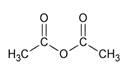
3.1. Acetic Anhydride Route: Influence of the Polymer Matrix Nature
The in situ non-hydrolytic sol-gel synthesis of TiO
2 from Ti(O
iPr)
4 and Ac
2O was already described in our previous work [
13]. The affinity between acetic anhydride and polypropylene or polystyrene matrices was investigated by the determination of their solubility parameters δ by the Fedors’ method, which requires only the knowledge of the compound structural formula [
17]. Calculated values of δ are given in
Table 3.
Hildebrand and Hansen solubility parameters of the polypropylene were estimated between 15.5 and 17.5 (MPa)
1/2 with a preferred value of 16.5 (MPa)
1/2 [
18] close to the calculated value of 16.0 (MPa)
1/2 by the Fedors’ method. For polystyrene, Hildebrand and Hansen solubility parameters are between 18.2 and 20.2 (MPa)
1/2 [
18], thus slightly lower than the value calculated from Fedor’s method. As the acetic anhydride has a calculated solubility parameter of 19.8 (MPa)
1/2, it may have more affinity with polystyrene (Δδ = 1.3 (MPa)
1/2) than with polypropylene (Δδ = 3.8 (MPa)
1/2). Based on this parameter, a better dispersion should be consequently expected for the PS-based composite in situ synthesized from acetic anhydride than for the PP-based composite.
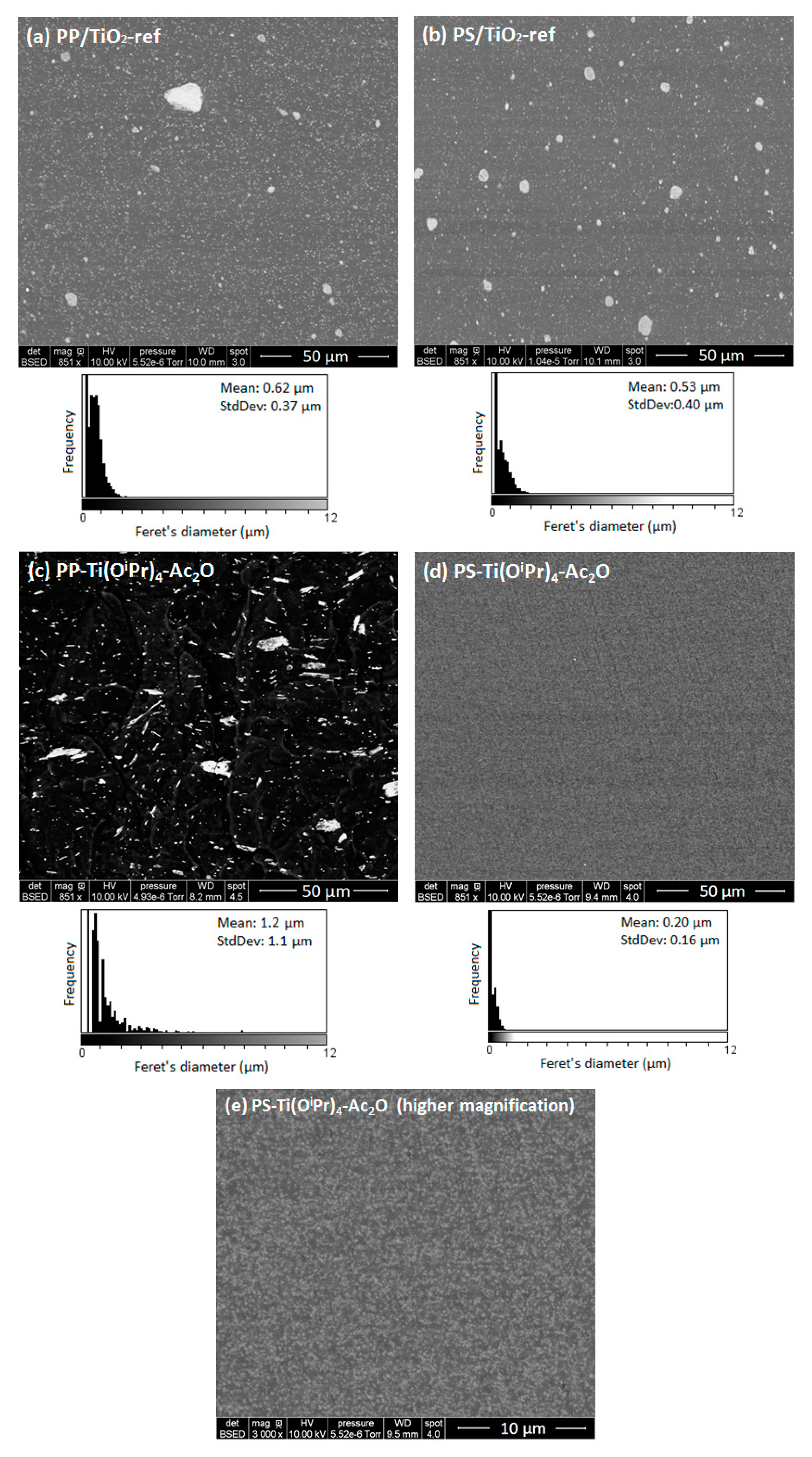
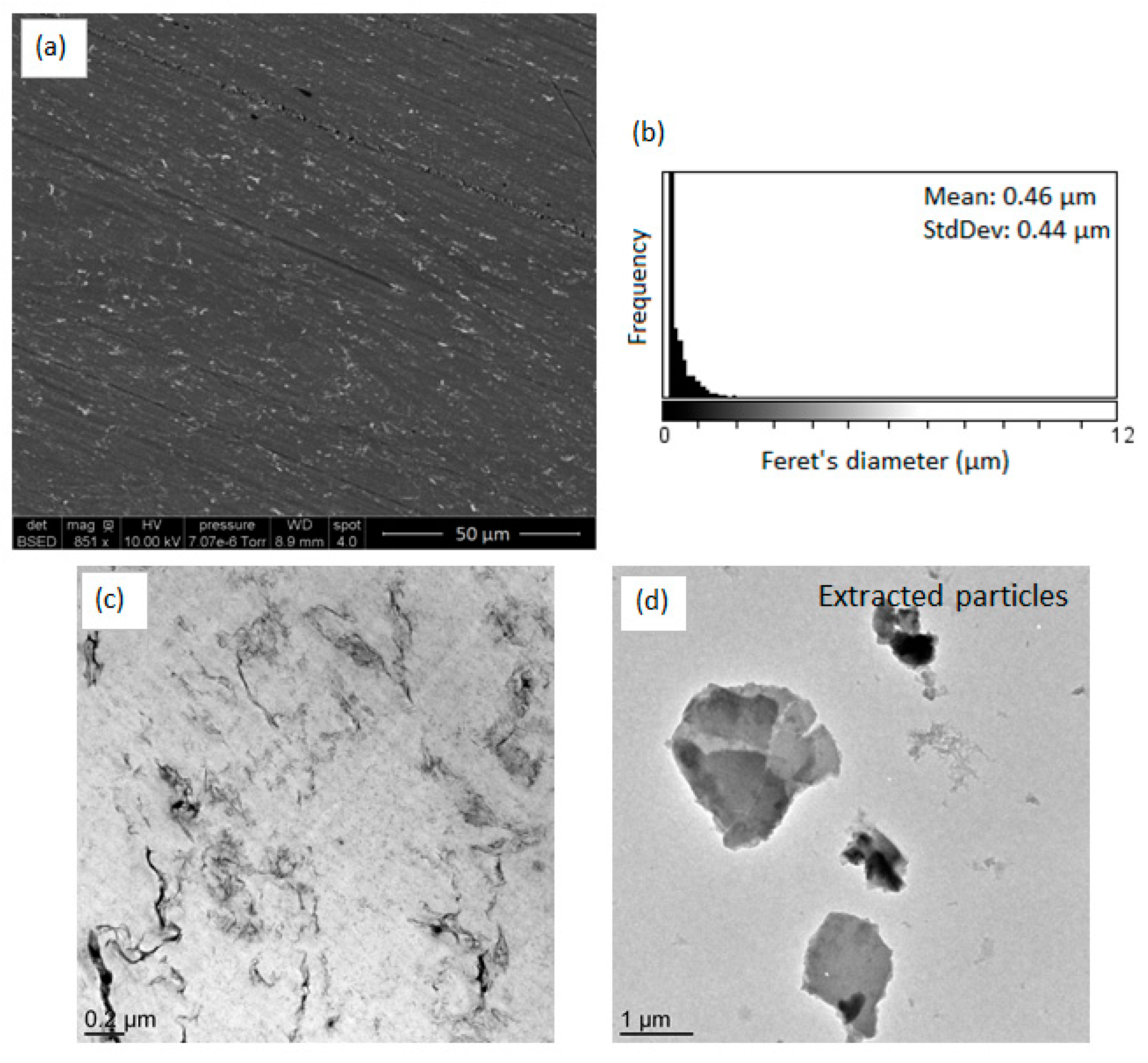
To evaluate this effect, the morphology of the in situ formed nanocomposites was investigated by electron microscopy (
Figure 1) and compared to the morphology of reference nanocomposites (e.g., nanocomposites prepared using a commercial TiO
2 filler). Concerning the morphologies of the two reference nanocomposites (PP/TiO
2-ref and PS/TiO
2-ref), we can observe that the TiO
2 particles distributed in both composites are mainly with a sub-micronic diameter with some large, micron-sized aggregates. The mean Feret’s diameter of observed aggregates is about 0.62 μm for PP/TiO
2-ref sample and 0.53 μm for PS/TiO
2-ref sample. Concerning the nanocomposites elaborated by the in situ NHSG synthesis of TiO
2 from the reaction between Ti(O
iPr)
4 and Ac
2O, morphologies are very different. For the PP-Ti(O
iPr)
4-Ac
2O composite, a wide distribution of fillers size is observed. The mean Feret’s diameter is about 1.18 µm but some filler particles are larger than 10 μm. Consequently, this in situ NHSG synthesis of TiO
2 in molten polypropylene does not lead to a better dispersion than the direct introduction of nanofillers by “top-down” approach.
