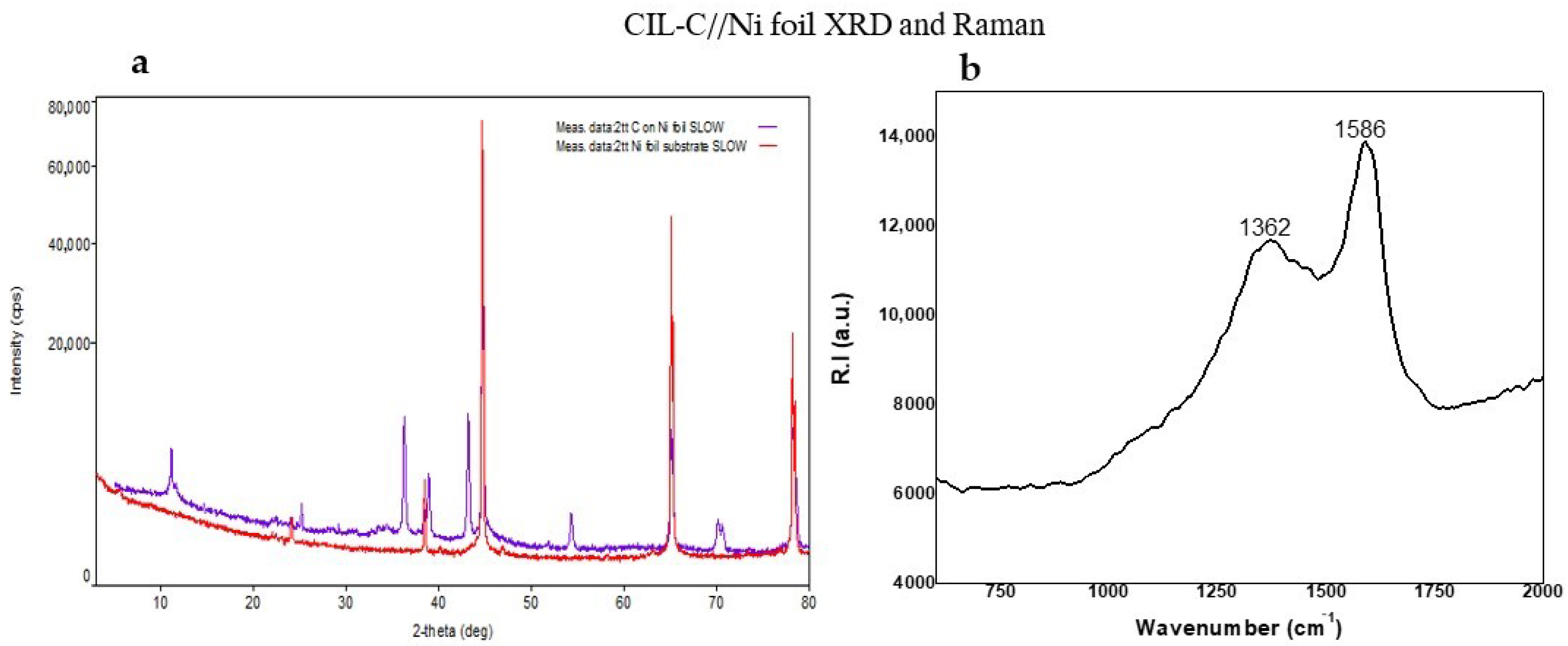
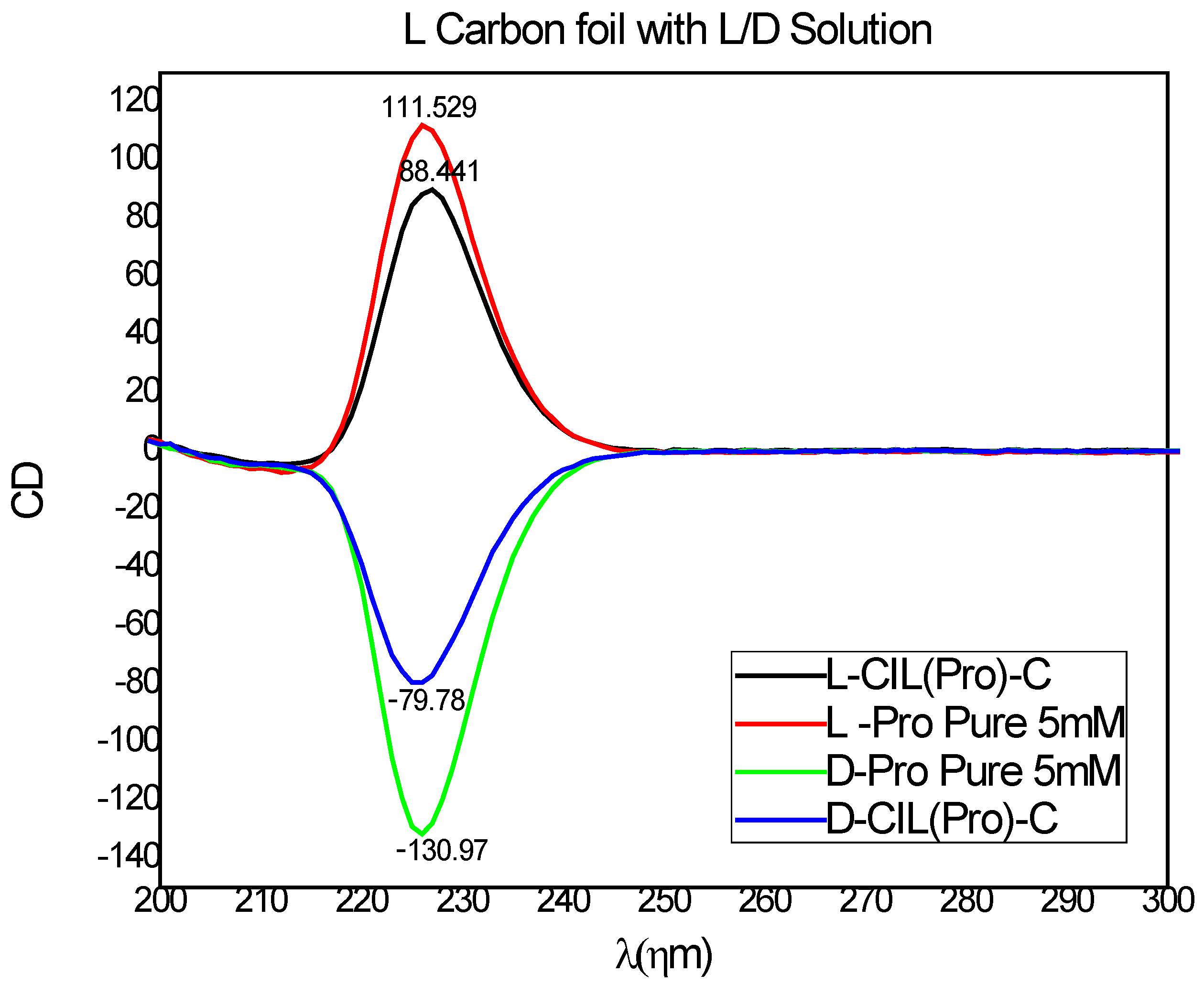
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlow, S.M.; Raval, R. Complex Organic Molecules at Metal Surfaces: Bonding, Organisation and Chirality. Surf. Sci. Rep. 2003, 50, 201–341. [Google Scholar] [CrossRef]
- Barlow, S.M.; Raval, R. Nanoscale Insights in the Creation and Transfer of Chirality in Amino Acid Monolayers at Defined Metal Surfaces. Curr. Opin. Colloid Interface Sci. 2008, 13, 65–73. [Google Scholar] [CrossRef]
- Gellman, A.J. Chiral Surfaces: Accomplishments and Challenges. ACS Nano 2010, 4, 5–10. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sholl, D.S. Chiral Selection on Inorganic Crystalline Surfaces. Nat. Mater. 2003, 2, 367–374. [Google Scholar] [CrossRef]
- Horvath, J.D.; Gellman, A.J. Naturally Chiral Surfaces. Top. Catal. 2003, 25, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Horvath, J.D.; Koritnik, A.; Kamakoti, P.; Sholl, D.S.; Gellman, A.J. Enantioselective Separation on a Naturally Chiral Surface. J. Am. Chem. Soc. 2004, 126, 14988–14994. [Google Scholar] [CrossRef]
- Mark, A.G.; Forster, M.; Raval, R. Recognition and Ordering at Surfaces: The Importance of Handedness and Footedness. ChemPhysChem 2011, 12, 1474–1480. [Google Scholar] [CrossRef]
- Kim, H.; Im, S.W.; Kim, R.M.; Cho, N.H.; Lee, H.-E.; Ahn, H.-Y.; Nam, K.T. Chirality Control of Inorganic Materials and Metals by Peptides or Amino Acids. Mater. Adv. 2020, 1, 512–524. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, Y.; Tang, Z. Chiral Inorganic Nanoparticles: Origin, Optical Properties and Bioapplications. Nanoscale 2011, 3, 1374–1382. [Google Scholar] [CrossRef]
- Dressler, D.H.; Mastai, Y. Chiral crystallization of glutamic acid on self assembled films of cysteine. Chirality 2007, 19, 358–365. [Google Scholar] [CrossRef]
