Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy
Abstract
:1. Introduction
2. Thermosensitive Hydrogel: A Brief Overview
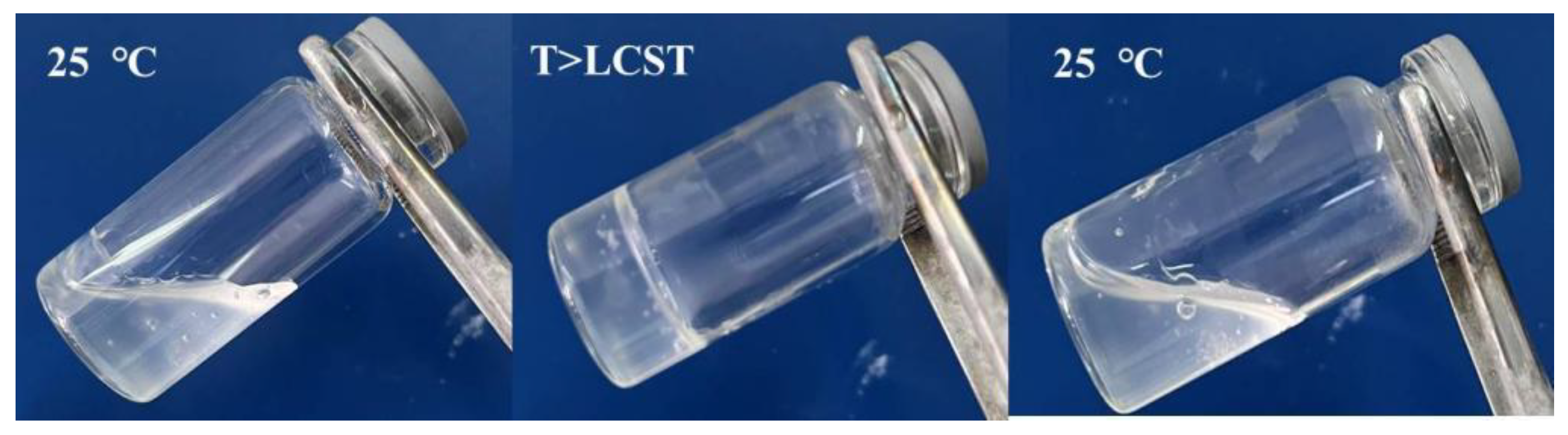
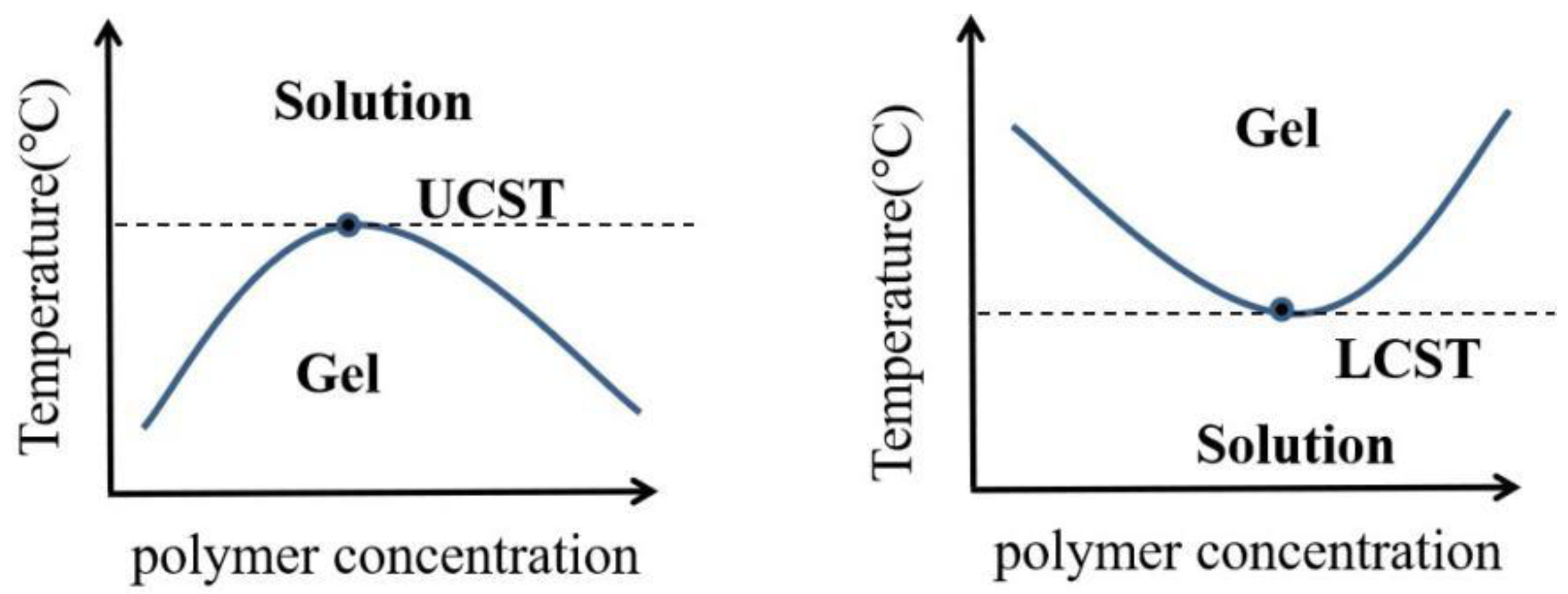
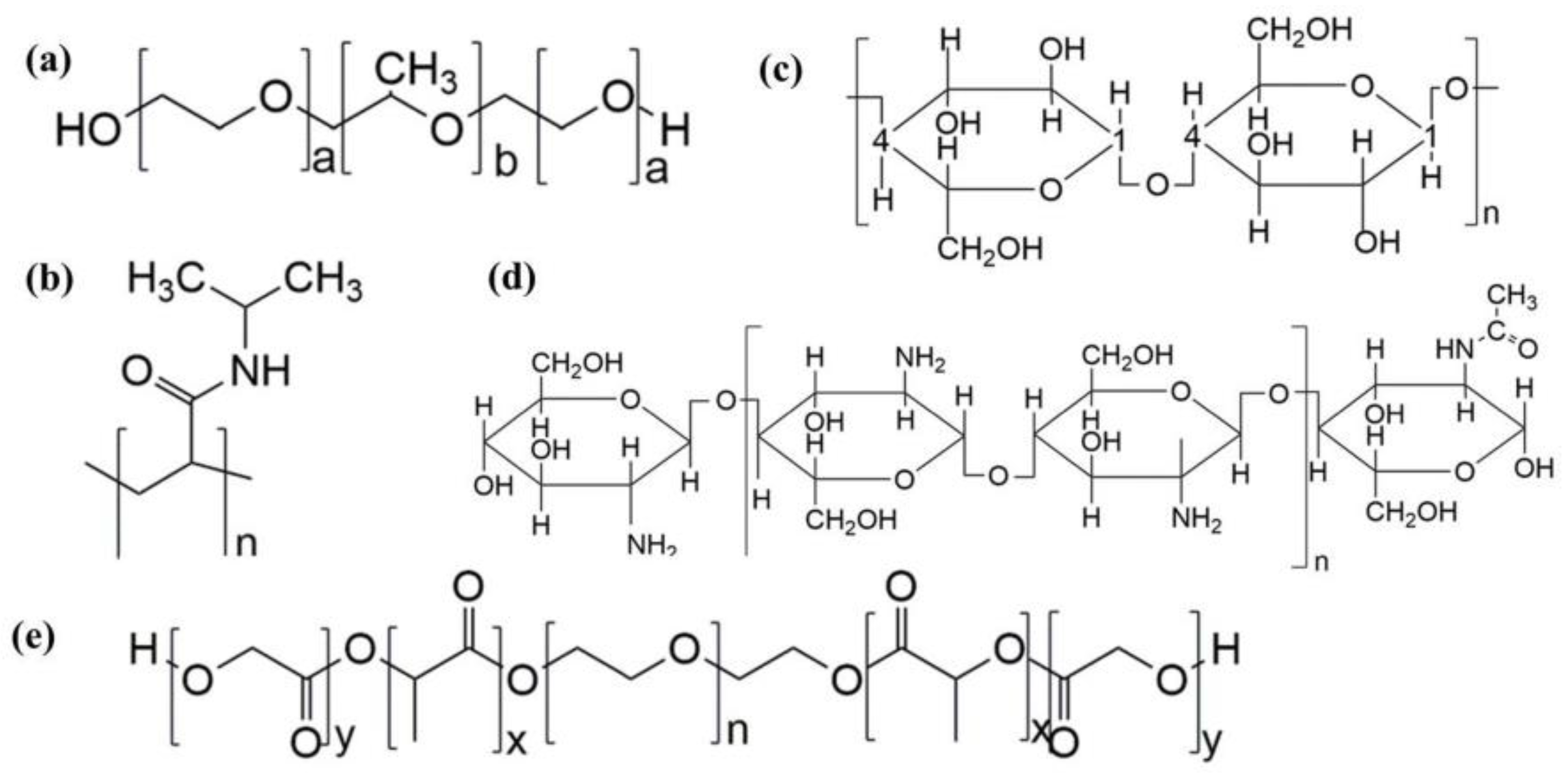
2.1. Common Temperature Sensitive Materials and Gelation Mechanism
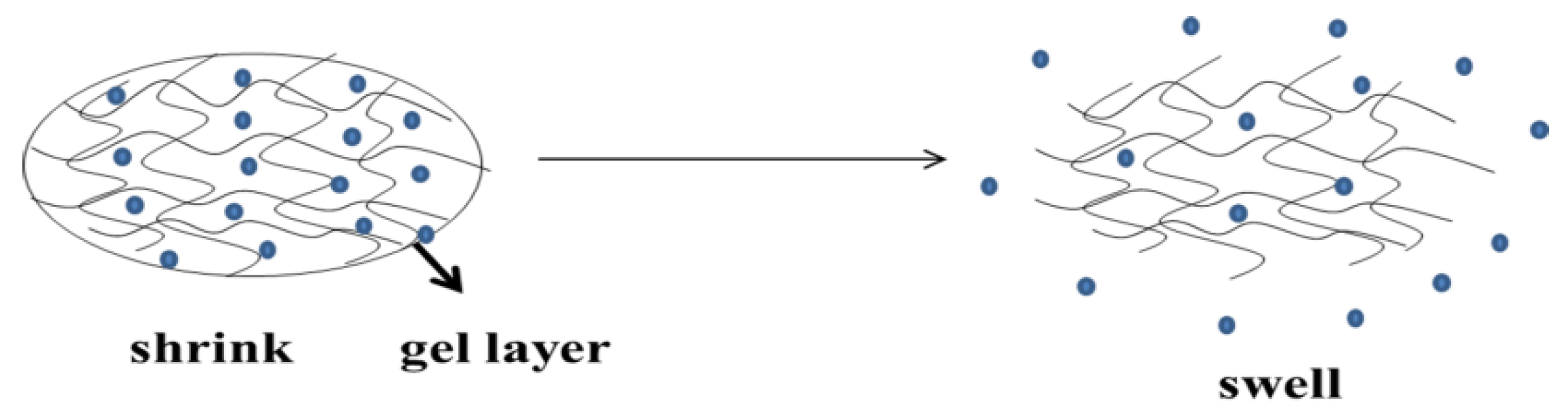
2.2. Drug Release Mechanism of Thermosensitive Hydrogel
2.3. Biodegradation and Adhesion
3. Application of Thermosensitive Hydrogel in Local Drug Delivery System
3.1. Cancer Treatment
3.1.1. Postoperative Recurrence of Tumor
3.1.2. Cancer Immunotherapy
3.2. Prevention of Postoperative Adhesion
3.3. Nasal Brain Targeting
3.4. Wound Healing
3.5. Osteoarthritis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Yoo, S.Y.; Kim, H.; Lee, J. Chitosan-Based Multifunctional Platforms for Local Delivery of Therapeutics. Mar. Drugs 2017, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Davila, G.W.; Daugherty, C.A.; Sanders, S.W. A short-term, multicenter, randomized double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence. J. Urol. 2001, 166, 140–145. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. A Publ. Can. Soc. Pharm. Sci. Soc. Can. Des Sci. Pharm. 2009, 12, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug Deliv. Rev. 2020, 158, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Suyash, I.; Varsha, T.; Baburao, C.; Vinod, M.; Onkar, K. A brief review onemulgel—A novel topical drug delivery system. Res. J. Pharm. Dos. Technol. 2021, 13, 25–30. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, B.; Bishnoi, S.; Mishra, S.K.; Nayak, D.; Kumar, A.; Sarma, T.K. Multifunctional Inosine Monophosphate Coordinated Metal–Organic Hydrogel: Multistimuli Responsiveness, Self-Healing Properties, and Separation of Water from Organic Solvents. ACS Sustain. Chem. Eng. 2018, 6, 8659–8671. [Google Scholar] [CrossRef]
- Bernhard, S.; Tibbitt, M.W. Supramolecular engineering of hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2021, 171, 240–256. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Gad, S.F.; Chobisa, D.; Li, Y.; Yeo, Y. Local drug delivery systems for inflammatory diseases: Status quo, challenges, and opportunities. J. Control. Release 2021, 330, 438–460. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Lee, D.S. Injectable biodegradable hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Goessl, A.; Tirelli, N.; Hubbell, J.A. A hydrogel system for stimulus-responsive, oxygen-sensitive in situ gelation. J. Biomater. Science. Polym. Ed. 2004, 15, 895–904. [Google Scholar] [CrossRef]
- Deng, H.; Dong, A.; Song, J.; Chen, X. Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery. J. Control. Release 2019, 297, 60–70. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Chung, P.-C.; Lin, H.-C.; Hung, S.-C. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Avila-Salas, F.; Duran-Lara, E.F. An Overview of Injectable Thermo-Responsive Hydrogels and Advances in their Biomedical Applications. Curr. Med. Chem. 2020, 27, 5773–5789. [Google Scholar] [CrossRef] [PubMed]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Park, M.H.; Joo, M.K.; Choi, B.G.; Jeong, B. Biodegradable thermogels. Acc. Chem. Res. 2012, 45, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Mandal, A.; Gote, V.; Pal, D.; Mitra, A.K. Thermosensitive hydrogel-based drug delivery system for sustained drug release. J. Polym. Res. 2019, 26, 131. [Google Scholar] [CrossRef]
- Ko, D.Y.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar] [CrossRef]
- Calderon, M.; Quadir, M.A.; Strumia, M.; Haag, R. Functional dendritic polymer architectures as stimuli-responsive nanocarriers. Biochimie 2010, 92, 1242–1251. [Google Scholar] [CrossRef]
- Kojima, C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010, 7, 307–319. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.e.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019, 216, 119220. [Google Scholar] [CrossRef]
