Lyophilic and Sorption Properties of Chitosan Aerogels Modified with Copolymers Based on Glycidyl Methacrylate and Alkyl Methacrylates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Chitosan Aerogel
2.3. Preparation of the Chitosan Films
2.4. Synthesis of GMA and AlMA Copolymers
2.5. Surface Modification of the Chitosan-Based Materials
2.6. Amination of the Free Surface Epoxy Groups of the Obtained Materials
2.7. Methods
3. Results and Discussion
3.1. Synthesis and Characterization of Chitosan Materials Modified by GMA and AlMA Copolymers
3.2. Wettability and Sorption Properties of Chitosan Aerogels Modified by GMA and AlMA Copolymers
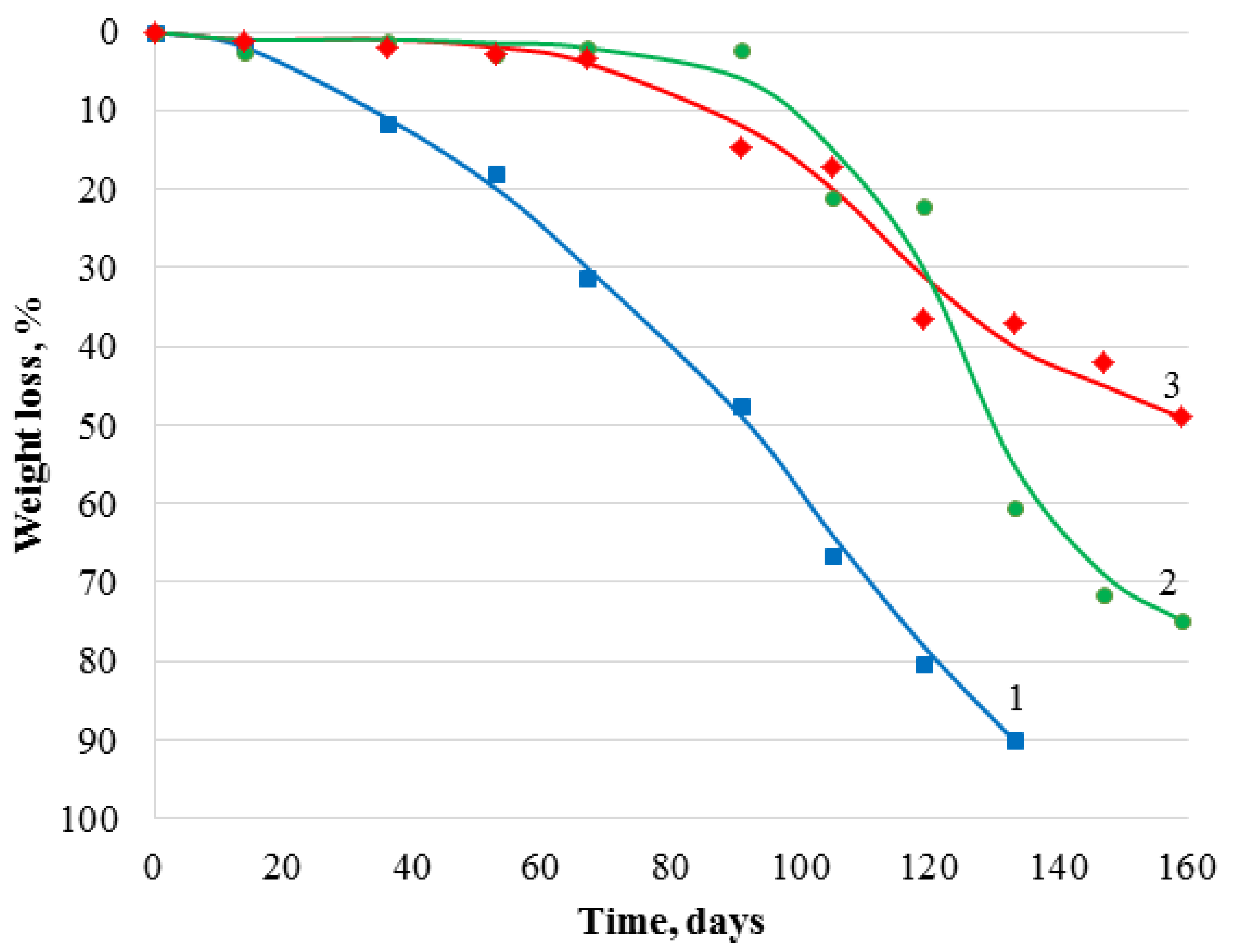
3.3. Biodegradability of Chitosan Materials Modified by GMA and AlMA Copolymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Dash, P.; Chang, Y.-H.; Yang, J.-M. Improvement of Chitosan Water Solubility by Fumaric Acid Modification. Mater. Lett. 2022, 316, 132046. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of Chitosan Aerogels as Promising Carriers for Drug Delivery: A Review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef] [PubMed]
- Vedaiyan, R.K.; Thyriyalakshmi, P. Utilization of Biodegradable Chitosan-Derived Sponges as Oil Retainers. Environ. Sci. Pollut. Res. 2020, 27, 28123–28131. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Patel, R. Wastewater Treatment by Polymeric Microspheres: A Review. Polymers 2022, 14, 1890. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Koh, J. Arginine Containing Chitosan-Graphene Oxide Aerogels for Highly Efficient Carbon Capture and Fixation. J. CO2 Util. 2022, 59, 101958. [Google Scholar] [CrossRef]
- Kas, H.S. Chitosan: Properties, Preparations and Application to Microparticulate Systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and Characterization of Water-Soluble N-Alkylated Chitosan. Carbohydr. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, F.; Wu, Z.; Jin, J.; Li, F.; Wang, Y.; Wang, Z.; Tang, S.; Wu, C.; Wang, Y. O-Acylation of Chitosan Nanofibers by Short-Chain and Long-Chain Fatty Acids. Carbohydr. Polym. 2017, 177, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Vasnev, V.A.; Tarasov, A.I.; Markova, G.D.; Vinogradova, S.V.; Garkusha, O.G. Synthesis and Properties of Acylated Chitin and Chitosan Derivatives. Carbohydr. Polym. 