Precise Fabrication of Porous Microspheres by Iso-Density Emulsion Combined with Microfluidics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
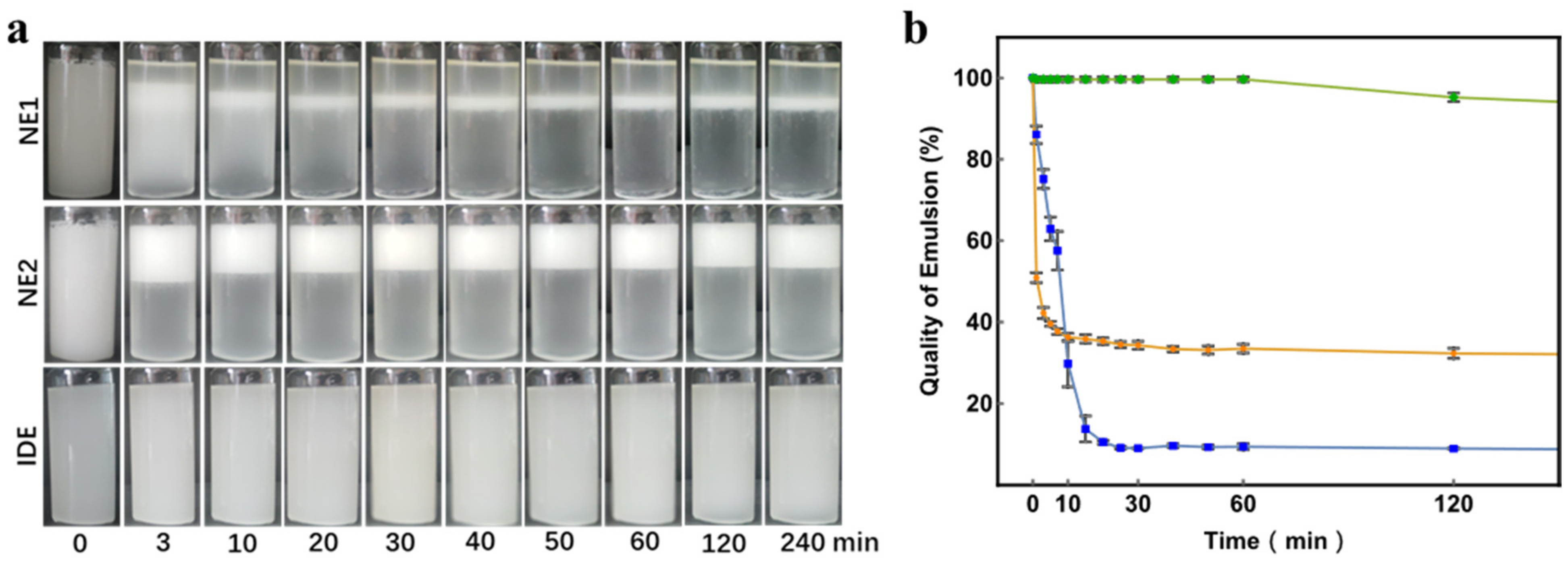
2.2. Emulsion Stability Test
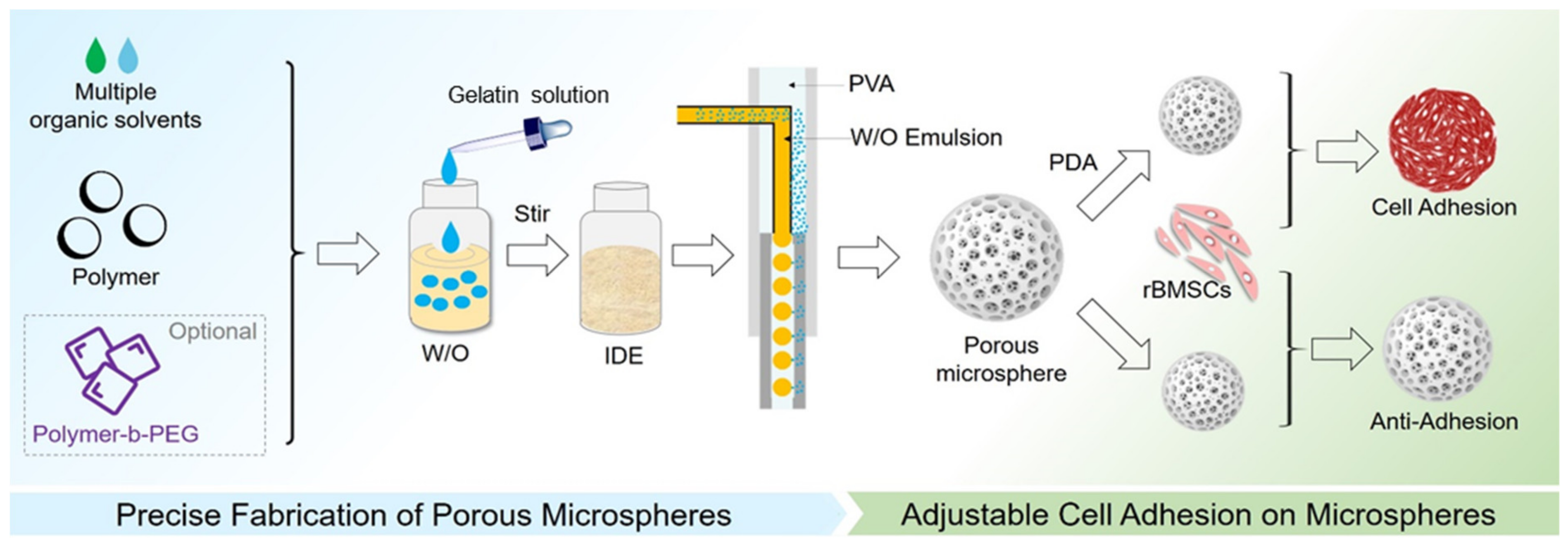
2.3. Preparation of Uniform Porous Microspheres
2.4. Preparation of PCL Porous Microspheres with Controllable Morphology
2.5. Characterization of PCL Porous Microspheres
2.6. Cytotoxicity Test of PCL Porous Microspheres
2.7. Dopamine Surface Modification and Cell Adhesion of PCL Porous Microspheres
2.8. In Vivo Compatibility and Degradation of PCL Porous Microspheres
3. Results and Discussion
3.1. Emulsion Stability
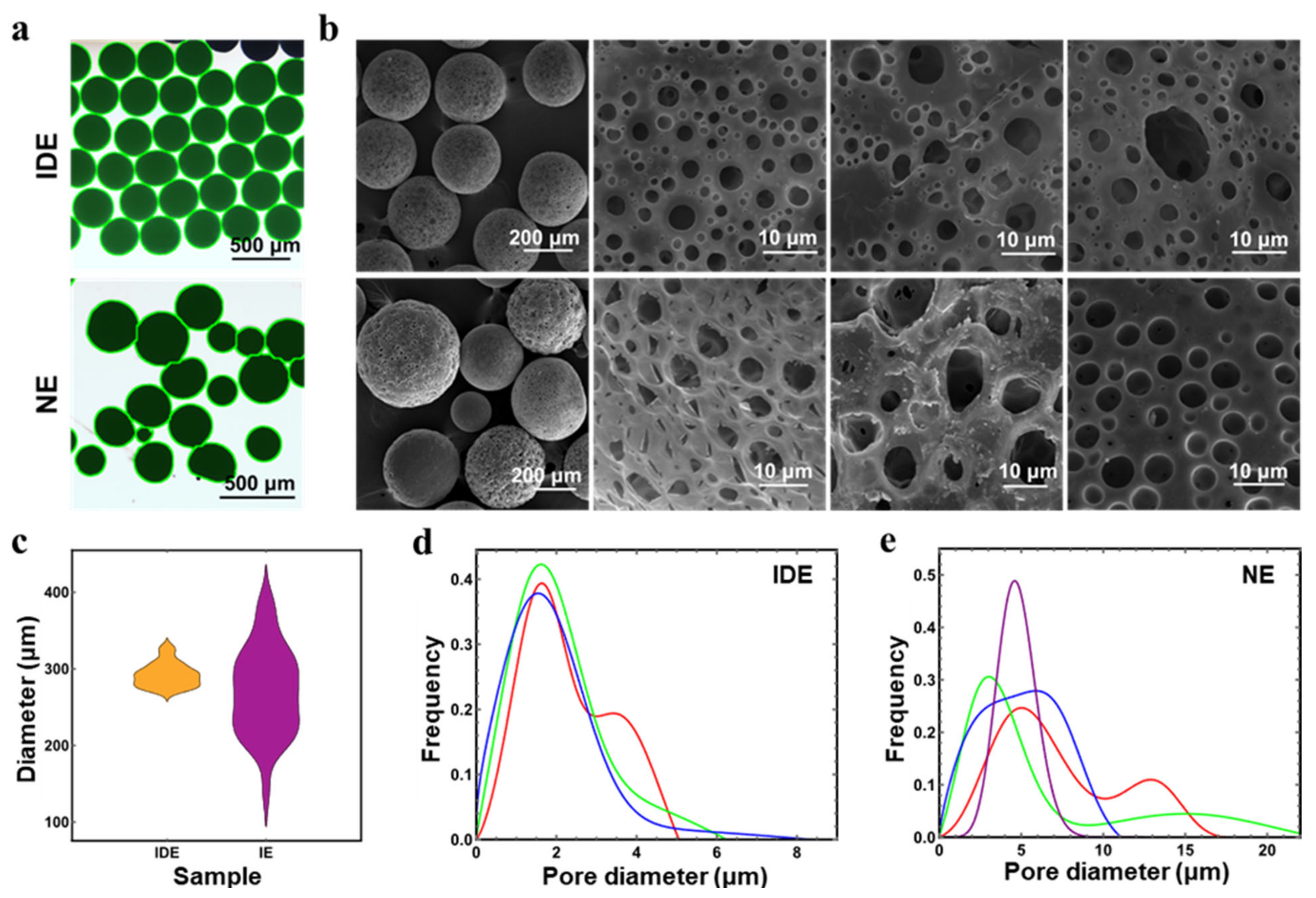
3.2. Uniformity of Porous Microspheres
3.3. PCL Porous Microspheres with Controllable Morphology
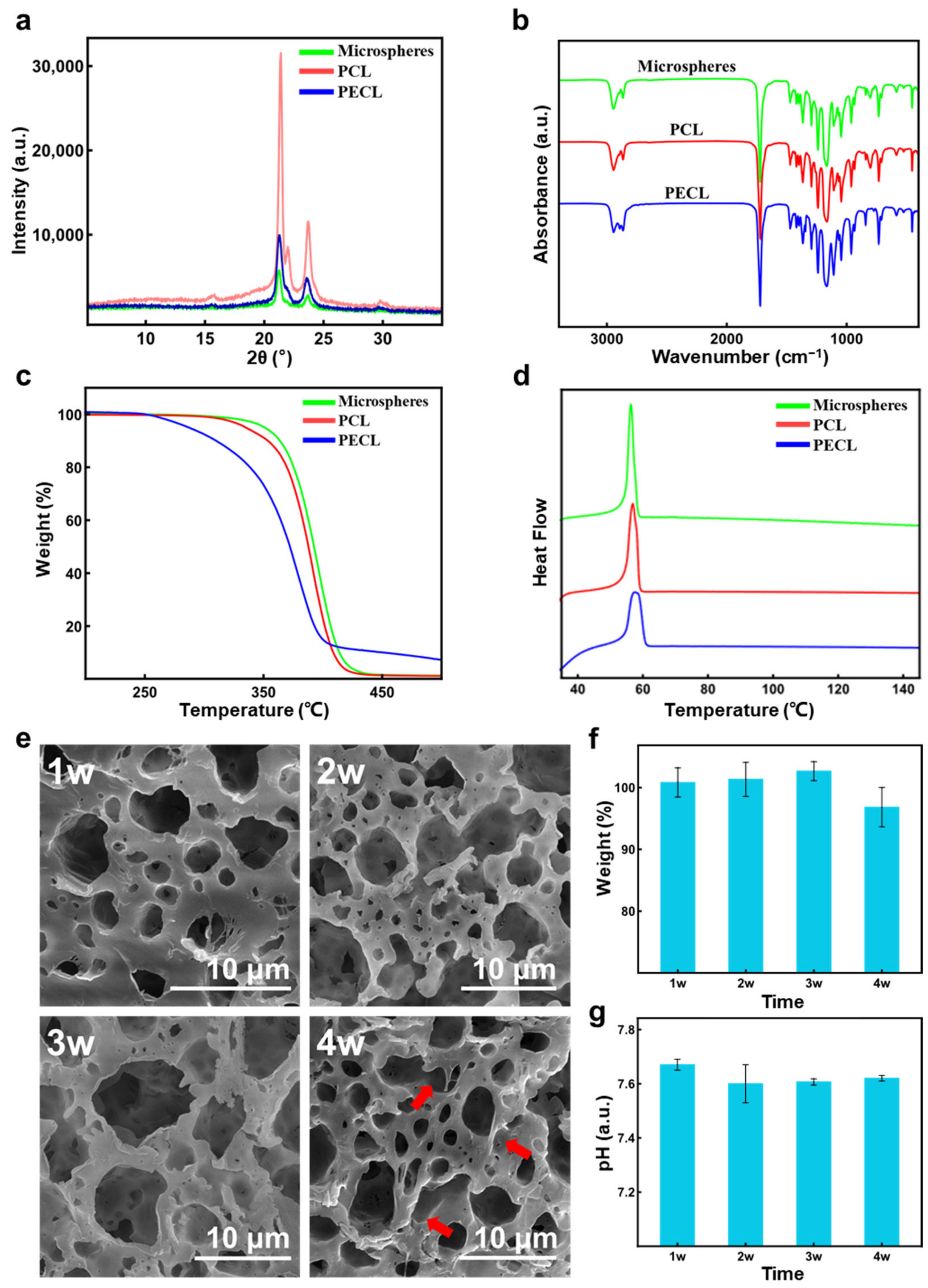
3.4. Characterization of PCL Porous Microspheres
3.5. Cytotoxicity Evaluation
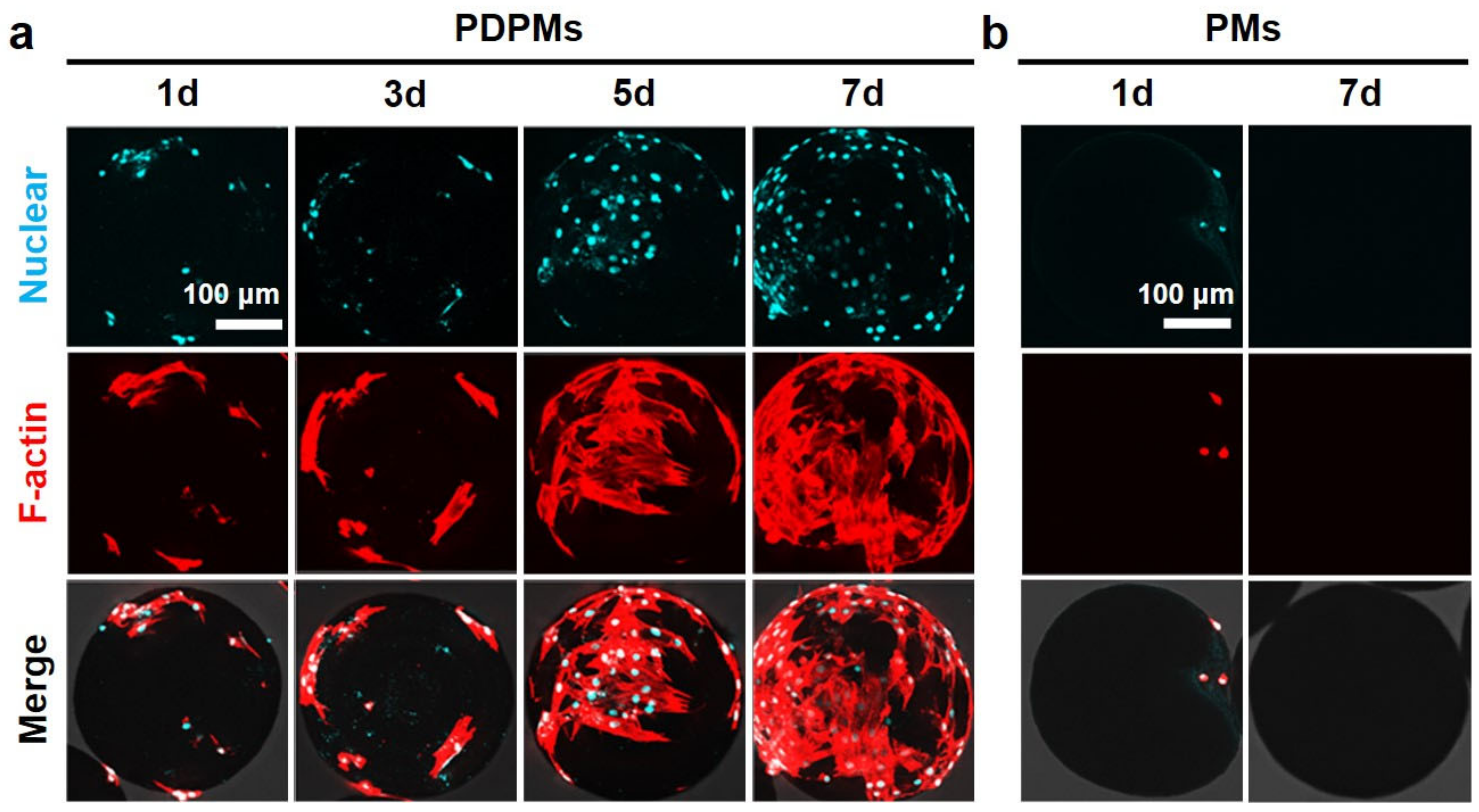
3.6. Polydopamine Modification and Cell Adhesion
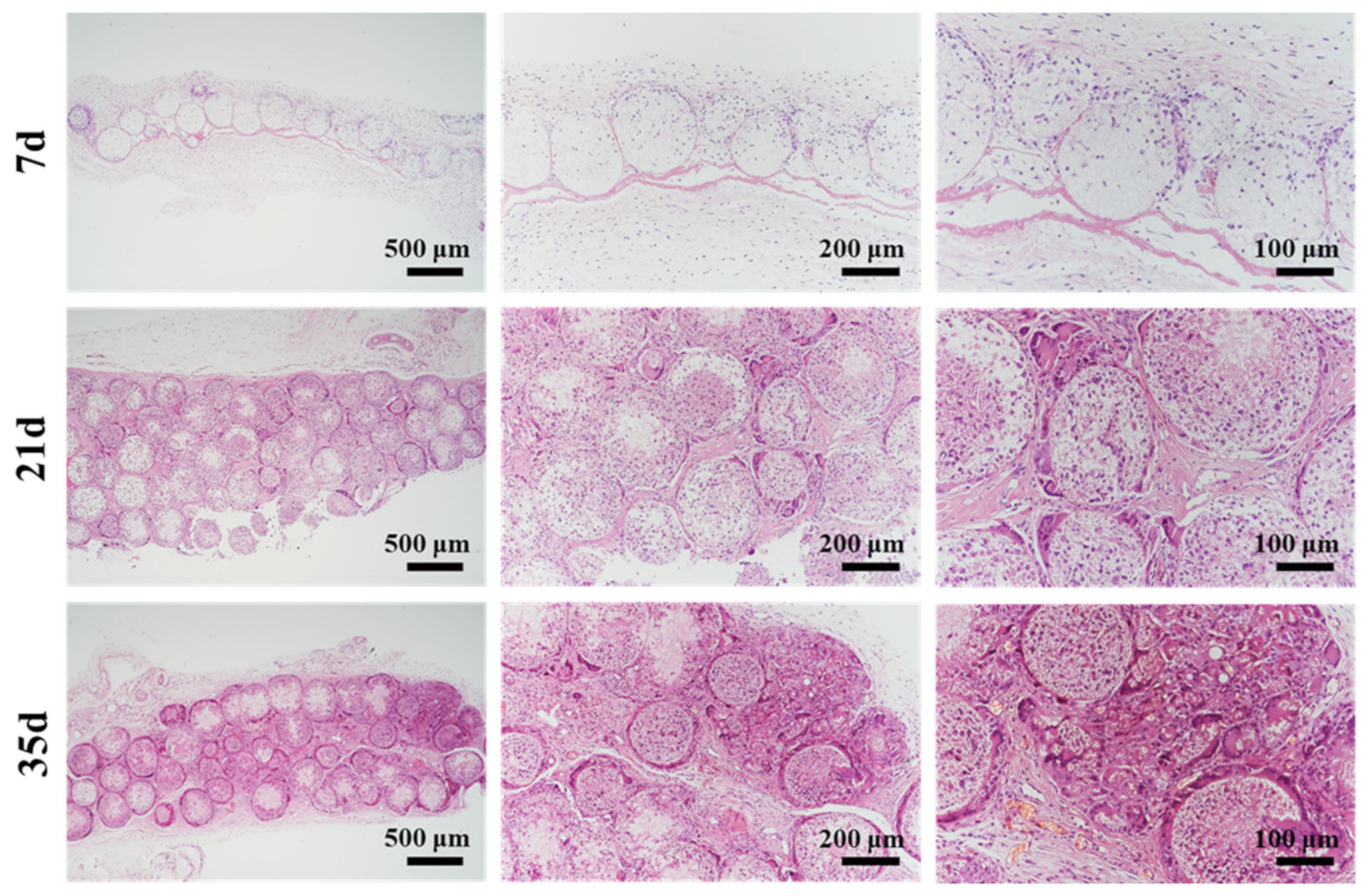
3.7. In Vivo Histocompatibility and Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Chen, Y.; Hong, X.; Liu, Z.; Yuan, W. Porous microsphere and its applications. Int. J. Nanomed. 2013, 8, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Dastidar, D.G.; Saha, S.; Chowdhury, M. Porous microspheres: Synthesis, characterisation and applications in pharmaceutical & medical fields. Int. J. Pharm. 2018, 548, 34–48. [Google Scholar] [CrossRef]
