Review of Phosphorus-Based Polymers for Mineral Scale and Corrosion Control in Oilfield
Abstract
:1. Introduction
2. Chemical Inhibition Mechanism
2.1. Scale Inhibition
2.1.1. Adsorption
2.1.2. Crystal Modification
2.1.3. Dispersion
2.1.4. Nanoimpurities’ Role in Scale Formation/Inhibition
2.2. Corrosion Inhibition
3. Synthesis and Evaluation of Phosphorus-Based Polymers
3.1. Introduction
3.2. Phosphino-Polycarboxylic Acid
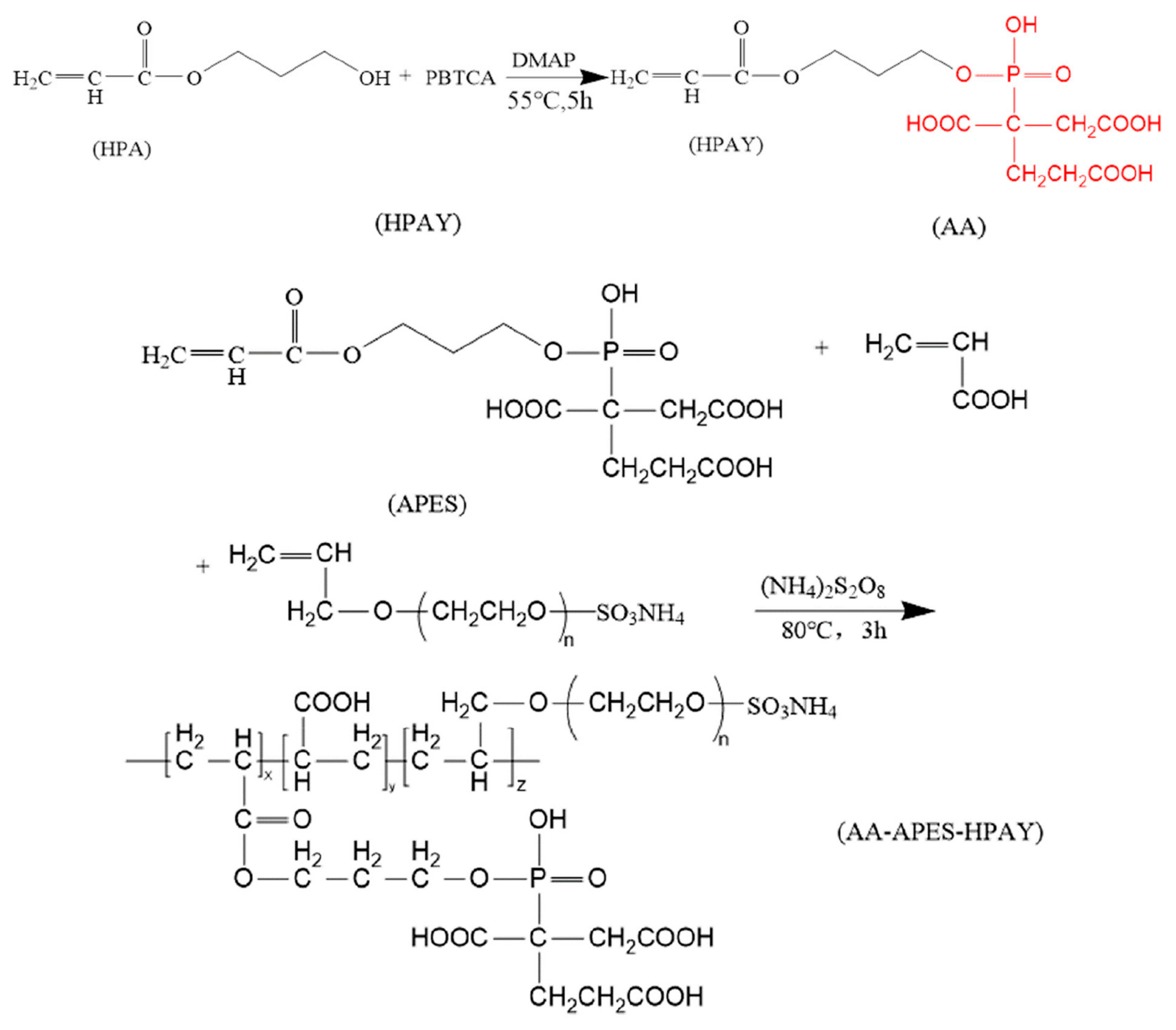
3.3. Phosphorus-Tagged (P-Tagged) Copolymer
3.4. Phosphonated Polyetheramines
3.5. Phosphonated Aliphatic Polycarbonates
3.6. Phosphonated Polyaspartic Acid
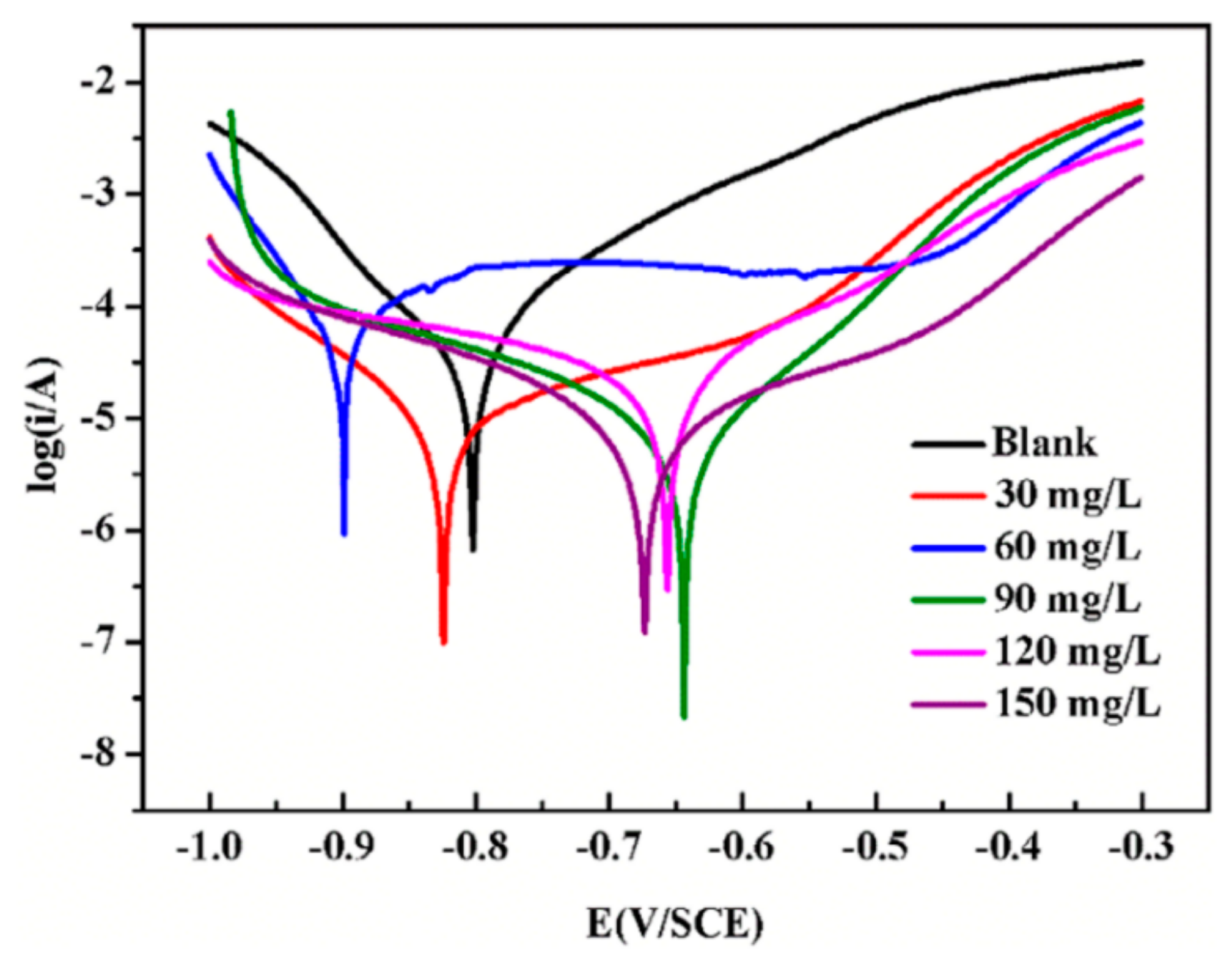
3.7. Grafted Copolymer
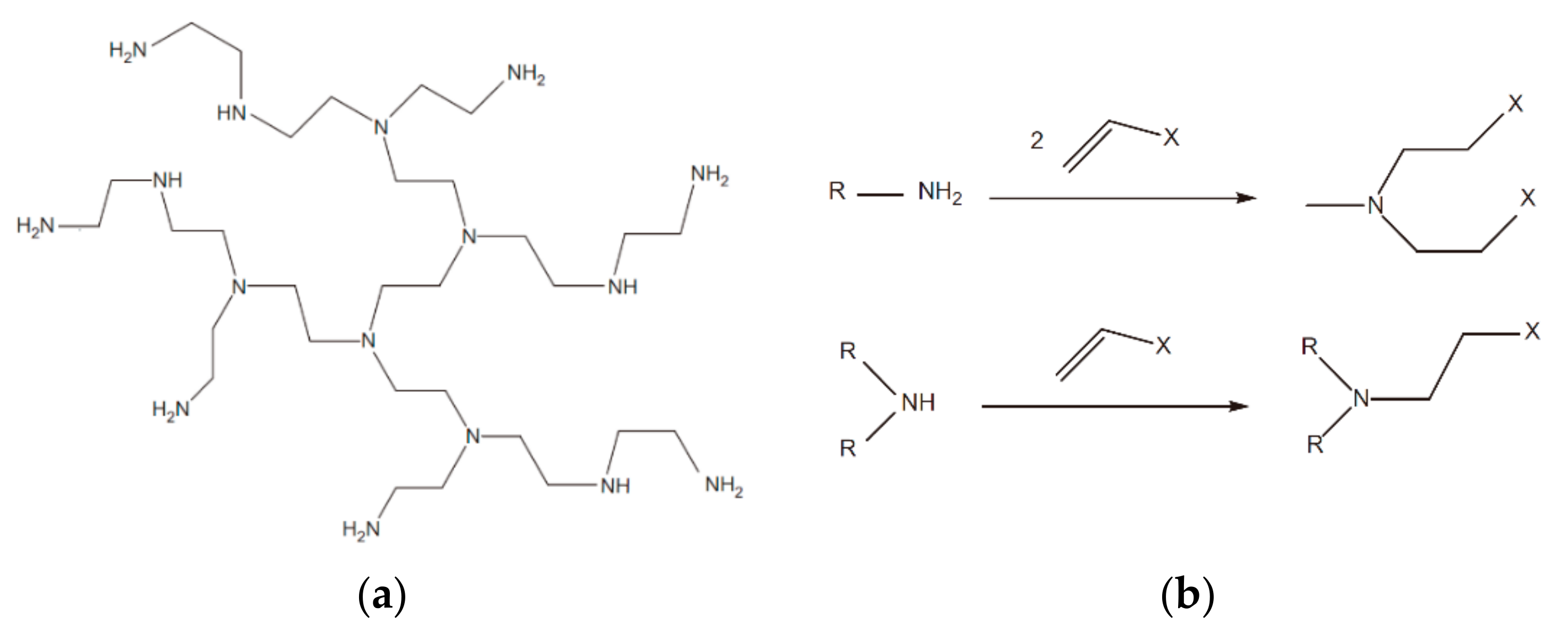
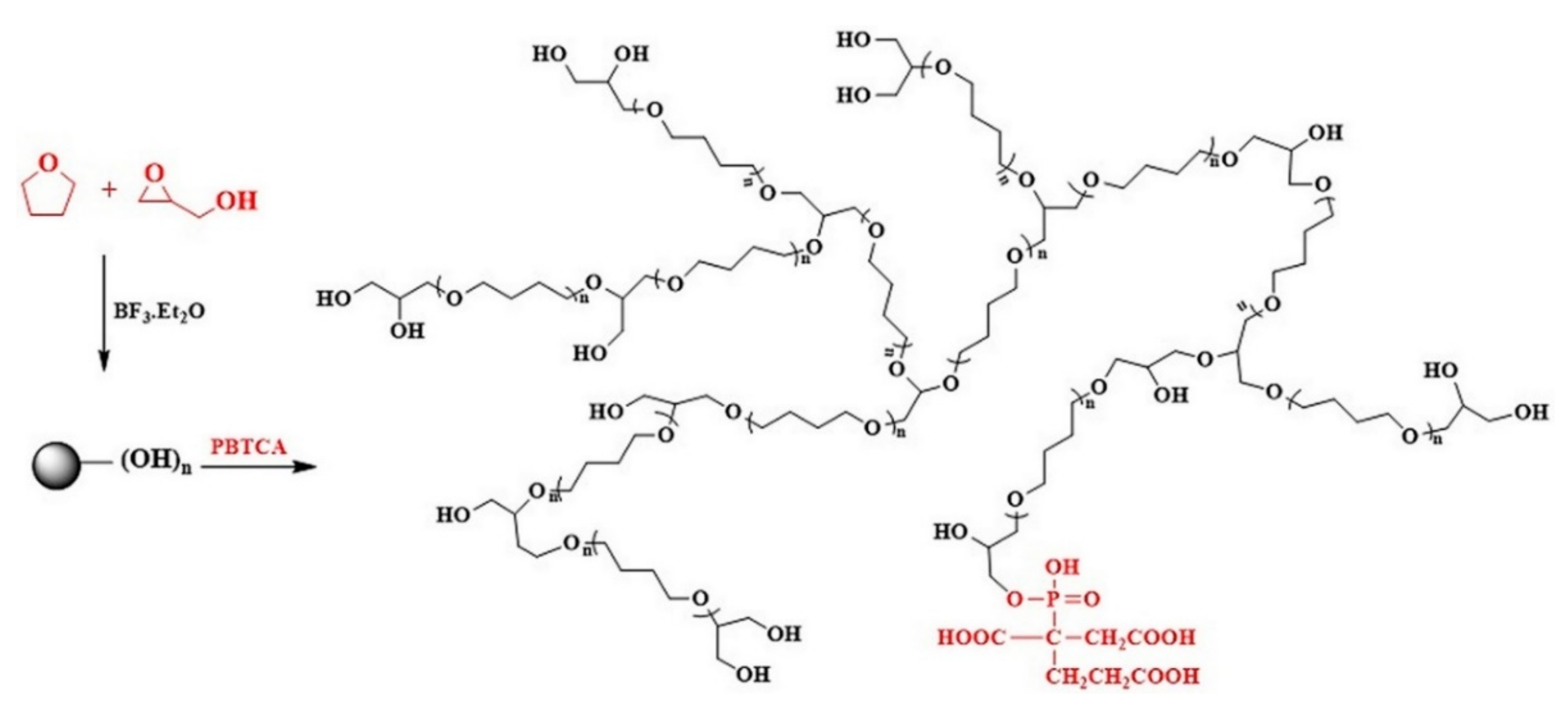
3.8. Dendrimeric or Hyperbranched Polymers
3.9. Other Terpolymer
4. Application for Oilfield Scale and Corrosion Control
4.1. Conventional Squeeze Procedure and Ideal Squeeze Inhibitor Selection
- Good inhibitory effectiveness at low levels of inhibitor concentrations, typically on the order of 1–50 mg L−1;
- Good compatibility with seawater, formation water, and other chemical additives for the application in oilfield flow assurance;
- Good adsorption/desorption properties allowing the long-term slow release of chemicals into production water at concentrations above the required scaling prevention level;
- High resistance to temperatures and pressures encountered downhole. It is not desirable to undergo thermal degradation under downhole conditions;
- More environmentally friendly than phosphonates;
- Balance between cost-effectiveness and affordability.
4.2. Retention/Release Mechanism of Inhibitor in the Formation
4.3. New Phosphorus-Based Inhibitors Used in Squeeze Treatment
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Nomenclature
| AA | acrylic acid |
| APES | allyl polyethoxyammonium sulfonate |
| BHPMP | bishexamethylenediamine penta (methylene phosphonic acid) |
| DTPMP | diethylenetriamine penta (methylene phosphonic acid) |
| EOR | enhanced oil recovery |
| HBP | hyperbranched polyether |
| HEDP | 1-hydroxyethane-1,1-bis (phosphonic acid) |
| HPAY | hydroxypropyl acrylate modified by 2-phosphonobutane-1,2,4-tricarboxylic acid |
| MAc-SS | maleic acid–sodium q-styrenesulfonate copolymer |
| NTMP | nitrilo tris (methylenephosphonic acid) |
| PAA | polyacrylic acid |
| PAPEMP | polyamino polyether methylene phosphonic acid |
| PASP | polyaspartic acid |
| PBTC/PBTCA | 2-phosphono-butane-1,2,4-tricarboxylic acid |
| PCA | polycarbonates |
| PCPA | phosphorus-containing polymer amine |
| PEG | polyethylene glycol |
| PESA | polyepoxysuccinic acid |
| PHOS | phosphonic acid |
| PMA | polymaleic acid |
| PMPA | phosphono methylated polyamine |
| POCA | phosphono carboxylic acid |
| PPCA | phosphino-polycarboxylate |
| SEM | scanning electron microscopy |
| SPCA | sulfonated polycarboxylic acid |
| VDPA | vinylidene diphosphonic acid |
| VPA | vinyl phosphonic acid |
References
- Ilyasov, I.; Koltsov, I.; Golub, P.; Tretyakov, N.; Cheban, A.; Thomas, A. Polymer retention determination in porous media for polymer flooding in unconsolidated reservoir. Polymers 2021, 13, 2737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Song, G.; Zhang, J. Corrosion control in CO2 enhanced oil recovery from a perspective of multiphase fluids. Front. Mater. 2019, 6, 272. [Google Scholar] [CrossRef]
- Amjad, Z.; Demadis, K.D. (Eds.) Water-Formed Deposits: Fundamentals and Mitigation Strategies; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Li, S.; Guo, C.; Wang, X.; Guan, C.; Chen, G. Corrosion inhibition coating based on the self-assembled polydopamine films and its anti-corrosion properties. Polymers 2022, 14, 794. [Google Scholar] [CrossRef] [PubMed]
- Assis, J.; Santos, A.; Midori, E.; Vieira, M.; Anna, S.; Araujo, M.; Pérez, C.; Griza, S. Corrosion damages of flow regulation valves for water injection in oil fields. Eng. Fail. Anal. 2019, 96, 362–373. [Google Scholar] [CrossRef]
- Rezaeizadeh, M.; Hajiabadi, S.H.; Aghaei, H.; Blunt, M.J. Pore-scale analysis of formation damage; a review of existing digital and analytical approaches. Adv. Colloid Interface Sci. 2021, 288, 102345. [Google Scholar] [CrossRef]
- Zhang, P.; Kan, A.T.; Tomson, M.B. Oil field mineral scale control. In Mineral Scales and Deposits: Scientific and Technological Approaches, 1st ed.; Amjad, Z., Demadis, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 603–617. [Google Scholar]
- Moghadasi, J.; Jamialahmadi, M.; Müller-Steinhagen, H.; Sharif, A.; Ghalambor, A.; Izadpanah, M.R.; Motaie, E. Scale Formation in Iranian Oil Reservoir and Production Equipment During Water Injection. In Proceedings of the 5th International Oilfield Scale Symposium and Exhibition, Aberdeen, UK, 29 January 2003. [Google Scholar] [CrossRef]
- Ruan, G.; Liu, Y.; Kan, A.T.; Tomson, M.B.; Zhang, P. Sodium chloride (Halite) mineral scale threat assessment and scale inhibitor evaluation by two common jar test based methods. J. Water Process Eng. 2021, 43, 102241. [Google Scholar] [CrossRef]
- Dai, Z.; Paudyal, S.; Dai, C.; Ko, S.; Zhao, Y.; Wang, X.; Li, W.; Lu, Y.-T.; Kan, A.T.; Tomson, M.B. A new CSTR method for scale inhibitor evaluation. Chem. Eng. J. 2022, 437, 135351. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Z.; Kan, A.T.; Tomson, M.B.; Zhang, P. Investigation of sorptive interaction between phosphonate inhibitor and barium sulfate for oilfield scale control. J. Pet. Sci. Eng. 2022, 208, 109425. [Google Scholar] [CrossRef]