2006, 64, 184–189. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of Sulfur Nanoparticle-Incorporated Antimicrobial Chitosan Films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Melo, L.V.; Saramago, B.; Barbosa, M.A. Functionalization of Chitosan Membranes through Phosphorylation: Atomic Force Microscopy, Wettability, and Cytotoxicity Studies. J. Appl. Polym. Sci. 2006, 102, 276–284. [Google Scholar] [CrossRef]
- Stepnova, E.A.; Tikhonov, V.E.; Babushkina, T.A.; Klimova, T.P.; Vorontsov, E.V.; Babak, V.G.; Lopatin, S.A.; Yamskov, I.A. New Approach to the Quaternization of Chitosan and Its Amphiphilic Derivatives. Eur. Polym. J. 2007, 43, 2414–2421. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan Derivatives Obtained by Chemical Modifications for Biomedical and Environmental Applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an Environment Friendly Biomaterial—A Review on Recent Modifications and Applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Lee, M.C.; Lim, J.W.; Pandey, S.; Garg, P. Synthesis, Characterization and Application of Chitosan-N-(4-Hydroxyphenyl)-Methacrylamide Derivative as a Drug and Gene Carrier. Int. J. Biol. Macromol. 2022, 195, 75–85. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Kennedy, J.F.; Nie, J. Synthesize and Characterization of Organic-Soluble Acylated Chitosan. Carbohydr. Polym. 2009, 75, 390–394. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Kulkarni, V.H.; Keshavayya, J.; Hukkeri, V.I.; Sung, H.-W. Anti-Microbial Activity and Film Characterization of Thiazolidinone Derivatives of Chitosan. Macromol. Biosci. 2005, 5, 490–493. [Google Scholar] [CrossRef] [PubMed]
- López-Mata, M.A.; Ruiz-Cruz, S.; de Jesús Ornelas-Paz, J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E.; Silva-Beltrán, N.P.; Cira-Chávez, L.A.; Burruel-Ibarra, S.E. Mechanical, Barrier and Antioxidant Properties of Chitosan Films Incorporating Cinnamaldehyde. J. Polym. Environ. 2018, 26, 452–461. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Elzein, T.; Dragoe, D.; Bejjani, A.; Lemée, F.; Levillain, J.; Bazin, P.; Roger, P.; Dez, I. Hydrophobization of Chitosan Films by Surface Grafting with Fluorinated Polymer Brushes. Carbohydr. Polym. 