- Wang, M.; Chen, M.; Niu, W.; Winston, D.D.; Cheng, W.; Lei, B. Injectable biodegradation-visual self-healing citrate hydrogel with high tissue penetration for microenvironment-responsive degradation and local tumor therapy. Biomaterials 2020, 261, 120301. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.D.; Cerankowski, L.D. Preparation of films exhibiting a balanced temperature dependence to permeation by aqueous solutions—a study of lower consolute behavior. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2551–2570. [Google Scholar] [CrossRef]
- Ruel-Gariepy, E.; Leroux, J.C. In situ-forming hydrogels--review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Suknuntha, K. Thermosensitive Poloxamer 407/Poly(Acrylic Acid) Hydrogels with Potential Application as Injectable Drug Delivery System. AAPS PharmSciTech 2018, 19, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.H.; Huynh, N.T.; Pham, N.O.; Nguyen, C.T.; Vu, M.T.; Dinh, V.T.; Le, V.T.; Tran, N.Q. Injectable nanocurcumin-dispersed gelatin–pluronic nanocomposite hydrogel platform for burn wound treatment. Bull. Mater. Sci. 2019, 42, 71. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Petkova-Olsson, Y.; Oelschlaeger, C.; Ullsten, H.; Järnström, L. Structural, microrheological and kinetic properties of a ternary silica-Pluronic F127-starch thermosensitive system. J. Colloid Interface Sci. 2018, 514, 459–467. [Google Scholar] [CrossRef]
- Ma, S.; Zheng, H.; Chen, Y.; Zou, J.; Zhang, C.; Wang, Y. Nanocomposite Polymer Hydrogels Reinforced by Carbon Dots and Hectorite Clay. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 287–292. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Tsai, Y.-t.; Bai, L.; Deng, J. Study on the Thermal Stability of Thermosensitive Hydrogel. Procedia Eng. 2016, 135, 501–509. [Google Scholar] [CrossRef]
- Goncharuk, O.; Samchenko, Y.; Sternik, D.; Kernosenko, L.; Poltorats’ka, T.; Pasmurtseva, N.; Abramov, M.; Pakhlov, E.; Derylo-Marczewska, A. Thermosensitive hydrogel nanocomposites with magnetic laponite nanoparticles. Appl. Nanosci. 2020, 10, 4559–4569. [Google Scholar] [CrossRef]
- Wu, W.-X.; Huang, Y.-C.; Lee, W.-F. Effect of poly(ethylene glycol)-derived crosslinkers on the properties of thermosensitive hydrogels. Iran. Polym. J. 2020, 29, 679–691. [Google Scholar] [CrossRef]
- Xu, W.-K.; Tang, J.-Y.; Yuan, Z.; Cai, C.-Y.; Chen, X.-B.; Cui, S.-Q.; Liu, P.; Yu, L.; Cai, K.-Y.; Ding, J.-D. Accelerated Cutaneous Wound Healing Using an Injectable Teicoplanin-loaded PLGA-PEG-PLGA Thermogel Dressing. Chin. J. Polym. Sci. 2019, 37, 548–559. [Google Scholar] [CrossRef]
- Patel, N.; Ji, N.; Wang, Y.; Li, X.; Langley, N.; Tan, C. Subcutaneous Delivery of Albumin: Impact of Thermosensitive Hydrogels. AAPS PharmSciTech 2021, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Maeda, T.; Hotta, A. PEG-based nanocomposite hydrogel: Thermo-responsive sol-gel transition and degradation behavior controlled by the LA/GA ratio of PLGA-PEG-PLGA. Polym. Degrad. Stab. 2018, 147, 222–228. [Google Scholar] [CrossRef]
- Velázquez, N.S.; Turino, L.N.; Luna, J.A.; Mengatto, L.N. Progesterone loaded thermosensitive hydrogel for vaginal application: Formulation and in vitro comparison with commercial product. Saudi Pharm. J. 2019, 27, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Tentor, F.R.; de Oliveira, J.H.; Scariot, D.B.; Lazarin-Bidóia, D.; Bonafé, E.G.; Nakamura, C.V.; Venter, S.A.S.; Monteiro, J.P.; Muniz, E.C.; Martins, A.F. Scaffolds based on chitosan/pectin thermosensitive hydrogels containing gold nanoparticles. Int. J. Biol. Macromol. 2017, 102, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.; Osman, S.K.; Saleh, K.I.; Samy, A.M. In Vitro Release of 5-Fluorouracil and Methotrexate from Different Thermosensitive Chitosan Hydrogel Systems. AAPS PharmSciTech 2020, 21, 131. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, J.; Xie, L.; Xie, H.; Chen, C.; Zhang, C.; Lin, D.; Cai, L. An injectable thermosensitive hydrogel for sustained release of apelin-13 to enhance flap survival in rat random skin flap. J. Mater. Sci. Mater. Med. 2019, 30, 106. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, W.; Zhang, J.; Peng, X.; Li, G.; Zhang, L.-M.; Yang, L. Thermosensitive hydrogels based on methylcellulose derivatives for prevention of postoperative adhesion. Cellulose 2019, 27, 1555–1571. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Gabrielli, V.; Muñoz-García, J.C.; Lewandowska, A.E.; Harniman, R.; Khimyak, Y.Z.; Angulo, J.; Eichhorn, S.J. Thermosensitive supramolecular and colloidal hydrogels via self-assembly modulated by hydrophobized cellulose nanocrystals. Cellulose 2019, 26, 529–542. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Salimi-Kenari, H.; Nasseri, R.; Knudsen, K.D.; Mirzadeh, H.; Nyström, B. Tunable viscoelastic features of aqueous mixtures of thermosensitive ethyl(hydroxyethyl)cellulose and cellulose nanowhiskers. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 590, 124489. [Google Scholar] [CrossRef]
- Fu, C.; Ren, F.; Zhang, Q.; Lao, G.; Zhang, L.-M. Effects of collagen incorporation on thermogelation and hydrogel characteristics of aqueous Pluronic F127 copolymer system. Colloid Polym. Sci. 2015, 293, 2191–2200. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, Y.; Li, H.; Zhang, Y.; Xia, X.; Yan, Q. Frontal polymerization and characterization of interpenetrating polymer networks composed of poly(N-isopropylacrylamide) and polyvinylpyrrolidone. Colloid Polym. Sci. 2017, 296, 165–172. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.Q. Postoperative anti-adhesion ability of a novel carboxymethyl chitosan from silkworm pupa in a rat cecal abrasion model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Schnell, C.N.; Galván, M.V.; Zanuttini, M.A.; Mocchiutti, P. Hydrogels from xylan/chitosan complexes for the controlled release of diclofenac sodium. Cellulose 2019, 27, 1465–1481. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Z.; Zhang, H.; Ding, J. Mixing a Sol and a Precipitate of Block Copolymers with. Biomacromolecules 2009, 10, 1547–1553. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Brian, A. Solute Diffusion within Hydrogels. Mechanisms and Models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
- Kristl, J.; Pecar, S.; Smid-Korbar, J.; Schara, M. Molecular motion of drugs in hydrocolloids measured by electron paramagnetic resonance. Pharm. Res. 1991, 8, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.L.; Peppas, N.A. Measurement of the swelling force in ionic polymer networks. III. Swelling force of interpolymer complexes. J. Control. Release 1995, 37, 277–280. [Google Scholar] [CrossRef]
- Heller, J. Controlled release of biologically active compounds from bioerodible polymers. Biomaterials 1980, 1, 51–57. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N. Chemical and Physical Structure of Polymers as Carriers for Controlled Release of Bioactive Agents: A Review. J. Macromol. Sci. Part C 2006, 23, 61–126. [Google Scholar] [CrossRef]
- Marvin, J.; Slepian, J.A.H. Polymeric endoluminal gel paving hydrogel systems for local barrier creation and site-specific drug delivery. Adv. Drug Deliv. Rev. 1996, 24, 11–30. [Google Scholar]
- Erthal, L.C.S.; Gobbo, O.L.; Ruiz-Hernandez, E. Biocompatible copolymer formulations to treat glioblastoma multiforme. Acta Biomater. 2021, 121, 89–102. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Nakayama, M.; Yamada, N.; Kumashiro, Y.; Kanazawa, H.; Yamato, M.; Okano, T. Thermoresponsive poly (N-isopropylacrylamide)-based block copolymer coating for optimizing cell sheet fabrication. Macromol. Biosci. 2012, 12, 751–760. [Google Scholar] [CrossRef]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.; Baum, M.; Gukas, I.D. The effects of surgery on tumor growth: A century of investigations. Ann. Oncol. 2008, 19, 1821–1828. [Google Scholar] [CrossRef]
- Shakhar, G.; Ben-Eliyahu, S. Potential prophylactic measures against postoperative immunosuppression: Could they reduce recurrence rates in oncological patients? Ann. Surg. Oncol. 2003, 10, 972–992. [Google Scholar] [CrossRef]