- Junqueira, M.V.; Bruschi, M.L. A review about the drug delivery from microsponges. AAPS PharmSciTech 2018, 19, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhao, J.; Liu, C.; Song, X.; Yang, D.; Zhu, K.; Wang, S.; Zhang, Y.S.; Chen, A. Highly porous microcarriers for minimally invasive in situ skeletal muscle cell delivery. Small 2019, 15, e1901397. [Google Scholar] [CrossRef]
- Huang, C.; Wei, H.; Yeh, Y.; Wang, J.; Lin, W.; Lee, T.; Hwang, S.; Choi, S.; Xia, Y.; Chang, Y.; et al. Injectable PLGA porous beads cellularized by hAFSCs for cellular cardiomyoplasty. Biomaterials 2012, 33, 4069–4077. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent progress in polymeric non-invasive insulin delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, L. Complex three-dimensional microparticles from microfluidic lithography. Electrophoresis 2020, 41, 1491–1502. [Google Scholar] [CrossRef]
- Pessi, J.; Santos, H.A.; Miroshnyk, I.; Yliruusi, J.; Weitz, D.A.; Mirza, S. Microfluidics-assisted engineering of polymeric microcapsules with high encapsulation efficiency for protein drug delivery. Int. J. Pharm. 2014, 472, 82–87. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.; Xu, J.; Zhang, A.; Lee, H. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef]
