Application of Fiber Biochar–MOF Matrix Composites in Electrochemical Energy Storage
Abstract
:1. Introduction
2. Experimental
2.1. Material Synthesis
2.1.1. Preparation of Biochar
2.1.2. Preparation of ZIF-67
2.1.3. Fiber Biochar and ZIF-67 Composite
2.1.4. Preparation of High-Temperature Charred ZIF-67 Material
2.2. Material Characterization
2.3. Electrochemical Performance Test
3. Results and Discussion
3.1. Properties of Charcoal-ZIF-67 Composite Material
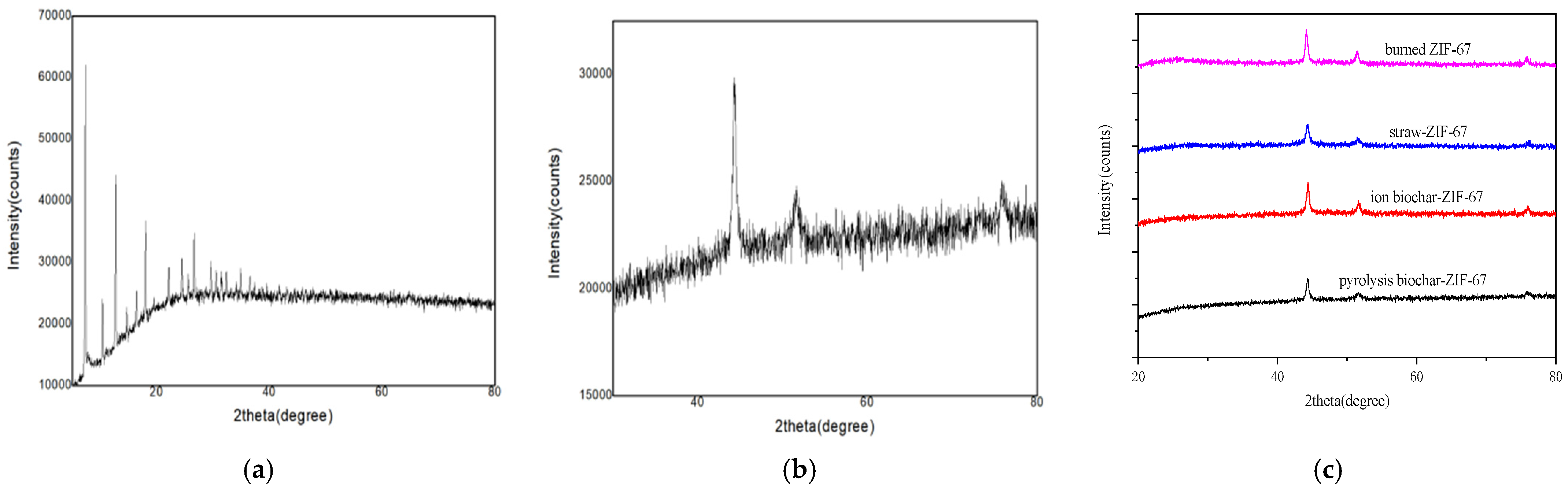
3.1.1. X-ray Powder Diffraction Studies
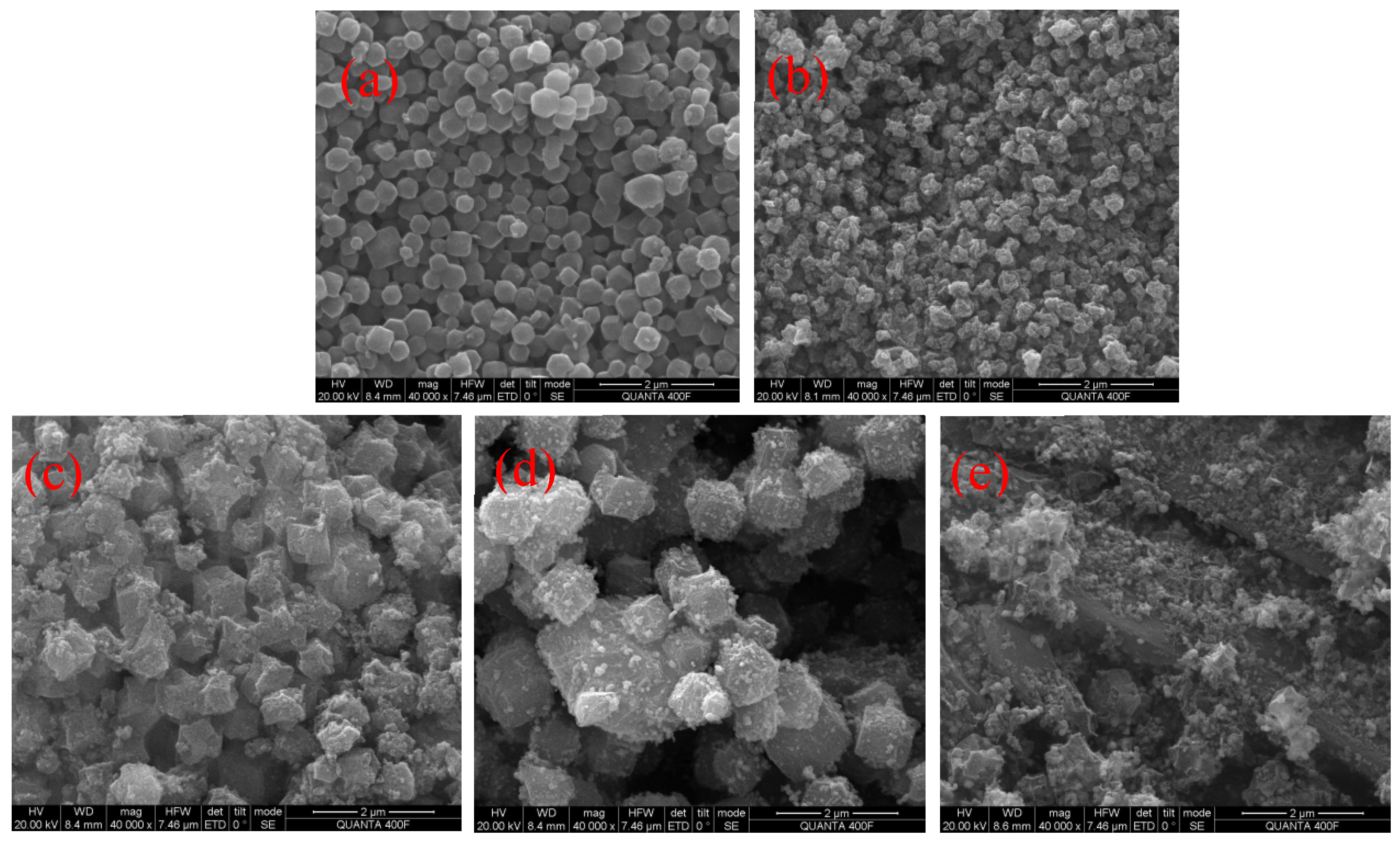
3.1.2. SEM Analysis
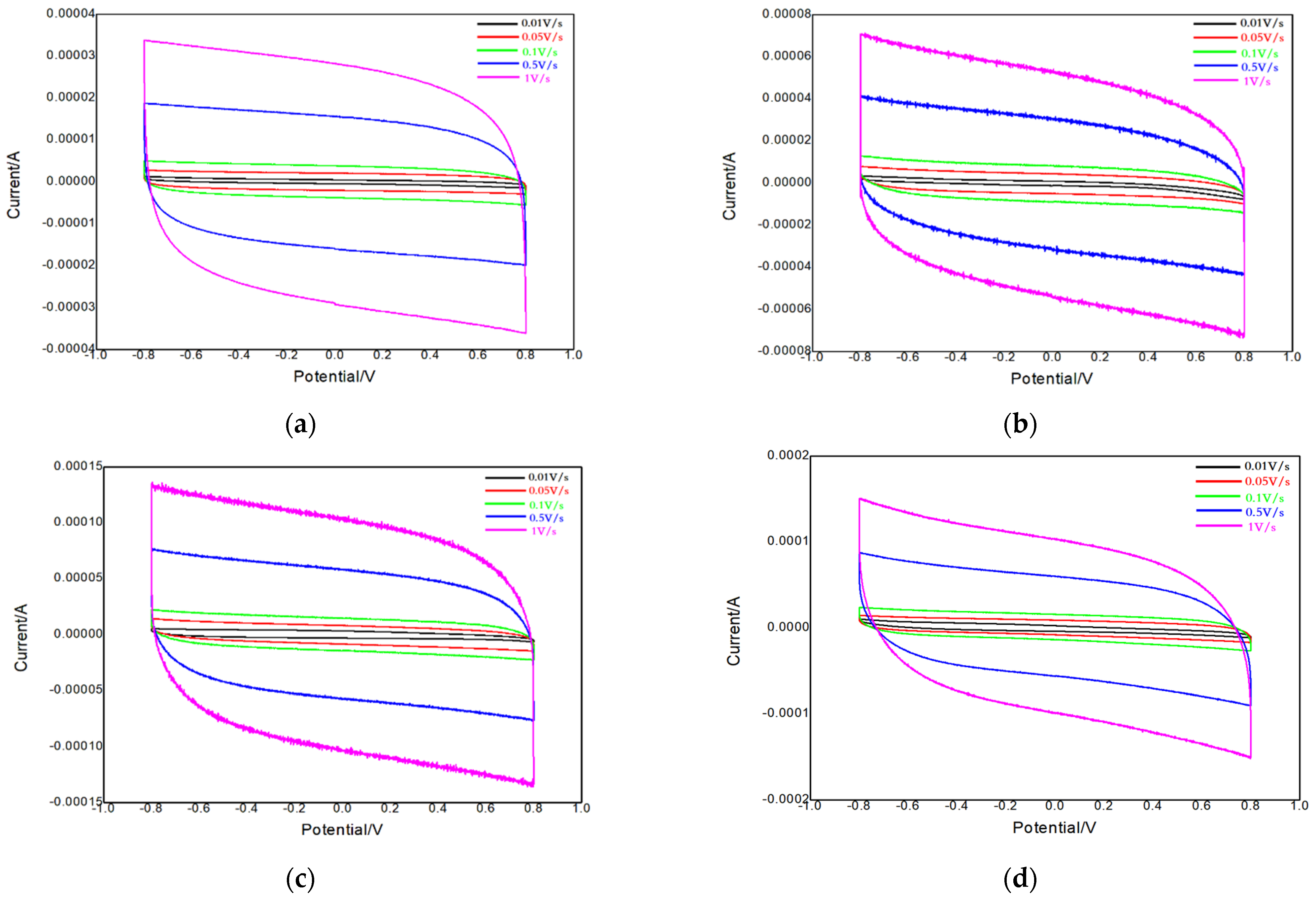
3.2. Electrochemical Performance Test for the Charcoal-ZIF-67 Composite Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wesley, R.J.; Durairaj, A.; Ramanathan, S.; Obadiah, A.; Justinabraham, R.; Lv, X.; Vasanthkumar, S. Potato peels biochar composite with copper phthalocyanine for energy storage application. Diam. Relat. Mater. 2021, 115, 108360. [Google Scholar] [CrossRef]
- Li, S.; Lin, J.; Xiong, W.; Guo, X.; Wu, D.; Zhang, Q.; Zhu, Q.-L.; Zhang, L. Design principles and direct applications of cobalt-based metal-organic frameworks for electrochemical energy storage. Coord. Chem. Rev. 2021, 438, 213872. [Google Scholar] [CrossRef]
- Ashourdan, M.; Semnani, A.; Hasanpour, F.; Moosavifard, S.E. Synthesis of nickel cobalt manganese metal organic framework@high quality graphene composites as novel electrode materials for high performance supercapacitors. J. Electroanal. Chem. 2021, 895, 115452. [Google Scholar] [CrossRef]