- Nichols, D.; Goodwin, N.; Graham, G.; Frigo, D. Scale Prediction and Mineral Solubility under HPHT Conditions. In Proceedings of the SPE International Conference on Oilfield Chemistry, Galveston, TX, USA, 8–9 April 2019. [Google Scholar] [CrossRef]
- Dai, Z.; Kan, A.T.; Shi, W.; Yan, F.; Zhang, F.; Bhandari, N.; Ruan, G.; Zhang, Z.; Liu, Y.; Alsaiari, H.A.; et al. Calcite and barite solubility measurements in mixed electrolyte solutions and development of a comprehensive model for water-mineral-gas equilibrium of the Na-K-Mg-Ca-Ba-Sr-Cl-SO4-CO3-HCO3-CO2 (aq)-H2O System up to 250 °C and 1500 bar. Ind. Eng. Chem. Res. 2017, 56, 6548–6561. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Z.; Ko, S.; Deng, G.; Zhao, Y.; Dai, C.; Li, W.; Paudyal, S.; Yao, X.; Kan, A.T.; et al. Iron sulfide solubility measurement and modeling over wide ranges of temperatures, ionic strength, and pH. SPE J. 2022, 27, 1263–1274. [Google Scholar] [CrossRef]
- Alhajri, I.H.; Alarifi, I.M.; Asadi, A.; Nguyen, H.M.; Moayedi, H. A general model for prediction of BaSO4 and SrSO4 solubility in aqueous electrolyte solutions over a wide range of temperatures and pressures. J. Mol. Liq. 2020, 299, 112142. [Google Scholar] [CrossRef]
- Smith, S.N.; Joosten, M.W. Corrosion of Carbon Steel by H2S in CO2 Containing Oilfield Environments-10 Year Update. In Proceedings of the CORROSION, Dallas, TX, USA, 15 March 2015. [Google Scholar]
- Kittel, J.; Ropital, F.; Grosjean, F.; Sutter, E.M.M.; Tribollet, B. Corrosion mechanisms in aqueous solutions containing dissolved H2S. Part 1: Characterisation of H2S reduction on a 316 L rotating disc electrode. Corros. Sci. 2013, 66, 324–329. [Google Scholar] [CrossRef] [Green Version]
- Mpelwa, M.; Tang, S.F. State of the art of synthetic threshold scale inhibitors for mineral scaling in the petroleum industry: A review. Pet. Sci. 2019, 16, 830–849. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.Q.; Zhang, P. Control of composite oilfield scales and deposits. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 353–468. [Google Scholar]
- Zhang, Q.H.; Hou, B.S.; Li, Y.Y.; Zhu, G.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. Dextran derivatives as highly efficient green corrosion inhibitors for carbon steel in CO2 -saturated oilfield produced water: Experimental and theoretical approaches. Chem. Eng. J. 2021, 424, 130519. [Google Scholar] [CrossRef]
- Mady, M.F.; Kelland, M.A. Overview of the synthesis of salts of organophosphonic acids and their application to the management of oil field scale. Energy Fuels 2017, 31, 4603–4615. [Google Scholar] [CrossRef]
- Zhang, P. Review of synthesis and evaluation of inhibitor nanomaterials for oilfield mineral scale control. Front. Chem. 2020, 8, 576055. [Google Scholar] [CrossRef]
- Kadhim, A.; Alazawi, R. Corrosion inhibitors. A review. Int. J. Corros. Scale Inhib. 2021, 10, 54–67. [Google Scholar] [CrossRef]
- Kelland, M.A.; Pomicpic, J.; Ghosh, R.; Undheim, C.; Hemmingsen, T.H.; Zhang, Q.; Varfolomeev, M.A.; Pavelyev, R.S.; Vinogradova, S.S. Multi-functional oilfield production chemicals: Maleic-based polymers for gas hydrate and corrosion inhibition. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1201, 012081. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.S.; Saad, M.A.S. Oilfield scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Kan, A.T.; Fu, G.; Tomson, M.B. Adsorption and precipitation of an aminoalkylphosphonate onto calcite. J. Colloid Interface Sci. 2005, 281, 275–284. [Google Scholar] [CrossRef]
- Nizio, K.D.; Harynuk, J.J. Analysis of alkyl phosphates in petroleum samples by comprehensive two-dimensional gas chromatography with nitrogen phosphorus detection and post-column deans switching. J. Chromatogra. A 2012, 1252, 171–176. [Google Scholar] [CrossRef]
- Kan, A.T.; Varughese, K.; Tomson, M.B. Determination of Low Concentrations of Phosphonate in Brines. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Anaheim, CA, USA, 20–22 February 1991. [Google Scholar] [CrossRef]
- Dai, C.; Dai, Z.; Zhang, F.; Zhao, Y.; Deng, G.; Harouaka, K.; Wang, X.; Lu, Y. A Unified Experimental Method and Model for Predicting Scale Inhibition. In Proceedings of the SPE International Conference on Oilfield Chemistry, Galveston, TX, USA, 8–9 April 2019. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, Z.; Dai, C.; Wang, X.; Paudyal, S.; Ko, S.; Yao, X.; Kan, A.T.; Tomson, M. A semiempirical model for barium-strontium-sulfate solid solution scale crystallization and inhibition kinetics at oilfield conditions. SPE J. 2021, 26, 4037–4050. [Google Scholar] [CrossRef]
- Djenane, M.; Chafaa, S.; Chafai, N.; Kerkour, R. Synthesis, spectral properties and corrosion inhibition efficiency of new ethyl hydrogen [(methoxyphenyl) (methylamino) methyl] phosphonate derivatives: Experimental and theoretical investigation. J. Mol. Struct. 2019, 1175, 398–413. [Google Scholar] [CrossRef]
- Migahed, M.A.; Alsabagh, A.M.; Abdou, M.I.; Abdel-rahman, A.A.; Aboulrous, A.A. Synthesis a novel family of phosphonate surfactants and their evaluation as corrosion inhibitors in formation water. J. Mol. Liq. 2019, 281, 528–541. [Google Scholar] [CrossRef]