- Choi, A.; Seo, K.D.; Kim, D.W.; Kim, B.C.; Kim, D.S. Recent advances in engineering microparticles and their nascent utilization in biomedical delivery and diagnostic applications. Lab Chip 2017, 17, 591–613. [Google Scholar] [CrossRef]
- Liu, H.; Singh, R.P.; Zhang, Z.; Han, X.; Liu, Y.; Hu, L. Microfluidic assembly: An innovative tool for the encapsulation, protection, and controlled release of nutraceuticals. J. Agric. Food Chem. 2021, 69, 2936–2949. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Yeh, Y.C.; Zhang, Y.; Sung, H.W.; Xia, Y. Uniform beads with controllable pore sizes for biomedical applications. Small 2010, 6, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, W.; Yu, H.; Wang, A. Preparation of porous adsorbent via Pickering emulsion template for water treatment: A review. J. Environ. Sci. 2020, 88, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Stubenrauch, C.; Menner, A.; Bismarck, A.; Drenckhan, W. Emulsion and foam templating-promising routes to tailor-made porous polymers. Angew. Chem. Int. Ed. Engl. 2018, 57, 10024–10032. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Aslam, H.Z.; Ansari, T.M.; Zhang, H.; Hussain, I. Fundamentals and design-led synthesis of emulsion-templated porous materials for environmental applications. Adv. Sci. 2021, 8, e2102540. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Claeyssens, F. Basic principles of emulsion templating and its use as an emerging manufacturing method of tissue engineering scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 875. [Google Scholar] [CrossRef]