- Faisal, M.M.; Ali, S.R.; Sanal, K.C.; Iqbal, M.W.; Iqbal, M.Z.; Saeed, A. Highly porous terpolymer-MOF composite electrode material for high performance supercapattery devices. J. Electroanal. Chem. 2021, 893, 115321. [Google Scholar] [CrossRef]
- Omar, N.; Abdullah, E.C.; Petrus, A.A.; Mubarak, N.M.; Khalid, M.; Agudosi, E.S.; Numan, A.; Aid, S.R. Single-route synthesis of binary metal oxide loaded coconut shell and watermelon rind biochar: Characterizations and cyclic voltammetry analysis. Biomass Convers. Biorefinery 2021, 1–13. [Google Scholar] [CrossRef]
- Karri, S.N.; Ega, S.P.; Perupogu, V.; Srinivasan, P. Enhancing the Electrochemical Performance of Polyaniline Using Fly Ash of Coal Waste for Supercapacitor Application. ChemistrySelect 2021, 6, 2576–2589. [Google Scholar] [CrossRef]
- Majumder, M.; Santosh, M.S.; Viswanatha, R.; Thakur, A.K.; Dubal, D.P.; Jayaramulu, K. Two-dimensional Conducting Metal-Organic Frameworks Enabled Energy Storage Devices. Energy Storage Mater. 2021, 37, 396–416. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.L.; Lu, Y.M.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent advances in poly (α-L-glutamic acid)-based nanomaterials for drug delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Yao, K.; Huang, W.; Wang, T. Facile synthesis of a neodymium doped metal organic frame modified antibacterial material and corrosion resistant coating. Inorg. Chim. Acta 2021, 528, 120599. [Google Scholar] [CrossRef]