- Telegdi, J. History of phosphorus-containing corrosion inhibitors: From the beginning till the present time. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–68. [Google Scholar]
- Mazumder, M.A.J. A review of green scale inhibitors: Process, types, mechanism and properties. Coatings 2020, 10, 928. [Google Scholar] [CrossRef]
- Amjad, Z.; Fellows, C.M. Polymers for industrial water systems: Synthesis, characterization, and applications. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 369–398. [Google Scholar]
- Plesu, N.; Macarie, L.; Popa, A.; Ilia, G. Polymeric supports for water treatment applications. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 399–434. [Google Scholar]
- Graham, G.M.; Munro, I.; Harvison, N.; Marshall, K.; Kyle, M. Improved Scale Inhibitor Assay for Sulphonated Polymers in Oilfield Brines. In Proceedings of the SPE International Conference on Oilfield Scale, Aberdeen, UK, 26 May 2010. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, A.; Zhang, Z.; Yan, C.; Yan, F.; Zhang, F.; Bhandari, N.; Dai, Z.; Ruan, G.; Wang, L.; et al. An assay method to determine mineral scale inhibitor efficiency in produced water. J. Pet. Sci. Eng. 2016, 143, 103–112. [Google Scholar] [CrossRef]
- Amjad, Z.; Landgraf, R.T.; Penn, J.L. Calcium sulfate dihydrate (Gypsum) scale inhibition by PAA, PAPEMP, and PAA/PAPEMP blend. Int. J. Corros. Scale Inhib. 2014, 3, 35–47. [Google Scholar] [CrossRef]
- Shaw, S.S.; Sorbie, K.S. Synergistic properties of phosphonate and polymeric scale-inhibitor blends for barium sulfate scale inhibition. SPE Prod. Oper. 2015, 30, 16–25. [Google Scholar] [CrossRef]
- Todd, M.J.; Thornton, A.R.; Wylde, J.; Strachan, C.J.; Clariant, G.M.; Services, O.; Goulding, J.R.; Goulding, J. Phosphorus Functionalised Polymeric Scale Inhibitors, Further Developments and Field Deployment. In Proceedings of the SPE International Conference on Oilfield Scale, Aberdeen, UK, 30 May 2012. [Google Scholar] [CrossRef]
- Wang, C.; Shen, T.; Li, S.; Wang, X. Investigation of in fluence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies. Desalination 2014, 348, 89–93. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures-synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Mady, M.F.; Rehman, A.; Kelland, M.A. Synthesis and study of modified polyaspartic acid coupled phosphonate and sulfonate moieties as green oilfield scale inhibitors. Ind. Eng. Chem. 2021, 60, 8331–8339. [Google Scholar] [CrossRef]
- Popov, K.I.; Kovaleva, N.E.; Rudakova, G.Y.; Kombarova, S.P.; Larchenko, V.E. Recent state-of-the-art of biodegradable scale inhibitors for cooling-water treatment applications (Review). Therm. Eng. 2016, 63, 122–129. [Google Scholar] [CrossRef]
- Shaw, S.S.; Sorbie, K.S.; Boak, L.S. The effects of barium sulfate saturation ratio, calcium, and magnesium on the inhibition efficiency-part ii: Polymeric scale inhibitors. SPE Prod. Oper. 2012, 27, 390–403. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Baek, S.H.; Ko, Y.G. Formation of stable coral reef-like structures via self-assembly of functionalized polyvinyl alcohol for superior corrosion performance of AZ31 Mg alloy. Mater. Des. 2020, 193, 108823. [Google Scholar] [CrossRef]
- Umoren, S.A. Polymers as corrosion inhibitors for metals in different media-a review. Open Corros. J. 2009, 2, 175–188. [Google Scholar] [CrossRef]
- Barber, M. Recent developments in oilfield scale control. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 295–306. [Google Scholar]
- Tomson, M.B.; Fu, G.; Watson, M.A.; Kan, A.T. Mechanisms of mineral scale inhibition. SPE Prod. Facil. 2003, 18, 192–199. [Google Scholar] [CrossRef]
- Sorbie, K.S.; Laing, N. How Scale Inhibitors Work: Mechanisms of Selected Barium Sulphate Scale Inhibitors Across a Wide Temperature Range. In Proceedings of the SPE International Symposium on Oilfield Scale, Aberdeen, UK, 26 May 2004. [Google Scholar] [CrossRef]
- Amjad, Z.; Koutsoukos, P.G. Evaluation of maleic acid based polymers as scale inhibitors and dispersants for industrial water applications. Desalination 2014, 335, 55–63. [Google Scholar] [CrossRef]
- Hoang, T.A. Mechanisms of scale formation and inhibition. In Water-Formed Deposits: Fundamentals and Mitigation Strategies, 1st ed.; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 14–48. [Google Scholar]
- Hoang, T.A. Mechanisms of scale formation and inhibition. In Mineral Scales and Deposits, Scientific and Technological Approaches, 1st ed.; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 47–83. [Google Scholar]
- Oshchepkov, M.S.; Popov, K.I. Mechanisms of scale inhibition derived from a fluorescent-tagged antiscalant visualization. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 765–782. [Google Scholar]
- Okocha, C.; Sorbie, K.S.; Boak, L.S. Inhibition Mechanisms for Sulphide Scales. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 13–15 February 2008. [Google Scholar] [CrossRef]
- Vazirian, M.M.; Charpentier, T.V.J.; Penna, M.D.O.; Neville, A. Surface inorganic scale formation in oil and gas industry: As adhesion and deposition processes. J. Pet. Sci. Eng. 2016, 137, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Kelland, M.A. Chapter 3. Scale Control. In Production Chemicals for the Oil and Gas Industry, 2nd ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2014; pp. 51–109. [Google Scholar]