- Ernst, K.-H. Molecular chirality at surfaces. Phys. Status Solidi B-Basic Solid State Phys. 2012, 249, 2057–2088. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities, and the Origins of Life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, J.D.; Gellman, A.J. Enantiospecific desorption of chiral compounds from chiral Cu(643) and achiral Cu(111) surfaces. J. Am. Chem. Soc. 2002, 124, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Humblot, V.; Haq, S.; Muryn, C.; Hofer, W.A.; Raval, R. From local adsorption stresses to chiral surfaces: (R,R)-tartaric acid on Ni(110). J. Am. Chem. Soc. 2002, 124, 503–510. [Google Scholar] [CrossRef]
- Moshe, H.; Levi, G.; Sharon, D.; Mastai, Y. Atomic layer deposition of enantioselective thin film of alumina on chiral self-assembled-monolayer. Surf. Sci. 2014, 629, 88–93. [Google Scholar] [CrossRef]
- Moshe, H.; Vanbel, M.; Valev, V.K.; Verbiest, T.; Dressler, D.; Mastai, Y. Chiral Thin Films of Metal Oxide. Chem. Eur. J. 2013, 19, 10295–10301. [Google Scholar] [CrossRef]
- Switzer, J.A.; Kothari, H.M.; Poizot, P.; Nakanishi, S.; Bohannan, E.W. Enantiospecific electrodeposition of a chiral catalyst. Nature 2003, 425, 490–493. [Google Scholar] [CrossRef]
- Kothari, H.M.; Kulp, E.A.; Boonsalee, S.; Nikiforov, M.P.; Bohannan, E.W.; Poizot, P.; Nakanishi, S.; Switzer, J.A. Enantiospecific electrodeposition of chiral CuO films from Copper(II) complexes of tartaric and amino acids on single-crystal Au(001). Chem. Mater. 2004, 16, 4232–4244. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Burla, N.; Bohannan, E.W.; Switzer, J.A. Enhancing enantioselectivity of electrodeposited CuO films by chiral etching. J. Am. Chem. Soc. 2007, 129, 8972. [Google Scholar] [CrossRef]
- Aloni, S.S.; Perovic, M.; Weitman, M.; Cohen, R.; Oschatz, M.; Mastai, Y. Amino acid-based ionic liquids as precursors for the synthesis of chiral nanoporous carbons. Nanoscale Adv. 2019, 1, 4981–4988. [Google Scholar] [CrossRef] [Green Version]