- Wang, D.; Dong, X.; Han, Y.; Liu, Y. Separation of hexane isomers by introducing “triangular-like and quadrilateral-like channels” in a bcu-type metal-organic framework. Nano Res. 2020, 14, 526–531. [Google Scholar] [CrossRef]
- Meng, L.; Yu, B.; Qin, Y. Templated interfacial synthesis of metal-organic framework (MOF) nano- and micro-structures with precisely controlled shapes and sizes. Commun. Chem. 2021, 4, 82. [Google Scholar] [CrossRef]
- Thapa, S.; Meng, L.Y.; Hettiarachchi, E.; Bader, Y.K.; Dickie, D.A.; Rubasinghege, G.; Ivanov, S.A.; Vreeland, E.C.; Qin, Y. Charge-separated and lewis paired metal–organic framework for anion exchange and CO2 chemical fixation. Chem. Eur. J. 2020, 26, 13788–13791. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, B.; Kelly, H. Comparative study between physicochemical characterization of biochar and metal organic frameworks (MOFs) as gas adsorbents. Can. J. Chem. Eng. 2016, 94, 2114–2120. [Google Scholar]
- Tang, J.; Hui, Z.-Z.; Hu, T.; Cheng, X.; Guo, J.-H.; Li, Z.-R.; Yu, H. A sensitive acetaminophen sensor based on Co metal–organic framework (ZIF-67) and macroporous carbon composite. Rare Met. 2021, 41, 189–198. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Peng, H.; Wang, D.; Fu, S. Cost-effective resource utilization for waste biomass: A simple preparation method of photo-thermal biochar cakes (BCs) toward dye wastewater treatment with solar energy. Environ. Res. 2021, 194, 110720. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Tian, R.; Dong, H.; Chen, J.; Li, R.; Xie, Q.; Li, L.; Li, Y.; Jin, Z.; Xiao, S.; Xiao, J. Electrochemical behaviors of biochar materials during pollutant removal in wastewater: A review. Chem. Eng. J. 2021, 425, 130585. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, D.; Wang, M.; Gong, T.; Sun, M.; Yang, T. A scientometric review of biochar preparation research from 2006 to 2019. Biochar 2021, 3, 283–298. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Huang, J.; Xing, L.; Hu, H.; Xiao, Y.; Dong, H.; Liu, Y.; Zheng, M. Mixed-biomass wastes derived hierarchically porous carbons for high-performance electrochemical energy storage. ACS Sustain. Chem. Eng. 2019, 7, 10393–10402. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, R.; Li, A.; Ji, G. In-situ self-activation strategy toward highly porous biochar for supercapacitors: Direct carbonization of marine algae. J. Electroanal. Chem. 2021, 882, 114986. [Google Scholar] [CrossRef]
- Altintas, C.; Altundal, O.F.; Keskin, S.; Yildirim, R. Machine Learning Meets with Metal Organic Frameworks for Gas Storage and Separation. J. Chem. Inf. Model. 2021, 61, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiao, Z.; Hou, B.; Jiang, H.; Gong, W.; Dong, J.; Li, H.-Y.; Cui, Y.; Liu, Y. Chiral metal-organic frameworks with tunable catalytic selectivity in asymmetric transfer hydrogenation reactions. Nano Res. 2020, 14, 466–472. [Google Scholar] [CrossRef]