- Zhang, Z.; Zhang, P.; Li, Z.; Kan, A.T.; Tomson, M.B. Laboratory evaluation and mechanistic understanding of the impact of ferric species on oilfield scale inhibitor performance. Energy Fuels 2018, 32, 8348–8357. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, A.T.; Tomson, M.B.; Zhang, P. Interactions of common scale inhibitors and formation mineral (calcium carbonate): Sorption and transportability investigations under equilibrium and dynamic conditions. J. Pet. Sci. Eng. 2022, 215, 110696. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Jafari, R.; Hamghalam, P.; Hajizadeh, A. Downhole corrosion inhibitors for oil and gas production—A review. Appl. Surf. Sci. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Graham, G.M.; Boak, L.S.; Sorbie, K.S. The influence of formation calcium and magnesium on the effectiveness of generically different barium sulphate oilfield scale inhibitors. SPE Prod. Facil. 2003, 18, 28–44. [Google Scholar] [CrossRef]
- Tomson, M.B.; Kan, A.T.; Fu, G.; Shen, D.; Nasr-el-din, H.A.; Al-saiari, H.; Al-thubaiti, M. Mechanistic understanding of rock/phosphonate interactions and the effect of metal ions on inhibitor retention. SPE J. 2008, 13, 325–336. [Google Scholar] [CrossRef]
- Xiao, J.; Kan, A.T.; Tomson, M.B. Prediction of BaSO4 precipitation in the presence and absence of a polymeric inhibitor: Phosphino-polycarboxylic acid. Langmuir 2001, 17, 4668–4673. [Google Scholar] [CrossRef]
- Tomson, M.B.; Kan, A.T.; Oddo, J.E. Acid/base and metal complex solution chemistry of the polyphosphonate DTPMP versus temperature and ionic strength. Langmuir 1994, 10, 1442–1449. [Google Scholar] [CrossRef]
- Frostman, L.M.; Kan, A.T.; Tomson, M.B. Mechanistic aspects of calcium phosphonates precipitation. In Calcium Phosphates in Biological and Industrial Systems; Amjad, Z., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1998; pp. 493–506. [Google Scholar]
- Al-Thubaiti, M.; Kan, A.T.; Tomson, M.B. The Temperature and Ionic Strength Dependence of the Solubility Product Constants of Acidic Calcium and Ferrous Phosphonate Phases in Oilfield Brine. In Proceedings of the NACE, New Orleans, LA, USA, 28 March–1 April 2004. [Google Scholar]
- Xiao, J.; Kan, A.T.; Tomson, M.B. Acid-base and metal complexation chemistry of phosphino-polycarboxylic acid under high ionic strength and high temperature. Langmuir 2001, 17, 4661–4667. [Google Scholar] [CrossRef]
- Pina, C.M.; Putnis, C.V.; Becker, U.; Biswas, S.; Carroll, E.C.; Bosbach, D.; Putnis, A. An atomic force microscopy and molecular simulations study of the inhibition of barite growth by phosphonates. Surf. Sci. 2004, 553, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.Y.-T.; Shi, W.; Wang, J.; Venkatesan, R.; Harouaka, K.; Paudyal, S.; Ko, S.; Dai, C.; Gao, S.; Deng, G.; et al. The mechanism of barium sulfate deposition inhibition and the prediction of inhibitor dosage. J. Chem. Eng. Data 2019, 64, 4968–4976. [Google Scholar] [CrossRef]
- Popov, K.; Rudakova, G.; Larchenko, V.; Tusheva, M.; Kamagurov, S.; Dikareva, J.; Kovaleva, N. A comparative performance evaluation of some novel (green) and traditional antiscalants in calcium sulfate scaling. Adv. Mater. Sci. Eng. 2016, 2016, 7635329. [Google Scholar] [CrossRef] [Green Version]
- Sheng, K.; Ge, H.; Huang, X.; Zhang, Y.; Song, Y.; Ge, F.; Zhao, Y. Formation and inhibition of calcium carbonate crystals under cathodic polarization conditions. Crystals 2020, 10, 275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Shen, D.; Kan, A.T.; Tomson, M.B. Phosphino-polycarboxylic acid modified inhibitor nanomaterial for oilfield scale control: Transport and inhibitor return in formation media. RSC Adv. 2016, 6, 59195–59205. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Jalab, R.; Saad, M.; Mahmoud, M. Review of iron sulfide scale removal and inhibition in oil and gas wells: Current status and perspectives. Energy Fuels 2021, 35, 14401–14421. [Google Scholar] [CrossRef]
- Ko, S.; Wang, X.; Kan, A.T.; Tomson, M.B. Growth inhibition and deposition prevention of sulfide scales using dispersants. J. Pet. Sci. Eng. 2021, 197, 108107. [Google Scholar] [CrossRef]
- Amjad, Z. Investigations on the influence of phosphonates in dispersing iron oxide (rust) by polymeric additives for industrial water applications. Int. J. Corros. Scale Inhib. 2014, 3, 89–100. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Kamagurov, S.; Tkachenko, S.; Ryabova, A.; Popov, K. Insight into the mechanisms of scale inhibition: A case study of a task-specific fluorescent-tagged scale inhibitor location on gypsum crystals. ChemNanoMat 2019, 5, 586–592. [Google Scholar] [CrossRef]
- Tkachenko, S.; Ryabova, A.; Oshchepkov, M.; Popov, K. Fluorescent-tagged antiscalants: A new look at the scale inhibition mechanism and antiscalant selection. ChemNanoMat 2022, 8, e202100370. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Liu, J.; Chen, H.; Chen, Q.; Wang, B. Fluorescent-tagged hyper-branched polyester for inhibition of caso4 scale and the scale inhibition mechanism. Mater. Today Commun. 2020, 25, 101359. [Google Scholar] [CrossRef]
