Honeycomb-Structured Porous Films from Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Physicochemical Characterization and Mesenchymal Stem Cells Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Synthesis
2.2. Preparation of Honeycomb-Patterned Films
2.3. Preparation of Patterned Films on Stamps
2.4. Characterization of Patterned Films
2.5. Differential Scanning Calorimetry
2.6. Cells
2.7. SEM Imaging
2.8. Statistical Analysis
3. Results
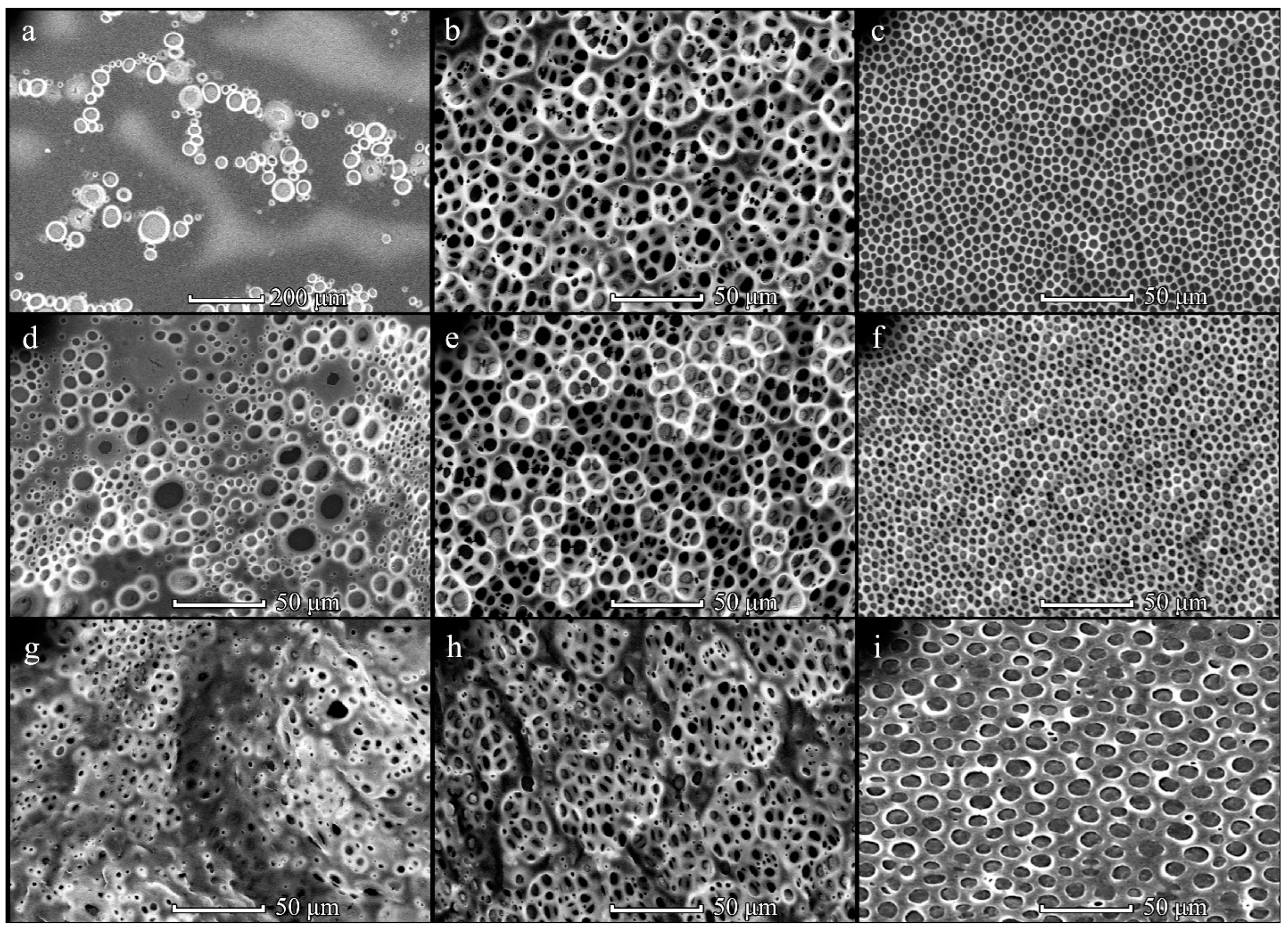
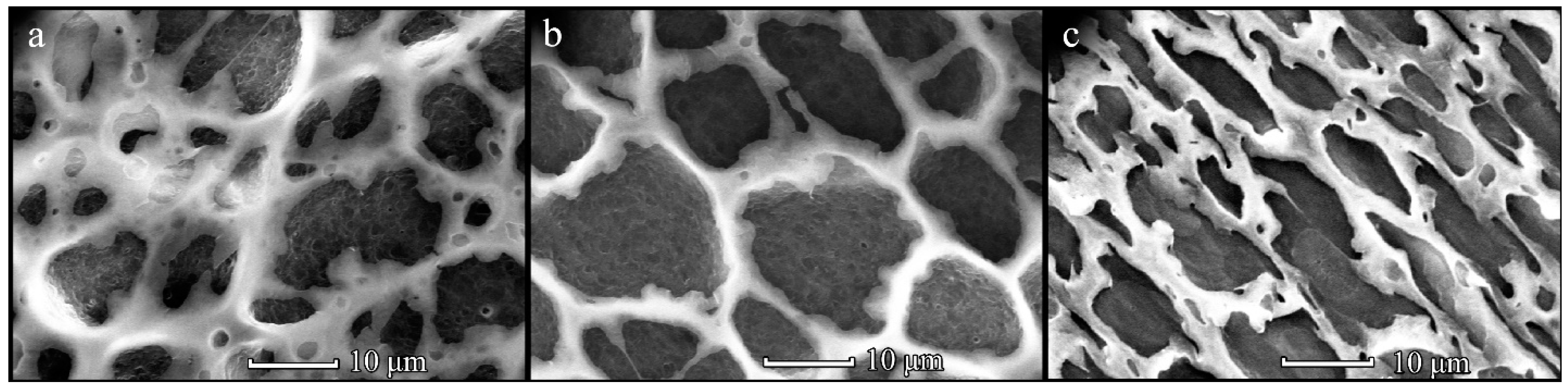
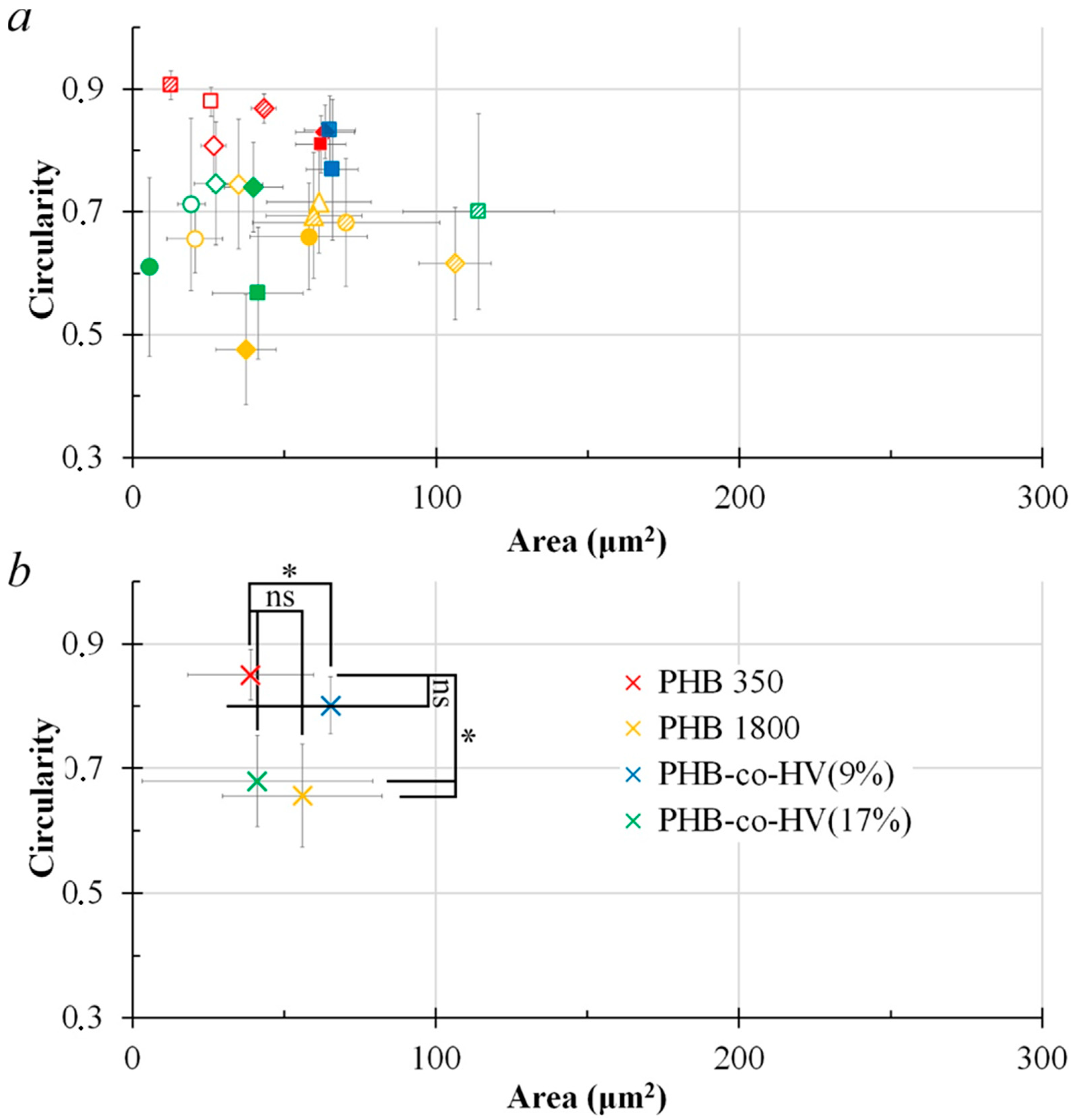
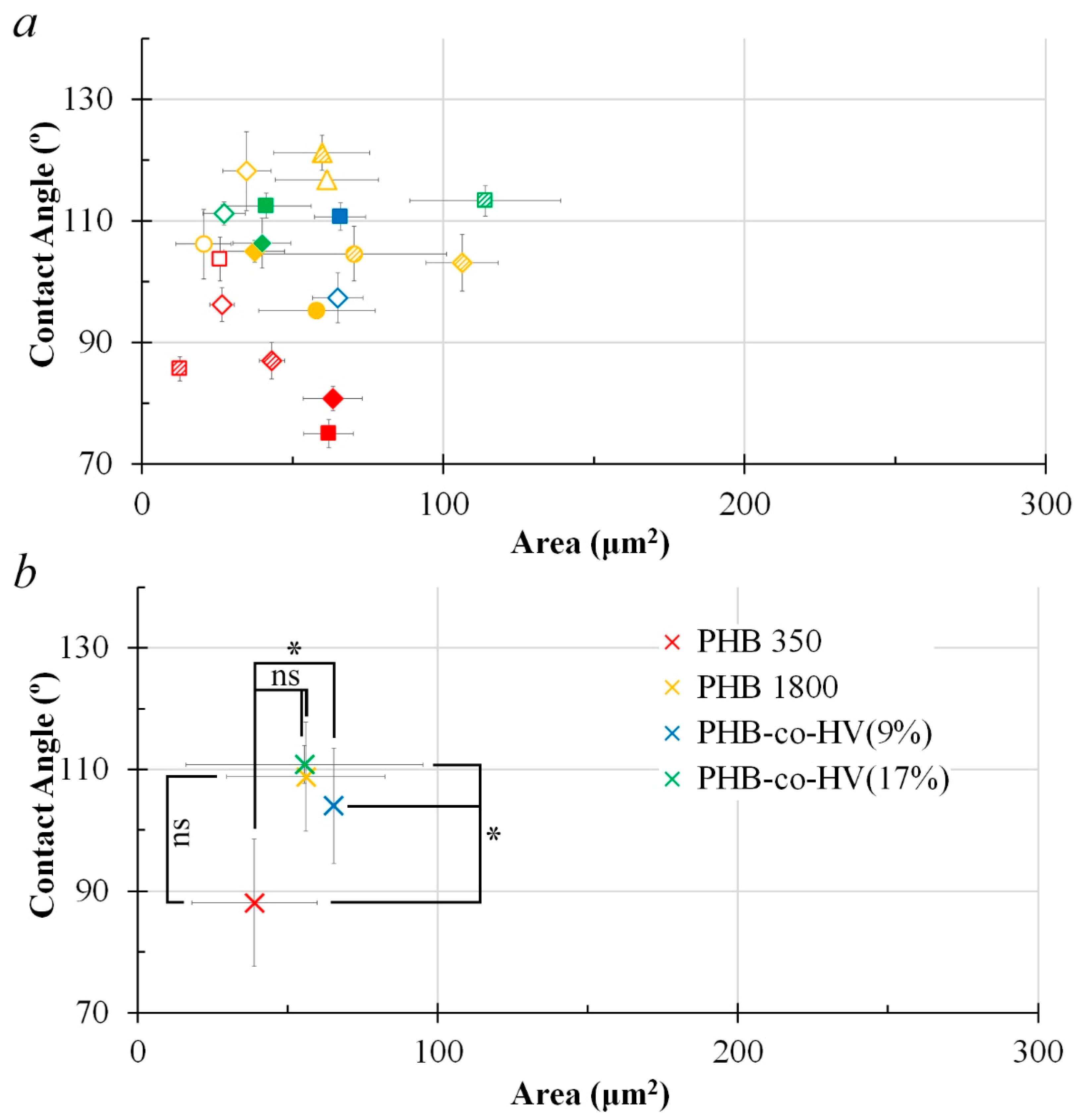
3.1. Formation of PHB Honeycomb-Patterned Films
3.2. Formation of PHB-co-HV Honeycomb-Patterned Films
3.3. Comparison of Polymer Films
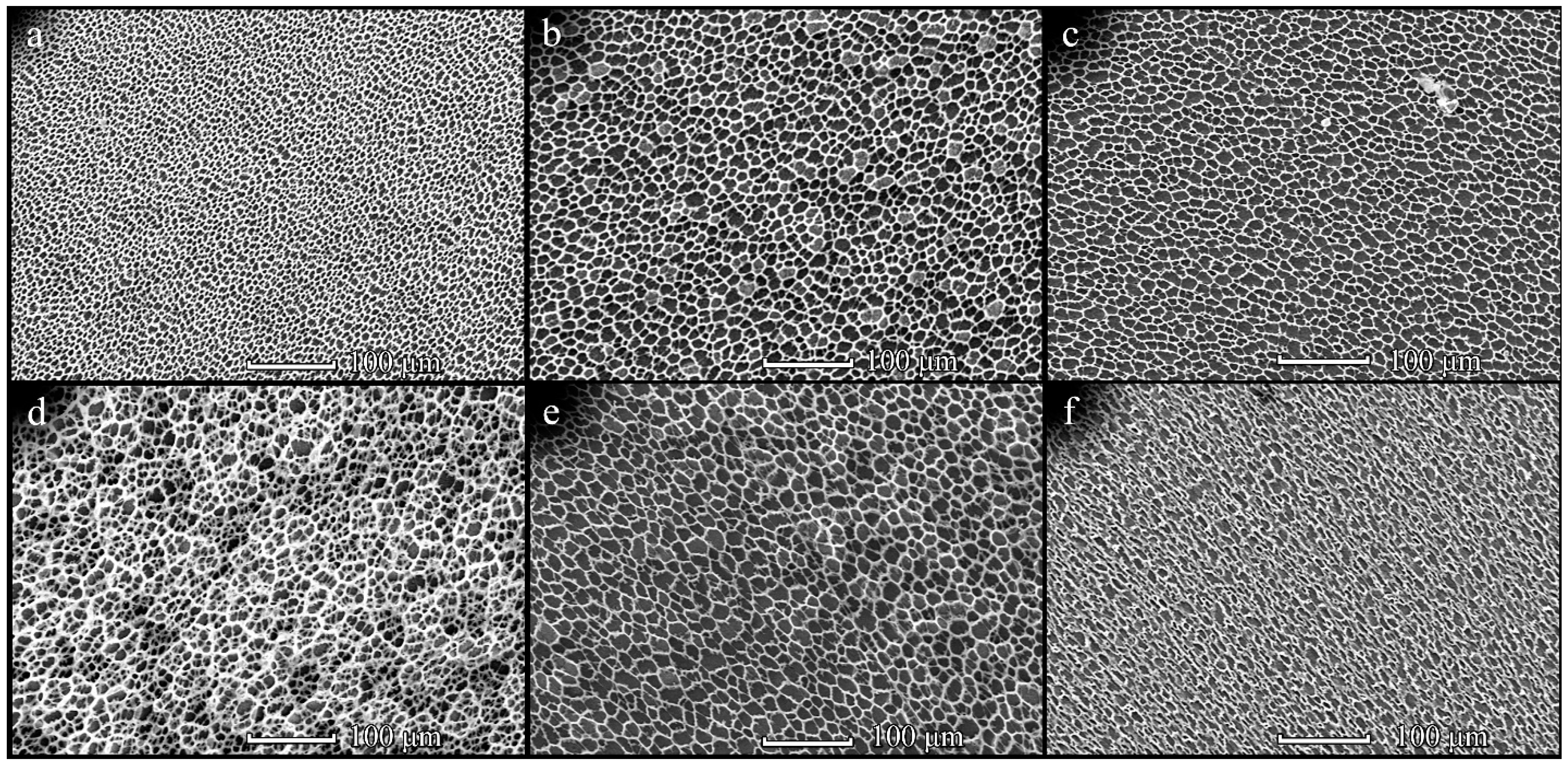
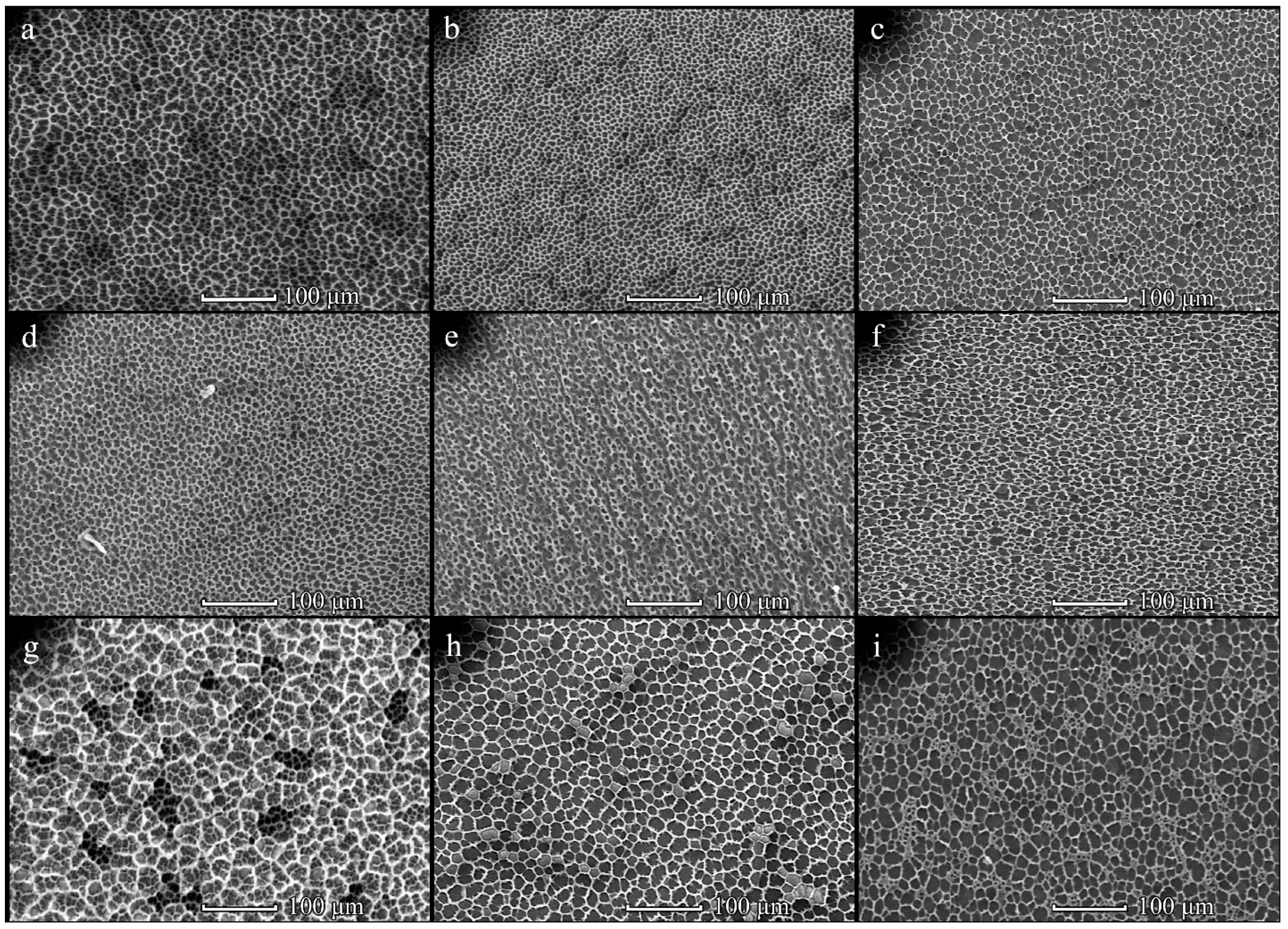
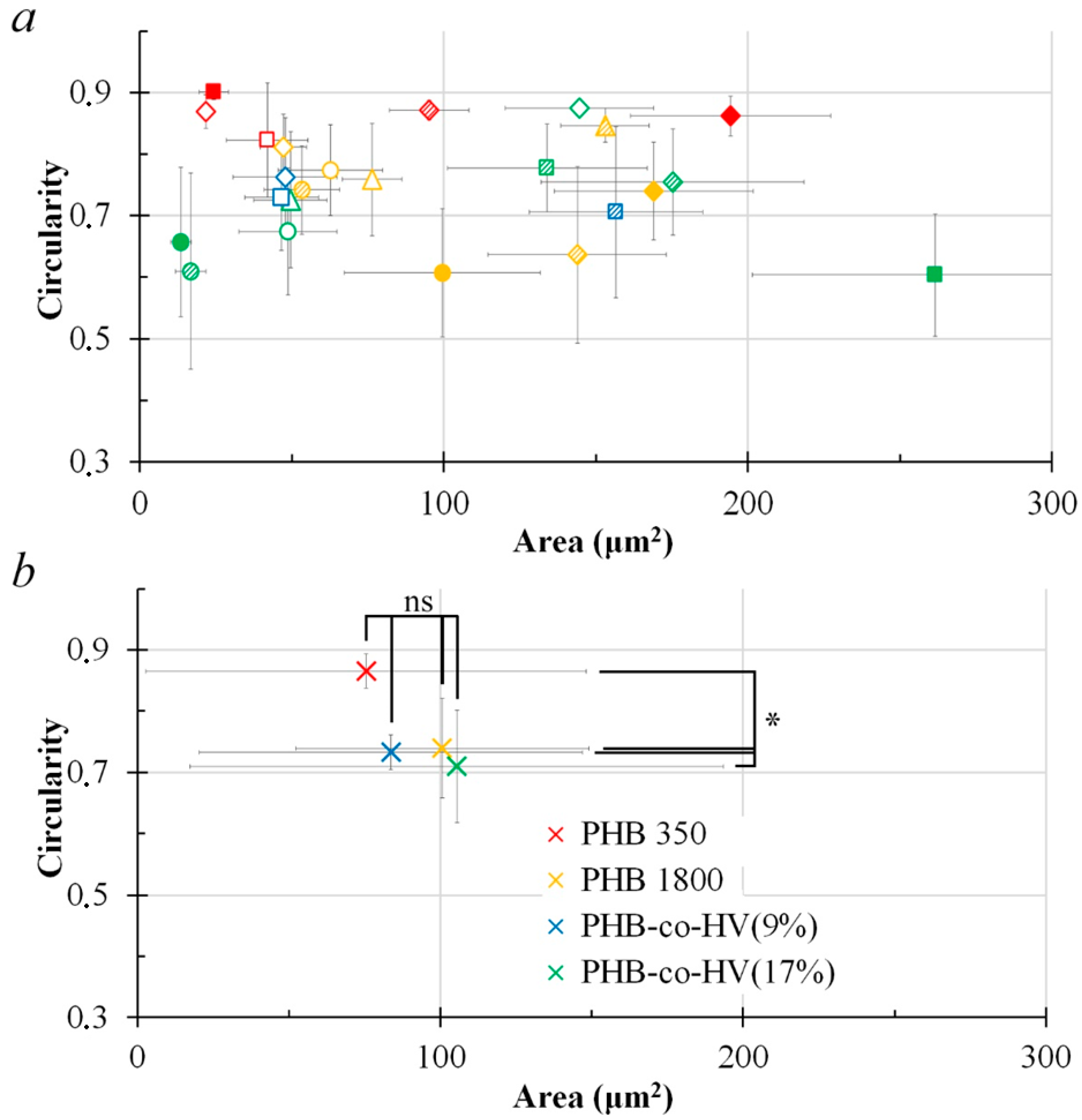
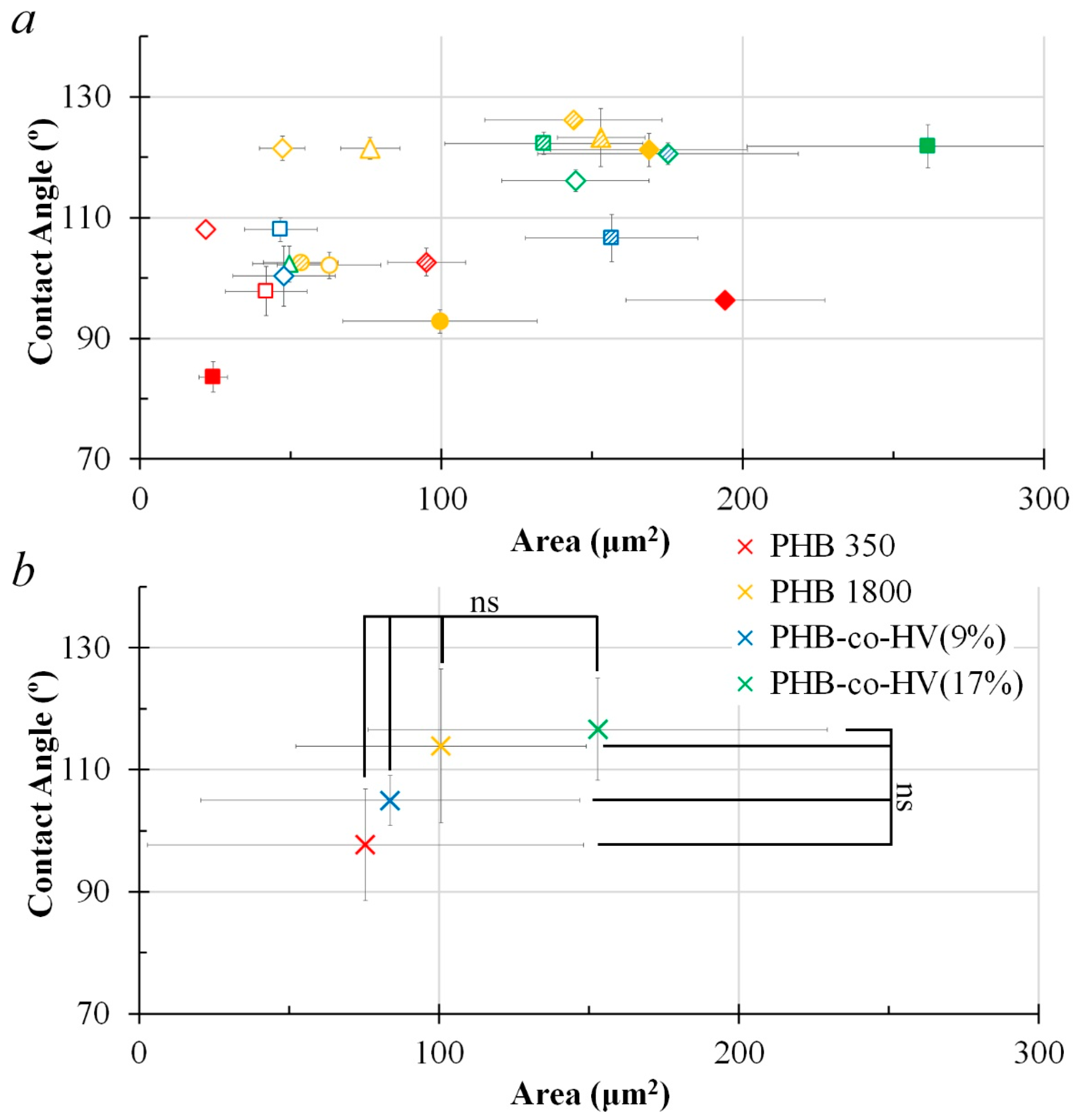
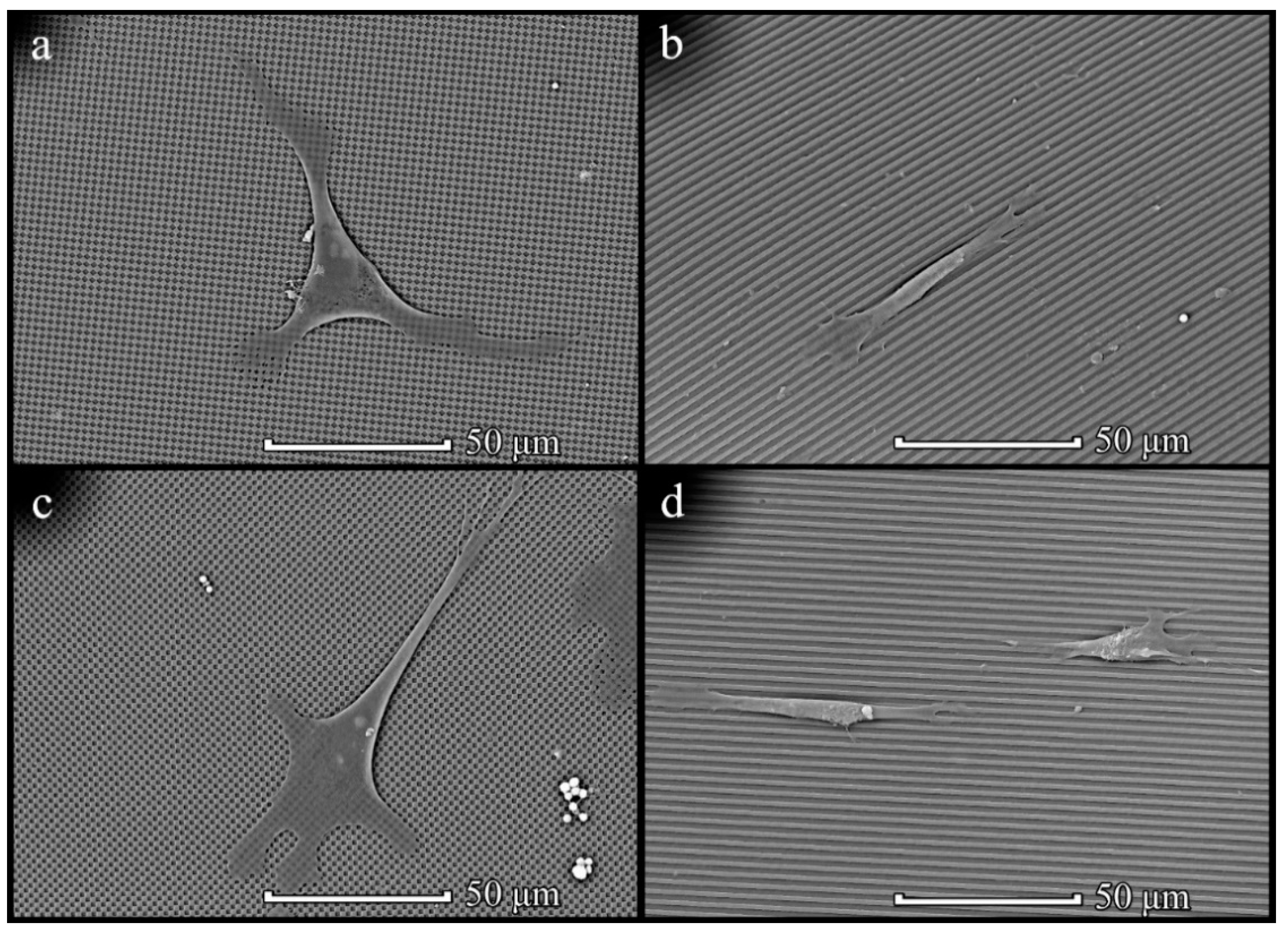
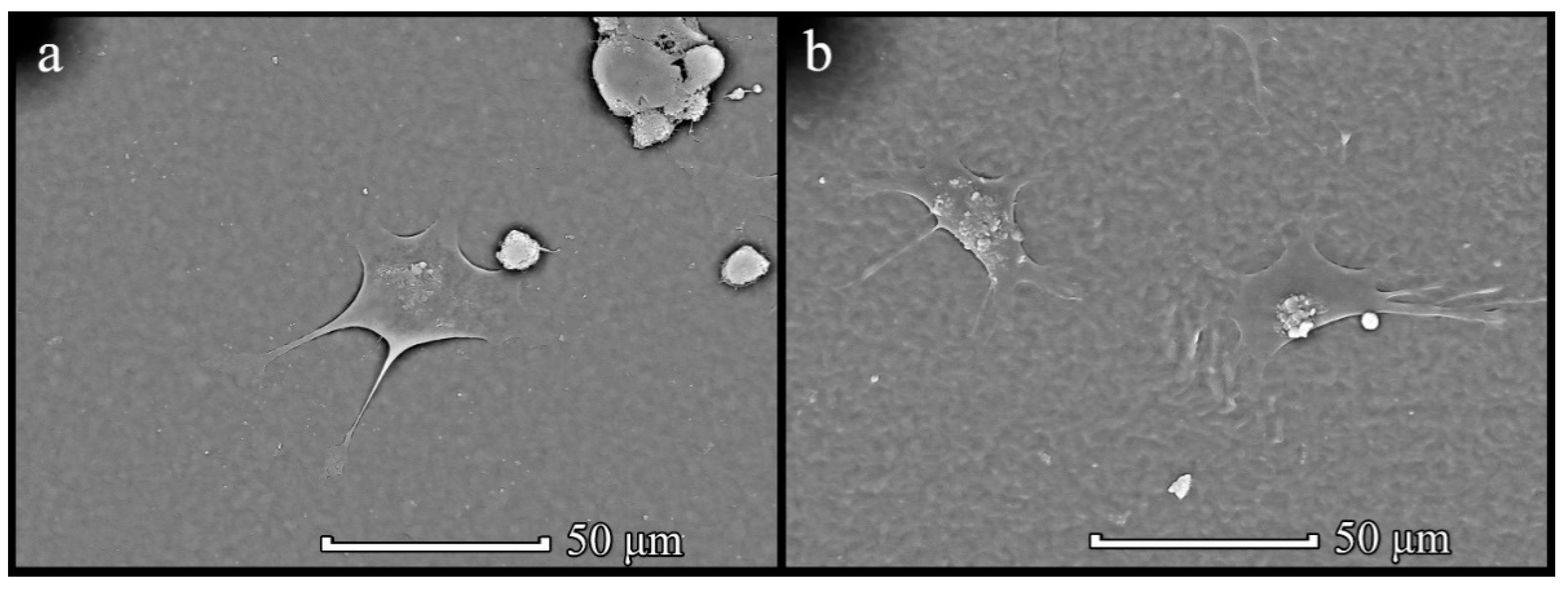
3.4. Cells on Patterned Films Formed on Stamps
3.5. Cells on Honeycomb Films
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Yan, C.; Zheng, Z. Functional Polymer Surfaces for Controlling Cell Behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Calejo, M.T.; Ilmarinen, T.; Skottman, H.; Kellomäki, M. Breath Figures in Tissue Engineering and Drug Delivery: State-of-the-Art and Future Perspectives. Acta Biomater. 