- Eisenhauer, E.; ten Bokkel Huinink, W.; Swenerton, K.; Gianni, L.; Myles, J.; van der Burg, M.; Kerr, I.; Vermorken, J.B.; Buser, K.; Colombo, N. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: High-dose versus low-dose and long versus short infusion. J. Clin. Oncol. 1994, 12, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Fentahun Darge, H.; Yibru Hanurry, E.; Simegniew Birhan, Y.; Worku Mekonnen, T.; Tizazu Andrgie, A.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem. Eng. J. 2021, 406, 126879. [Google Scholar] [CrossRef]
- Wu, J.; Qu, Y.; Shi, K.; Chu, B.; Jia, Y.; Xiao, X.; He, Q.; Qian, Z. Camptothecin@HMSNs/thermosensitive hydrogel composite for applications in preventing local breast cancer recurrence. Chin. Chem. Lett. 2018, 29, 1819–1823. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Yang, X.; Wang, Y.; Yu, L.; Sun, J.; Ding, J. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+ breast cancer after breast-conserving surgery. Theranostics 2019, 9, 6080–6098. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and In Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef]
- Hamard, L.; Ratel, D.; Selek, L.; Berger, F.; van der Sanden, B.; Wion, D. The brain tissue response to surgical injury and its possible contribution to glioma recurrence. J. Neuro-Oncol. 2016, 128, 1–8. [Google Scholar] [CrossRef]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, J.; Sun, H.; Li, J.; Wang, X. Polymer-based hydrogels with local drug release for cancer immunotherapy. Biomed. Pharmacother. 2021, 137, 111333. [Google Scholar] [CrossRef]
- He, T.; Liang, X.; Li, L.; Gong, S.; Li, X.; Zhang, M.; Zhu, S.; Xiao, H.; Wu, Q.; Gong, C. A spontaneously formed and self-adjuvanted hydrogel vaccine triggers strong immune responses. Mater. Des. 2021, 197, 109232. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.; Yang, Y.; Chen, X.; Ge, F.; Wang, J.; Deng, J. Inhibition of tumor recurrence and metastasis via a surgical tumor-derived personalized hydrogel vaccine. Biomater. Sci. 2022, 10, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, M.; Liu, Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev. 2019, 48, 5506–5526. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Hao, Y.; Jia, Y.; Liu, Q.; Chen, Y.; Pan, M.; Yuan, L.; Yu, Y.; Qian, Z. Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner. Bioact. Mater. 2021, 6, 3036–3048. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Jia, Y.; Hao, Y.; Peng, J.; Yuan, L.; Chen, Y.; Pan, M.; Qian, Z. A biodegradable thermosensitive hydrogel vaccine for cancer immunotherapy. Appl. Mater. Today 2020, 19, 100608. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Yang, C.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Subcutaneous vaccination using injectable biodegradable hydrogels for long-term immune response. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102056. [Google Scholar] [CrossRef]
- Wei, P.-S.; Chen, Y.-J.; Lin, S.-Y.; Chuang, K.-H.; Sheu, M.-T.; Ho, H.-O. In situ subcutaneously injectable thermosensitive PEG-PLGA diblock and PLGA-PEG-PLGA triblock copolymer composite as sustained delivery of bispecific anti-CD3 scFv T-cell/anti-EGFR Fab Engager (BiTEE). Biomaterials 2021, 278, 121166. [Google Scholar] [CrossRef] [PubMed]
- Bruggmann, D.; Tchartchian, G.; Wallwiener, M.; Munstedt, K.; Tinneberg, H.R.; Hackethal, A. Intra-abdominal adhesions: Definition, origin, significance in surgical practice, and treatment options. Dtsch. Arztebl. Int. 2010, 107, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Control and prevention of peritoneal adhesions in gynecologic surgery. Fertil. Steril. 2006, 86, S1–S5. [Google Scholar] [CrossRef]