- Popov, K.; Oshchepkov, M.; Afanas’eva, E.; Koltinova, E.; Dikareva, Y.; Rönkkömäki, H.A. New insight into the mechanism of the scale inhibition: Dls study of gypsum nucleation in presence of phosphonates using nanosilver dispersion as an internal light scattering intensity reference. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 122–129. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Gha, S.; Hajizadeh, A. Film former corrosion inhibitors for oil and gas pipelines—A technical review. J. Nat. Gas Sci. Eng. 2018, 58, 92–114. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Z.; Yuan, M.; Huang, G.; Guo, H. Trigger and response mechanisms for controlled release of corrosion inhibitors from micro/nanocontainers interpreted using endogenous and exogenous stimuli: A review. J. Mater. Sci. Technol. 2022, 125, 67–80. [Google Scholar] [CrossRef]
- Gao, X.; Liu, S.; Lu, H.; Gao, F.; Ma, H. Corrosion inhibition of iron in acidic solutions by monoalkyl phosphate esters with different chain lengths with different chain lengths. Ind. Eng. Chem. Res. 2015, 54, 1941–1952. [Google Scholar] [CrossRef]
- Guo, W.; Talha, M.; Lin, Y.; Ma, Y.; Kong, X. Effect of phosphonate functional group on corrosion inhibition of imidazoline derivatives in acidic environment. J. Colloid Interface Sci. 2021, 597, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations). Integr. Med. Res. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Yohai, L.; Vázquez, M.; Valcarce, M.B. Phosphate ions as corrosion inhibitors for reinforcement steel in chloride-rich environments. Electrochim. Electrochim. Acta 2013, 102, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Kelland, M.A. Chapter 8. Corrorsion Control during Production. In Production Chemicals for the Oil and Gas Industry, 2nd ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2014; pp. 194–195. [Google Scholar]
- Achour, M.; Kolts, J. Corrosion Control by Inhibition Part I: Corrosion Control by Film Forming Inhibitors. In Proceedings of the CORROSION, Dallas, TX, USA, 15 March 2015. [Google Scholar]
- Tiu, B.D.B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Shamsa, A.; Barker, R.; Hua, Y.; Barmatov, E.; Hughes, T.L.; Neville, A. Performance evaluation of an imidazoline corrosion inhibitor in a CO2-saturated environment with emphasis on localised corrosion. Corros. Sci. 2020, 176, 108916. [Google Scholar] [CrossRef]
- Mady, M.F. Oilfield scale inhibitors: Synthetic and performance aspects. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 325–352. [Google Scholar]
- Smith, M.J.; Miles, P.; Richardson, N.; Finan, M.A. Inhibiting Scale Formation in Aqueous Systems. UK Patent GB 1458235, 8 December 1976. [Google Scholar]
- Laubender, M.; Heintz, E.; Seidl, C.; Urtel, B.; Berger, A. Copolymers of Monocarboxylic Acids and Dicarboxylic Acids, Their Preparation and Use. U.S. Patent Application 20120004383, 5 January 2012. [Google Scholar]
- Benbakhti, A.; Bachir-Bey, T. Synthesis and characterization of maleic acid polymer for use as scale deposits inhibitors. J. Appl. Polym. Sci. 2010, 116, 3095–3102. [Google Scholar] [CrossRef]
- Malaie, K.; Shojaei, O.; Iranpour, S.; Taherkhani, Z. Crystal growth inhibition of gypsum under normal conditions and high supersaturations by a copolymer of phosphino-polycarboxylic acid. Heliyon 2021, 7, e06064. [Google Scholar] [CrossRef]
- Fleming, N.; Bourne, H.M.; Strachan, C.J.; Buckley, A.S. Development of an ecofreindly scale inhibitor for harsh scaling environments. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13 February 2001. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Li, T. Calcium carbonate inhibition by a phosphonate-terminated poly (maleic-co-sulfonate) polymeric inhibitor. Desalination 2009, 249, 1–4. [Google Scholar] [CrossRef]
- Emmons, D.H.; Fong, D.W.; Kinsella, M.A. Phosphonate-Containing Polymers for Controlling Scale in Underground Petroleum-Containing Formations and Equipment Associated Therewith. U.S. Patent 5213691, 25 May 1993. [Google Scholar]
- Herrera, T.L.; Guzmann, M.; Neubecker, K.; Goöthlich, A. Process and Polymer for Preventing Ba/Sr Scale with Incorporated Detectable Phosphorus Functionality. International Patent Application WO 2008/095945, 14 August 2008. [Google Scholar]
- Todd, M.J.; Strachan, C.J.; Moir, G.; Services, C.O.; Goulding, J.; Goulding, J. Development of The Next Generation of Phosphorus Tagged Polymeric Scale Inhibitors. In Proceedings of the SPE International Conference on Oilfield Scale, Aberdeen, UK, 26 May 2010. [Google Scholar] [CrossRef]
- Woodward, G.; Otter, G.P.; Davis, K.P.; Huan, K. Biodegradable polymers. International Patent Application WO 2004/056886, 8 July 2004. [Google Scholar]
- Davis, K.P.; Walker, D.R.E.; Woodward, G.; Smith, A.C. Novel phosphino derivatives. European Patent Application EP 0861846, 21 February 1998. [Google Scholar]
- Davis, K.P.; Fidoe, S.D.; Otter, G.P.; Talbot, R.E.; Veale, M.A. Novel Scale Inhibitor Polymers with Enhanced Adsorption Properties. In Proceedings of the SPE International Symposium on Oilfield Scale, Aberdeen, UK, 29 January 2003. [Google Scholar] [CrossRef]
- De Campo, F.; Colaco, A.; Kesavan, S. Scale Squeeze Treatment Methods and Systems. WO 2008/066918, 5 June 2008. [Google Scholar]
- Chen, S.T.; Matz, G.F. Polyether Polyamino Methylene Using Phosphonates Method for High pH Scale Control. U.S. Patent 5358642A, 25 October 1994. [Google Scholar]
- Ramon, A.M.; Nancy, S.S. Synergistic Antimicrobial Combination of Polyether Phosphonates and Non-Oxidizing Biocides. U.S. Patent 5457083A, 10 October 1995. [Google Scholar]
- Shen, D.; Perkins, R.; Schielke, D.; Shcolnik, D. Method for Preventing Scale Formation in the Presence of Dissolved Iron. U.S. Patent 8236734 B1, 7 August 2012. [Google Scholar]