- Kramer, S.; Cameron, N.R.; Krajnc, P. Porous polymers from high internal phase emulsions as scaffolds for biological applications. Polymers 2021, 13, 1786. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid. Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, X.; Gan, Z.; Wang, F. Modification of porous PLGA microspheres by poly-l-lysine for use as tissue engineering scaffolds. Colloids Surf. B Biointerfaces 2018, 161, 162–168. [Google Scholar] [CrossRef]
- Qutachi, O.; Vetsch, J.R.; Gill, D.; Cox, H.; Scurr, D.J.; Hofmann, S.; Müller, R.; Quirk, R.A.; Shakesheff, K.M.; Rahman, C.V. Injectable and porous PLGA microspheres that form highly porous scaffolds at body temperature. Acta. Biomater. 2014, 10, 5090–5098. [Google Scholar] [CrossRef] [Green Version]
- Amoyav, B.; Benny, O. Microfluidic based fabrication and characterization of highly porous polymeric microspheres. Polymers 2019, 11, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Tan, K.; Ye, Z.; Zhang, Y.; Tan, W.; Lang, M. Preparation of open porous polycaprolactone microspheres and their applications as effective cell carriers in hydrogel system. Mat. Sci. Eng. C 2012, 32, 2589–2595. [Google Scholar] [CrossRef]
