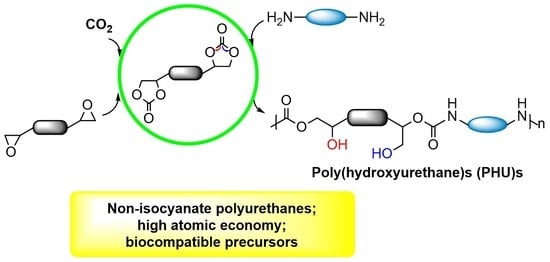
Synthesis of Nonisocyanate Poly(hydroxy)urethanes from Bis(cyclic carbonates) and Polyamines
Abstract
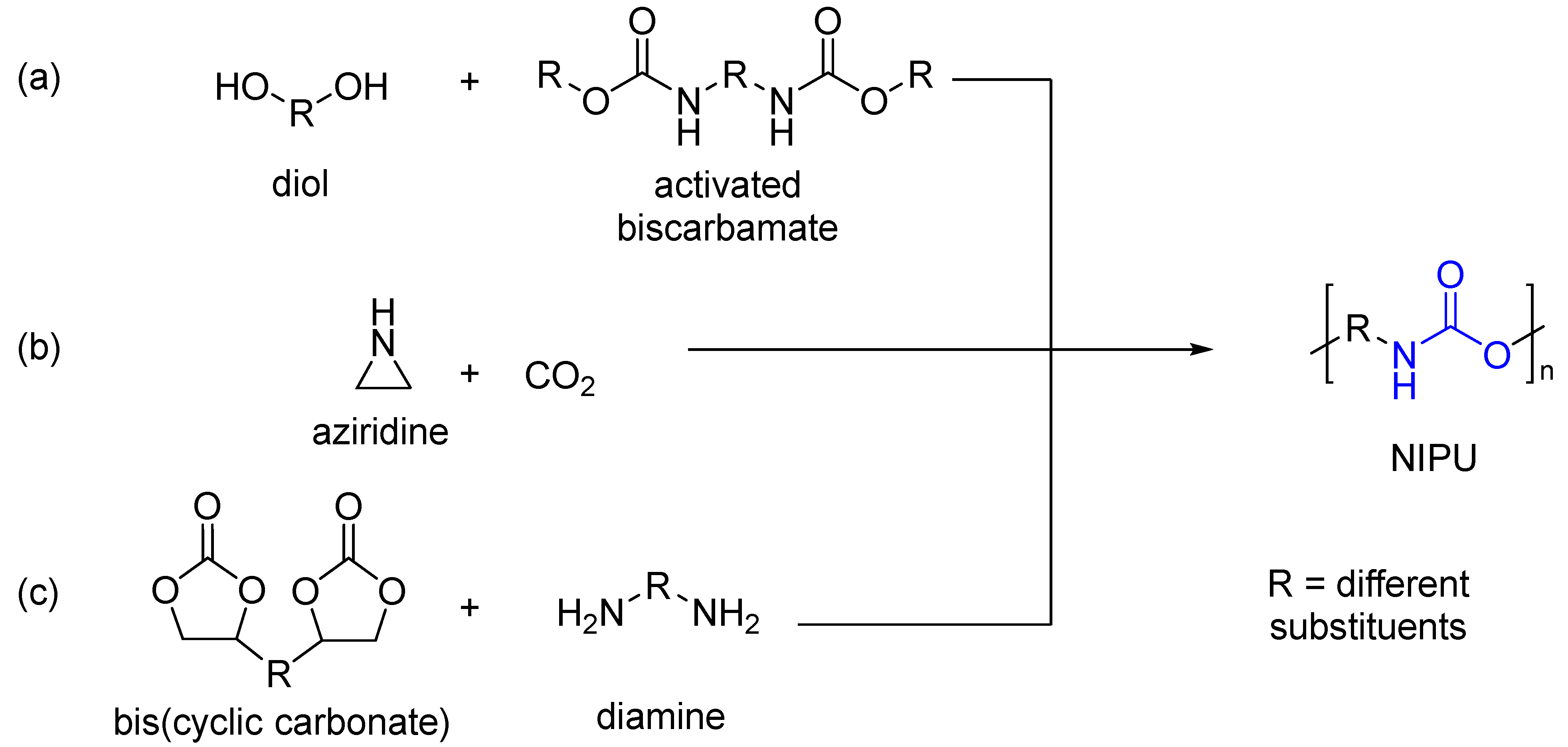
:1. Introduction
2. Experimental Details
2.1. Materials and Methods
2.2. Materials and Reagents
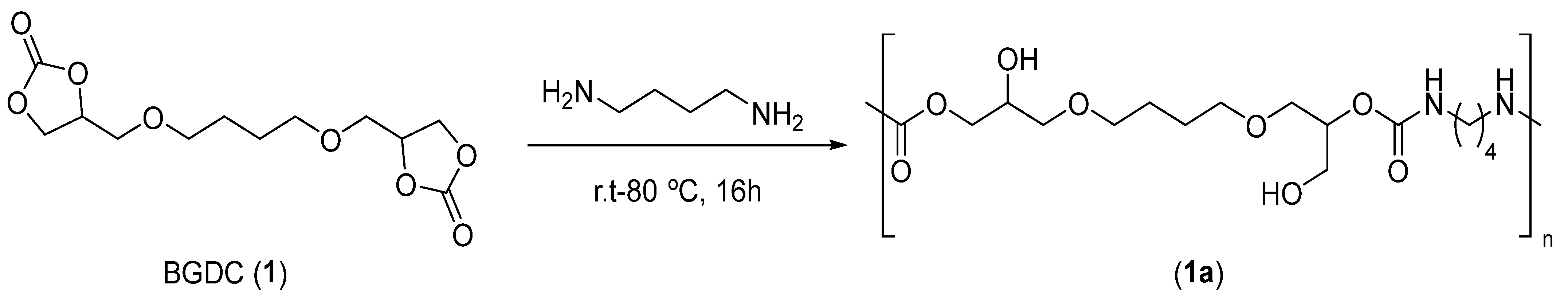
2.3. General Procedure for the Synthesis of 1,4-Butanediol bis(glycidyl ether carbonate) 1
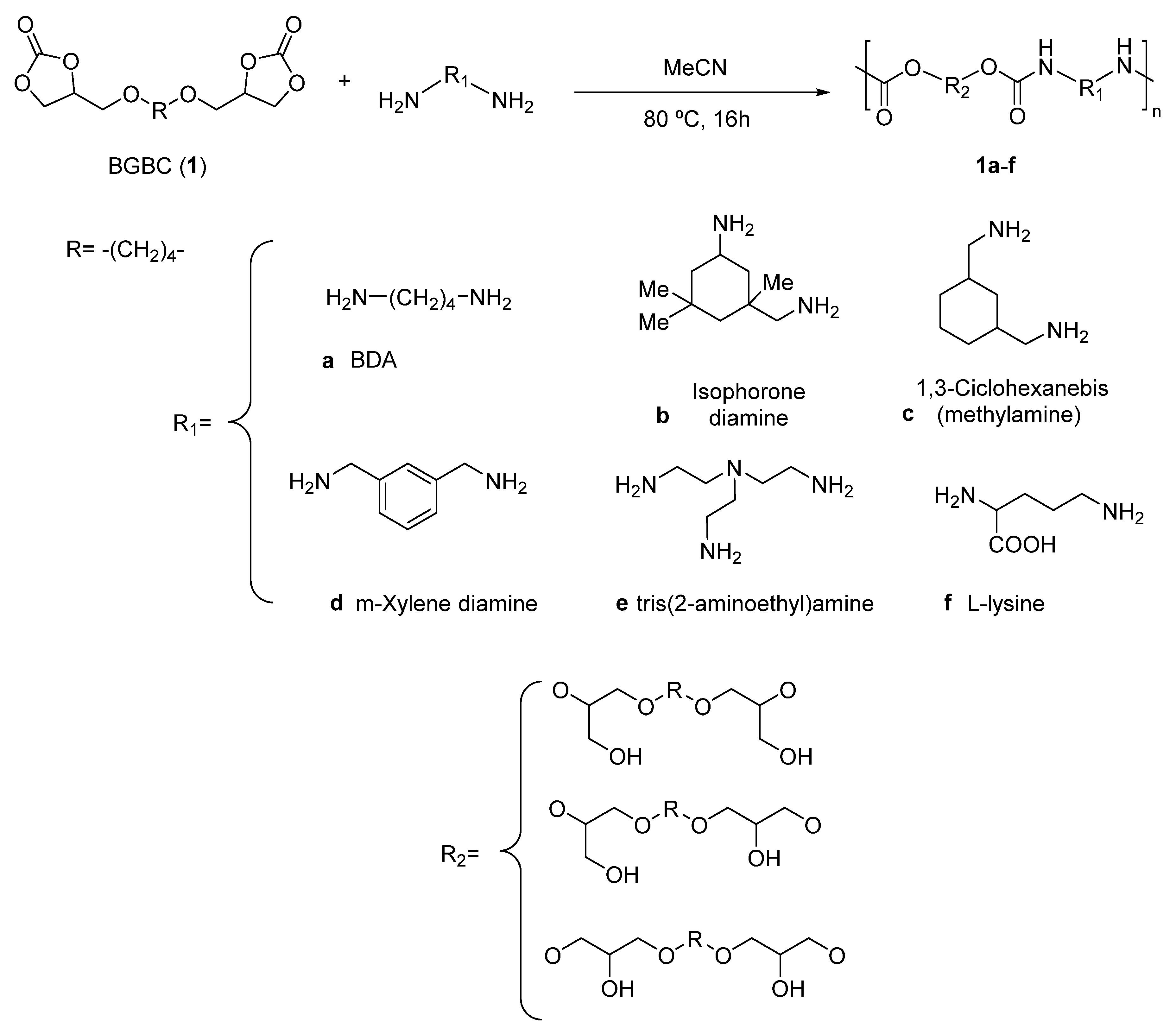
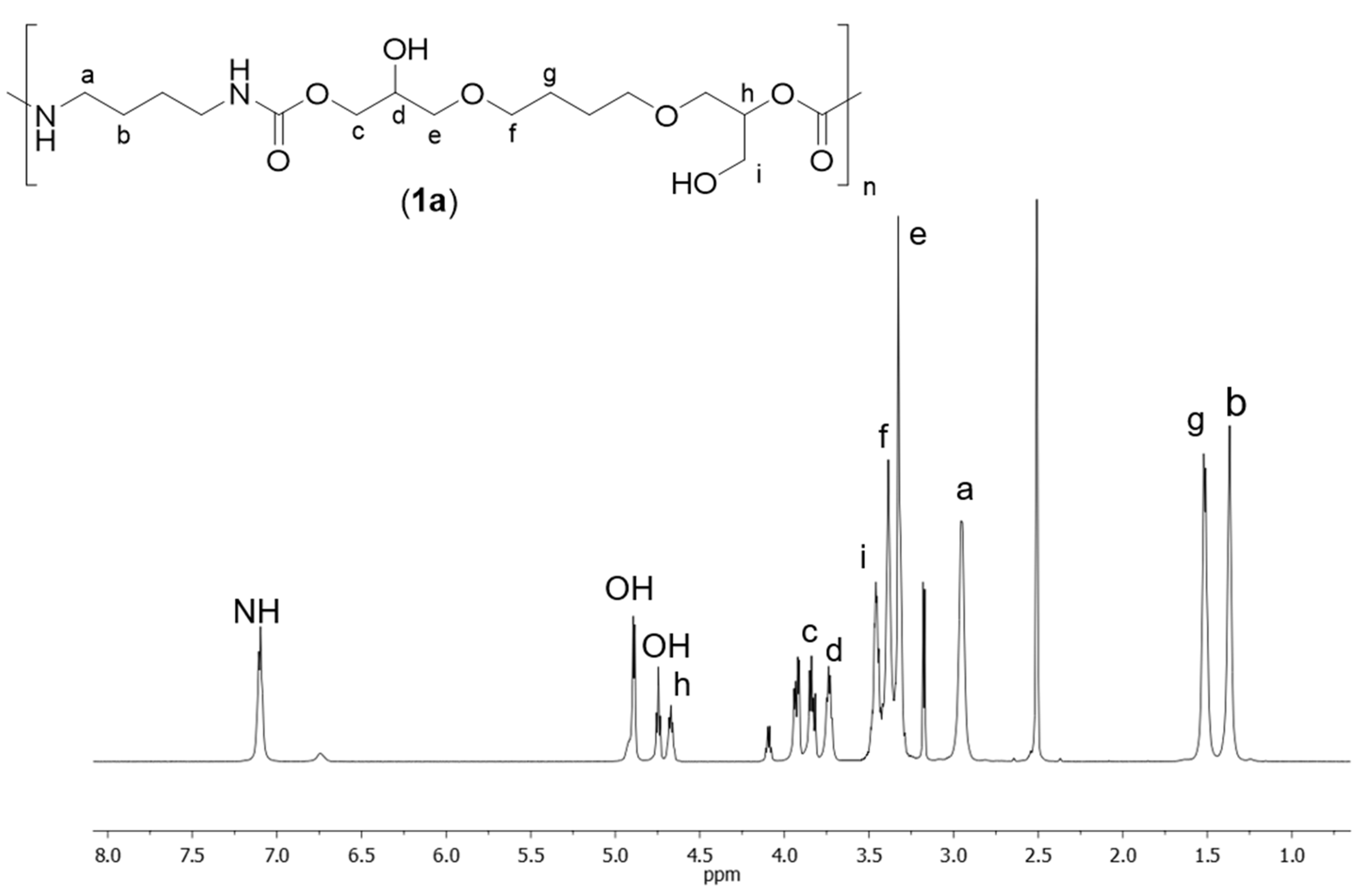
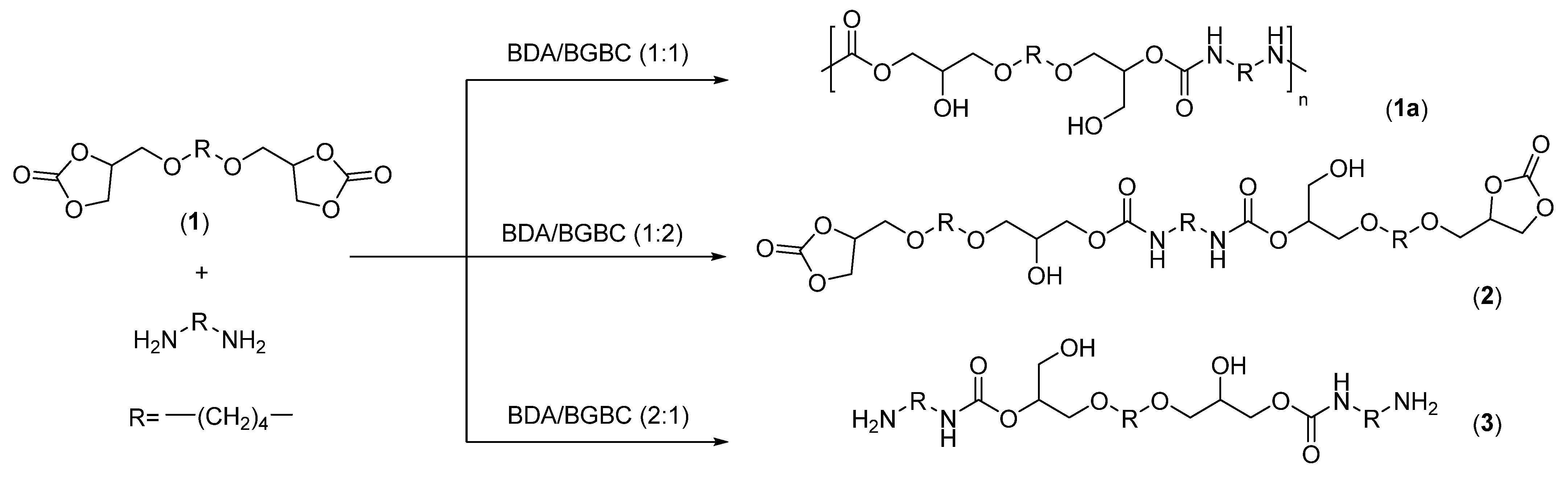
2.4. General Procedure for the Synthesis of Poly(hydroxyurethane)s 1a-e
2.5. Synthesis of Poly(hydroxyurethane) 1f
2.6. General Procedure for the Synthesis of Hydroxycarbamates 2 and 3
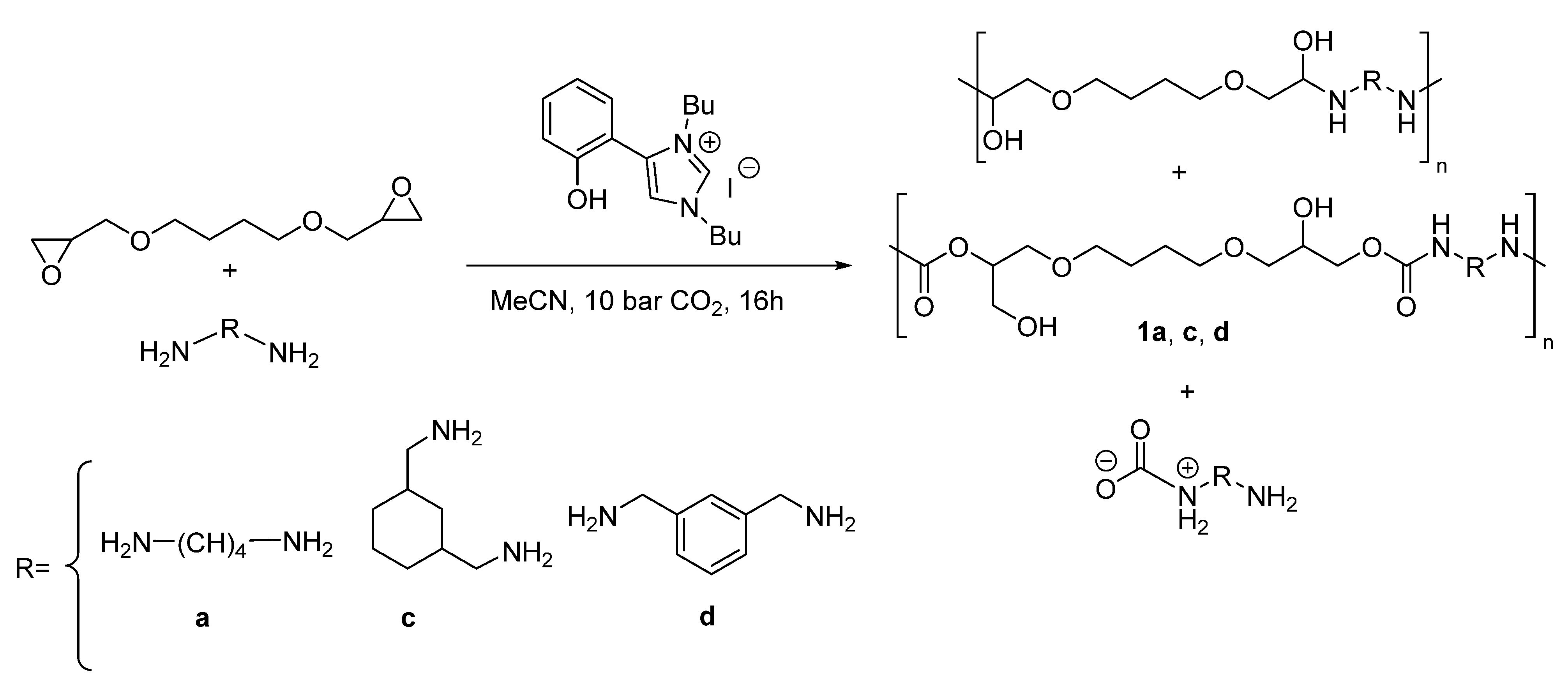
2.7. General Procedure for the One-Pot Synthesis of Poly(hydroxyurethane)s 1a,c,d
2.8. General Procedure for the Sequential Synthesis of Poly(hydroxyurethane)s 1a,c,d
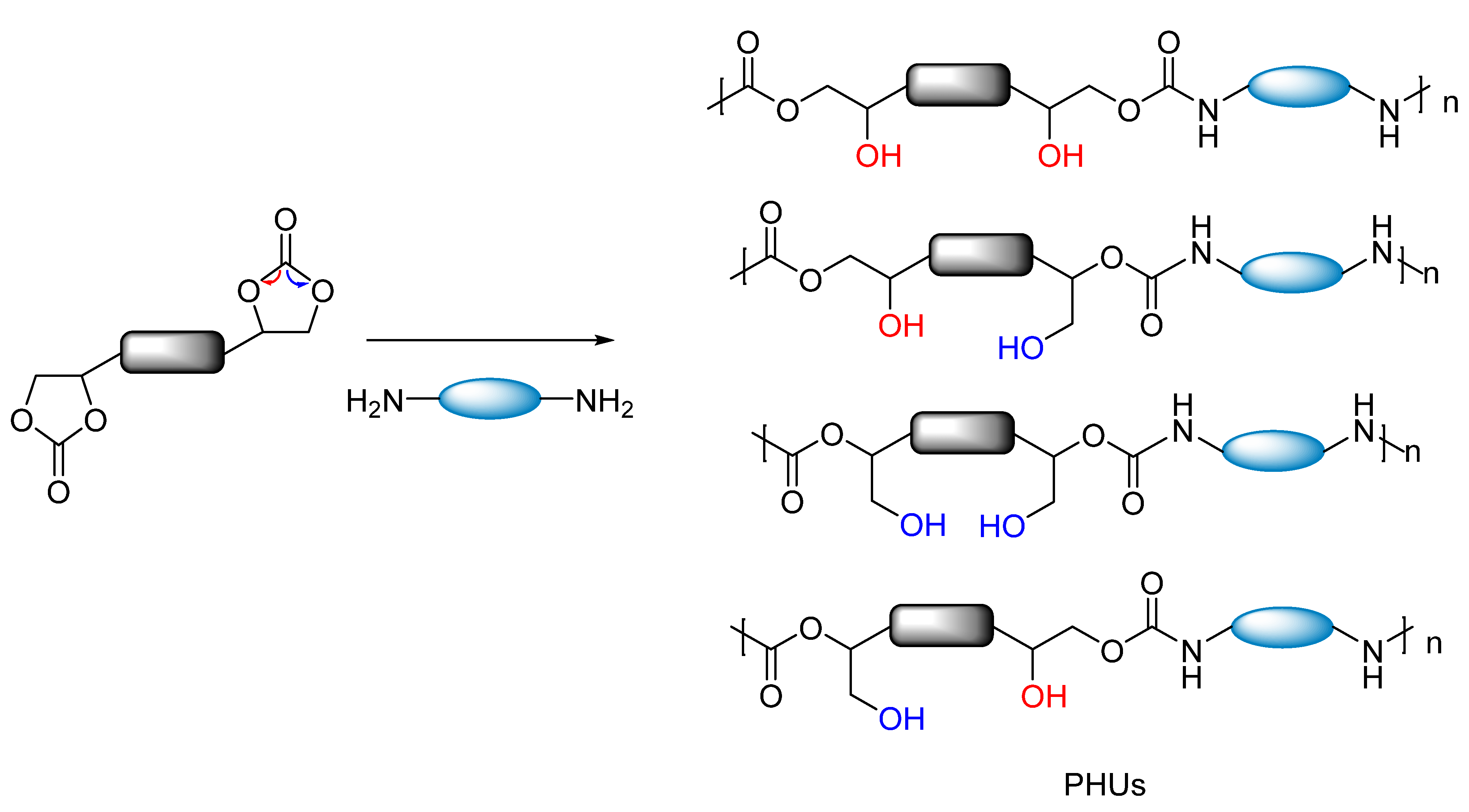
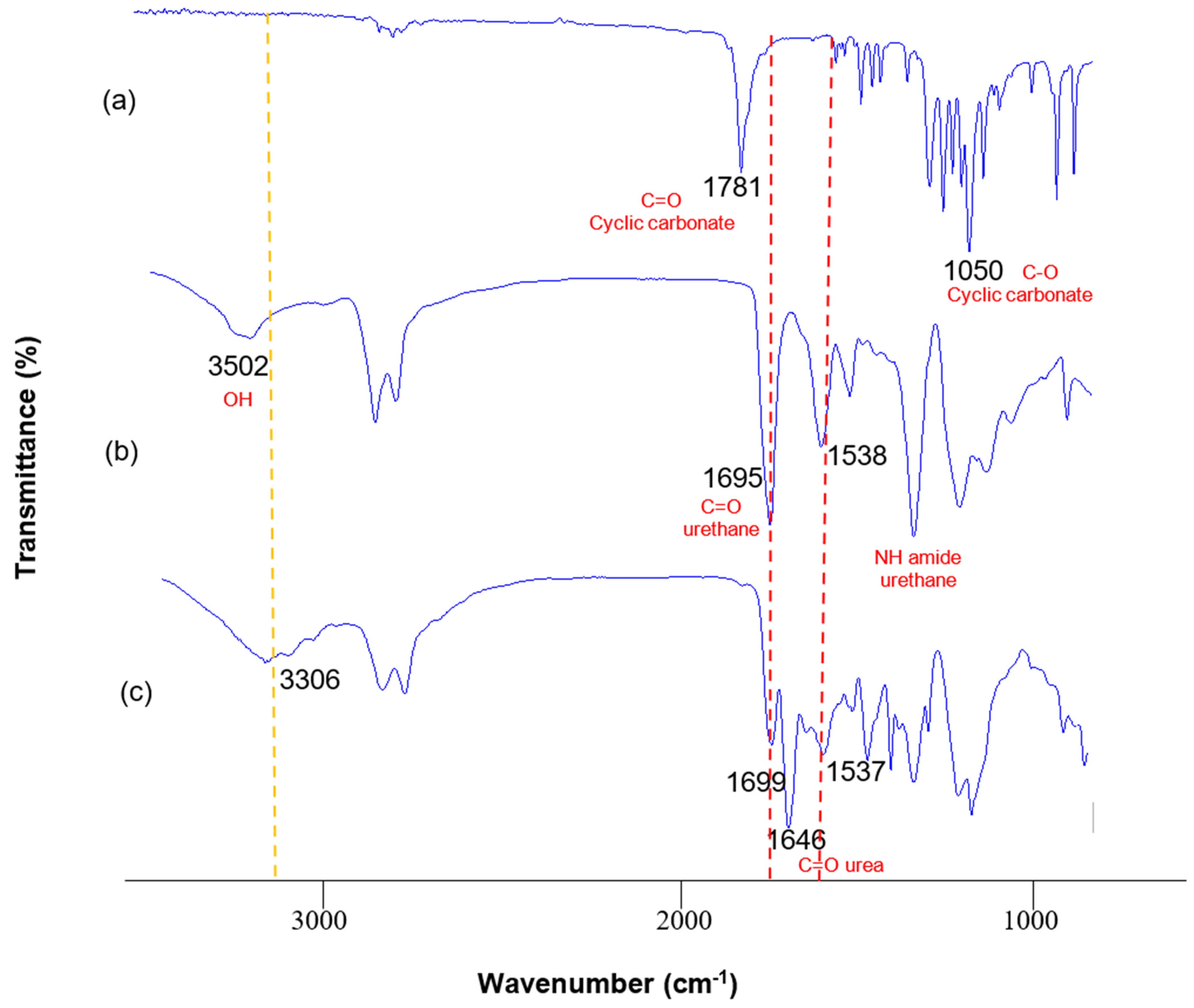
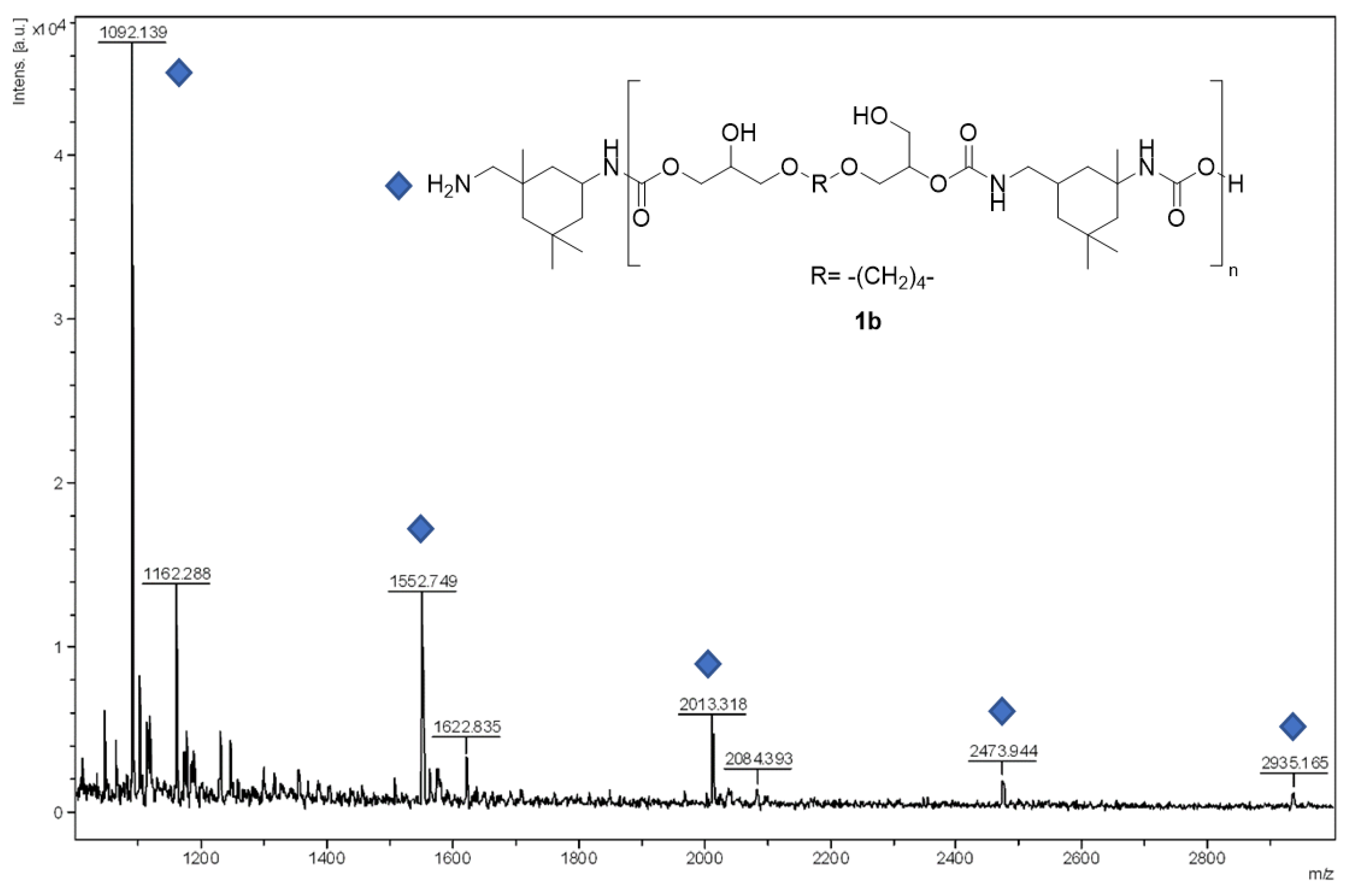
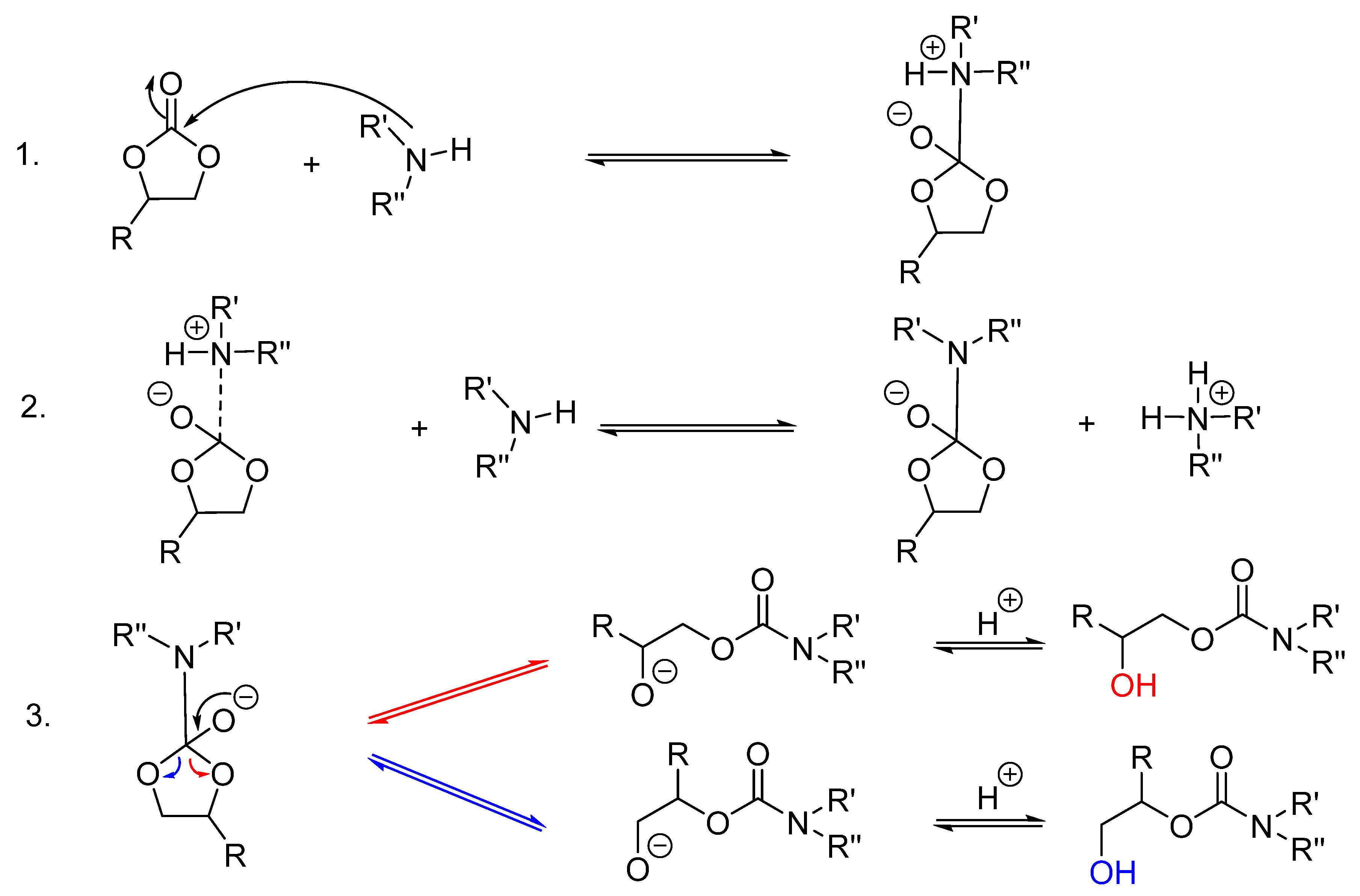
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rafiee, Z.; Keshavarz, V. Synthesis and Characterization of Polyurethane/Microcrystalline Cellulose Bionanocomposites. Prog. Org. Coat. 2015, 86, 190–193. [Google Scholar] [CrossRef]
- Zia, K.M.; Anjum, S.; Zuber, M.; Mujahid, M.; Jamil, T. Synthesis and Molecular Characterization of Chitosan Based Polyurethane Elastomers Using Aromatic Diisocyanate. Int. J. Biol. Macromol. 2014, 66, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, S.A.; Otaigbe, J.U. Recent Advances in Synthesis, Characterization and Rheological Properties of Polyurethanes and POSS/Polyurethane Nanocomposites Dispersions and Films. Prog. Polym. Sci. 2009, 34, 1283–1332. [Google Scholar] [CrossRef]