- Mady, M.F.; Bayat, P.; Kelland, M.A. Environmentally friendly phosphonated polyetheramine scale inhibitors-excellent calcium compatibility for oil fi eld applications. Ind. Eng. Chem. Res. 2020, 59, 9808–9818. [Google Scholar] [CrossRef]
- Mady, M.F.; Charoensumran, P.; Ajiro, H.; Kelland, M.A. Synthesis and characterization of modified aliphatic polycarbonates as environmentally friendly oilfield scale inhibitors. Energy Fuels 2018, 32, 6746–6755. [Google Scholar] [CrossRef]
- Thombre, S.M.; Sarwade, B.D. Synthesis and biodegradability of polyaspartic acid: A critical review. J. Macromol. Sci. Part A 2005, 42, 1299–1315. [Google Scholar] [CrossRef]
- Hasson, D.; Shemer, H.; Sher, A. State of the art of friendly “green” scale control inhibitors: A review article. Ind. Eng. Chem. Res. 2011, 50, 7601–7607. [Google Scholar] [CrossRef]
- Fu, L.; Lv, J.; Zhou, L.; Li, Z.; Tang, M.; Li, J. Study on corrosion and scale inhibition mechanism of polyaspartic acid grafted b -cyclodextrin. Mater. Lett. 2020, 264, 127276. [Google Scholar] [CrossRef]
- Migahed, M.A.; Rashwan, S.M.; Kamel, M.M.; Habib, R.E. Synthesized polyaspartic acid derivatives as corrosion and scale inhibitors in desalination operations. Cogent Eng. 2017, 4, 1366255. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Guo, H.; Xu, T.; Zhang, J.; Zhao, W.; Zhang, J.; Lin, C.; Zhang, L. A multifunctional anti-fog, antibacterial, and self-cleaning surface coating based on poly(NVP-co-MA). Chem. Eng. J. 2018, 351, 409–417. [Google Scholar] [CrossRef]
- Wang, C.; Zou, C.; Cao, Y. Electrochemical and isothermal adsorption studies on corrosion inhibition performance of β-cyclodextrin grafted polyacrylamide for X80 steel in oil and gas production. J. Mol. Struct. 2021, 1228, 129737. [Google Scholar] [CrossRef]
- Spinthaki, A.; Kamaratou, M.; Skordalou, G.; Petratos, G.; Petrou, I.; Tramaux, A.; David, G.; Demadis, K.D. Searching for a universal scale inhibitor: A multi-scale approach towards inhibitor efficiency. Geothermics 2021, 89, 101954. [Google Scholar] [CrossRef]
- Spinthaki, A.; Kamaratou, M.; Skordalou, G.; Petratos, G.; Tramaux, A.; David, G.; Demadis, K.D. A universal scale inhibitor: A dual inhibition/dispersion performance evaluation under difficult brine stresses. Geothermics 2021, 89, 101972. [Google Scholar] [CrossRef]
- Parisi, O.I.; Curcio, M.; Puoci, F. Polymer chemistry and synthetic polymers. In Advanced Polymers in Medicine; Puoci, F., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–31. [Google Scholar]
- Zhang, Z.; Liang, T.; Liu, J.; Ding, K.; Chen, H. Hyperbranched polyesters with carboxylic acid functional groups for the inhibition of the calcium carbonate scale. J. Appl. Polym. Sci. 2018, 135, 46292. [Google Scholar] [CrossRef]
- Dong, S.; Yuan, X.; Chen, S.; Zhang, L.; Huang, T. A novel HPEI-based hyperbranched scale and corrosion inhibitor: Construction, performance, and inhibition mechanism. Ind. Eng. Chem. Res. 2018, 57, 13952–13961. [Google Scholar] [CrossRef]
- Jensen, M.K.; Kelland, M.A. A new class of hyperbranched polymeric scale inhibitors. J. Pet. Sci. Eng. 2012, 94, 66–72. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhang, Z.; Huang, H.; Liu, B.; Ding, K. Synthesis and characterization of PBTCA-modified hyperbranched polyether corrosion and scale inhibitors. J. Appl. Polym. Sci. 2019, 136, 48041. [Google Scholar] [CrossRef]
- Zuo, Y.; Sun, Y.; Yang, W.; Zhang, K.; Chen, Y.; Yin, X.; Liu, Y. Performance and mechanism of 1-hydroxy ethylidene-1, 1-diphosphonic acid and 2-phosphonobutane-1, 2, 4-tricarboxylic acid in the inhibition of calcium carbonate scale. J. Mol. Liq. 2021, 334, 116093. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yao, Q.; Bu, Y.; Wang, H.; Wu, W.; Sun, W. Evaluation of a low-phosphorus terpolymer as calcium scales inhibitor in cooling water. Desalin. Water Treat. 2015, 55, 945–955. [Google Scholar] [CrossRef]
- Kahraman, G.; Wang, D.-Y.; von Irmer, J.; Gallei, M.; Hey-Hawkins, E.; Eren, T. Synthesis and characterization of phosphorus- and carborane-containing polyoxanorbornene block copolymers. Polymers 2019, 11, 613. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, B.; Quintero-Jaime, A.F.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Synthesis of phosphorus-containing polyanilines by electrochemical copolymerization. Polymers 2020, 12, 1029. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, X.; Yao, Q.; Zhao, L.; Xu, Z.; Zhou, Y. Synthesis of a new type of 2-phosphonobutane-1, 2, 4-tricarboxylic- acid-modified terpolymer scale inhibitor and its application in the oil field. Energy Fuels 2021, 35, 6136–6143. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Tkachenko, S.; Redchuk, A.; Rönkkömäki, H.; Rudakova, G.; Pervov, A.; Popov, K. Visualization of a novel fluorescent-tagged bisphosphonate behavior during reverse osmosis desalination of water with high sulfate content. Sep. Purif. Technol. 2021, 255, 117382. [Google Scholar] [CrossRef]
- Chen, T.; Liang, F.; Chang, F.; Mukhles, A. Evaluation of Corrosion Inhibitor Batch Treatment Using Newly Developed Downhole Monitoring Tool in Sour Gas Wells. In Proceedings of the SPE Conference at Oman Petroleum & Energy Show, Muscat, Oman, 21 March 2022. [Google Scholar] [CrossRef]
- Vazquez, O.; Fursov, I.; Mackay, E. Automatic optimization of oilfield scale inhibitor squeeze treatment designs. J. Pet. Sci. Eng. 2016, 147, 302–307. [Google Scholar] [CrossRef]