2019, 205, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Bryuzgina, E.; Yartseva, V.; Bryuzgin, E.; Tuzhikov, O.; Navrotskiy, A. Surface Modification of Film Chitosan Materials with Aldehydes for Wettability and Biodegradation Control. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A Review on the Synthesis of Graft Copolymers of Chitosan and Their Potential Applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Detchprohm, S.; Aoi, K.; Okada, M. Synthesis of a Novel Chitin Derivative Having Oligo(ɛ-Caprolactone) Side Chains in Aqueous Reaction Media. Macromol. Chem. Phys. 2001, 202, 3560–3570. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Takayama, T.; Tsubokawa, N. Grafting Reaction of Living Polymer Cations with Amino Groups on Chitosan Powder. J. Appl. Polym. Sci. 1998, 68, 1883–1889. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Takayama, T. Surface Modification of Chitosan Powder by Grafting of ‘Dendrimer-like’ Hyperbranched Polymer onto the Surface. React. Funct. Polym. 2000, 43, 341–350. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Chen, B.; Shi, S.; Nie, J.; Ma, G. Functionalized Chitosan Electrospun Nanofiber Membranes for Heavy-Metal Removal. Polymer 2019, 163, 74–85. [Google Scholar] [CrossRef]
- Francis, R.; Baby, D.K.; Gnanou, Y. Synthesis and Self-Assembly of Chitosan-g-Polystyrene Copolymer: A New Route for the Preparation of Heavy Metal Nanoparticles. J. Colloid Interface Sci. 2015, 438, 110–115. [Google Scholar] [CrossRef]
- Anbinder, P.; Macchi, C.; Amalvy, J.; Somoza, A. Chitosan-Graft-Poly(n-Butyl Acrylate) Copolymer: Synthesis and Characterization of a Natural/Synthetic Hybrid Material. Carbohydr. Polym. 2016, 145, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Enescu, D.; Hamciuc, V.; Ardeleanu, R.; Cristea, M.; Ioanid, A.; Harabagiu, V.; Simionescu, B.C. Polydimethylsiloxane Modified Chitosan. Part III: Preparation and Characterization of Hybrid Membranes. Carbohydr. Polym. 2009, 76, 268–278. [Google Scholar] [CrossRef]
- Chernyshova, E.B.; Berezin, A.S.; Tuzhikov, O.I. Hydrophobization of Chitosan-Based Films with Acrolein. Russ. J. Appl. Chem. 2017, 90, 1165–1170. [Google Scholar] [CrossRef]