- Llevot, A.; Meier, M. Perspective: Green Polyurethane Synthesis for Coating Applications. Polym. Int. 2019, 68, 826–831. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural Engineering of Polyurethane Coatings for High Performance Applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-Isocyanate Polyurethanes: From Chemistry to Applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-Based Routes to Synthesize Cyclic Carbonates and Polyamines Precursors of Non-Isocyanate Polyurethanes: A Review. Eur. Polym. J. 2019, 118, 668–684. [Google Scholar] [CrossRef]
- Bayer, O. E Das Di-Lsocganat-Poluadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–272. [Google Scholar] [CrossRef]
- Labunska, I.; Stephenson, A.; Brigden, K.; Stringer, R.; Santillo, D.; Johnston, P.A. The Bhopal Legacy: Toxic Contaminants at the Former Union Carbide Factory Site, Bhopal, India: 15 Years after the Bhopal Accident; Greenpeace Research Laboratories: Exeter, UK, 1999. [Google Scholar]
- Hahn, C.; Keul, H.; Möller, M. Hydroxyl-Functional Polyurethanes and Polyesters: Synthesis, Properties and Potential Biomedical Application. Polym. Int. 2012, 61, 1048–1060. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-Isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Guan, J.; Song, Y.; Lin, Y.; Yin, X.; Zuo, M.; Zhao, Y.; Tao, X.; Zheng, Q. Progress in Study of Non-Isocyanate Polyurethane. Ind. Eng. Chem. Res. 2011, 50, 6517–6527. [Google Scholar] [CrossRef]
- Persson, P.V.; Casas, J.; Iversen, T.; Córdova, A. Direct Organocatalytic Chemoselective Synthesis of a Dendrimer-like Star Polyester. Macromolecules 2006, 39, 2819–2822. [Google Scholar] [CrossRef]