- Fan, H.; Jin, Z. Hierarchical porous polycaprolactone microspheres generated via a simple pathway combining nanoprecipitation and hydrolysis. Chem. Commun. 2015, 51, 15114–15117. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, F.; Wang, Q.; Su, Z.; Ma, G. Double emulsion-templated microspheres with flow-through pores at micrometer scale. Colloid Polym. Sci. 2013, 291, 117–126. [Google Scholar] [CrossRef]
- Ku, K.H.; Shin, J.M.; Klinger, D.; Jang, S.G.; Hayward, R.C.; Hawker, C.J.; Kim, B.J. Particles with tunable porosity and morphology by controlling interfacial instability in block copolymer emulsions. ACS Nano 2016, 10, 5243–5251. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Yin, Y.; Jiang, R.; Li, B. Formation mechanisms of porous particles from self-assembly of amphiphilic diblock copolymers inside an oil-in-water emulsion droplet upon solvent evaporation. Langmuir 2019, 35, 5902–5910. [Google Scholar] [CrossRef]
- Del Grosso, C.A.; Leng, C.; Zhang, K.; Hung, H.C.; Jiang, S.; Chen, Z.; Wilker, J.J. Surface hydration for antifouling and bio-adhesion. Chem. Sci. 2020, 11, 10367–10377. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, S.; Zhang, D.; Zhang, W.; Zhang, H.; Zou, J.; Mao, Z.; Yuan, Y.; Gao, C.; Liu, R. Impact of antifouling PEG layer on the performance of functional peptides in regulating cell behaviors. J. Am. Chem. Soc. 2019, 141, 16772–16780. [Google Scholar] [CrossRef]