- Klimov, V.V.; Bryuzgin, E.V.; Le, M.D.; Zelenova, E.A.; Nguyen, T.H.; Navrotskii, A.V.; Nishide, H.; Novakov, I.A. An Investigation of the Hydrophobic Property Stability of Grafted Polymeric Coatings on a Cellulose Material Surface. Polym. Sci.-Ser. D 2016, 9, 364–367. [Google Scholar] [CrossRef]
- Bryuzgin, E.; Klimov, V.; Le, M.D.; Navrotskiy, A.; Novakov, I. The Superhydrophobic State Stability of Coatings Based on Copolymers of Glycidyl Methacrylate and Alkyl Methacrylates on Cotton Fabric Surface. Fibers Polym. 2020, 21, 1032–1038. [Google Scholar] [CrossRef]
- Stamm, M. (Ed.) Polymer Surfaces and Interfaces; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-73864-0. [Google Scholar]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Springer: Dordrecht, The Netherlands, 1975; ISBN 978-94-011-6019-3. [Google Scholar]
- Kildeeva, N.R.; Perminov, P.A.; Vladimirov, L.V.; Novikov, V.V.; Mikhailov, S.N. About Mechanism of Chitosan Cross-Linking with Glutaraldehyde. Russ. J. Bioorg. Chem. 2009, 35, 360–369. [Google Scholar] [CrossRef]
- Bruchet, A.; Dugas, V.; Laszak, I.; Mariet, C.; Goutelard, F.; Randon, J. Synthesis and Characterization of Ammonium Functionalized Porous Poly(Glycidyl Methacrylate-Co-Ethylene Dimethacrylate) Monoliths for Microscale Analysis and Its Application to DNA Purification. J. Biomed. Nanotechnol. 2011, 7, 415–425. [Google Scholar] [CrossRef]
- Druzhinina, T.V.; Nazar’ina, L.A. Chemisorption Fibres Based on Graft Copolymers: Fabrication and Properties. A Review. Fibre Chem. 1999, 31, 252–262. [Google Scholar] [CrossRef]
- Druzhinina, T.V.; Nazar’ina, L.A.; Kardash, K.V. Sorption-Active Modified Chemical Fibres. Fibre Chem. 2000, 32, 407–410. [Google Scholar] [CrossRef]
- Qian, X.; Fan, H.; Wang, C.; Wei, Y. Preparation of High-Capacity, Weak Anion-Exchange Membranes by Surface-Initiated Atom Transfer Radical Polymerization of Poly(Glycidyl Methacrylate) and Subsequent Derivatization with Diethylamine. Appl. Surf. Sci. 2013, 271, 240–247. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1995; ISBN 10: 096481241X; ISBN 13: 9780964812413. [Google Scholar]