- Hellebrekers, B.; Trimbos-Kemper, T.; Trimbos, J.; Emeis, J.; Kooistra, T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil. Steril. 2000, 74, 203–212. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Chen, S.-H.; Chen, C.-H.; Chen, S.-H.; Fong, Y.T.; Chen, J.-P. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater. 2017, 63, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, S.H.; Mao, S.H.; Tsai, M.J.; Chou, P.Y.; Liao, C.H.; Chen, J.P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Lee, M.; Kim, S.I.; Seol, A.; Lee, E.J.; Kim, H.S.; Song, Y.S. A Randomized Controlled Trial of Thermo-Sensitive Sol–Gel Anti-Adhesion Agent after Gynecologic Surgery. J. Clin. Med. 2020, 9, 2261. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.; Bowley, C. Multidisciplinary and multiagency contributions to care for those with learning disability who have epilepsy. Epilepsia 2001, 42, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In Situ-Based Gels for Nose to Brain Delivery for the Treatment of Neurological Diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Z.; Liu, M.; Tao, Y.; Li, Z.; Wu, Z.; Gui, S. Facile nose-to-brain delivery of rotigotine-loaded polymer micelles thermosensitive hydrogels: In vitro characterization and in vivo behavior study. Int. J. Pharm. 2020, 577, 119046. [Google Scholar] [CrossRef]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef]
- Xu, D.; Qiu, C.; Wang, Y.; Qiao, T.; Cui, Y.-L. Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int. J. Pharm. 2021, 603, 120667. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Liao, J.-F.; Kong, Z.-C.; Xu, Y.-F.; Chen, Z.-J.; Chen, H.-Y.; Kuang, D.-B.; Fenske, D.; Su, C.-Y. Conformal coating of ultrathin metal-organic framework on semiconductor electrode for boosted photoelectrochemical water oxidation. Appl. Catal. B Environ. 2018, 237, 9–17. [Google Scholar] [CrossRef]
- Lan, B.; Zhang, L.; Yang, L.; Wu, J.; Li, N.; Pan, C.; Wang, X.; Zeng, L.; Yan, L.; Yang, C.; et al. Sustained delivery of MMP-9 siRNA via thermosensitive hydrogel accelerates diabetic wound healing. J. Nanobiotechnol. 2021, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Andrgie, A.T.; Darge, H.F.; Mekonnen, T.W.; Birhan, Y.S.; Hanurry, E.Y.; Chou, H.-Y.; Wang, C.-F.; Tsai, H.-C.; Yang, J.M.; Chang, Y.-H. Ibuprofen-Loaded Heparin Modified Thermosensitive Hydrogel for Inhibiting Excessive Inflammation and Promoting Wound Healing. Polymers 2020, 12, 2619. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhao, X.; Wang, C.; Geng, Y.; Zhao, J.; Xu, J.; Zuo, B.; Zhao, C.; Wang, C.; Zhang, X. MicroRNA-145 attenuates TNF-alpha-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017, 8, e3140. [Google Scholar] [CrossRef]
- Chen, Z.P.; Liu, W.; Liu, D.; Xiao, Y.Y.; Chen, H.X.; Chen, J.; Li, W.; Cai, H.; Li, W.; Cai, B.C.; et al. Development of brucine-loaded microsphere/thermally responsive hydrogel combination system for intra-articular administration. J. Control. Release 2012, 162, 628–635. [Google Scholar] [CrossRef]
- Wang, Q.S.; Xu, B.X.; Fan, K.J.; Fan, Y.S.; Teng, H.; Wang, T.Y. Dexamethasone-loaded thermo-sensitive hydrogel attenuates osteoarthritis by protecting cartilage and providing effective pain relief. Ann. Transl. Med. 2021, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Shu, X.; Wu, M.; Liu, J.; Hao, T.; Cui, H.; Zheng, L. Intra-articular delivery of flurbiprofen sustained release thermogel: Improved therapeutic outcome of collagenase II-induced rat knee osteoarthritis. Drug Deliv. 2020, 27, 1034–1043. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Dou, H.; Liu, Q.; Shu, G.; Lin, J.; Zhang, W.; Peng, G.; Zhong, Z.; Fu, H. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111352. [Google Scholar] [CrossRef] [PubMed]
- Verekar, R.R.; Gurav, S.S.; Bolmal, U. Thermosensitive mucoadhesive in situ gel for intranasal delivery of Almotriptan malate: Formulation, characterization, and evaluation. J. Drug Deliv. Sci. Technol. 2020, 58, 101778. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Palve, V.K. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285. [Google Scholar] [CrossRef]