- Lundberg, R.D.; Albans, S.; Montgomery, D.R. Carbon Dioxide Polymers. U.S. Patent US3523924A, 11 August 1970. [Google Scholar]
- Ihata, O.; Kayaki, Y.; Ikariya, T. Synthesis of Thermoresponsive Polyurethane from 2-Methylaziridine and Supercritical Carbon Dioxide. Angew. Chem. Int. Ed. 2004, 43, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Chiang, W.-Y.; Ikeda, S. Copolymerization of Carbon Dioxide with Propyleneimine. J. Polym. Sci. Polym. Chem. 1974, 12, 121–131. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(Hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef] [Green Version]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and Phosgene-Free Routes to Polyfunctional Cyclic Carbonates and Green Polyurethanes by Fixation of Carbon Dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From Petrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef] [Green Version]
- Camp, J.E. Bio-Available Solvent Cyrene: Synthesis, Derivatization, and Applications. ChemSusChem 2018, 11, 3048–3055. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.A.; Rudenko, A.E.; Waymouth, R.M. Zwitterionic Ring-Opening Polymerization of N-Substituted Eight-Membered Cyclic Carbonates to Generate Cyclic Poly(Carbonate)s. ACS Macro Lett. 2016, 5, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Büttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Recent Developments in the Synthesis of Cyclic Carbonates from Epoxides and CO2. Top. Curr. Chem. 2017, 375, 89–144. [Google Scholar]
- Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Castro-Osma, J.A.; Lara-Sánchez, A.; Otero, A. An Efficient and Versatile Lanthanum Heteroscorpionate Catalyst for Carbon Dioxide Fixation into Cyclic Carbonates. ChemSusChem 2017, 10, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- de La Cruz-Martínez, F.; Martínez, J.; Gaona, M.A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Rodríguez, A.M.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Bifunctional Aluminum Catalysts for the Chemical Fixation of Carbon Dioxide into Cyclic Carbonates. ACS Sustain. Chem. Eng. 2018, 6, 5322–5332. [Google Scholar] [CrossRef]
- de La Cruz-Martínez, F.; Martínez De Sarasa Buchaca, M.; Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Lara-Sánchez, A. Synthesis of Bio-Derived Cyclic Carbonates from Renewable Resources. ACS Sustain. Chem. Eng. 2019, 7, 20126–20138. [Google Scholar] [CrossRef]
- Gaona, M.A.; de La Cruz-Martínez, F.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Alonso-Moreno, C.; Rodríguez, A.M.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Synthesis of Helical Aluminium Catalysts for Cyclic Carbonate Formation. Dalton Trans. 2019, 48, 4218–4227. [Google Scholar] [CrossRef]
- Khattak, Z.A.K.; Younus, H.A.; Ahmad, N.; Ullah, H.; Suleman, S.; Hossain, M.S.; Elkadi, M.; Verpoort, F. Highly Active Dinuclear Cobalt Complexes for Solvent-Free Cycloaddition of CO2 to Epoxides at Ambient Pressure. Chem. Commun. 2019, 55, 8274–8277. [Google Scholar] [CrossRef]
- Figovsky, O.; Shapovalov, L.; Leykin, A.; Birukova, O.; Potashnikova, R. Advacnes in the field of nonisocyanate polyurethanes based on cyclic carbonates. Chem. Chem. Technol. 2013, 7, 79–87. [Google Scholar] [CrossRef]
- Bunco, O.; Yuriko, S.; Takeshi, E. Polyaddition of Bifunctional Cyclic Carbonate with Diamine in Ionic Liquids: In Situ Ion Composite Formation and Simple Separation of Ionic Liquid. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4629–4635. [Google Scholar]