- Lv, L.; Zhang, J.; Yuan, S.; Huang, L.; Tang, S.; Liang, B.; Pehkonen, S.O. Enhanced Adsorption of Cu(II) Ions on Chitosan Microspheres Functionalized with Polyethylenimine-Conjugated Poly(Glycidyl Methacrylate) Brushes. RSC Adv. 2016, 6, 78136–78150. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Vilsinski, B.H.; Bonafé, E.G.; Monteiro, J.P.; Kipper, M.J.; Martins, A.F. Chitosan Content Modulates Durability and Structural Homogeneity of Chitosan-Gellan Gum Assemblies. Int. J. Biol. Macromol. 2019, 128, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Stevie, F.A.; Donley, C.L. Introduction to X-ray Photoelectron Spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 063204. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Hydrophobic Materials and Coatings: Principles of Design, Properties and Applications. Russ. Chem. Rev. 2008, 77, 583–600. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A. A Wetting Experiment as a Tool to Study the Physicochemical Processes Accompanying the Contact of Hydrophobic and Superhydrophobic Materials with Aqueous Media. Adv. Colloid Interface Sci. 2012, 179–182, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic Zeolites from Fly Ash as Effective Mineral Sorbents for Land-Based Petroleum Spills Cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Mojžiš, M.; Bubeníková, T.; Zachar, M.; Kačíková, D.; Štefková, J. Comparison of Natural and Synthetic Sorbents’ Efficiency at Oil Spill Removal. BioResources 2019, 14, 8738–8752. [Google Scholar] [CrossRef]
- Angelova, D.; Uzunov, I.; Uzunova, S.; Gigova, A.; Minchev, L. Kinetics of Oil and Oil Products Adsorption by Carbonized Rice Husks. Chem. Eng. J. 2011, 172, 306–311. [Google Scholar] [CrossRef]
- Ceylan, D.; Dogu, S.; Karacik, B.; Yakan, S.D.; Okay, O.S.; Okay, O. Evaluation of Butyl Rubber as Sorbent Material for the Removal of Oil and Polycyclic Aromatic Hydrocarbons from Seawater. Environ. Sci. Technol. 2009, 43, 3846–3852. [Google Scholar] [CrossRef]
- Rengasamy, R.S.; Das, D.; Praba Karan, C. Study of Oil Sorption Behavior of Filled and Structured Fiber Assemblies Made from Polypropylene, Kapok and Milkweed Fibers. J. Hazard. Mater. 2011, 186, 526–532. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.; Le, A.T.T.; Hoang, N.; Tan, V.B.C.; Duong, H.M. Cellulose Aerogel from Paper Waste for Crude Oil Spill Cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Shi, M.; Tang, C.; Yang, X.; Zhou, J.; Jia, F.; Han, Y.; Li, Z. Superhydrophobic Silica Aerogels Reinforced with Polyacrylonitrile Fibers for Adsorbing Oil from Water and Oil Mixtures. RSC Adv. 2017, 7, 4039–4045. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Lin, R.; Lin, C.; He, B.; Zheng, T.; Lu, L.; Cao, Y. An Environment-Friendly and Multi-Functional Absorbent from Chitosan for Organic Pollutants and Heavy Metal Ion. Carbohydr. Polym. 2016, 148, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.; Wilson, S.; Sy, J.C. Biodegradation of Polymers. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 547–560. [Google Scholar]

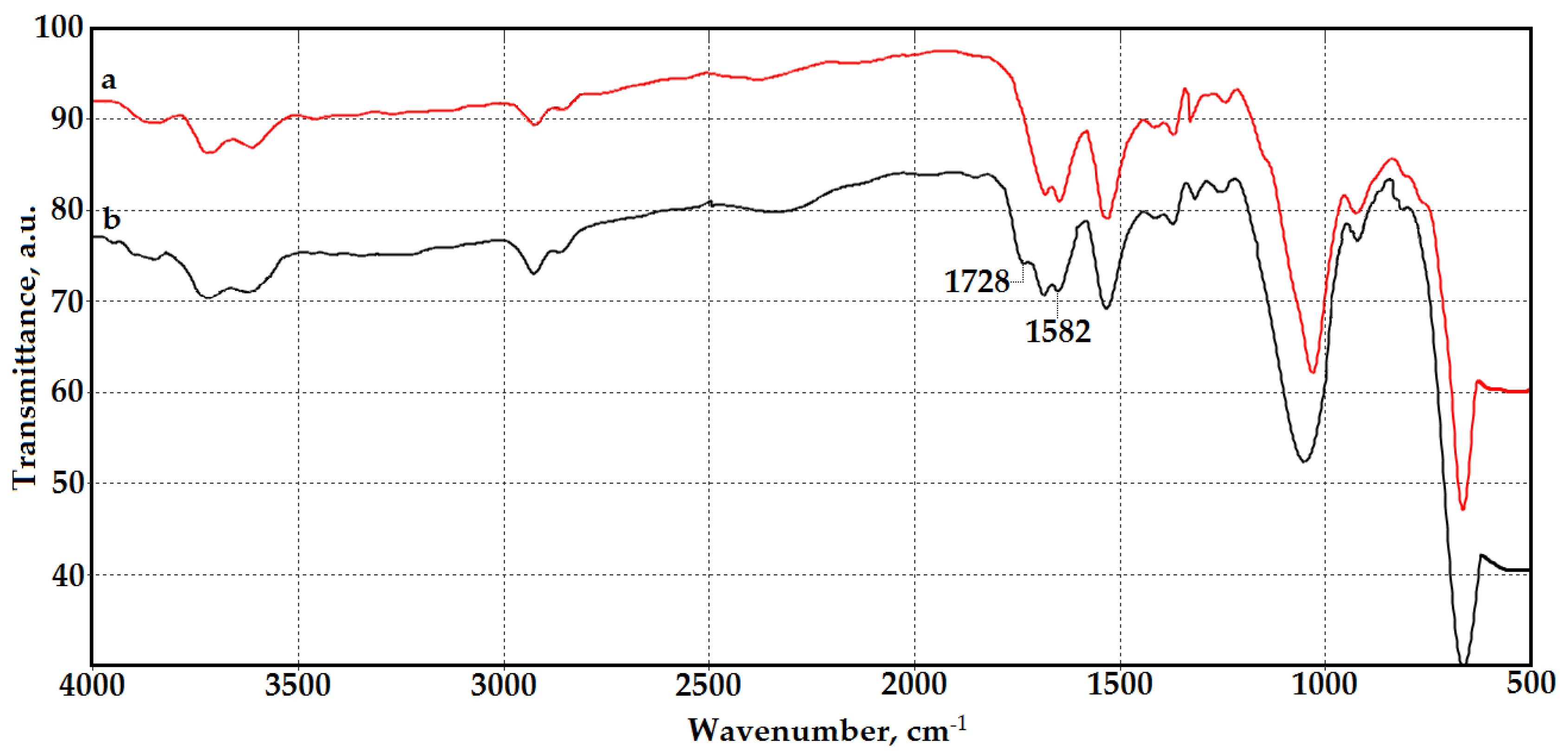
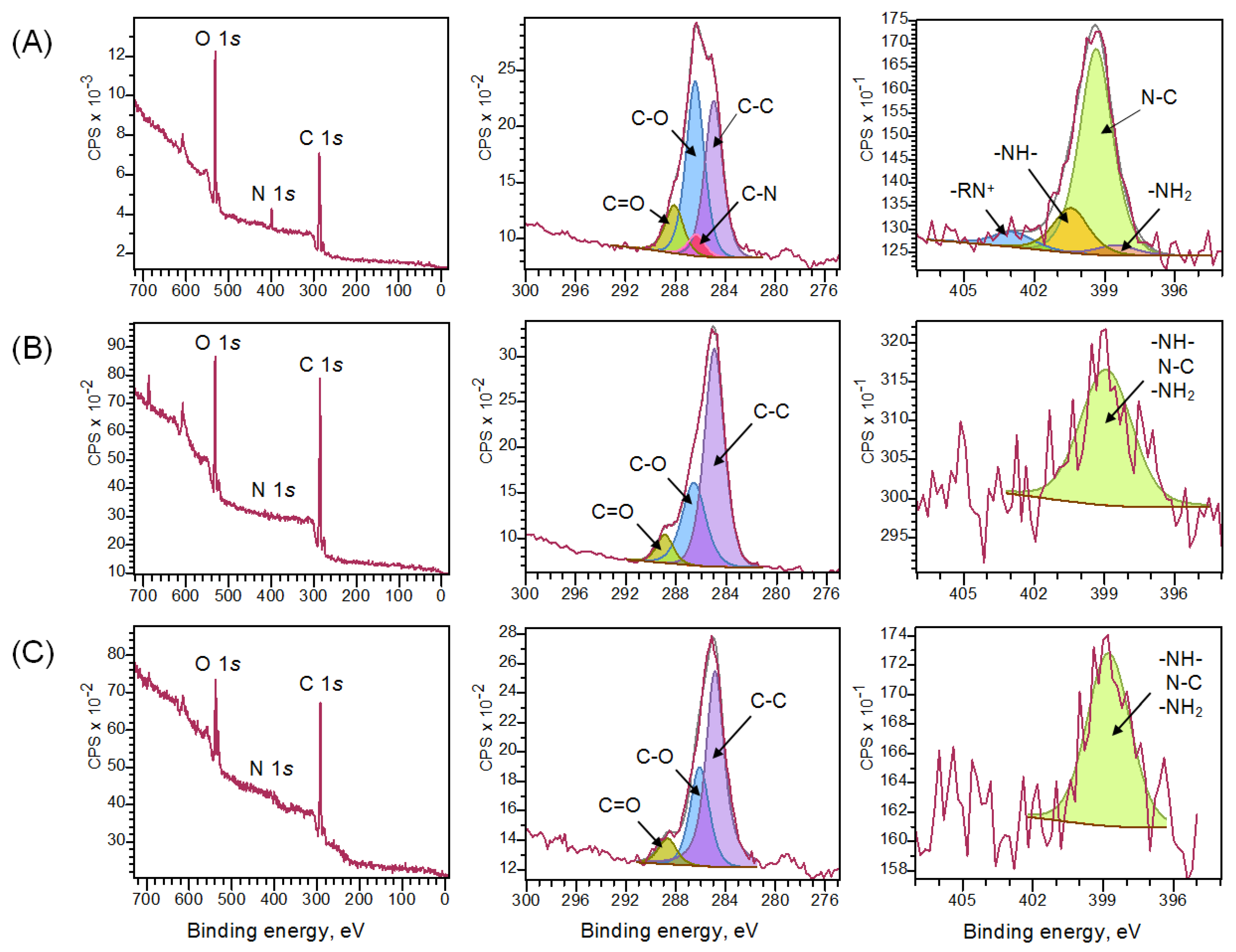
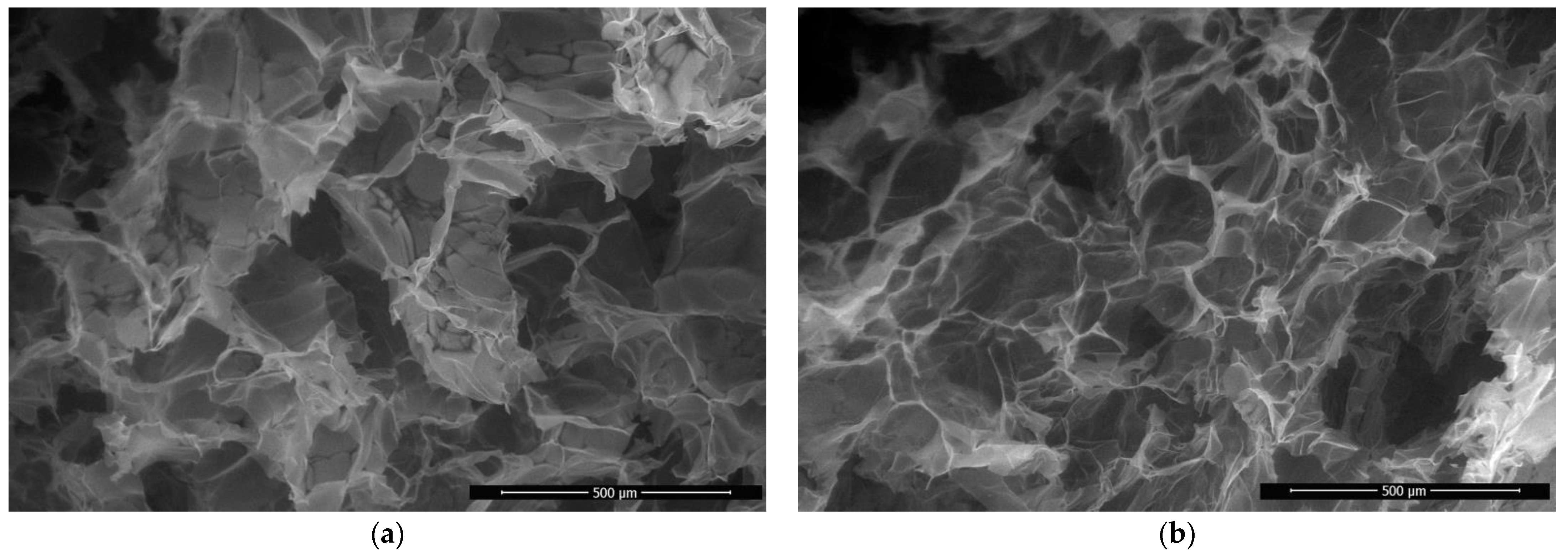
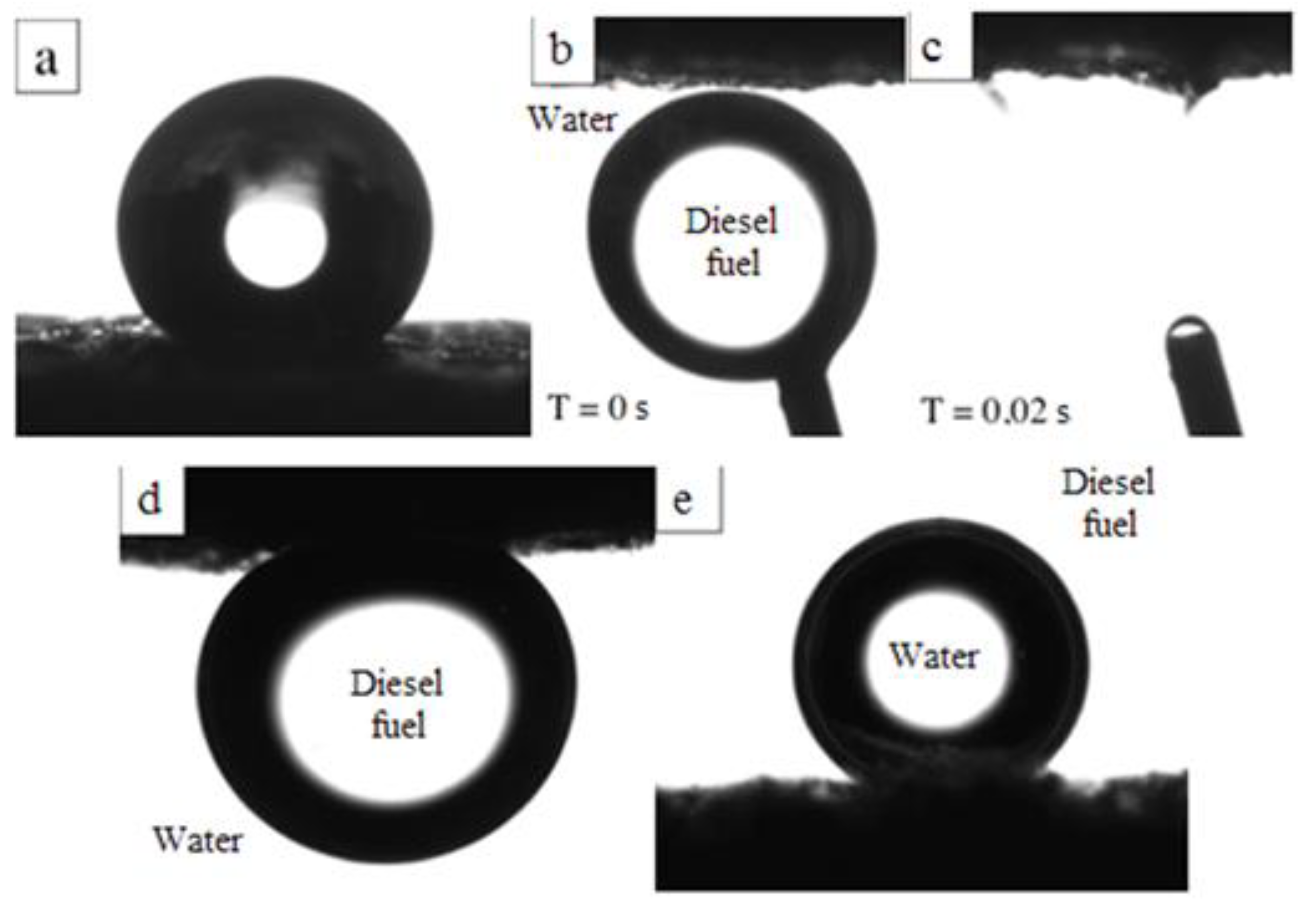

| Modifier | Molar Ratio | Mn × 10−3 | Mw × 10−3 | Mw/Mn | |
|---|---|---|---|---|---|
| Theoretical | Experimental | ||||
| Poly(GMA-co-HeMA) | 2.3:1 | 2.0:1 | 39.8 | 77.8 | 1.9 |
| Poly(GMA-co-DMA) | 1.9:1 | 51.6 | 92.8 | 1.8 | |
| Poly(GMA-co-LMA) | 2.2:1 | 71.1 | 159.5 | 2.2 | |
| Poly(GMA-co-TDMA) | 1.9:1 | 44.6 | 81.8 | 1.8 | |
| Poly(GMA-co-SMA) | 2.0:1 | 64.6 | 155.7 | 2.4 | |
| Chitosan Material | Elemental Composition, wt% | C/N | Modifier Content, wt% | ||
|---|---|---|---|---|---|
| C | H | N | |||
| Film | 44.74 | 7.072 | 8.31 | 5.38 | - |
| Aerogel | 45.29 | 7.055 | 7.26 | 6.24 | 10.38 * |
| Aerogel, modified by poly(GMA-co-HeMA) | 45.67 | 6.944 | 7.01 | 6.51 | 3.04 |
| Aerogel, modified by poly(GMA-co-DMA) | 45.76 | 6.919 | 7.07 | 6.47 | 2.49 |
| Aerogel, modified by poly(GMA-co-TDMA) | 45.51 | 6.895 | 7.02 | 6.48 | 2.50 |
| Aerogel, modified by poly(GMA-co-TDMA) and diethylamine | 45.61 | 6.733 | 7.05 | 6.47 | 0.13 ** |
| Chitosan Aerogel | Elemental Composition, at.% | ||
|---|---|---|---|
| O | N | C | |
| Unmodified | 28.2 | 5.8 | 66.0 |
| With grafted poly(GMA-co-TDMA) | 20.4 | 1.0 | 78.6 |
| With grafted poly(GMA-co-TDMA) and diethylamine | 19.3 | 2.3 | 78.4 |
| Chitosan Aerogel | C 1s | N 1s | ||||||
|---|---|---|---|---|---|---|---|---|
| C–C | C–O | C=O | C–N | N–C | –RN+ | –NH– | –NH2 | |
| Unmodified | 34.9 | 43.8 | 12.0 | 9.2 | 72.3 | 6.4 | 16.9 | 4.3 |