For the PS-Ti(O
iPr)
4-Ac
2O sample, the filler particles are homogeneously dispersed in the polymer with a mean diameter of 200 nm. The size distribution is narrow in comparison with the PS/TiO
2-ref sample. These first results are consistent with the prediction conducted from the determination of the solubility parameters (
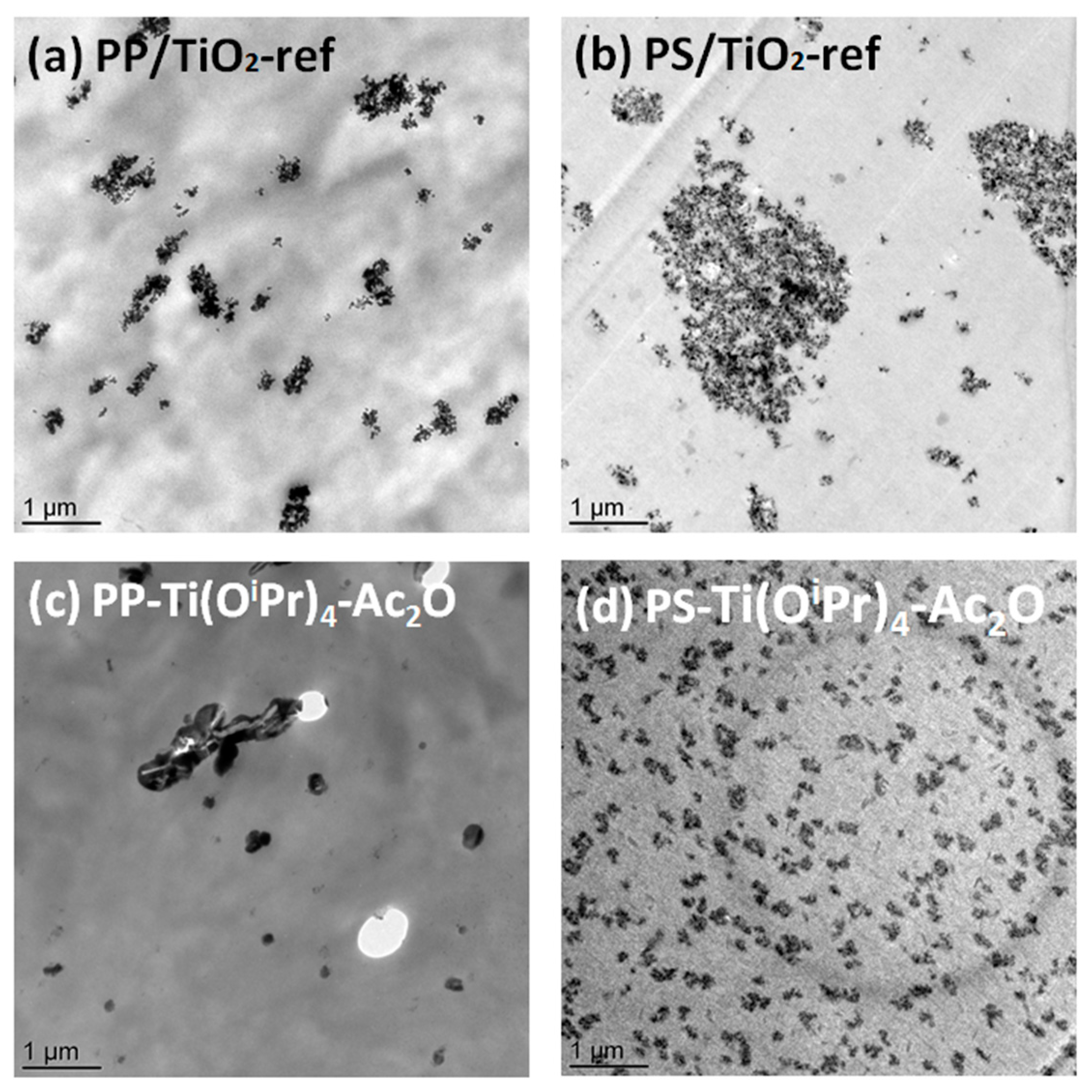
Table 3) where a better dispersion of the filler was expected in the PS matrix. To go further in the morphology analysis, TEM analysis was performed. The transmission electron micrographs presented in
Figure 2 show that the dispersed domains observed at the SEM scale for PP/TiO
2-ref, PS/TiO
2-ref and PS-Ti(O
iPr)
4-Ac
2O samples are in fact composed of aggregates of TiO
2 nanoparticles. In situ synthesized TiO
2 primary nanoparticles in PS-Ti(O
iPr)
4-Ac
2O nanocomposite have diameters below 10 nm, smaller than Aeroxide P25 TiO
2 primary particles which have an average diameter of 21 nm. These nanoparticles are assembled to form well-dispersed aggregates of around 200 nm. For the PP-Ti(O
iPr)
4-Ac
2O sample observed by TEM (
Figure 2c), the morphology is more complex and a wide range of particle sizes is observed. Small fillers seem rather spherical whereas big filler particles have a rod-shaped morphology. There are also some holes in the observed section, induced by large filler particles torn from the polypropylene matrix indicating a poor adhesion between the TiO
2 filler and the PP matrix.
These observations demonstrated that NHSG chemistry allowed the in situ formation of TiO2 filler in both PP and PS matrices, with a morphology that is highly dependent on the polymer structure. The determination of the solubility parameter gave a first clue to explain this difference in morphologies. However as detailed in the introduction, other parameters can contribute to this difference in morphology such as, for example, the condensation degree.