- Yu, S.; Zuo, X.; Shen, T.; Duan, Y.; Mao, Z.; Gao, C. A density gradient of VAPG peptides on a cell-resisting surface achieves selective adhesion and directional migration of smooth muscle cells over fibroblasts. Acta Biomater. 2018, 72, 70–81. [Google Scholar] [CrossRef]
- Du, W.; Gao, C. Selective adhesion and directional migration of endothelial cells guided by Cys-Ala-Gly peptide density gradient on antifouling polymer brushes. Macromol. Biosci. 2019, 19, e1900292. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, H.; Geng, K.; Shen, J.; Feng, X.; Xu, P.; Duan, Y.; Li, Y.; Wu, R.; Gou, Z.; et al. Large fuzzy biodegradable polyester microspheres with dopamine deposition enhance cell adhesion and bone regeneration in vivo. Biomaterials 2021, 272, 120783. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Emmenegger, C.; Preuss, C.M.; Yameen, B.; Pop-Georgievski, O.; Bachmann, M.; Mueller, J.O.; Bruns, M.; Goldmann, A.S.; Bastmeyer, M.; Barner-Kowollik, C. Controlled cell adhesion on poly(dopamine) interfaces photopatterned with non-fouling brushes. Adv. Mater. 2013, 25, 6123–6127. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; Schattling, P.; Städler, B. Recent developments in poly(dopamine)-based coatings for biomedical applications. Nanomedicine 2015, 10, 2725–2742. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Finkenstadt, V.L.; Gordon, S.H.; Biresaw, G.; Palmquist, D.E.; Rayas-Duarte, P. Thermal properties of PCL/gluten bioblends characterized by TGA, DSC, SEM, and infrared-PAS. J. Appl. Polym. Sci. 2008, 110, 3256–3266. [Google Scholar] [CrossRef]