2018, 66, 44–66. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L.; Li, X.; Zhou, C.; Li, B. Three-Dimensional Honeycomb-Patterned Chitosan/Poly(L-Lactic Acid) Scaffolds with Improved Mechanical and Cell Compatibility. J. Biomed. Mater. Res. Part A 2011, 98A, 434–441. [Google Scholar] [CrossRef]
- Cheng, K.-Y.; Chang, C.-H.; Yang, Y.-W.; Liao, G.-C.; Liu, C.-T.; Wu, J.-S. Enhancement of Cell Growth on Honeycomb-Structured Polylactide Surface Using Atmospheric-Pressure Plasma Jet Modification. Appl. Surf. Sci. 2017, 394, 534–542. [Google Scholar] [CrossRef]
- Foldberg, S.; Petersen, M.; Fojan, P.; Gurevich, L.; Fink, T.; Pennisi, C.P.; Zachar, V. Patterned Poly(Lactic Acid) Films Support Growth and Spontaneous Multilineage Gene Expression of Adipose-Derived Stem Cells. Colloids Surf. B Biointerfaces 2012, 93, 92–99. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Li, J.; Zhang, A.; Liu, X.; Xu, B.; Gao, S.; Jin, G.; Ma, Z. Robust and Hydrophilic Polymeric Films with Honeycomb Pattern and Their Cell Scaffold Applications. J. Mater. Chem. 2009, 19, 2789. [Google Scholar] [CrossRef]
- Tsuruma, A.; Tanaka, M.; Yamamoto, S.; Shimomura, M. Control of Neural Stem Cell Differentiation on Honeycomb Films. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 536–540. [Google Scholar] [CrossRef]