To estimate the condensation degree of TiO
2 synthesized in situ by non-hydrolytic sol-gel reactions, XPS measurements were performed on PP-Ti(O
iPr)
4-Ac
2O and PS-Ti(O
iPr)
4-Ac
2O composites. The atomic concentrations are the following: 98.9% C, 0.8% O and 0.3% Ti for the PP-Ti(O
iPr)
4-Ac
2O composite and 95.0% C, 3.9% O and 0.2% Ti for the PS-Ti(O
iPr)
4-Ac
2O composite. According to the Ti/O ratio, a higher condensation degree is expected for PP-Ti(O
iPr)
4-Ac
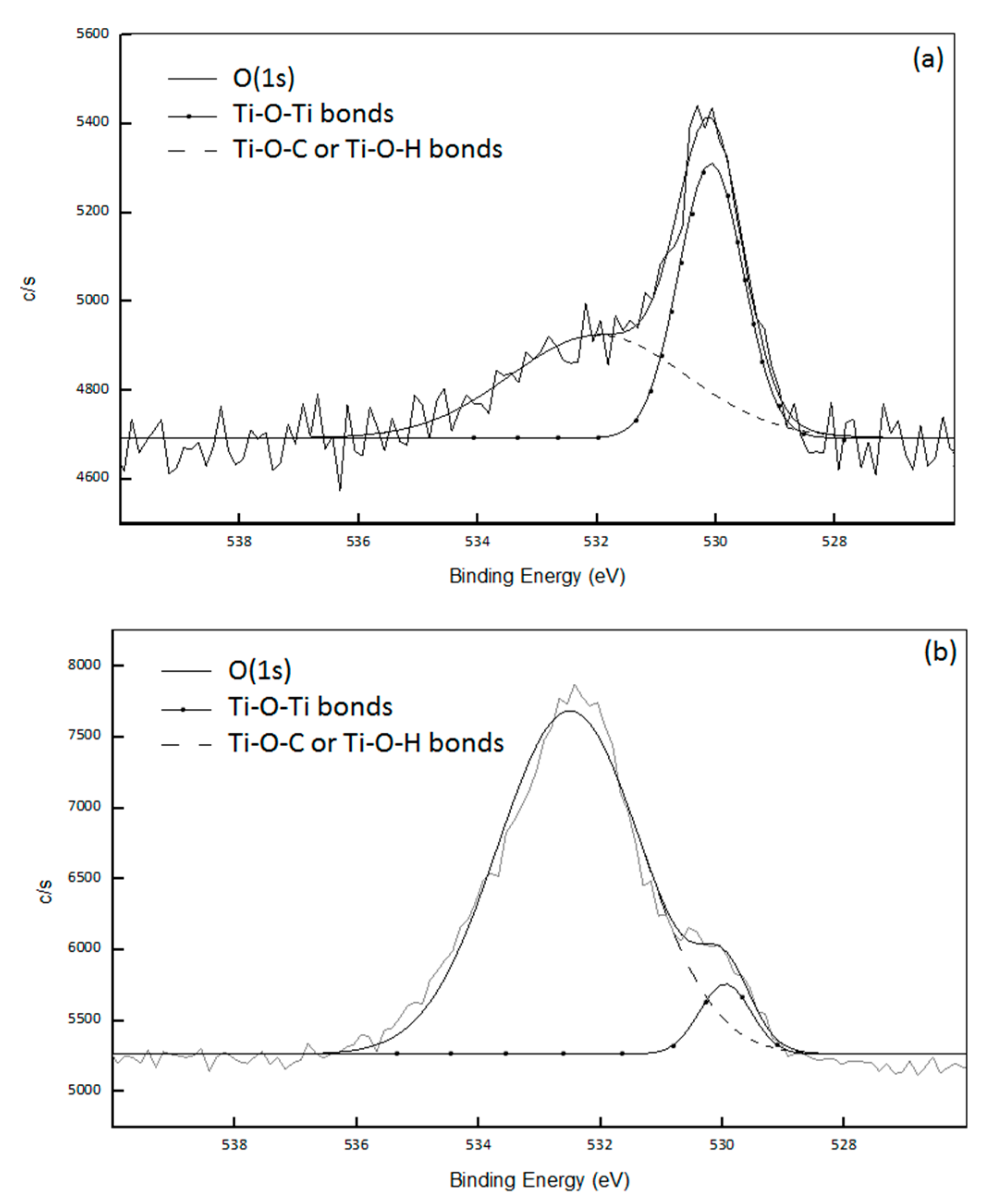
2O composite. Actually, the XPS analysis provides information about the condensation degree of synthesized titanium dioxide through the quantification of –Ti–O–Ti–, –Ti–O–C– or –Ti–O–H bonds respectively. Deconvolution of the O(1s) signal for the two composites (
Figure 3) allowed to determine the different environments of oxygen atoms in the created nanofillers: the signal at 529.9 eV corresponded to –Ti–O–Ti– and the one at 531.8 eV to –Ti–O–H or –Ti–O–C [
19,
20,
21,
22,
23,
24]. For PP-Ti(O
iPr)
4-Ac
2O sample, 65% of oxygen atoms were in a –Ti–O–Ti environment and 35% of oxygen atoms were in a –Ti–O–C or –Ti–O–H environment. The mean formula of the Ti domain is therefore Ti(O)
1.58(OR)
0.84 which implies a condensation degree of 79%. The condensation degree calculation was already detailed in a previous publication [
13] For the PS-Ti(O
iPr)
4-Ac
2O nanocomposite, the deconvolution of the O(1s) signal (
Figure 3b) allowed to determine that 11% of O atoms were involved in –Ti–O–Ti bonds and 89% were involved in -Ti–O–C or –Ti–O–H bonds, giving a condensation degree of 20%.
This difference in condensation degree: 79% for the PP-Ti(O
iPr)
4-Ac
2O sample and 20% for PS-Ti(O
iPr)
4-Ac
2O sample explained also the finest size of the filler in the PS matrix as also pointed out by Jiang et al. [
15]. The large difference in viscosity (higher for the PS compared to PP one) can contribute to the decrease of the condensation degree by limiting the probability of reactions between chemical species.
However, other contributions such as the initial miscibility of the reactants and also the phase separation scenario depending on the NHSG reactions advancement may be involved.
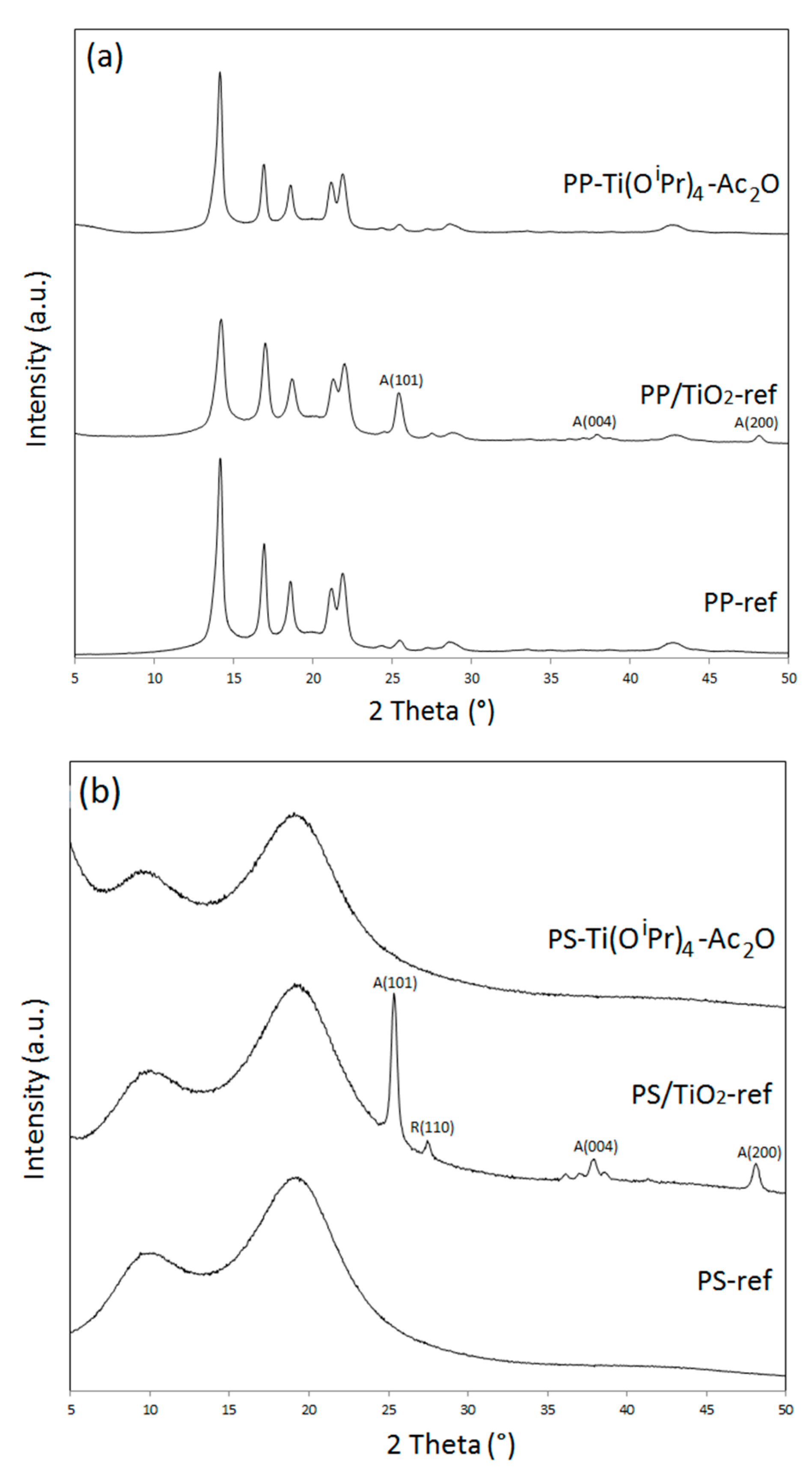
The structure of the filler was then studied through XRD analysis. The powder XRD patterns of the neat polymers and their respective polymer/TiO
2 nanocomposites at wide angles, i.e., between 5 and 50°, are displayed in
Figure 4. Polypropylene is a semi-crystalline polymer and the diffractogram of PP-ref corresponds to the α-monoclinic form of isotactic polypropylene [
25]. On the PP/TiO
2-ref diffractogram in
Figure 4a, three new signals at 25.3°, 37.8°, and 47.9° are distinctly observed. These three signals are respectively related to the (101), (004), and (200) crystal planes of anatase TiO
2 [
26]. However, in the PP-Ti(O
iPr)
4-Ac
2O composite pattern, the absence of signals other than those of the polypropylene ones evidenced that the in situ synthesized TiO
2 was in an amorphous state. These observations are consistent with previous results obtained for in situ hydrolytic sol-gel synthesis of TiO
2 in molten polypropylene [
9,
19] and also with our first study dedicated to the in situ NHSG synthesis of TiO
2 in polypropylene [
13].