- Perovic, M.; Aloni, S.S.; Mastai, Y.; Oschatz, M. Mesoporous carbon materials with enantioselective surface obtained by nanocasting for selective adsorption of chiral molecules from solution and the gas phase. Carbon 2020, 170, 550–557. [Google Scholar] [CrossRef]
- Fuchs, I.; Fechler, N.; Antonietti, M.; Mastai, Y. Enantioselective Nanoporous Carbon Based on Chiral Ionic Liquids. Angew. Chem. Int. Ed. 2016, 55, 408–412. [Google Scholar] [CrossRef]
- Bucknum, M.J.; Castro, E.A. The Carbon Allotrope Hexagonite and Its Potential Synthesis from Cold Compression of Carbon Nanotubes. J. Chem. Theory Comput. 2006, 2, 775–781. [Google Scholar] [CrossRef]
- Huo, Q.; Zhao, D.; Feng, J.; Waston, K.; Buratto, S.K.; Stucky, G.D.; Schacht, S.; Schüth, F. Room temperature growth of mesoporous silica fibers: A new high-surface-area optical waveguide. Adv. Mater. 1997, 9, 974–978. [Google Scholar] [CrossRef]
- Chen, S.; Xing, W.; Duan, J.; Hu, X.; Qiao, S.Z. Nanostructured morphology control for efficient supercapacitor electrodes. J. Mater. Chem. 2013, 1, 2941–2954. [Google Scholar] [CrossRef]
- Liu, X.; Fechler, N.; Antonietti, M. Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem. Soc. Rev. 2013, 42, 8237–8265. [Google Scholar] [CrossRef] [Green Version]
- Fayos, J. Possible 3D Carbon Structures as Progressive Intermediates in Graphite to Diamond Phase Transition. J. Solid State Chem. 1999, 148, 278–285. [Google Scholar] [CrossRef]
- Ownby, P.D.; Yang, X.; Liu, J. Calculated X-ray Diffraction Data for Diamond Polytypes. J. Am. Ceram. Soc. 1992, 75, 1876–1883. [Google Scholar] [CrossRef]
- Tontini, G.; Koch, A., Jr.; Schmachtenberg, V.A.; Binder, C.; Klein, A.N.; Drago, V. Synthesis and Magnetic Properties of Nickel Micro Urchins. Mater. Res. Bull. 2015, 61, 177–182. [Google Scholar] [CrossRef]