- Kawano, T.; Sato, M.; Yabu, H.; Shimomura, M. Honeycomb-Shaped Surface Topography Induces Differentiation of Human Mesenchymal Stem Cells (HMSCs): Uniform Porous Polymer Scaffolds Prepared by the Breath Figure Technique. Biomater. Sci. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The Control of Human Mesenchymal Cell Differentiation Using Nanoscale Symmetry and Disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Sunami, H.; Ito, E.; Tanaka, M.; Yamamoto, S.; Shimomura, M. Effect of Honeycomb Film on Protein Adsorption, Cell Adhesion and Proliferation. Colloids Surf. A Physicochem. Eng. Asp. 2006, 284–285, 548–551. [Google Scholar] [CrossRef] [Green Version]
- Beattie, D.; Wong, K.H.; Williams, C.; Poole-Warren, L.A.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Honeycomb-Structured Porous Films from Polypyrrole-Containing Block Copolymers Prepared via RAFT Polymerization as a Scaffold for Cell Growth. Biomacromolecules 2006, 7, 1072–1082. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nonomura, M.; Arai, K.; Hayashi, J.; Sawadaishi, T.; Nishiura, Y.; Hara, M.; Shimomura, M. Micropatterns Based on Deformation of a Viscoelastic Honeycomb Mesh. Langmuir 2003, 19, 6193–6201. [Google Scholar] [CrossRef]