- Wylde, J.J.; Reid, M.; Kirkpatrick, A.; Obeyesekere, N.; Glasgow, D. When To Batch and When Not to Batch: An Overview of Integrity Management and Batch Corrosion Inhibitor Testing Methods and Application Strategies. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8 April 2013. [Google Scholar] [CrossRef]
- Alharooni, K.; Pack, D.; Iglauer, S.; Gubner, R.; Ghodkay, V.; Barifcani, A. Effects of thermally degraded monoethylene glycol with methyl diethanolamine and film-forming corrosion inhibitor on gas hydrate kinetics. Energy Fuels 2017, 31, 6397–6412. [Google Scholar] [CrossRef]
- Lehmann, M.N.; Lamm, A.; Nguyen, H.M.; Bowman, C.W.; Mok, W.Y.; Salasi, M.; Gubner, R. Corrosion Inhibitor and Oxygen Scavenger for Use as MEG Additives in The Inhibition of Wet Gas Pipelines. In Proceedings of the Offshore Technology Conference-Asia, Kuala Lumpur, Malaysia, 25 March 2014. [Google Scholar] [CrossRef]
- Graham, G.M.; Dyer, S.J.; Sorbie, K.S.; Sablerolle, W.R.; Shone, P.; Frigo, D. Scale Inhibitor Selection for Continuous and Downhole Squeeze Application in HP/HT Conditions. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 27 September 1998. [Google Scholar] [CrossRef]
- Kan, A.T.; Fu, G.; Xiao, J.; Tomson, M.B. A New Approach to Inhibitor Squeeze Design. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5 February 2003. [Google Scholar] [CrossRef]
- Kan, A.T.; Dai, Z.; Tomson, M.B. The state of the art in scale inhibitor squeeze treatment. Pet. Sci. 2020, 17, 1579–1601. [Google Scholar] [CrossRef]
- Kan, A.T.; Fu, G.; Tomson, M.B. Prediction of Scale Inhibitor Squeeze and Return in Calcite-Bearing Formation. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Woodlands, TX, USA, 2 February 2005. [Google Scholar] [CrossRef]
- Sorbie, K.S.; Gdanski, R.D. A Complete Theory of Scale-Inhibitor Transport and Adsorption/Desorption in Squeeze Treatments. In Proceedings of the SPE International Symposium on Oilfield Scale, Aberdeen, UK, 11 May 2005. [Google Scholar] [CrossRef]
- Jordan, M.M.; Sutherland, L. Assessment of Formation Damage Potential of Corrosion Inhibitor Squeeze Applications. In Proceedings of the CORROSION, Vancouver, BC, Canada, 6 March 2016. [Google Scholar]
- Kan, A.; Yan, L.; Bedient, P.B.; Oddo, J.E.; Tomson, M.B. Sorption and Fate of Phosphonate Scale Inhibitors in the Sandstone Reservoir: Studied by Laboratory Apparatus with Core Material. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 7 April 1991. [Google Scholar] [CrossRef]
- Jordan, M.M.; Mackay, E.J.; Vazquez, O. The Influence of Overflush Fluid Type on Scale Squeeze Lifetime: Field Examples and Placement Simulation Evaluation. In Proceedings of the Corrosion, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Zhang, P.; Ruan, G.; Kan, A.T.; Tomson, M.B. Functional scale inhibitor nanoparticle capsule delivery vehicles for oilfield mineral scale control. RSC Adv. 2016, 6, 43016–43027. [Google Scholar] [CrossRef]
- Tomson, M.B.; Kan, A.T.; Fu, G. Control of inhibitor squeeze through mechanistic understanding of inhibitor chemistry. SPE J. 2006, 11, 283–293. [Google Scholar] [CrossRef]
- Farooqui, N.M.; Sorbie, K.S.; Boak, L.S. The Solubility and Dissolution of PPCA_Ca Complex in Precipitation. In Proceedings of the SPE International Oilfield Scale Conference and Exhibition Held in Aberdeen, Scotland, UK, 11 May 2016. [Google Scholar] [CrossRef]
- Kan, A.T.; Fu, G.M.; Tomson, M.B.; Al-Thubaiti, M.; Xiao, A.J. Factors affecting scale inhibitor retention in carbonate-rich formation during squeeze treatment. SPE J. 2004, 9, 280–289. [Google Scholar] [CrossRef]
- Jordan, M.M.; Sutherland, L.; Johnston, C. Developments in Squeeze Treatments Design-Why not Apply 24 Month Duration Squeeze to Offshore Production Wells? In Proceedings of the SPE International Oilfield Scale Conference and Exhibition, Virtual, 24 June 2020. [CrossRef]
- Farooqui, N.M.; Sorbie, K.S. The use of PPCA in scale-inhibitor precipitation squeezes: Solubility, inhibition efficiency, and molecular-weight effects. SPE Prod. Oper. 2016, 31, 258–269. [Google Scholar] [CrossRef]
- Andrei, M.; Gagliardi, F. Redissolution studies in bulk and in coreflood for PPCA scales inhibitor. J. Pet. Sci. Eng. 2004, 43, 35–55. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, F.; Bhandari, N.; Wang, L.; Dai, Z.; Zhang, Z.; Liu, Y.; Ruan, G.; Kan, A.; Tomson, M.; et al. Adsorption and precipitation of scale inhibitors on shale formations. J. Pet. Sci. Eng. 2015, 136, 32–40. [Google Scholar] [CrossRef]
- Jarrahian, K.; Sorbie, K.S. Mechanistic investigation of adsorption behavior of two scale inhibitors on carbonate formations for application in squeeze treatments. Energy Fuels 2020, 34, 4484–4496. [Google Scholar] [CrossRef]
- Selle, O.M.; Wat, R.M.S.; Vikane, O.; Nasvik, H.; Chen, P.; Hagen, T.; Montgomerie, H.; Bourne, H. A Way Beyond Scale Inhibitors–Extending Scale Inhibitor Squeeze Life Through Bridging, In Proceedings of the SPE 5th International Symposium on Oilfield Scale in Aberdeen, Scotland, UK, 29 January 2003. [CrossRef]
- Jordan, M.M.; Sorhaug, E.; Marlow, D. Red vs. green scale inhibitors for extending squeeze life-a case study from the North Sea, Norwegian sector—Part II. SPE Prod. Oper. 2012, 27, 404–413. [Google Scholar] [CrossRef]

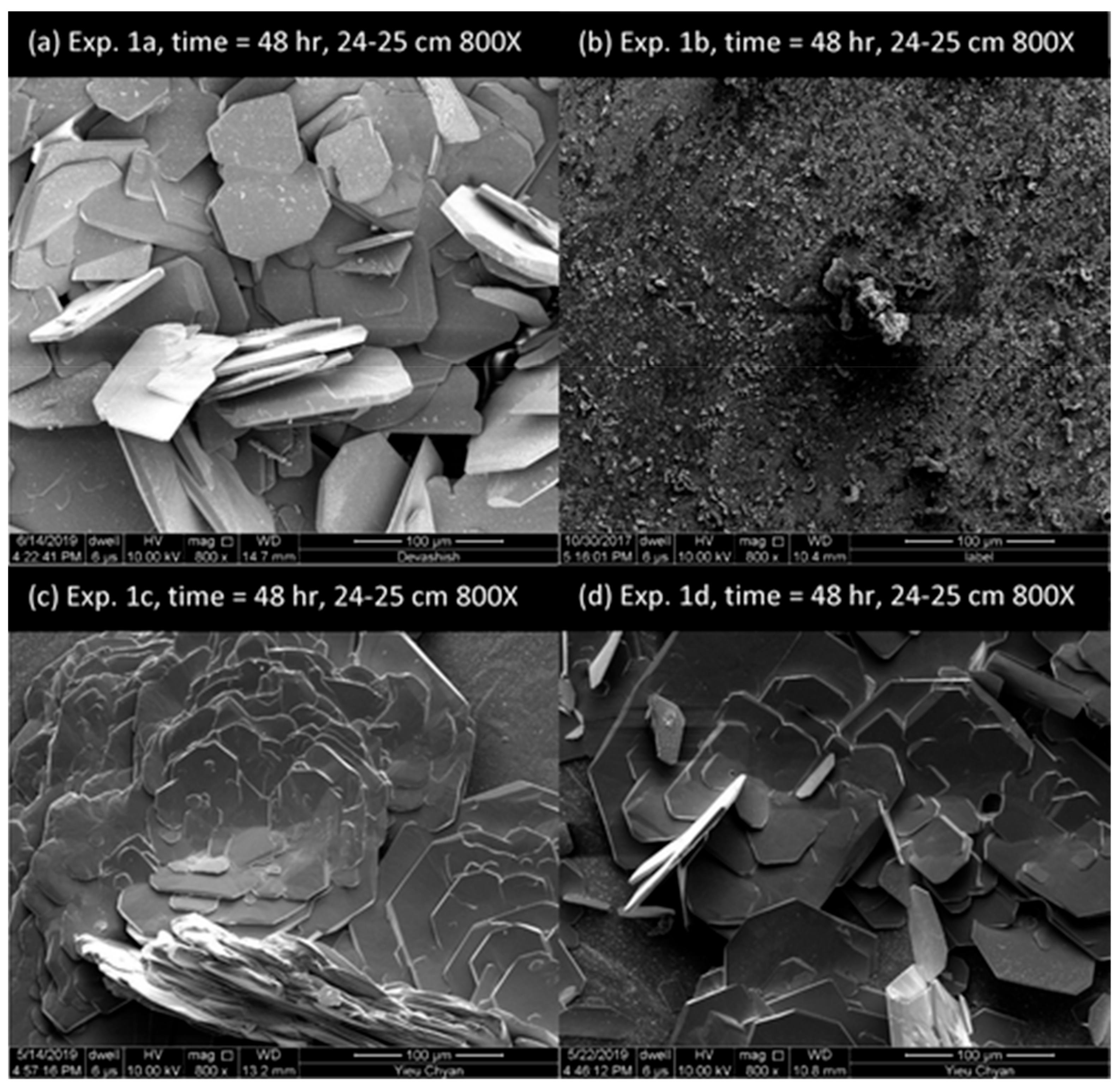


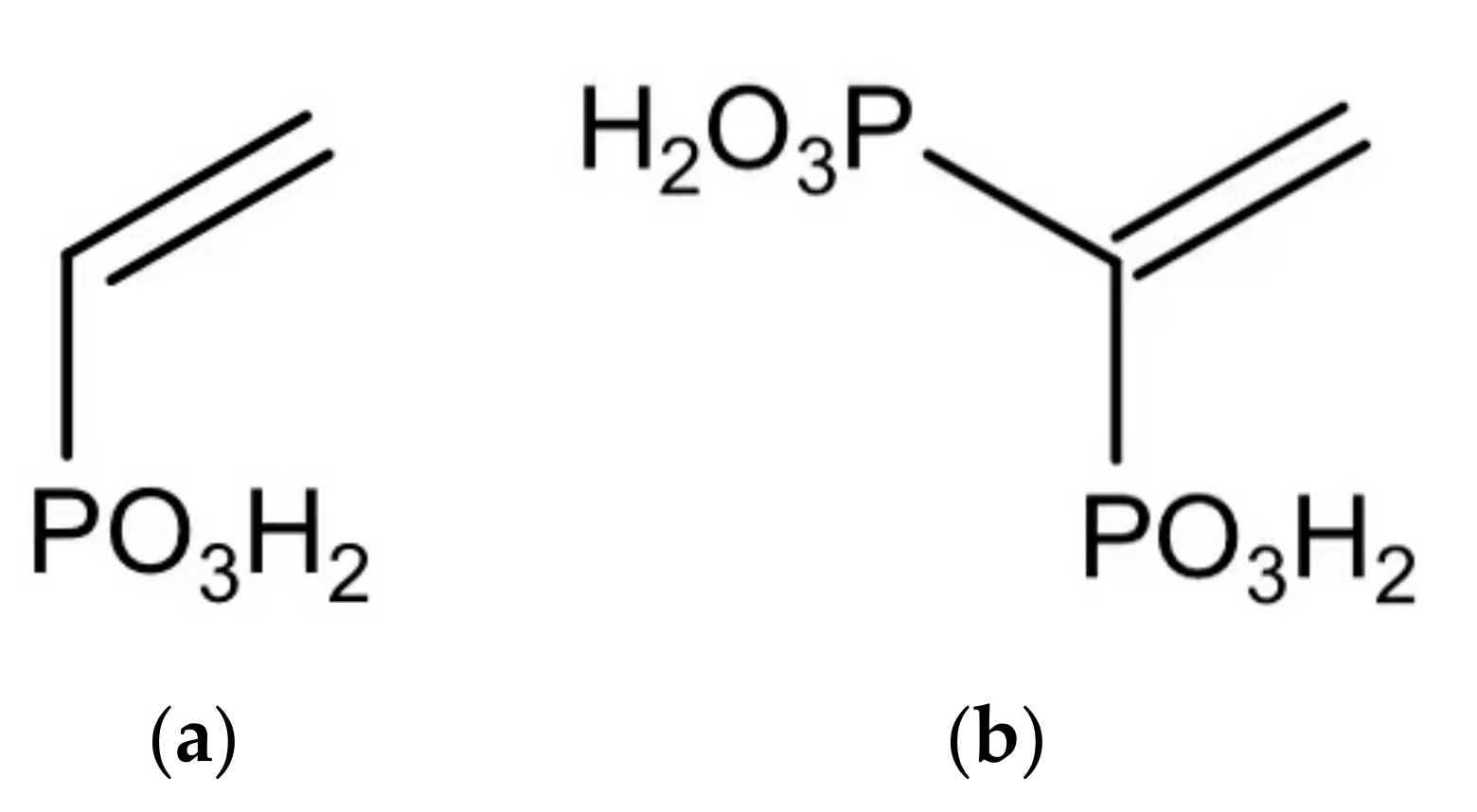
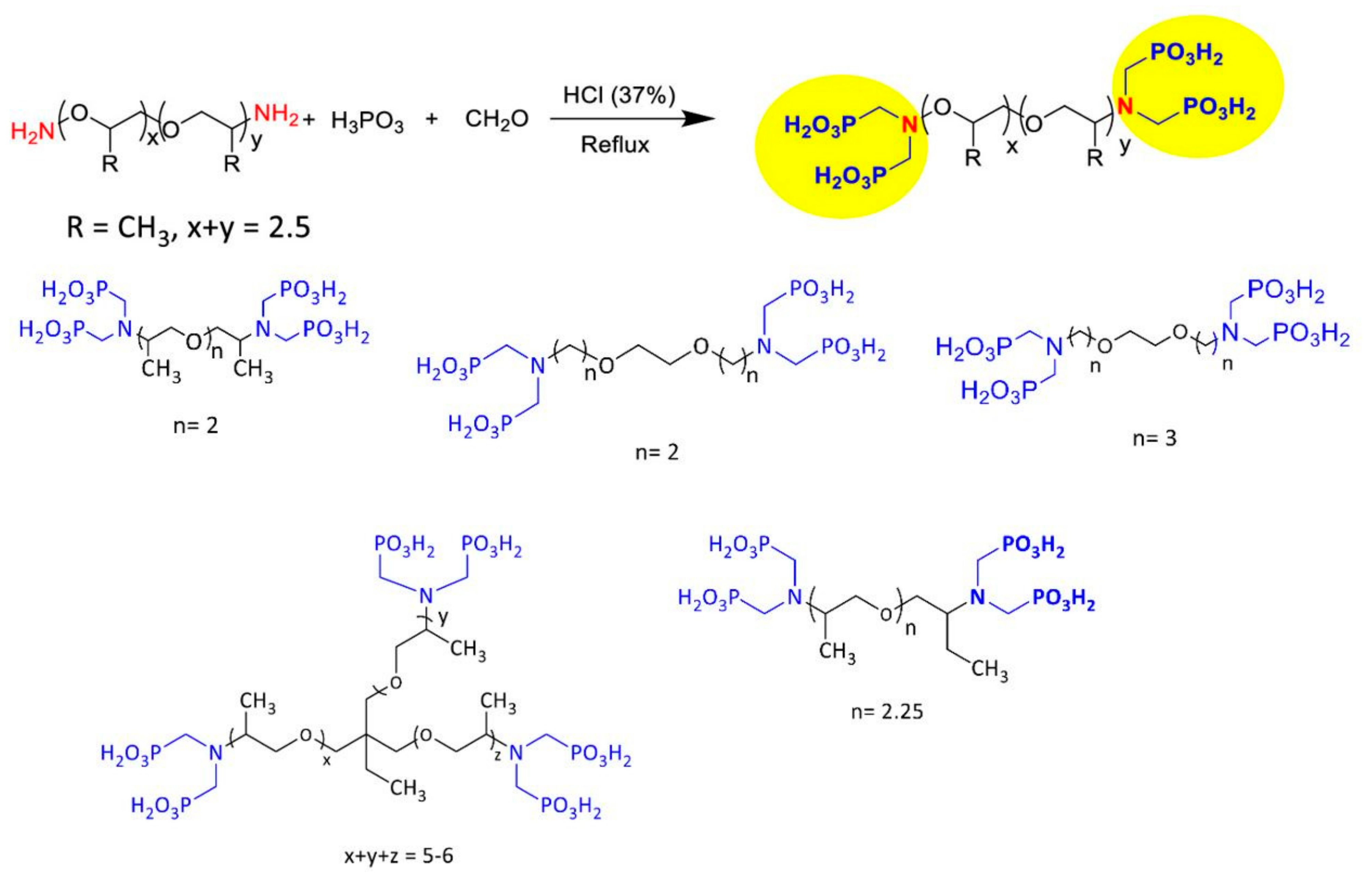