- Li, J.; Pan, H.; Qiao, S.; Li, Y.; Wang, J.; Liu, W.; Pan, W. The utilization of low molecular weight heparin-poloxamer associated Laponite nanoplatform for safe and efficient tumor therapy. Int. J. Biol. Macromol. 2019, 134, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pastor, Y.; Ting, I.; Martinez, A.L.; Irache, J.M.; Gamazo, C. Intranasal delivery system of bacterial antigen using thermosensitive hydrogels based on a Pluronic-Gantrez conjugate. Int. J. Pharm. 2020, 579, 119154. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; Chen, Y. Dual dynamically crosslinked thermosensitive hydrogel with self-fixing as a postoperative anti-adhesion barrier. Acta Biomater. 2020, 110, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yu, D.; Li, D.; Wang, X. Prevention of Local Tumor Recurrence After Surgery by Thermosensitive Gel-Based Chemophotothermal Therapy in Mice. Lasers Surg. Med. 2020, 52, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, M.; Yang, X.; Sun, W.; Lu, C.; Hui, Q.; Shi, C.; Li, X.; Wang, X. Modified poloxamer 407 and hyaluronic acid thermosensitive hydrogel-encapsulated keratinocyte growth factor 2 improves knee osteoarthritis in rats. Mater. Des. 2021, 210, 110086. [Google Scholar] [CrossRef]
- Yurtdaş-Kırımlıoğlu, G. A promising approach to design thermosensitive in situ gel based on solid dispersions of desloratadine with Kolliphor® 188 and Pluronic® F127. J. Therm. Anal. Calorim. 2021, 147, 1307–1327. [Google Scholar] [CrossRef]
- Saeednia, L.; Yao, L.; Cluff, K.; Asmatulu, R. Sustained Releasing of Methotrexate from Injectable and Thermosensitive Chitosan–Carbon Nanotube Hybrid Hydrogels Effectively Controls Tumor Cell Growth. ACS Omega 2019, 4, 4040–4048. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cai, X.; Chen, R.; Zhang, H.; Jiang, T.; Wang, Y. A thermosensitive chitosan-based hydrogel for sealing and lubricating purposes in dental implant system. Clin. Implant. Dent. Relat. Res. 2019, 21, 324–335. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S.; Yang, X.; Pang, S.; Wang, X.; Yang, S. Preparation of a recombinant collagen-peptide (RHC)-conjugated chitosan thermosensitive hydrogel for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111555. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Messerotti, E.; Pozzoli, M.; Cheng, S.; Traini, D.; Young, P.; Kourmatzis, A.; Caramella, C.; Ong, H.X. Application of a Thermosensitive In Situ Gel of Chitosan-Based Nasal Spray Loaded with Tranexamic Acid for Localised Treatment of Nasal Wounds. AAPS PharmSciTech 2019, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Li, M.X.; Cho, W.K.; Joung, Y.K.; Huh, K.M. Thermosensitive gallic acid-conjugated hexanoyl glycol chitosan as a novel wound healing biomaterial. Carbohydr. Polym. 2021, 260, 117808. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Hsiao, C.Y.; Tsai, K.L.; Cheng, Y.H. Injectable thermosensitive chitosan-based hydrogel containing ferulic acid for treating peripheral arterial disease. J. Tissue Eng. Regen. Med. 2020, 14, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ruiz, J.L.; Calpena-Campmany, A.C.; Silva-Abreu, M.; Halbout-Bellowa, L.; Bozal-de Febrer, N.; Rodriguez-Lagunas, M.J.; Clares-Naveros, B. Design and evaluation of a multifunctional thermosensitive poloxamer-chitosan-hyaluronic acid gel for the treatment of skin burns. Int. J. Biol. Macromol. 2020, 142, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hao, J.; Ding, F.; Ren, X. Nanocatalyst doped bacterial cellulose-based thermosensitive nanogel with biocatalytic function for antibacterial application. Int. J. Biol. Macromol. 2022, 195, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, Y.; Yang, L.; Yi, J.Z.; Deng, F.; Zhang, L.M. Injectable and bioactive methylcellulose hydrogel carrying bone mesenchymal stem cells as a filler for critical-size defects with enhanced bone regeneration. Colloids Surf. B Biointerfaces 2020, 194, 111159. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Giovagnoli, S.; Perioli, L.; Tiralti, M.C.; Ricci, M. Development and characterization of mucoadhesive-thermoresponsive gels for the treatment of oral mucosa diseases. Eur. J. Pharm. Sci. 2020, 142, 105125. [Google Scholar] [CrossRef]
- Sultana, T.; Gwon, J.G.; Lee, B.T. Thermal stimuli-responsive hyaluronic acid loaded cellulose based physical hydrogel for post-surgical de novo peritoneal adhesion prevention. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110661. [Google Scholar] [CrossRef]