- Wei, S.; Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Tang, Z.; Wei, J. Effect of Surface Roughness on Osteogenesis In Vitro and Osseointegration in Vivo of Carbon Fiber-Reinforced Polyetheretherketone–Nanohydroxyapatite Composite. Int. J. Nanomed. 2015, 10, 1425–1427. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Boehm, C.A.; Mata, A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. Post Microtextures Accelerate Cell Proliferation and Osteogenesis. Acta Biomater. 2010, 6, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Koh, L.B.; Rodriguez, I.; Venkatraman, S.S. The Effect of Topography of Polymer Surfaces on Platelet Adhesion. Biomaterials 2010, 31, 1533–1545. [Google Scholar] [CrossRef]
- Widawski, G.; Rawiso, M.; François, B. Self-Organized Honeycomb Morphology of Star-Polymer Polystyrene Films. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- François, B.; Pitois, O.; François, J. Polymer Films with a Self-Organized Honeycomb Morphology. Adv. Mater. 1995, 7, 1041–1044. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, Y.; Yan, D.; Mai, Y.; He, L.; Jin, C. Honeycomb-Structured Microporous Films Made from Hyperbranched Polymers by the Breath Figure Method. Langmuir 2009, 25, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Tokaruk, W.A.; Molteno, T.C.A.; Morris, S.W. Bénard-Marangoni Convection in Two-Layered Liquids. Phys. Rev. Lett. 2000, 84, 3590–3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.H.; Hernández-Guerrero, M.; Granville, A.M.