A similar behavior is observed for the PS-based composites (
Figure 4b). The in situ synthesized TiO
2 was in an amorphous state in the PS-Ti(O
iPr)
4-Ac
2O nanocomposite as in the case of PP-Ti(O
iPr)
4-Ac
2O. As polystyrene is an amorphous polymer, its X-ray diffractogram features two broad signals at 10° and 20°. In the PS/TiO
2-ref sample, diffraction peaks due to the presence of nano-crystalline TiO
2 are therefore easily distinguished. The same three peaks corresponding to anatase structure as previously observed are present, as well as a weak peak at 27.4° corresponding to the (110) crystal plane of rutile form [
26]. In the PP/TiO
2-ref diffractogram, this peak was not observed due to the overlap with the crystalline PP peaks. Actually, the commercial TiO
2 filler used includes about 80% of anatase phase and 20% of rutile phase [
27].
The amorphous nature of the filler created by NHSG reactions is consistent with our previous results [
7,
9,
13,
19] and the present processing conditions: short reactions times and viscous reactional medium.
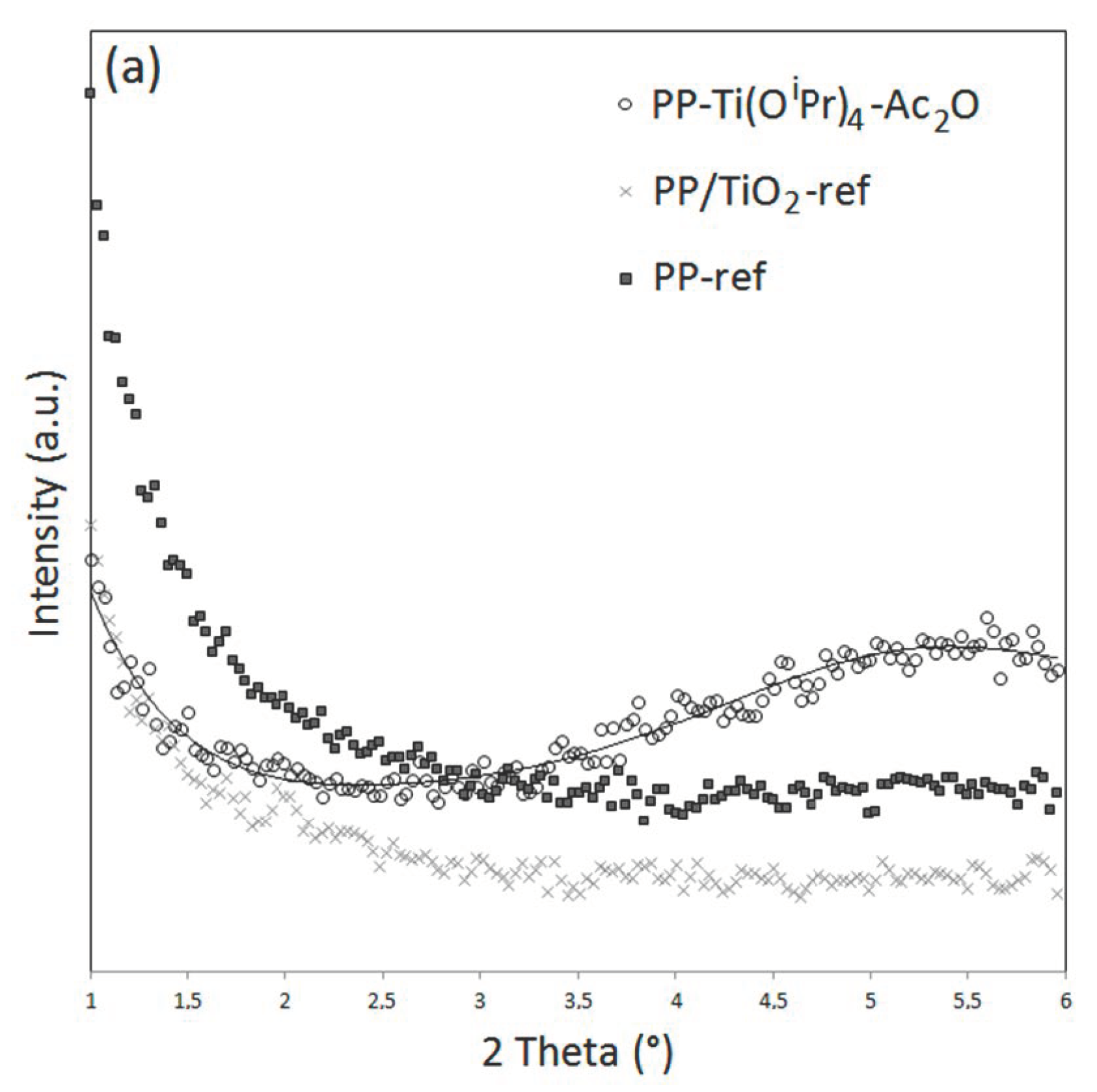
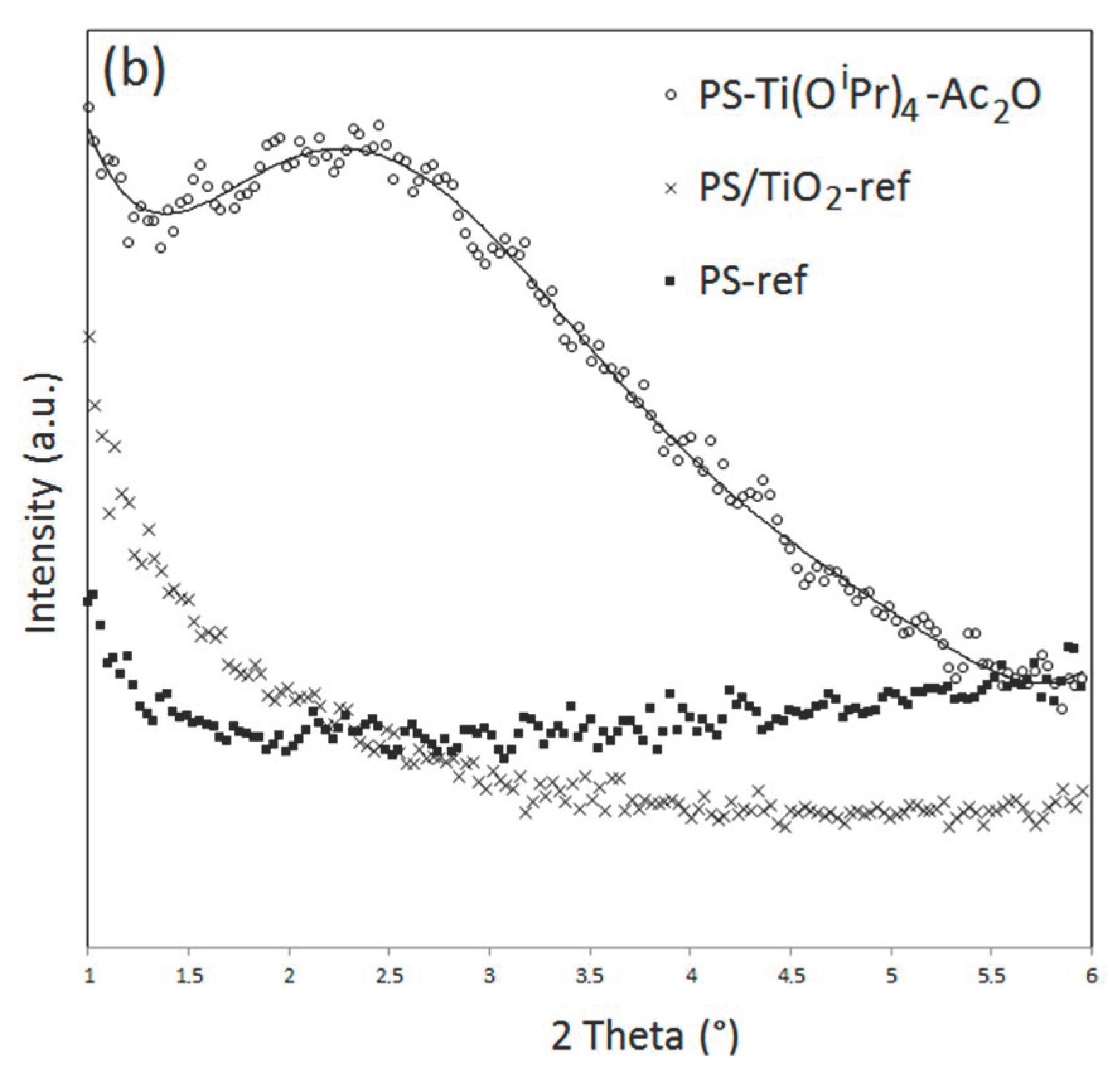
In addition, a rise in signal intensity at a low angle is observed on the diffractograms of PS-Ti(O
iPr)
4-Ac
2O. In order to investigate the material’s behavior at a low angle, XRD measurements were performed in the 2Ɵ range between 1 and 6° (
Figure 5). These analyses confirmed the presence of a broad intense signal centered at 2.2° for the PS-Ti(O
iPr)
4-Ac
2O nanocomposite but not for the PS and PS/TiO
2 references. For PP-Ti(O
iPr)
4-Ac
2O sample, a broad less intense signal centered at 5.5° can be also observed for the composites obtained in situ. This signal has already been observed in other studies concerned with the in situ generation of TiO
2 nanofillers [
19,
28] and related to a nanofiller dispersion state in the polymer matrix. Actually, Wu [
28] by studying the XRD pattern of PCL/TiO
2 and PCL-g-AA/TiO
2 elaborated by the in situ synthesis of TiO
2 fillers into the molten polymer evidenced a signal shift from 2.9° for PCL/TiO
2 down to 2.6° for PCL-g-AA/TiO
2. The authors explained the shift of angle by a better nanofiller dispersion state in the PCL-g-AA/TiO
2 nanocomposite thanks to the grafted polymer. Based on these observations, our results could corroborate the most homogeneous dispersion observed by electron microscopy analysis for the PS-based system. Note that the signal at a very low angle is that of the direct beam.
All these data confirmed that the in situ synthesis of TiO2 filler by NHSG led to finer and more homogeneous dispersed fillers in a viscous PS compared to the ones created in a PP matrix due to the difference of affinity between the inorganic precursor and the matrix, the polymer viscosity, and the condensation degree. These observations are very interesting and agree with that is observed classically for NHSG carried out in liquid media. It confirmed that our viscous polymer can be considered as a specific “solvent” medium impacting the final morphology of the nanocomposites.