| With grafted poly(GMA-co-TDMA) | 61.0 | 32.3 | 6.7 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| With grafted poly(GMA-co-TDMA) and diethylamine | 64.9 | 26.3 | 8.8 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| Chitosan Aerogel | Apparent Density, g/cm3 | True Density, g/cm3 | Porosity, % | Pore Diameter, µm | Pore Wall Thickness, µm |
|---|---|---|---|---|---|
| Unmodified | 0.021 ± 0.003 | 1.357 ± 0.011 | 98.5 ± 0.4 | 100–300 | 0.6–1 |
| With grafted poly(GMA-co-TDMA) | 0.022 ± 0.003 | 1.364 ± 0.009 | 98.4 ± 0.4 |
| Concentration of the Modifier in Solution, wt% | Initial Contact Angle, ° | Water Absorption (After 24 h), g/g |
|---|---|---|
| 0.01 | 150 ± 7 | 26.4 |
| 0.02 | 151 ± 4 | 7.6 |
| 0.04 | 152 ± 3 | 5.5 |
| 0.08 | 155 ± 4 | 2.4 |
| 0.1 | 157 ± 2 | 2.1 |
| 0.2 | 157 ± 2 | 2.2 |
| 0.5 | 157 ± 2 | 2.2 |
| 1 | 157 ± 2 | 2.1 |
| Modification Temperature, °C | Initial Contact Angle, ° | Water Absorption (After 24 h), g/g |
|---|---|---|
| 20 | 149 ± 5 | 18.4 |
| 40 | 145 ± 7 | 18.7 |
| 60 | 152 ± 5 | 18.1 |
| 80 | 154 ± 4 | 17.2 |
| 100 | 153 ± 4 | 3.5 |
| 120 | 154 ± 4 | 2.8 |
| 140 | 157 ± 2 | 2.1 |
| 150 | 157 ± 2 | 2.3 |
| 160 | 157 ± 2 | 2.2 |
| Chitosan Aerogel with Grafted GMA and AlMA Copolymers | Contact Angle in the “Wetting Agent/Medium” System, ° | ||
|---|---|---|---|
| Deionized Water in air | Deionized Water in Diesel Fuel | Diesel Fuel in Deionized Water | |
| unmodified | Wetted | 160 ± 2 | 162 ± 2 |
| Poly(GMA-co-HeMA) | 146 ± 2 | 162 ± 2 | Wetted |
| Poly(GMA-co-DMA) | 152 ± 2 | ||
| Poly(GMA-co-LMA) | 153 ± 3 | ||
| Poly(GMA-co-TDMA) | 157 ± 2 | ||
| Poly(GMA-co-SMA) | 157 ± 3 | ||
| Chitosan Aerogel with Grafted GMA and AlMA Copolymers | Sorption Capacity, g/g | |||||||
|---|---|---|---|---|---|---|---|---|
| Distilled Water | Synthetic Motor Oil | Diesel Fuel | Light Oil | |||||
| 15 min | 24 h | 15 min | 24 h | 15 min | 24 h | 15 min | 24 h | |
| Unmodified | 53.7 | 56.4 | 42.6 | 44.3 | 37.1 | 37.5 | 35.7 | 36.3 |
| Poly(GMA-co-HeMA) | 1.3 | 4.6 | 37.9 | 39.6 | 35.2 | 35.7 | 31.8 | 32.3 |
| Poly(GMA-co-DMA) | 1.0 | 2.1 | 41.4 | 43.5 | 32.8 | 33.5 | 30.9 | 32.3 |
| Poly(GMA-co-LMA) | 1.0 | 2.2 | 35.4 | 37.7 | 33.4 | 33.6 | 34.4 | 34.7 |
| Poly(GMA-co-TDMA) | 0.9 | 2.1 | 42.2 | 42.5 | 31.5 | 31.7 | 31.1 | 33.1 |
| Poly(GMA-co-SMA) | 0.8 | 1.8 | 43.0 | 44.0 | 34.3 | 34.6 | 34.3 | 34.9 |
| Sorbent | Type of Sorbent Liquid | Sorption Capacity, g/g | Reference |