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Water-Assisted Formation of Honeycomb Structured Porous Films. J. Porous Mater. 2006, 13, 213–223. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Shaitan, K.V.; Kirpichnikov, M.P. Application of Polyhydroxyalkanoates in Medicine and the Biological Activity of Natural Poly(3-Hydroxybutyrate). Acta Naturae 2019, 11, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative Structure-Property Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s Films under Hydrolytic and Enzymatic Degradation: Finding a Transition Point in 3-Hydroxyvalerate Content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The Growth of 3T3 Fibroblasts on PHB, PLA and PHB/PLA Blend Films at Different Stages of Their Biodegradation In Vitro. Polymers 2020, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Mahalingam, S.; Edirisinghe, M. Creating “Hotels” for Cells by Electrospinning Honeycomb-like Polymeric Structures. Mater. Sci. Eng. C 2013, 33, 4384–4391. [Google Scholar] [CrossRef]
- Kong, L.; Dong, R.; Ma, H.; Hao, J. Au NP Honeycomb-Patterned Films with Controllable Pore Size and Their Surface-Enhanced Raman Scattering. Langmuir 2013, 29, 4235–4241. [Google Scholar] [CrossRef]
- Mir, S.H.; Ochiai, B. Conductive Polymer-Ag Honeycomb Thin Film: The Factors Affecting the Complexity of the Microstructure. J. Electrochem. Soc. 2018, 165, B3030–B3034. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Takebayashi, M.; Shimomura, M. Fabrication of Ordered Arrays of Biodegradable Polymer Pincushions Using Self-Organized Honeycomb-Patterned Films. Macromol. Symp. 2009, 279, 175–182. [Google Scholar] [CrossRef]
- Shadrina, V.I.; Bashmakov, I.A.; Gracheva, E.A.; Agabekov, V.E.; Kaputskii, F.N. Influence of the Conditions of Formation of Nitrocellulose Honeycomb-Structure Films on Their Morphology. Russ. J. Appl. Chem. 2010, 83, 1318–1323. [Google Scholar] [CrossRef]
- Peng, J.; Han, Y.; Yang, Y.; Li, B. The Influencing Factors on the Macroporous Formation in Polymer Films by Water Droplet Templating. Polymer 2004, 45, 447–452. [Google Scholar] [CrossRef]
- Kulikouskaya, V.; Chyshankou, I.; Pinchuk, S.; Vasilevich, I.; Volotovski, I.; Agabekov, V. Fabrication and Characterization of Ultrathin Spin-Coated Poly(L-Lactic Acid) Films Suitable for Cell Attachment and Curcumin Loading. Biomed. Mater. 2020, 15, 065022. [Google Scholar] [CrossRef]
- Galeotti, F.; Calabrese, V.; Cavazzini, M.; Quici, S.; Poleunis, C.; Yunus, S.; Bolognesi, A. Self-Functionalizing Polymer Film Surfaces Assisted by Specific Polystyrene End-Tagging. Chem. Mater. 