- Jahromi, S.P.; Ghenaatian, H.R. The Synthesis NiO/GO Nano Composite for High Performance Supercapacitors. In Proceedings of the 6th International Conference on Nanostructures (ICNS6), Kish Island, Iran, 7–10 March 2016; pp. 1–2. [Google Scholar]
- Ahmed, S.; Shim, J.; Park, G. Electrochemical Performances of Nickel Deposited Zeolitic-Imidazolate Framework-67 for Oxygen Reduction and Evolution Reactions in Alkaline Medium. Mater. Lett. 2020, 275, 128144. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman spectroscopic investigations of activated carbon materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Attard, G.A.; Ahmadi, A. Anion—Surface Interactions Part 3. N2O Reduction as a Chemical Probe of the Local Potential of Zero Total Charge. J. Electroanal. Chem. 1995, 389, 175–190. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, N.; Kumar Chopra, H. Chiral Recognition Methods in Analytical Chemistry: Role of the Chiral Ionic Liquids. Crit. Rev. Anal. Chem. 2019, 49, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chopra, H.K. Synthesis and Applications of Carbohydrate Based Chiral Ionic Liquids as Chiral Recognition Agents and Organocatalysts. J. Mol. Liq. 2020, 298, 111994. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Attard, G.A. Electrochemical Studies of Enantioselectivity at Chiral Metal Surfaces. J. Phys. Chem. B 2001, 105, 3158–3167. [Google Scholar] [CrossRef]
- Wattanakit, C.; Saint Côme, Y.B.; Lapeyre, V.; Bopp, P.A.; Heim, M.; Yadnum, S.; Nokbin, S.; Warakulwit, C.; Limtrakul, J.; Kuhn, A. Enantioselective Recognition at Mesoporous Chiral Metal Surfaces. Nat. Commun. 2014, 5, 3325. [Google Scholar] [CrossRef]