- Fu, X.; Zeng, H.; Guo, J.; Liu, H.; Shi, Z.; Chen, H.; Li, D.; Xie, X.; Kuang, C. A PLGA–PEG–PLGA Thermosensitive Gel Enabling Sustained Delivery of Ropivacaine Hydrochloride for Postoperative Pain Relief. Chem. Pharm. Bull. 2017, 65, 229–235. [Google Scholar] [CrossRef]
- Chan, P.S.; Li, Q.; Zhang, B.; To, K.K.W.; Leung, S.S.Y. In vivo biocompatibility and efficacy of dexamethasone-loaded PLGA-PEG-PLGA thermogel in an alkali-burn induced corneal neovascularization disease model. Eur. J. Pharm. Biopharm. 2020, 155, 190–198. [Google Scholar] [CrossRef]
- Norouzi, M.; Firouzi, J.; Sodeifi, N.; Ebrahimi, M.; Miller, D.W. Salinomycin-loaded injectable thermosensitive hydrogels for glioblastoma therapy. Int. J. Pharm. 2021, 598, 120316. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, J.; Lu, Y. Doxorubicin and CDCUR inclusion complex coloaded in thermosensitive hydrogel PLGAPEGPLGA localized administration for osteosarcoma. Int. J. Oncol. 2020, 57, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Fu, Q.; Gu, Z.; Zhang, Z.; Lv, H. Injectable corilagin/low molecular weight chitosan/PLGA-PEG-PLGA thermosensitive hydrogels for localized cancer therapy and promoting drug infiltration by modulation of tumor microenvironment. Int. J. Pharm. 2020, 589, 119772. [Google Scholar] [CrossRef] [PubMed]
- Navara, A.M.; Kim, Y.S.; Xu, Y.; Crafton, C.L.; Diba, M.; Guo, J.L.; Mikos, A.G. A dual-gelling poly(N-isopropylacrylamide)-based ink and thermoreversible poloxamer support bath for high-resolution bioprinting. Bioact. Mater. 2022, 14, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Perçin, I.; Idil, N.; Denizli, A. Molecularly imprinted poly(N-isopropylacrylamide) thermosensitive based cryogel for immunoglobulin G purification. Process Biochem. 2019, 80, 181–189. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lu, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 2014, 35, 5679–5688. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-incorporated crosslinked sodium alginate-g-poly (N-isopropyl acrylamide) thermo-responsive hydrogel as an in-situ forming injectable dressing for wound healing: In vitro characterization and in vivo evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Yang, I.H.; Kuan, C.Y.; Chen, Z.Y.; Li, C.H.; Chi, C.Y.; Lin, Y.Y.; Liang, Y.J.; Kuo, W.T.; Li, Y.A.; Lin, F.H. Engineered cell-laden thermosensitive poly(N-isopropylacrylamide)-immobilized gelatin microspheres as 3D cell carriers for regenerative medicine. Mater. Today Bio. 2022, 15, 100266. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, X.Y.; Li, Z.Y.; Wang, M.L.; Chen, P.P.; Liu, Y.; Zhang, X.Z.; Jiang, X.J. Intra-myocardial Delivery of a Novel Thermosensitive Hydrogel Inhibits Post-infarct Heart Failure After Degradation in Rat. J. Cardiovasc. Transl. Res. 2020, 13, 677–685. [Google Scholar] [CrossRef]




| Classification | Characteristic | Polymer | Drug and Application | Objective of the Study | Reference |
|---|---|---|---|---|---|
| Natural | Excellent biocompatibility, low toxicity, good compatibility with other chemical reagents and strong solubilization, which can delay drug release | Poloxamer407/188 | Almotriptan malate | Deliver drugs through the nose to the brain | [110] |
| Poloxamer407/188 | Voriconazole | Vaginal infection | [111] | ||
| Poloxamer407 | Doxorubicin | Antitumor and antiangiogenic efficacy | [112] | ||
| Pluronic®F127 | OMV-antigenic complex | Intranasal delivery system of bacterial antigen | [113] | ||
| Poloxamer407 | Amikacin | Accelerated wound healing | [99] | ||
| Pluronic®F127 oxidized hyaluronic acid | / | Postoperative antiadhesion barrier | [114] | ||
| Poloxamer407 | Doxorubicin and ICG. | Prevention of local tumor Recurrence after surgery | [115] | ||
| Poloxamer 407 and hyaluronic acid | keratinocyte growth factor 2 | Knee osteoarthritis | [116] | ||
| Poloxamer407/188 | Desloratadine | Antiallergic agent through the nose | [117] | ||