- Prömpers, G.; Keul, H.; Höcker, H. Polyurethanes with Pendant Hydroxy Groups: Polycondensation of D-Mannitol-1,2:5,6-Dicarbonate with Diamines. Des. Monomers Polym. 2005, 8, 547–569. [Google Scholar] [CrossRef]
- Blain, M.; Jean-Gérard, L.; Auvergne, R.; Benazet, D.; Caillol, S.; Andrioletti, B. Rational Investigations in the Ring Opening of Cyclic Carbonates by Amines. Green Chem. 2014, 16, 4286–4291. [Google Scholar] [CrossRef]
- Groszos, S.J.; Drechsel, E.K. Method of Preparing a Polyurethane. U.S. Patent US2802022A, 6 August 1957. [Google Scholar]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. Salt Effect on Polyaddition of Bifunctional Cyclic Carbonate and Diamine. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6282–6286. [Google Scholar] [CrossRef]
- Bossion, A.; Aguirresarobe, R.H.; Irusta, L.; Taton, D.; Cramail, H.; Grau, E.; Mecerreyes, D.; Su, C.; Liu, G.; Müller, A.J.; et al. Unexpected Synthesis of Segmented Poly(Hydroxyurea-Urethane)s from Dicyclic Carbonates and Diamines by Organocatalysis. Macromolecules 2018, 51, 5556–5566. [Google Scholar] [CrossRef]
- Ochiai, B.; Satoh, Y.; Endo, T. Nucleophilic Polyaddition in Water Based on Chemo-Selective Reaction of Cyclic Carbonate with Amine. Green Chem. 2005, 7, 765–767. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Polyaddition Behavior of Bis(Five-and Six-Membered Cyclic Carbonate)s with Diamine. J. Polym. Sci. Part A 2001, 39, 860–867. [Google Scholar] [CrossRef]