2010, 22, 2764–2769. [Google Scholar] [CrossRef]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological Responses to Physicochemical Properties of Biomaterial Surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Mizuno, M.; Fujisawa, R.; Katano, H.; Endo, K.; Ozeki, N.; Sakamaki, Y.; Koga, H.; Sekiya, I. Optimal Pore Size of Honeycomb Polylactic Acid Films for In Vitro Cartilage Formation by Synovial Mesenchymal Stem Cells. Stem Cells Int. 2021, 2021, 9239728. [Google Scholar] [CrossRef] [PubMed]



| Initial Films before Incubation/Cell Culture | Films after Incubation in Culture Medium without Cells for 1 Week | Films after 1 Week of Cell Growth on Their Surface | ||||
|---|---|---|---|---|---|---|
| Polymer Type | Melting Point, °C | Degree of Crystallinity, % | Melting Point, °C | Degree of Crystallinity, % | Melting Point, °C | Degree of Crystallinity, % |
| 1 | 174 | 61.0 | 166 | 62.3 | 174 | 66.2 |
| 2 | 164 | 56.0 | 162 | 62.3 | 176 | 65.8 |
| 3 | 166 | 67.2 | 176 | 66.2 | 180 | 70.6 |
| 4 | 162/170 | 66.6 | 178 | 63.5 | 180 | 74.9 |
| 5 | 170 | 63.2 | 176 | 63.8 | 176 | 68.4 |
| 6 | 174 | 61.0 | 165 | 54.9 | 160 | 59.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikouskaya, V.I.; Nikalaichuk, V.V.; Bonartsev, A.P.; Akoulina, E.A.; Belishev, N.V.; Demianova, I.V.; Chesnokova, D.V.; Makhina, T.K.; Bonartseva, G.A.; Shaitan, K.V.; et al. Honeycomb-Structured Porous Films from Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Physicochemical Characterization and Mesenchymal Stem Cells Behavior. Polymers 2022, 14, 2671. https://doi.org/10.3390/polym14132671