Finally, the thermal stability of the nanocomposites was evaluated. TGA thermograms in
Figure S1 evidenced the absence of mass loss before 300 °C for both PP-Ti(O
iPr)
4-Ac
2O and PS-Ti(O
iPr)
4-Ac
2O, confirming the complete evaporation of by-products during the synthesis, as previously postulated. A slight mass loss between 310 and 360 °C was observed in PP-Ti(O
iPr)
4-Ac
2O and PS-Ti(O
iPr)
4-Ac
2O samples thermograms, which could correspond to by-products elimination throughout the residual condensation reactions occurring during the TGA analysis.
Overlay of PS-ref, PS/TiO
2-ref and PS-Ti(O
iPr)
4-Ac
2O thermograms (
Figure S1b) evidenced a modification of the degradation temperature range with the introduction of TiO
2 in the PS matrix. Actually, the polystyrene degradation for the neat polymer occurs between 350 and 440 °C, whereas the polystyrene matrix degradation for the two PS/TiO
2 composites starts at 380 °C. The presence of TiO
2 fillers leads to an improvement of the thermal stability of the PS matrix. This behavior has already been reported for PS/TiO
2 nanocomposites [
29,
30,
31]. According to the authors, the presence of TiO
2 nanofillers results in reduced mobility of the polymer chains and therefore enhances the energy needed for polystyrene chain breakage. A slight increase in polypropylene degradation temperature was also observed for PP-Ti(O
iPr)
4-Ac
2O in comparison with the PP-ref sample in
Figure S1a. Actually, PP degradation starts at 385 °C for the PP-Ti(O
iPr)
4-Ac
2O composite whereas for neat PP, the mass loss starts at a lower temperature around 350 °C. It is noteworthy that it remains about 5wt% of inorganic moieties after 500 °C in each nanocomposite, as expected.
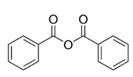
3.2. Impact of Aromatic Oxygen Donors Nature with Polystyrene Matrix
Based on these previous results and the literature analysis [
13,
16], it appears interesting, complementary to the influence of the polymer nature, to study the impact of other oxygen donors on the nanofiller formation and resulting nanocomposite morphology. In particular, oxygen donors with aromatic groups should be suitable to promote interactions between TiO
2 fillers and polystyrene matrix. For that purpose, two aromatic molecules were selected as oxygen donors to react with Ti(O
iPr)
4: benzoic anhydride and acetophenone. To estimate the affinity of these two molecules with the polystyrene matrix, solubility parameters were calculated according to Fedor’s method [
17]. The theoretical values for benzoic anhydride and acetophenone are respectively 22.9 and 21.1 (MPa)
1/2, thus close to the value of polystyrene (δ
PS = 21.1 (MPa)
1/2) in particular for acetophenone.
First, the system with benzoic anhydride was discussed by successively evaluating the reaction mechanism and advancement and more specifically the condensation degree followed by the morphology analysis.