- Kihara, N.; Kushida, Y.; Endo, T. Optically Active Poly(Hydroxyurethane)s Derived from Cyclic Carbonate and L-Lysine Derivatives. J. Polym. Sci. Part A 1996, 34, 2173–2179. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Martínez, J.; de La Cruz-Martínez, F.; Caballero, M.P.; Fernández-Baeza, J.; Rodríguez-López, J.; Otero, A.; Lara-Sánchez, A.; Tejeda, J. Development of Hydroxy-Containing Imidazole Organocatalysts for CO2 Fixation into Cyclic Carbonates. Catal. Sci. Technol. 2018, 8, 1981–1987. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Fleischer, M.; Blattmann, H.; Mulhaupt, R. Glycerol-, pentaerythritol- and trimethylolpropane-based polyurethanes and their cellulose carbonate composites prepared via the non-isocyanate route with catalytic carbon dioxide fixation. Green Chem. 2013, 15, 934–942. [Google Scholar] [CrossRef]
- Lambeth, R.H.; Henderson, T.J. Organocatalytic Synthesis of (Poly)Hydroxyurethanes from Cyclic Carbonates and Amines. Polymer 2013, 54, 5568–5573. [Google Scholar] [CrossRef]
- Steblyanko, A.; Choi, W.; Sanda, F.; Endo, T. Addition of Five-Membered Cyclic Carbonate with Amine and Its Application to Polymer Synthesis. J. Polym. Sci. Part A 2000, 38, 2375–2380. [Google Scholar] [CrossRef]
- Benyahya, S.; Boutevin, B.; Caillol, S.; Lapinte, V.; Habas, J.P. Optimization of the Synthesis of Polyhydroxyurethanes Using Dynamic Rheometry. Polym. Int. 2012, 61, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Benyahya, S.; Habas, J.P.; Auvergne, R.; Lapinte, V.; Caillol, S. Structure-Property Relationships in Polyhydroxyurethanes Produced from Terephthaloyl Dicyclocarbonate with Various Polyamines. Polym. Int. 2012, 61, 1666–1674. [Google Scholar] [CrossRef]