- Silva, D.F.; Lima, K.T.; Bastos, G.N.T.; Oliveira, J.A.R.; do Nascimento, L.A.S.; Costa, C.E.F.; Filho, G.N.R.; Concha, V.O.C.; Passos, M.F. PCL/andiroba oil (Carapa guianensis Aubl.) hybrid film for wound healing applications. Polymers 2021, 13, 1591. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lima, A.; Botelho, G.; Silva, M.M.; Machado, A.V. Durability of PCL nanocomposites under different environments. J. Polym. Environ. 2013, 21, 710–717. [Google Scholar] [CrossRef]
- Allaf, R.M.; Albarahmieh, E.; AlHamarneh, B.M. Solid-state compounding of immiscible PCL-PEO blend powders for molding processes. J. Mech. Behav. Biomed. Mater. 2019, 97, 198–211. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. Fabrication and characterisation of melt-extruded chitosan/keratin/PCL/PEG drug-eluting sutures designed for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111696. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, Z.; Yuan, X.; Wan, Z.; Yu, Y.; Zhan, Q.; Zhao, Y.; Han, J.; Huang, J.; Xiong, C.; et al. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact. Mater. 2022, 14, 377–388. [Google Scholar] [CrossRef]
- Gu, X.; Zha, Y.; Li, Y.W.; Chen, J.; Liu, S.; Du, Y.; Zhang, S.; Wang, J. Integrated polycaprolactone microsphere-based scaffolds with biomimetic hierarchy and tunable vascularization for osteochondral repair. Acta Biomater. 2022, 141, 190–197. [Google Scholar] [CrossRef] [PubMed]