- Casiraghi, G.; Zanardi, F.; Appendino, G.; Rassu, G. The Vinylogous Aldol Reaction: A Valuable, yet Understated Carbon− Carbon Bond-Forming Maneuver. Chem. Rev. 2000, 100, 1929–1972. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P. The Direct Catalytic Asymmetric Cross-aldol Reaction of Aldehydes. Angew. Chem. Int. Ed. 2003, 42, 858–860. [Google Scholar] [CrossRef]
- Ube, H.; Shimada, N.; Terada, M. Asymmetric Direct Vinylogous Aldol Reaction of Furanone Derivatives Catalyzed by an Axially Chiral Guanidine Base. Angew. Chem. 2010, 122, 1902–1905. [Google Scholar] [CrossRef]
- Machajewski, T.D.; Wong, C. The Catalytic Asymmetric Aldol Reaction. Angew. Chem. Int. Ed. 2000, 39, 1352–1375. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, S.; Ghosh, S.K.; Ghosh, A.; Saha, R.; Banerjee, S.; Saha, B. Review of the Aldol Reaction. Synth. Commun. 2016, 46, 1327–1342. [Google Scholar] [CrossRef]
- Minakuchi, H.; Nakanishi, K.; Soga, N.; Ishizuka, N.; Tanaka, N. Octadecylsilylated porous silica rods as separation media for reversed-phase liquid chromatography. Anal. Chem. 1996, 68, 3497–3501. [Google Scholar] [CrossRef]



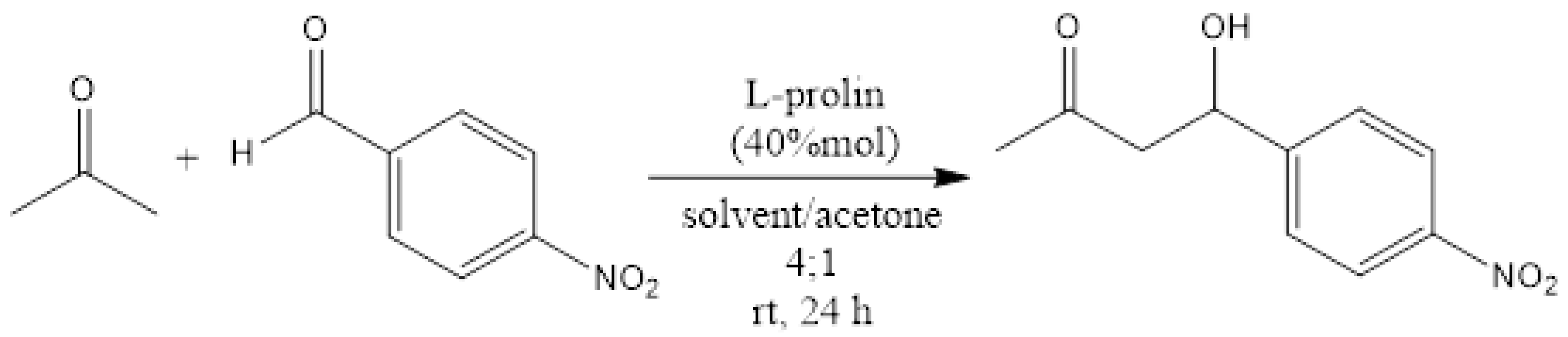
| No | Solvent | Yield (%) |
|---|---|---|
| 1 | DMSO | N.O 1 |
| 2 | THF | 60 |
| 3 | DMF | 16 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloni, S.S.; Nassir, M.; Mastai, Y. Chiral Porous Carbon Surfaces for Enantiospecific Synthesis. Polymers 2022, 14, 2765. https://doi.org/10.3390/polym14142765
Aloni SS, Nassir M, Mastai Y. Chiral Porous Carbon Surfaces for Enantiospecific Synthesis. Polymers. 2022; 14(14):2765. https://doi.org/10.3390/polym14142765
Chicago/Turabian StyleAloni, Sapir Shekef, Molhm Nassir, and Yitzhak Mastai. 2022. "Chiral Porous Carbon Surfaces for Enantiospecific Synthesis" Polymers 14, no. 14: 2765. https://doi.org/10.3390/polym14142765
APA StyleAloni, S. S., Nassir, M., & Mastai, Y. (2022). Chiral Porous Carbon Surfaces for Enantiospecific Synthesis. Polymers, 14(14), 2765. https://doi.org/10.3390/polym14142765