- Garipov, R.M.; Sysoev, V.A.; Mikheev, V.V.; Zagidullin, A.I.; Deberdeev, V.R.; Irzhak, Y.I.; Berlin, A.A. Reactivity of cyclocarbonate groups in modified epoxy-amine compositions. Dokl. Phys. Chem. 2003, 393, 289–292. [Google Scholar] [CrossRef]
- Xu, L.; Fu, J.H.; Schlup, J.R. In Situ Near-Infrared Spectroscopic Investigation of Epoxy Resin-Aromatic Amine Cure Mechanisms. J. Am. Chem. Soc. 1994, 116, 2821–2826. [Google Scholar] [CrossRef]
- Saddique, F.A.; Zahoor, A.F.; Faiz, S.; Naqvi, S.A.R.; Usman, M.; Ahmad, M. Recent Trends in Ring Opening of Epoxides by Amines as Nucleophiles. Synth. Commun. 2016, 46, 831–868. [Google Scholar] [CrossRef]
- Lee, B.; Lee, K.H.; Lim, B.W.; Cho, J.; Nam, W.; Hur, N.H. Direct Synthesis of Imines via Solid State Reactions of Carbamates with Aldehydes. Adv. Synth. Catal. 2013, 355, 389–394. [Google Scholar]


| Entry | Solvent | Time (h) | T (°C) | Conversion (%) 1 |
|---|---|---|---|---|
| 1 | DMF | 16 | 80 | 100 |
| 2 | MEK | 16 | 80 | 100 |
| 3 | EtOAc | 16 | 80 | 100 |
| 4 | MeCN | 16 | 80 | 100 |
| 5 | MeCN | 16 | 60 | 93 |
| 6 | MeCN | 16 | 40 | 83 |
| 7 | MeCN | 16 | r.t | 67 |
| Entry | Diamine (PHU) | Conversion (%) 2 | Primary OH: Secondary OH | Mn, exp 3 | PDI 3 |
|---|---|---|---|---|---|
| 1 | 1,4-diaminobutane (1a) | 100 | 32:68 | 14500 | 2.2 |
| 2 | Isophorone diamine (1b) | 100 | 36:64 | 13250 | 1.5 |
| 3 | 1,3-cyclohexanebis (methanamine) (1c) | 100 | 36:64 | 17800 | 1.6 |
| 4 | m-Xylene diamine (1d) | 100 | 38:62 | 9050 | 2.2 |
| 5 | tris(2-aminoethyl)amine (1e) | 100 | 28:72 | 17200 | 2.1 |
| 6 4 | tris(2-aminoethyl)amine (1e) | 100 | - | - | - |
| 7 | L-lysine (1f) | 0 | - | - | - |
| 8 5 | L-lysine (1f) | 88 | - | 39200 | 2.1 |
| 9 6 | L-lysine (1f) | 90 | - | 40325 | 2.2 |
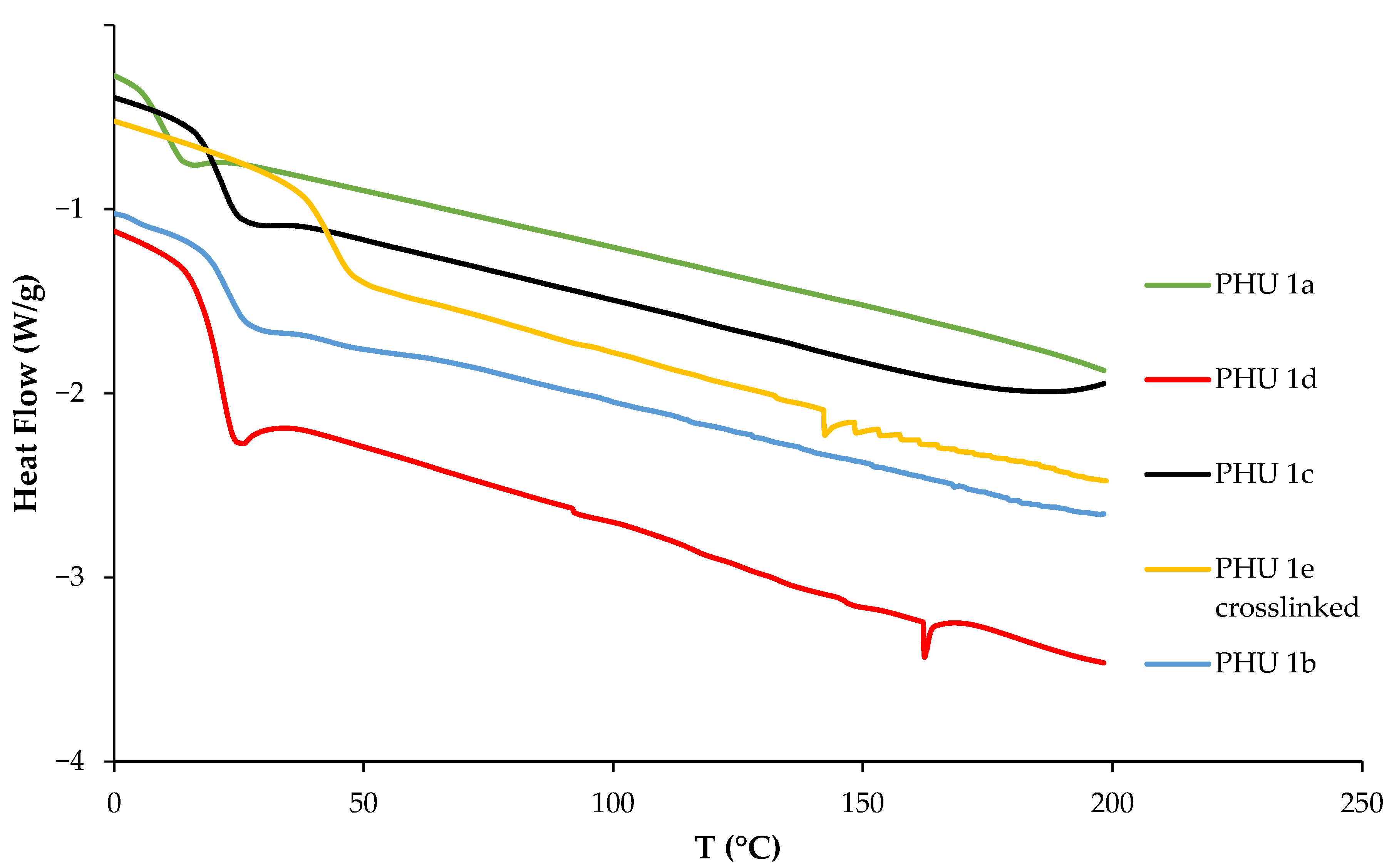