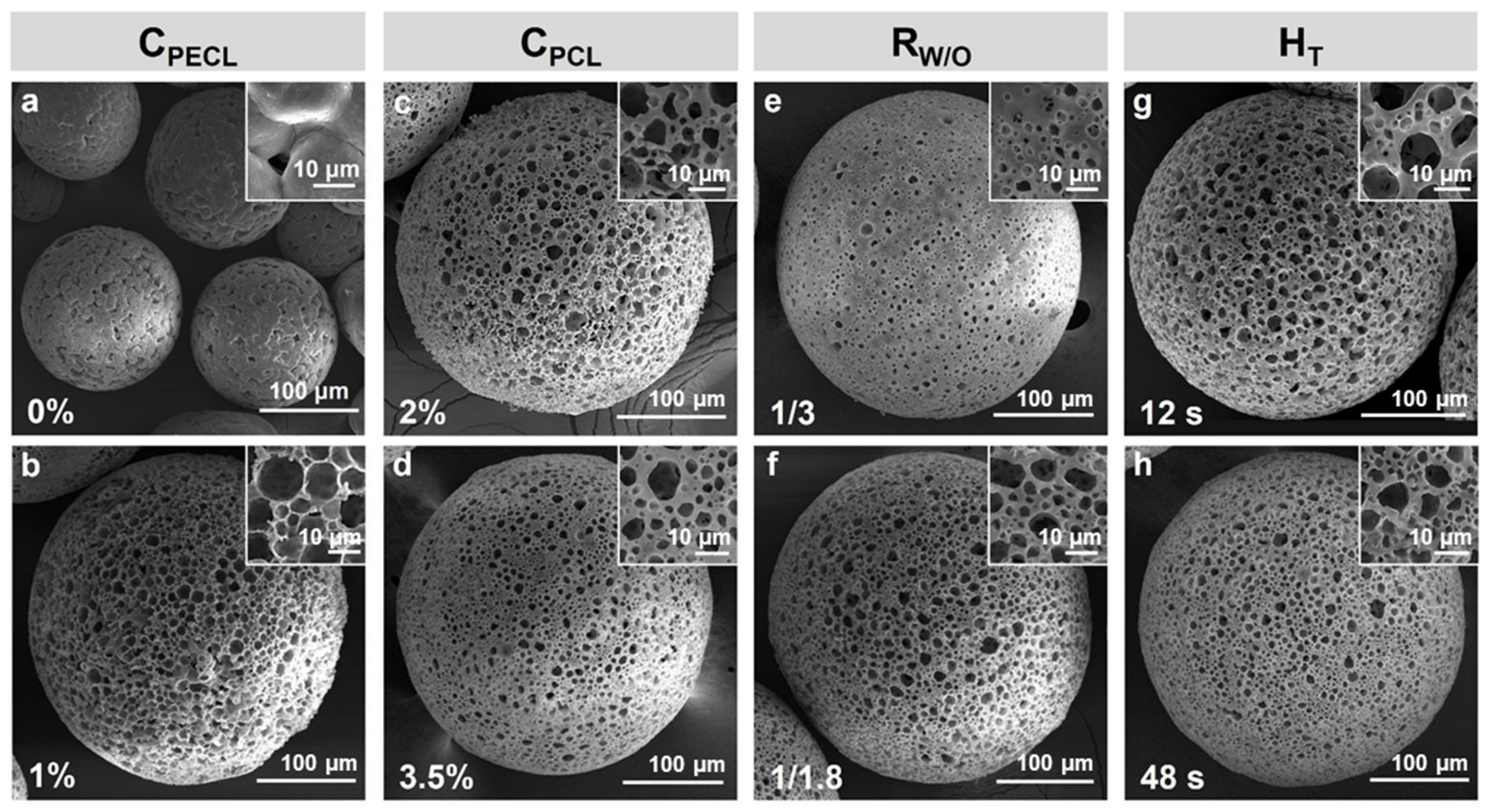


| Microspheres | CPECL (%) | CPCL (%) | RW/O | TH (%) |
|---|---|---|---|---|
| a | 0 | 2.5 | 1/2.4 | 24 |
| b | 1 | |||
| c | 0.5 | 2 | 1/2.4 | 24 |
| d | 3.5 | |||
| e | 0.5 | 2.5 | 1/3 | 24 |
| f | 1/1.8 | |||
| g | 0.5 | 2.5 | 1/2.4 | 12 |
| h | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhang, X.; Mu, K.; Wang, Y.; Jiang, T.; Jiang, S.; Zhang, S.; Du, Y. Precise Fabrication of Porous Microspheres by Iso-Density Emulsion Combined with Microfluidics. Polymers 2022, 14, 2687. https://doi.org/10.3390/polym14132687
Shi Y, Zhang X, Mu K, Wang Y, Jiang T, Jiang S, Zhang S, Du Y. Precise Fabrication of Porous Microspheres by Iso-Density Emulsion Combined with Microfluidics. Polymers. 2022; 14(13):2687. https://doi.org/10.3390/polym14132687
Chicago/Turabian StyleShi, Yuxiao, Xin Zhang, Ketao Mu, Yifan Wang, Ting Jiang, Shangtong Jiang, Shengmin Zhang, and Yingying Du. 2022. "Precise Fabrication of Porous Microspheres by Iso-Density Emulsion Combined with Microfluidics" Polymers 14, no. 13: 2687. https://doi.org/10.3390/polym14132687
APA StyleShi, Y., Zhang, X., Mu, K., Wang, Y., Jiang, T., Jiang, S., Zhang, S., & Du, Y. (2022). Precise Fabrication of Porous Microspheres by Iso-Density Emulsion Combined with Microfluidics. Polymers, 14(13), 2687. https://doi.org/10.3390/polym14132687