|---|---|---|---|
| Zeolite | Engine oil | 0.4–0.9 | [49] |
| Moss | Engine oil | 28.4 | [50] |
| Rice husk | Gasoline | 3.7 | [51] |
| Diesel | 5.5 | ||
| Light crude oil | 6.0 | ||
| Motor oil | 7.5 | ||
| Heavy crude oil | 9.2 | ||
| Butyl rubber | Toluene | 17.8 | [52] |
| Gasoline | 16.7 | ||
| Diesel | 20.3 | ||
| Fuel oil | 15.4 | ||
| Crude oil | 23.0 | ||
| Olive oil | 7.9 | ||
| Polypropylene fiber | Diesel | 17.1 | [53] |
| High-density oil | 18.8 | ||
| Cellulose aerogel | Crude oil | 18.4–20.5 | [54] |
| Silica aerogel | Diesel oil | 9.6 | [55] |
| Chitosan aerogel | Crude oil | 41.1 | [56] |
| Diesel | 31.1 | ||
| Chitosan aerogel | Synthetic motor oil | 44.0 | Current study |
| Diesel fuel | 35.7 | ||
| Light oil | 34.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yartseva, V.M.; Makevnina, O.A.; Bryuzgina, E.B.; Bryuzgin, E.V.; Klimov, V.V.; Kolyaganova, O.V.; Nikolitchev, D.E.; Navrotsky, A.V.; Novakov, I.A. Lyophilic and Sorption Properties of Chitosan Aerogels Modified with Copolymers Based on Glycidyl Methacrylate and Alkyl Methacrylates. Polymers 2022, 14, 2711. https://doi.org/10.3390/polym14132711
Yartseva VM, Makevnina OA, Bryuzgina EB, Bryuzgin EV, Klimov VV, Kolyaganova OV, Nikolitchev DE, Navrotsky AV, Novakov IA. Lyophilic and Sorption Properties of Chitosan Aerogels Modified with Copolymers Based on Glycidyl Methacrylate and Alkyl Methacrylates. Polymers. 2022; 14(13):2711. https://doi.org/10.3390/polym14132711
Chicago/Turabian StyleYartseva, Vitalia M., Olga A. Makevnina, Ekaterina B. Bryuzgina, Evgeny V. Bryuzgin, Viktor V. Klimov, Olga V. Kolyaganova, Dmitry E. Nikolitchev, Alexander V. Navrotsky, and Ivan A. Novakov. 2022. "Lyophilic and Sorption Properties of Chitosan Aerogels Modified with Copolymers Based on Glycidyl Methacrylate and Alkyl Methacrylates" Polymers 14, no. 13: 2711. https://doi.org/10.3390/polym14132711
APA StyleYartseva, V. M., Makevnina, O. A., Bryuzgina, E. B., Bryuzgin, E. V., Klimov, V. V., Kolyaganova, O. V., Nikolitchev, D. E., Navrotsky, A. V., & Novakov, I. A. (2022). Lyophilic and Sorption Properties of Chitosan Aerogels Modified with Copolymers Based on Glycidyl Methacrylate and Alkyl Methacrylates. Polymers, 14(13), 2711. https://doi.org/10.3390/polym14132711