| Natural linear polymer, high porosity, biodegradable, nontoxic, antibacterial, good biocompatibility, good temperature sensitivity and antibacterial hemostatic properties, has a powerful function in promoting wound healing | Chitosan | Methotrexate | Controls tumor cell growth | [118] | |
| Chitosan | / | Sealing and lubricating purposes in dental implant system | [119] | ||
| Chitosan | Recombinant human collagen-peptide (RHC) | Cell encapsulation and wound repair | [119] | ||
| Chitosan | Tranexamic Acid | Localized treatment of nasal wounds | [120] | ||
| Chitosan | Gallic acid | Wound healing | [121] | ||
| Chitosan | Ferulic acid | Peripheral arterial disease | [122] | ||
| Poloxamer-chitosan | Vitamins A, D and E | Skin burns | [123] | ||
| Natural-derived polymers, through the introduction of hydrophobic groups through chemical modification, make cellulose have temperature-sensitive properties. Different synthetic polymers are mixed with cellulose to adjust its drug release characteristics. They are biodegradable and biocompatible | Cellulose | carbon-based nanozyme | Antibacterial application | [124] | |
| Methylcellulose | Bone mesenchymal stem cells | Bone regeneration | [125] | ||
| Poloxamer 407, sodium carboxymethyl cellulose, chitosan | Benzydamine hydrochloride | Oral mucosa diseases | [126] | ||
| Methyl cellulose (MC), Hyaluronic acid (HA) | / | Postsurgical de novo peritoneal adhesion | [127] | ||
| Poloxamer 407, chitosan (CS), methyl cellulose (MC) | L-carnosine | Wound healing effect | [128] | ||
| Synthesis | Polyethylene glycol has very high hydrophilicity and can crosslink degradable polyester at the end to obtain multi block copolymer. The change of molecular weight and block ratio of the copolymer can realize the intelligent adjustment of the hydrophilic and hydrophobic properties and temperature sensitivity of the material | PLGA–PEG–PLGA | Ropivacaine Hydrochloride | Postoperative pain relief | [129] |
| PLGA–PEG–PLGA | Dexamethasone | Alkali-burn-induced corneal neovascularization | [130] | ||
| Pluronic®F127, PLGA–PEG–PLGA | Salinomycin | Glioblastoma therapy | [131] | ||
| PCL-PEG-PCL | Diclofenac sodium | Anti-inflammatory and analgesic | [132] | ||
| PLGA-PEG-PLGA | Curcumin, Doxorubicin | Localized administration for osteosarcoma | [133] | ||
| PLGA-PEG-PLGA | Corilagin, chitosan | Localized cancer therapy | [134] | ||
| Good temperature sensitivity, simple preparation, easy availability of materials, numerous modified monomers, crosslinking with a variety of temperature-sensitive materials, and rich functional properties | poly(N-isopropylacrylamide) | Cell | High-resolution bioprinting | [134] | |
| poly(N-isopropylacrylamide) | / | Immunoglobulin G (IgG) purification | [135] | ||
| poly(N-isopropylacrylamide) | Diclofenac sodium (DS) | Skin reinfection | [136] | ||
| poly(N-isopropylacrylamide) | Brown adipose-derived stem cells | Stem cell transplantation in myocardial repair | [137] | ||
| Sodium alginate-g-poly (N-isopropylacrylamide) | Curcumin | Wound healing | [24] | ||
| poly(N-isopropylacrylamide) | Wharton′s jelly-derived mesenchymal stem cells | 3D cell carriers for regenerative medicine | [138] | ||
| poly(N-isopropylacrylamide) | Dextran | Postinfarct heart failure After degradation | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. https://doi.org/10.3390/polym14122379
Fan R, Cheng Y, Wang R, Zhang T, Zhang H, Li J, Song S, Zheng A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers. 2022; 14(12):2379. https://doi.org/10.3390/polym14122379
Chicago/Turabian StyleFan, Ranran, Yi Cheng, Rongrong Wang, Ting Zhang, Hui Zhang, Jianchun Li, Shenghan Song, and Aiping Zheng. 2022. "Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy" Polymers 14, no. 12: 2379. https://doi.org/10.3390/polym14122379
APA StyleFan, R., Cheng, Y., Wang, R., Zhang, T., Zhang, H., Li, J., Song, S., & Zheng, A. (2022). Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers, 14(12), 2379. https://doi.org/10.3390/polym14122379