- Chen, X.; Kong, X.; Wang, S.; Zhang, Y.; Zhong, W.; Lu, A.; Xu, W.; Liu, L.; Liu, Y. Facile preparation of Metal/Metal-organic frameworks decorated phase change composite materials for thermal energy storage. J. Energy Storage 2021, 40, 102711. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Majumder, M. Recent advances on redox active composites of metal-organic framework and conducting polymers as pseudocapacitor electrode material. Renew. Sustain. Energy Rev. 2021, 145, 110854. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Dewage, N.B.; Karunanayake, A.G.; Farmer, E.L.; Perez, F.; Hassan, E.B.; Mlsna, T.E.; Pittman, C.U. Rhodamine B Adsorptive Removal and Photocatalytic Degradation on MIL-53-Fe MOF/Magnetic Magnetite/Biochar Composites. J. Inorg. Organomet. Polym. Mater. 2019, 30, 214–229. [Google Scholar] [CrossRef]
- Xi, J.; Li, H.; Xi, J.; Tan, S.; Zheng, J.; Tan, Z. Preparation of high porosity biochar materials by template method: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 20675–20684. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wu, J.; Chen, W.F. Review on Prepation and Application of Biochar. Adv. Mater. Res. 2014, 898, 456–460. [Google Scholar] [CrossRef]
- Ma, Z.W.; Liu, H.Q.; Lü, Q.F. Porous biochar derived from tea saponin for supercapacitor electrode: Effect of preparation technique. J. Energy Storage 2021, 40, 102773. [Google Scholar] [CrossRef]
- Huang, S.; Ding, Y.; Li, Y.; Han, X.; Xing, B.; Wang, S. Nitrogen and Sulfur Co-doped Hierarchical Porous Biochar Derived from the Pyrolysis of Mantis Shrimp Shell for Supercapacitor Electrodes. Energy Fuels 2021, 35, 1557–1566. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, C.; Kong, F.; Zhao, Q.; Kong, A.; Shan, Y. Activated biochar derived from peanut shells as the electrode materials with excellent performance in Zinc-air battery and supercapacitance. Waste Manag. 2021, 125, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Vakros, J.; Manariotis, I.D.; Dracopoulos, V.; Mantzavinos, D.; Lianos, P. Biochar from Spent Malt Rootlets and Its application to an energy conversion and storage device. Chemosensors 2021, 9, 57. [Google Scholar] [CrossRef]
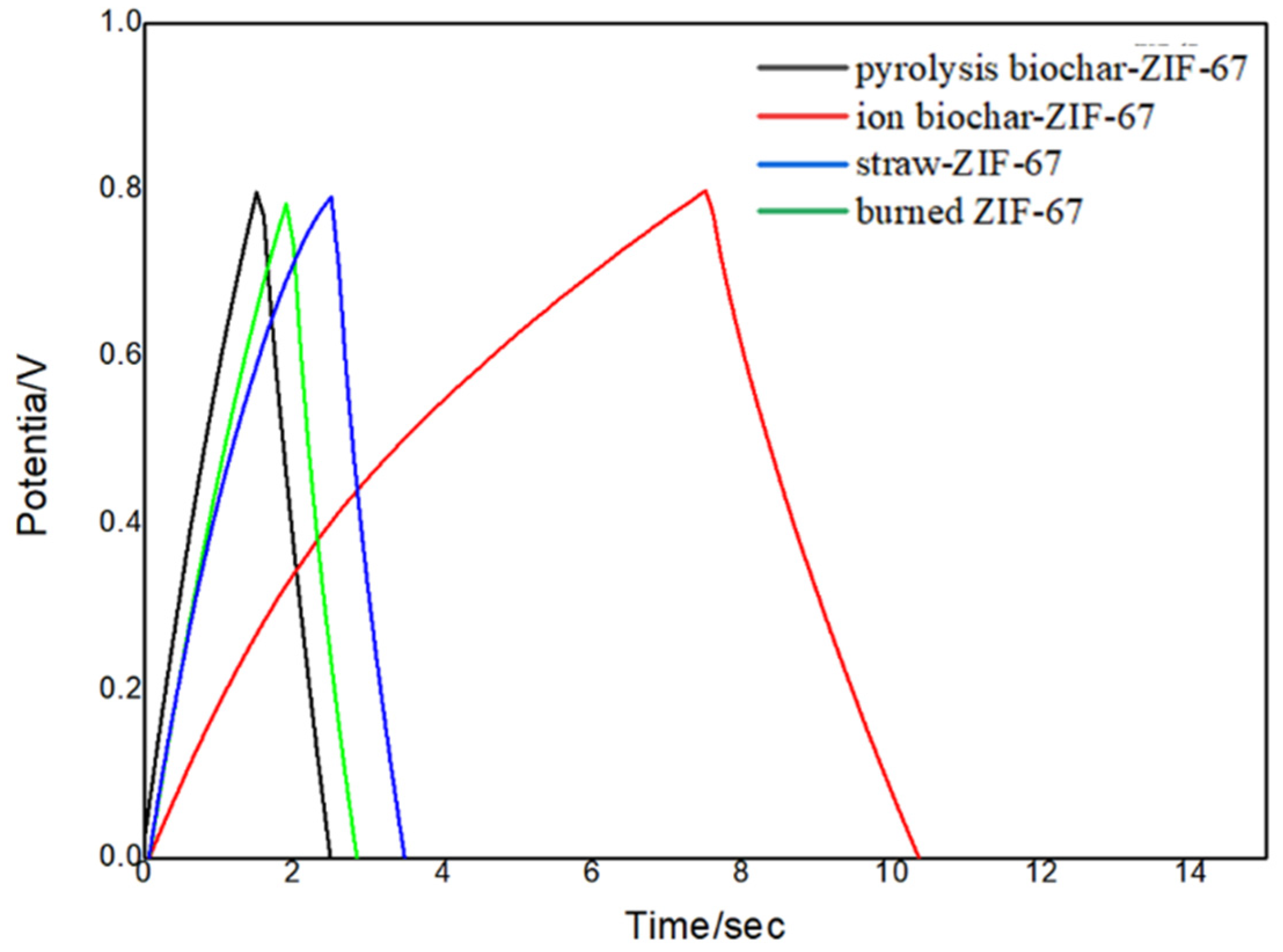

| Sample | Values (ohm) |
|---|---|
| Pyrolysis biochar-ZIF-67 | 12 |
| Ion biochar | 10 |
| Straw-ZIF-67 | 95 |
| Burned ZIF-67 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Lu, M.; Zhang, X.; Luo, Z.; Xiao, J. Application of Fiber Biochar–MOF Matrix Composites in Electrochemical Energy Storage. Polymers 2022, 14, 2419. https://doi.org/10.3390/polym14122419
Gao M, Lu M, Zhang X, Luo Z, Xiao J. Application of Fiber Biochar–MOF Matrix Composites in Electrochemical Energy Storage. Polymers. 2022; 14(12):2419. https://doi.org/10.3390/polym14122419
Chicago/Turabian StyleGao, Meixiang, Meng Lu, Xia Zhang, Zhenhui Luo, and Jiaqi Xiao. 2022. "Application of Fiber Biochar–MOF Matrix Composites in Electrochemical Energy Storage" Polymers 14, no. 12: 2419. https://doi.org/10.3390/polym14122419
APA StyleGao, M., Lu, M., Zhang, X., Luo, Z., & Xiao, J. (2022). Application of Fiber Biochar–MOF Matrix Composites in Electrochemical Energy Storage. Polymers, 14(12), 2419. https://doi.org/10.3390/polym14122419