| Entry | Diamine (PHU) | Tg (°C) 1 | Td, 5% (°C) 2 |
|---|---|---|---|
| 1 | 1,4-diaminobutane (1a) | 11 | 252 |
| 2 | Isophorone diamine (1b) | 22 | 272 |
| 3 | 1,3-cyclohexanebis(methylamine) (1c) | 22 | 263 |
| 4 | m-Xylene diamine (1d) | 22 | 231 |
| 5 | tris(2-aminoethyl)amine (1e) | −3 | 229 |
| 6 3 | tris(2-aminoethyl)amine (1e) | 43 | 261 |
| 7 | L-lysine (1f) | −13 | 128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Francés-Poveda, E.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Castro-Osma, J.A.; Lara-Sánchez, A. Synthesis of Nonisocyanate Poly(hydroxy)urethanes from Bis(cyclic carbonates) and Polyamines. Polymers 2022, 14, 2719. https://doi.org/10.3390/polym14132719
Martínez de Sarasa Buchaca M, de la Cruz-Martínez F, Francés-Poveda E, Fernández-Baeza J, Sánchez-Barba LF, Garcés A, Castro-Osma JA, Lara-Sánchez A. Synthesis of Nonisocyanate Poly(hydroxy)urethanes from Bis(cyclic carbonates) and Polyamines. Polymers. 2022; 14(13):2719. https://doi.org/10.3390/polym14132719
Chicago/Turabian StyleMartínez de Sarasa Buchaca, Marc, Felipe de la Cruz-Martínez, Enrique Francés-Poveda, Juan Fernández-Baeza, Luis F. Sánchez-Barba, Andrés Garcés, José A. Castro-Osma, and Agustín Lara-Sánchez. 2022. "Synthesis of Nonisocyanate Poly(hydroxy)urethanes from Bis(cyclic carbonates) and Polyamines" Polymers 14, no. 13: 2719. https://doi.org/10.3390/polym14132719
APA StyleMartínez de Sarasa Buchaca, M., de la Cruz-Martínez, F., Francés-Poveda, E., Fernández-Baeza, J., Sánchez-Barba, L. F., Garcés, A., Castro-Osma, J. A., & Lara-Sánchez, A. (2022). Synthesis of Nonisocyanate Poly(hydroxy)urethanes from Bis(cyclic carbonates) and Polyamines. Polymers, 14(13), 2719. https://doi.org/10.3390/polym14132719