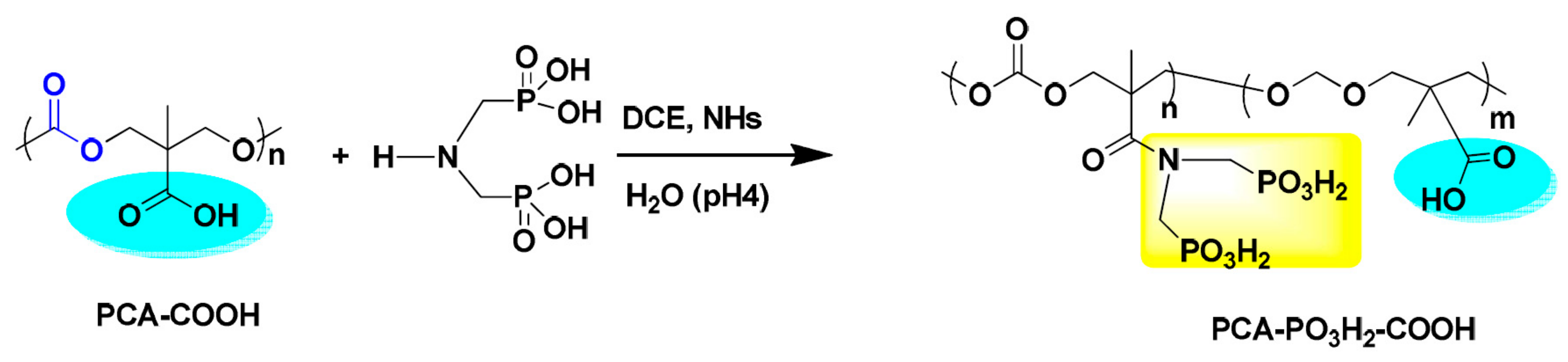




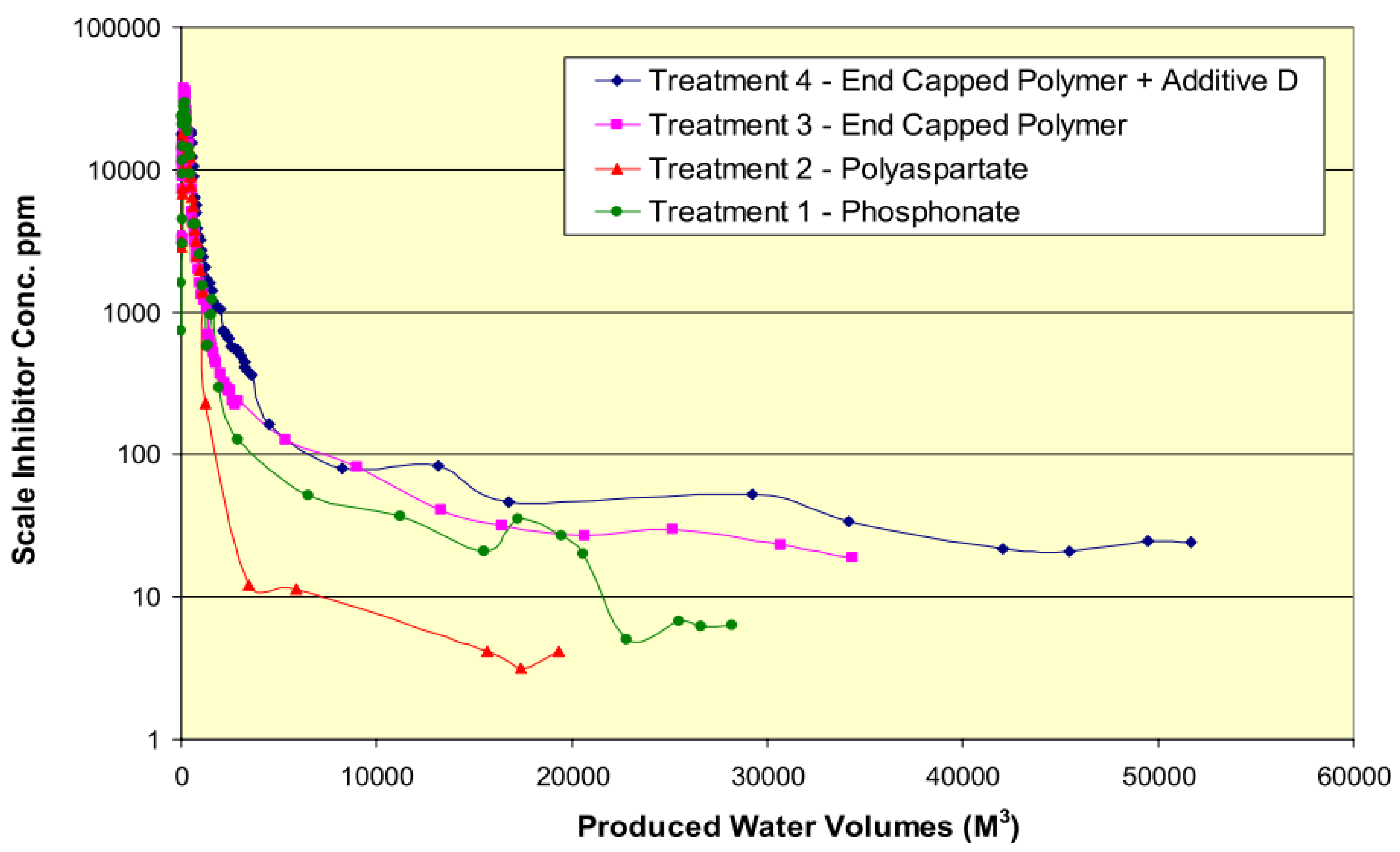
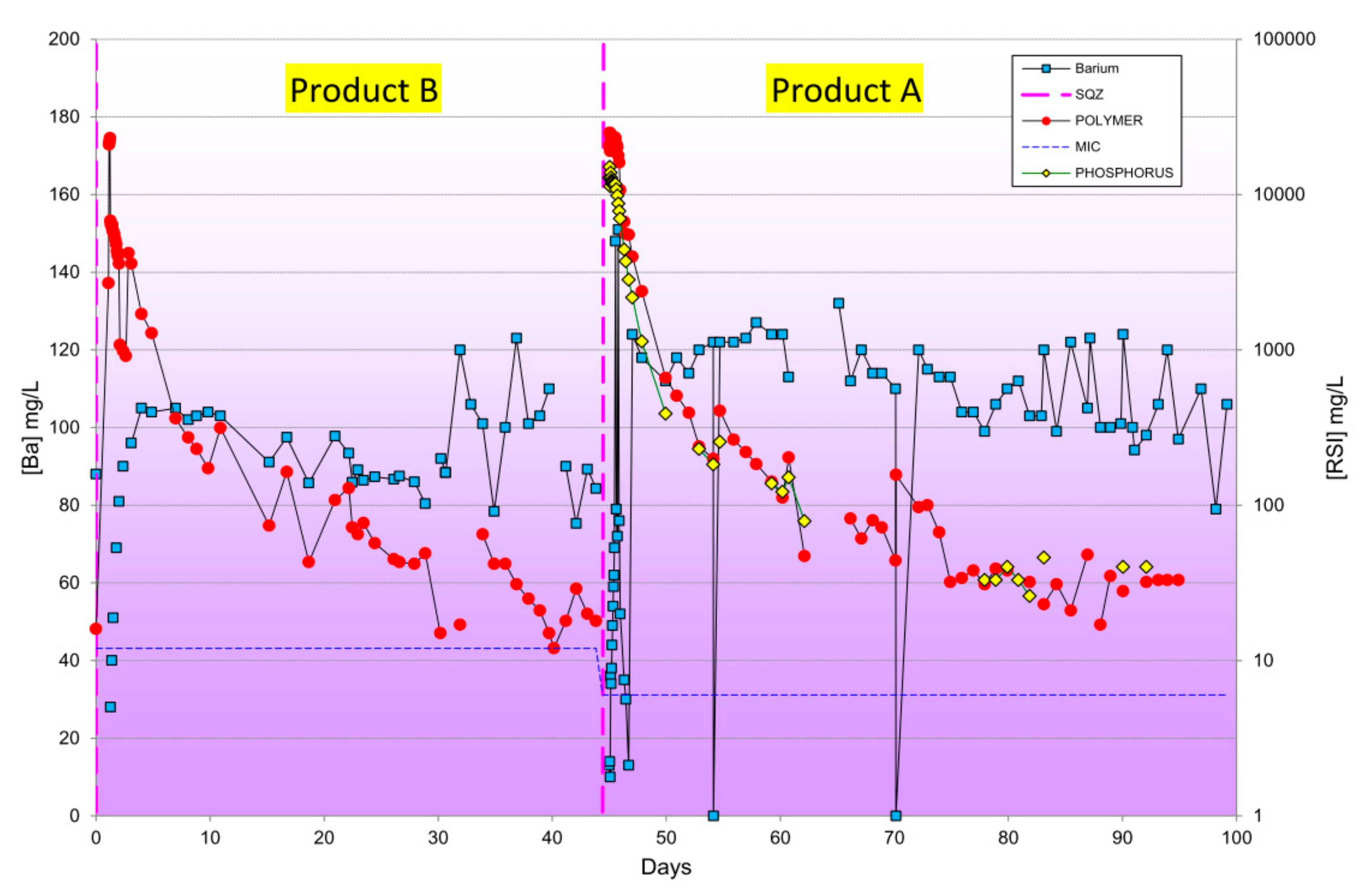
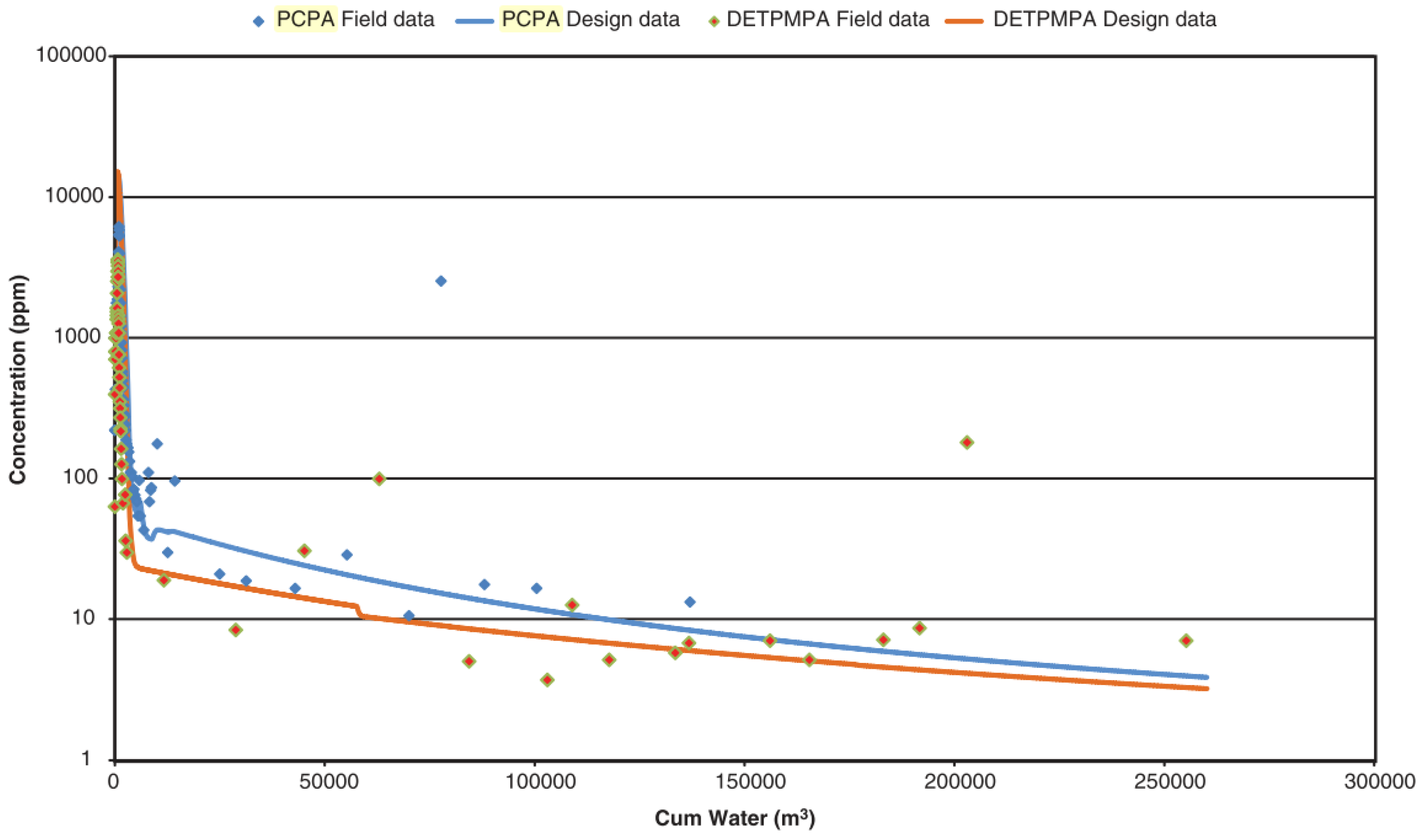
| Type | Scale Inhibitor | Advantage | Disadvantage |
|---|---|---|---|
| Polyphosphates | Sodium tripolyphosphate, Sodium hexametaphosphate | Effective scale and corrosion inhibition | Low solubility; lower thermal stabilities than phosphonates; Easily hydrolysed into orthophosphates to form insoluble calcium salts |
| Phosphonates | BHPMP, DTPMP, HEDP, NTMP, and PBTC | Good scale and corrosion inhibition; Good adsorption | Poor biodegradability; Less thermally stable than polymeric species; Poor compatibilities with the production system |
| Phosphino-polycarboxylic acid | PPCA | Excellent calcium carbonate and calcium sulfate inhibition; Great barium sulfate inhibitor; High calcium tolerance and halogen resistant; Hydrothermally stable | Less adsorption than phosphonates |
| Polyacrylates | PAA | Good scale inhibition; Good dispersion | Poor biodegradation; Poor adsorption |
| Polymaleates | PMA | Fairly biodegradable | Poor adsorption |
| Polyepoxysuccinic acid | PESA | Highly biodegradable; Good corrosion inhibition | |
| Polyaspartates | PASP | Highly biodegradable |
| Phosphorus-Based Polymers | Abbreviation | Mol. Mass (Dalton) | Structure |
|---|---|---|---|
| Polyamino polyether methylene phosphonic acid | PAPEMP | 600 | 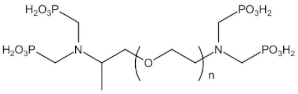 |
| Phosphono carboxylic acid | POCA | 2000 | 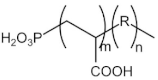 |
| Phosphino-polycarboxylic acid | PPCA | 3800 | 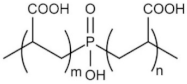 |
| Maleic acid–sodium q-styrenesulfonate copolymer | MAc-SS | 1.86 × 105 |  |
| N-phosphonomethylated amino-2-hydroxypropylene polymer | PMPA | 300–5000 |  |
| Structure | Notes |
|---|---|
 | wherein the scale inhibitor comprises a phosphonic acid terminated polymer according to formula [104]. |
 | wherein X corresponds to H or an anion, and x + y is an integer between 2 and 500 [104]. |
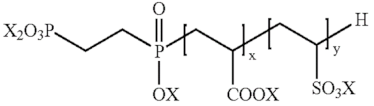 | wherein X denotes H or an anion, and x + y is an integer between 2 and 500. [104] |
 | [97] |
 | wherein R represents H or PO3H2, and m, n, and p can be either zeroor any number [58]. |
| Phosphorus-Based Polymer Inhibitor | Efficiency | Toxicity a | Other Properties |
|---|---|---|---|
| Phosphino poly carboxylic acid (PPCA) [95] | Complete gypsum inhibition at SI = 0.31 at any pH and T; 24% inhibition with NaCl at an extremely high SI of 1.47 | N.A. b | N.A. b |
| P-tagged copolymer [97] | 98.2% inhibition effect on CaCO3 with 16 ppm inhibitor at 80 °C for 10 h | Environmentally safe | N.A. b |
| P-tagged copolymer [100] | Greater inhibition performance than DTPMP and sulphonated copolymer | N.A. b | Excellent thermal stability; Comparable Ca tolerance relative to standard sulphonated copolymer |
| P-tagged copolymer [102] | Similar inhibition performance with the best conventional polymeric scale inhibitor under extreme conditions | N.A. b | Enhancement of adsorption property; Stable at least 200 °C; 30% biodegradation in 28 days in seawater by OECD306 test |
| Phosphonated polyetheramines [108] | Improved inhibition performance against both calcite and barite compared to common commercial phosphonated inhibitors | Environmentally friendly | Superior calcium tolerance; good thermal stability at 130 °C 47% biodegradation in 28 days in seawater by OECD306 test |
| Phosphonated aliphatic polycarbonates [109] | Better inhibition performance against calcite and barite compared to carboxylated homopolymer | Environmentally friendly | Enhancement of water thermal stability; 36% biodegradation in 28 days in seawater by OECD306 test |
| Phosphonated polyaspartic acid [44] | Excellent calcite scale inhibition property under high pressure high temperature conditions | Environmentally friendly | Desirable thermal stability under harsh oilfield conditions (at 130 °C for 7 days) compared to PASP and other modified compounds; Calcium tolerance ability with Ca2+ ions up to 100 mg L−1 |
| Grafted copolymer [117,118] | Able to control multiple scales in any process field, in particular under harsh conditions; Poor inhibition performance with brines in high scaling tendency systems | N.A. b | N.A. b |
| Dendrimeric or hyperbranched polymers [123] | 99% CaCO3 and 97% CaSO4 inhibition, respectively at 20 mg L−1; ca. 73% corrosion inhibition efficiency at 150 mg L−1 | Environmentally friendly | N.A. b |
| Other terpolymer [128] | 89.2% CaCO3 inhibition at 21 mg L−1; 92.4% CaSO4 inhibition at the 3 mg L−1 | N.A. b | Good hydrolytic stability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, P. Review of Phosphorus-Based Polymers for Mineral Scale and Corrosion Control in Oilfield. Polymers 2022, 14, 2673. https://doi.org/10.3390/polym14132673
Liu Y, Zhang P. Review of Phosphorus-Based Polymers for Mineral Scale and Corrosion Control in Oilfield. Polymers. 2022; 14(13):2673. https://doi.org/10.3390/polym14132673
Chicago/Turabian StyleLiu, Yuan, and Ping Zhang. 2022. "Review of Phosphorus-Based Polymers for Mineral Scale and Corrosion Control in Oilfield" Polymers 14, no. 13: 2673. https://doi.org/10.3390/polym14132673
APA StyleLiu, Y., & Zhang, P. (2022). Review of Phosphorus-Based Polymers for Mineral Scale and Corrosion Control in Oilfield. Polymers, 14(13), 2673. https://doi.org/10.3390/polym14132673