Kulikouskaya VI, Nikalaichuk VV, Bonartsev AP, Akoulina EA, Belishev NV, Demianova IV, Chesnokova DV, Makhina TK, Bonartseva GA, Shaitan KV, et al. Honeycomb-Structured Porous Films from Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Physicochemical Characterization and Mesenchymal Stem Cells Behavior. Polymers. 2022; 14(13):2671. https://doi.org/10.3390/polym14132671
Chicago/Turabian StyleKulikouskaya, Viktoryia I., Viktoryia V. Nikalaichuk, Anton P. Bonartsev, Elizaveta A. Akoulina, Nikita V. Belishev, Irina V. Demianova, Dariana V. Chesnokova, Tatiana K. Makhina, Garina A. Bonartseva, Konstantin V. Shaitan, and et al. 2022. "Honeycomb-Structured Porous Films from Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Physicochemical Characterization and Mesenchymal Stem Cells Behavior" Polymers 14, no. 13: 2671. https://doi.org/10.3390/polym14132671
APA StyleKulikouskaya, V. I., Nikalaichuk, V. V., Bonartsev, A. P., Akoulina, E. A., Belishev, N. V., Demianova, I. V., Chesnokova, D. V., Makhina, T. K., Bonartseva, G. A., Shaitan, K. V., Hileuskaya, K. S., & Voinova, V. V. (2022). Honeycomb-Structured Porous Films from Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Physicochemical Characterization and Mesenchymal Stem Cells Behavior. Polymers, 14(13), 2671. https://doi.org/10.3390/polym14132671