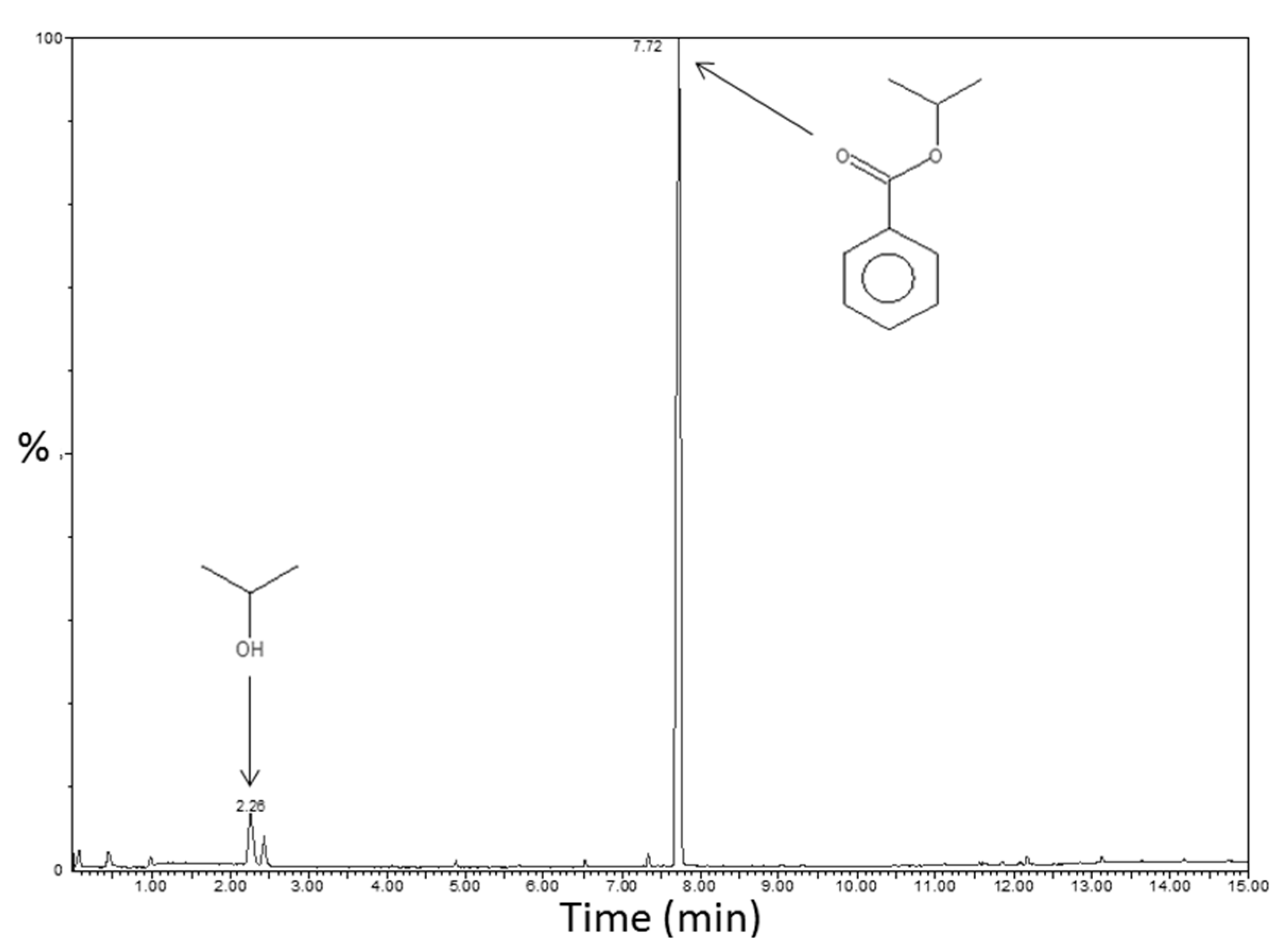
The expected non-hydrolytic sol-gel reaction is described in
Figure S2. It leads to isopropyl benzoate as a by-product. TDA-GC-MS coupling analyses were performed on the sample PS-Ti(O
iPr)
4-BzA with the aim to confirm the proposed reaction mechanism thanks to the identification of the molecules contained in the system (especially the created by-product). The chromatogram with the identified molecules is displayed in
Figure 6. The predominant molecule in PS-Ti(O
iPr)
4-BzA nanocomposite was the expected isopropyl benzoate, at a retention time of 7.7 min. A small signal was also present at a retention time of about 2.3 min and corresponds to isopropanol, due to a marginal amount of titanium isopropoxide hydrolysis reactions. No signal attributable to benzoic anhydride was present on the chromatogram, suggesting a complete conversion for the first reaction step.
To confirm the complete conversion of benzoic anhydride, by-products were extracted from the polystyrene matrix by immersion of the nanocomposite grounded into powder in glacial acetic acid for 24 h under stirring. The purified nanocomposite was recovered by filtration and analyzed. FTIR analyses on PS-Ti(O
iPr)
4-BzA sample before and after extraction allowed confirming the efficiency of the extraction (
Figure S3). Total disappearance of signals corresponding to ester by-product (C = O stretching vibration of ester close to an aromatic group at 1712 cm
−1, C-O stretching vibrations of ester at 1272 cm
−1 and at 1090 cm
−1) [
32] was evidenced and confirmed the efficiency of the purification. Moreover, the absence of a signal at 1770 cm
−1 confirms the complete reaction of benzoic anhydride.
Another way to ascertain the by-products extraction in PS-Ti(O
iPr)
4-BzA nanocomposite was to perform thermogravimetric analyses. TGA thermograms before and after extraction are displayed in
Figure S4. Before extraction, a mass loss of 14wt% was observed between 100 °C and 350 °C that could be assigned to isopropyl benzoate. For a complete condensation, the theoretical amount of isopropyl benzoate in composite was 29wt%. However, as the boiling point of this ester is 218 °C, i.e., lower than the reaction temperature, a part of the by-product could have evaporated during the nanocomposite elaboration in the internal mixer. Consequently, no conclusion on the condensation degree can be drawn from TGA results. After by-products extraction, a low mass loss of 2.6% still occurred before 350 °C, potentially due to condensation reactions occurring during the analysis. It was noteworthy that after the polystyrene degradation, 3.5wt% of inorganic residue was obtained in the nanocomposite before by-products extraction which is lower than the expected amount of inorganic fraction of 5wt%. Moreover, a part of the inorganic compound was also probably extracted from the nanocomposite during the immersion in glacial acetic acid. Indeed, the amount of inorganic residue at 525 °C (2.3wt%) is lower than that measured before extraction (3.5wt%).
As previously described, the condensation degree was determined by XPS analysis on purified sample, according to the respective area of the two signals at 530 eV and 532 eV (
Figure S5). A condensation degree value of 26% was obtained, slightly higher than the condensation degree of 20% determined for TiO
2 synthesized from acetic anhydride in the PS-Ti(O
iPr)
4-Ac
2O nanocomposite. Wang et al. [
16] also obtained a low condensation degree for the synthesis of TiO
2 fillers by the benzoic anhydride route in toluene. Indeed, a calcination yield of 53% was determined for this sample whereas the calcination yields for samples prepared by the ether and acetophenone routes were much higher (>90%).
As elucidated in the first part of this study, the influence of the inorganic precursor/polymer affinity and of the condensation degree on the morphology was evidenced. The dispersion state of TiO
2 fillers in PS-Ti(O
iPr)
4-BzA sample was investigated by electron microscopy. SEM and TEM micrographs are displayed in
Figure 7. The in situ synthesis of TiO
2 from Ti(O
iPr)
4 and benzoic anhydride led to the formation of nano-objects with a homogeneous dispersion according to SEM images. TEM observations of the nanocomposite revealed a platelet-like morphology, with fillers observed on the edge, which was also evidenced by the TEM image of extracted fillers. The thickness of fillers appeared lower than 50 nm on the TEM micrograph of PS-Ti(O
iPr)
4-BzA nanocomposite, and their length was in the micrometer range. This filler shape was very different compared with PS-Ti(O
iPr)
4-Ac
2O sample. For the first time, we demonstrated the possibility to control a specific filler shape by the association of NHSG and reactive polymer processing. Actually, three factors had a significant influence on the morphology of obtained fillers: the nature of residual groups, the synthesis route, and the solvent [
15]. The presence of phenyl groups in the case of PS-Ti(O
iPr)
4-BzA sample could lead to the formation of π-π interactions between aromatic rings of polystyrene matrix and aromatic rings of benzoate groups which could influence the growth and shape of the TiO
2 fillers.
As previously observed, TiO
2 synthesized in situ by the benzoic anhydride route in our experimental conditions was amorphous, as shown by X-ray diffractograms in
Figure S6. A broad signal was present at a low angle (at 5.01°) in the PS-Ti(O
iPr)
4-BzA diffractogram. As seen for PP-Ti(O
iPr)
4-Ac
2O and PS-Ti(O
iPr)
4-Ac
2O nanocomposites, the presence of this signal may suggest a fillers organization at a long distance.
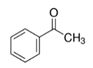
In the literature, another aromatic oxygen donor was used for the synthesis of TiO
2 and BaTiO
3 which is the acetophenone [
16,
33,
34]. The reaction mechanism between Ti(O
iPr)
4 and ketones was proposed by Garnweitner et al. [
33] (
Figure S7). This reaction involves the elimination of 1,3-diephenylbut-2-en-1-one (dypnone) and isopropanol. Another mechanism has been proposed by Pazik et al. [
35] and leads to the formation of the ketal compound PhCMe(O
iPr)
2 considering two equivalent of acetophenone in relation to Ti atoms.
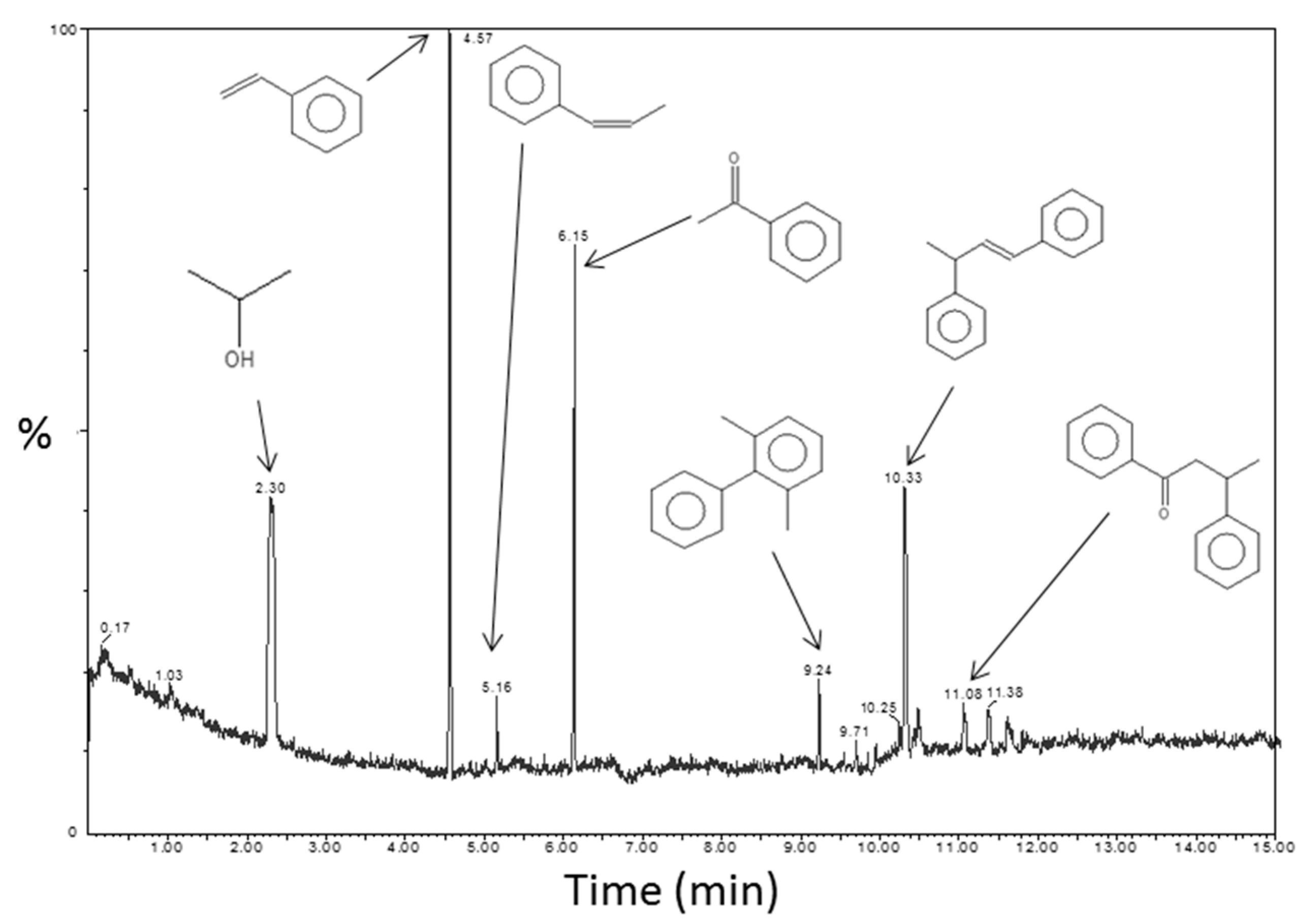
TDA-GC-MS coupling analyses were performed on the PS-Ti(O
iPr)
4-Aph nanocomposite for confirming the proposed reaction mechanism. A chromatogram with corresponding molecules identified by mass spectrometry is displayed in
Figure 8. The first signal at 2.3 min corresponding to isopropanol was one of the expected by-products of the reaction mechanism described in
Figure S7. The highest signal was attributable to styrene and can be the result of residual monomers from the polystyrene matrix or a reductive dehydration of acetophenone with isopropanol [
36]. A small amount of styrene was actually present in the chromatogram of the PS matrix (not shown) but the presence of styrene can be explained by the contribution of the two phenomena mentioned above. The presence of a signal for prop-1-en-1-ylbenzene at 5.2 min can be explained in the same way. A part of acetophenone did not react, as evidenced by the presence of the signal at 6.2 min. Neither dypnone nor PhCMe(O
iPr)
2 were present in the PS-Ti(O
iPr)
4-Aph composite according to this analysis. Indeed, the formation of dypnone would involve the presence of a signal at around 12.3 min in the chromatogram. However, some other molecules with two aromatic groups were identified: 1,3-dimethyl-2-phenylbenzene, 3-phenylbut-1-en-1-ylbenzene and 1,3-diphenylpropan-1-one. These molecules could result from reactions between intermediate reactants and by-products. The reaction mechanism was not actually as simple as described previously by Garnweitner et al. [
33].
The residual reactants and by-products were extracted from the polystyrene matrix by immersion of nanocomposites grounded into powder in glacial acetic acid for 24 h under stirring, as described for PS-Ti(O
iPr)
4-BzA sample. TGA analyses before and after extraction were performed (
Figure S8) to confirm the total elimination of residual acetophenone as well as other by-products.
Before extraction, the mass loss between 100 °C and 350 °C was around 7.3wt%. As seen by TDA-GC-MS coupling analyses, this mass loss corresponded to residual acetophenone and by-products. The expected amount of by-product for a complete condensation was around 16wt% but the presence of unreacted oxygen donor and the possible evaporation of isopropanol and dypnone during the synthesis prevented the determination of a condensation degree. The residual inorganic part at 525 °C was also lower than expected (3.2wt% instead of 5.0wt%). The immersion of nanocomposite in glacial acetic acid allowed extracting acetophenone and by-products (2.1wt% of mass loss between 100 °C and 350 °C instead of 7.3wt%). Besides a lower inorganic residue measured at high temperature after purification may be as in the case of PS-Ti(OiPr)4-BzA nanocomposite due to a partial extraction of low condensed titanium-based species from the nanocomposite. These analyses confirmed the uncomplete reaction of the acetophenone contrary to the previously used benzoic anhydride.
All further following characterizations were performed on purified PS-Ti(OiPr)4-Aph sample, i.e., after extraction step of by-products by immersion in glacial acetic acid.
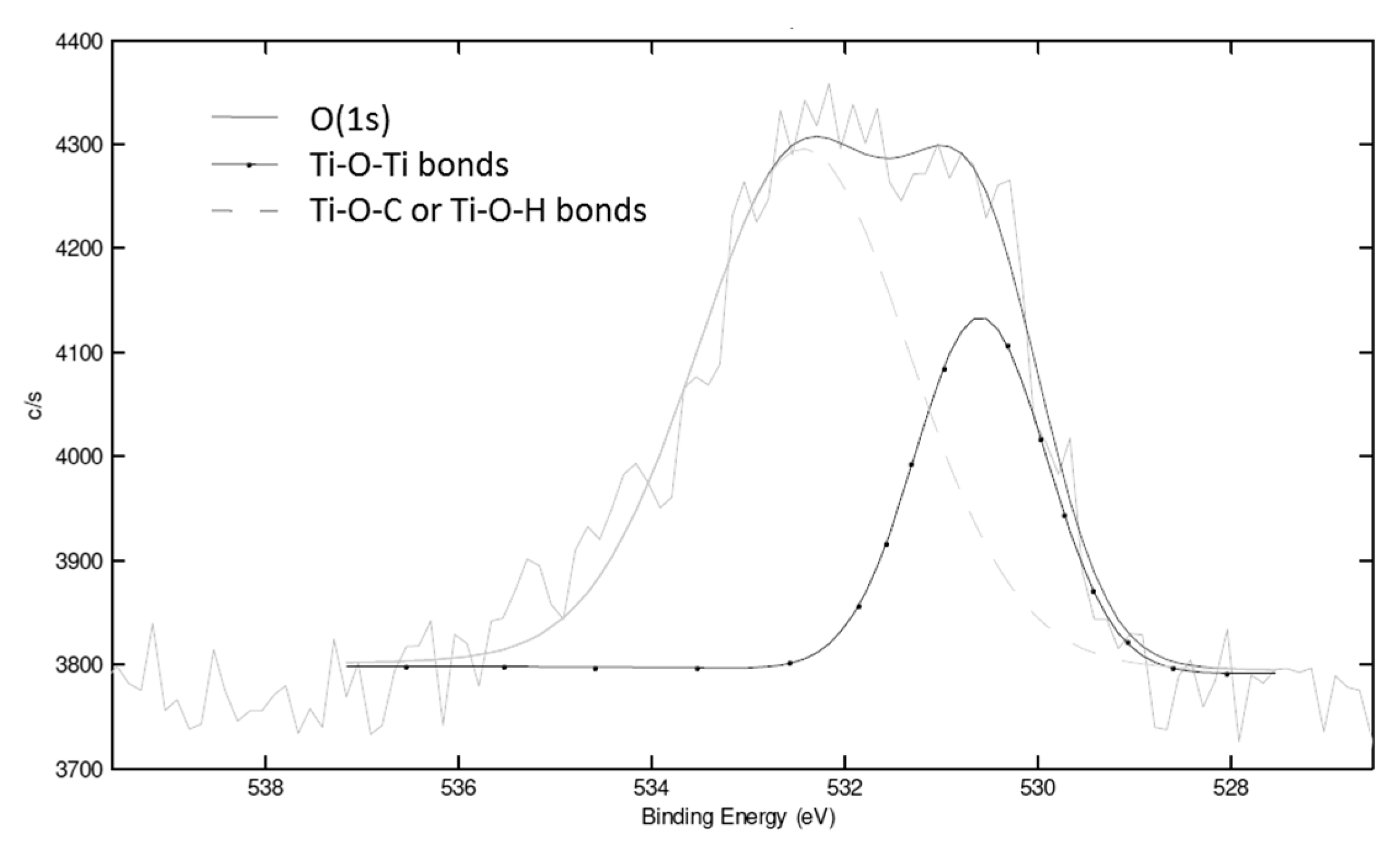
XPS analysis was used to determine the condensation degree of the NHSG reaction. From the C, O and Ti atomic concentrations of 97.5, 1.1 and 0.3%, respectively, and from the area of the O(1s) signal at around 530 eV and 532 eV (-Ti-O-Ti- and -Ti-O-H or -Ti-O-C respectively) (
Figure 9), a condensation degree of 45% was determined which was higher than for PS-Ti(O
iPr)
4-BzA sample (26%).
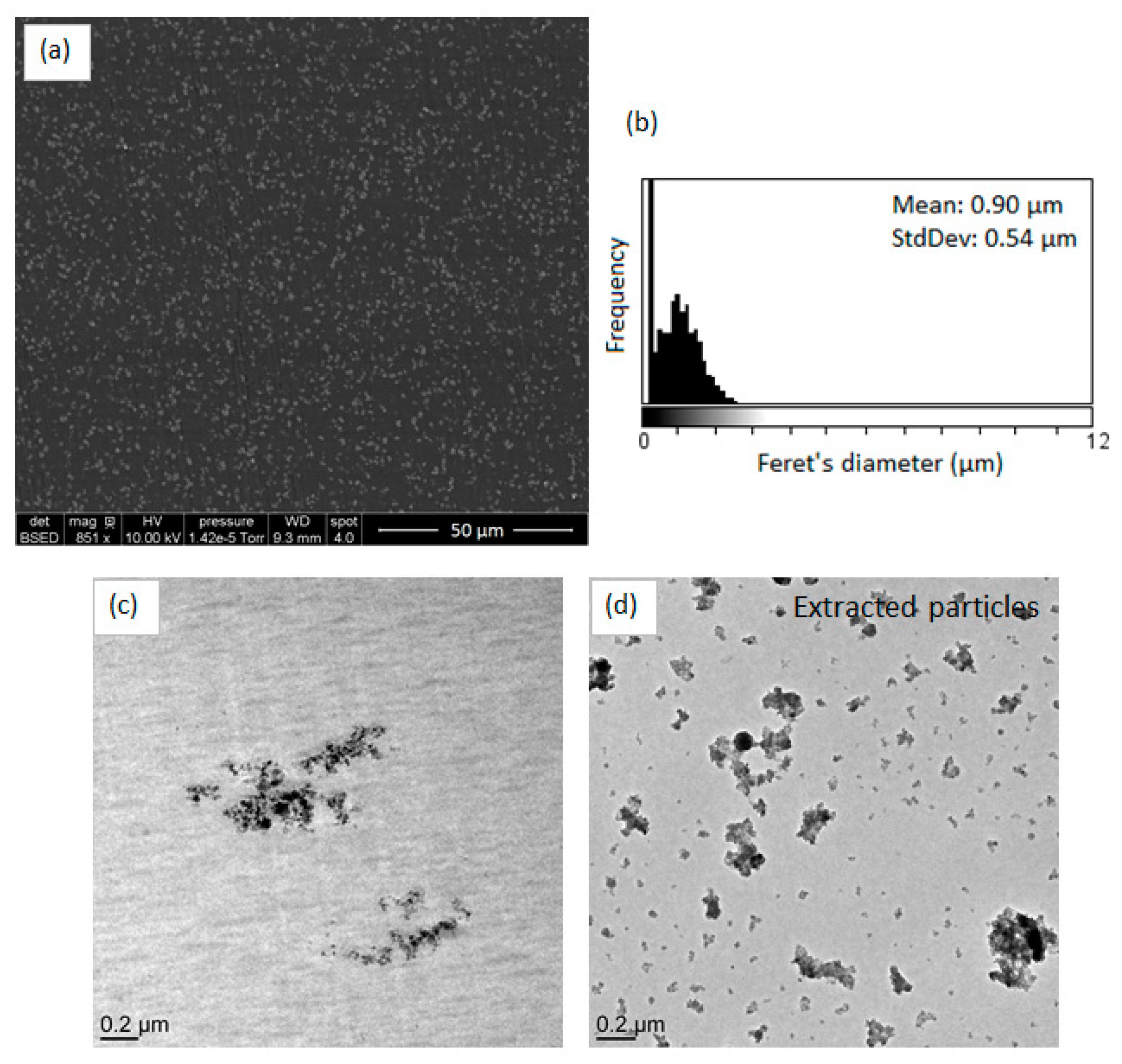
SEM and TEM micrographs of the PS-Ti(O
iPr)
4-Aph nanocomposite and extracted fillers are displayed in
Figure 10. We can observe that the use of acetophenone as an oxygen donor led to the formation of TiO
2 nanofillers forming homogeneously dispersed aggregates with a mean diameter of approximately 900 nm. The morphology and the organization of TiO
2 fillers in the PS matrix were close to the ones observed for PS-Ti(O
iPr)
4-Ac
2O composite. The TEM micrograph of extracted fillers (
Figure 10d) showed the presence of single fillers and aggregates. The mean Feret’s diameter of primary fillers, determined by ImageJ software, was 15.0 nm ± 4.2 nm. Although the oxygen donor contains an aromatic group, the TiO
2 fillers morphology is quite different from the TiO
2 fillers synthesized from benzoic anhydride evidencing again also the role of the condensation degree on the final morphology.
As shown by X-ray diffractogram at wide angle (
Figure S9), TiO
2 synthesized in situ in viscous polystyrene is amorphous. A slight increase of the intensity was observed in the PS-Ti(O
iPr)
4-Aph pattern at a low angle that could again suggest a better organization of fillers at a long distance as seen previously for the other PS based-systems.
As already described, oxygen donor played an essential role in the particles’ morphology and dispersion in non-hydrolytic sol-gel reactions in the solvent medium [
16,
37,
38]. We depicted a similar effect in this work while the polystyrene acts as a solvent. In our case, the selection of the non-hydrolytic sol-gel route and the oxygen donor associated to the polymer processing conditions allowed tuning the particles’ morphology. We have underlined this potentiality and this richness by comparing two aromatic oxygen donors, leading to distinct morphologies.