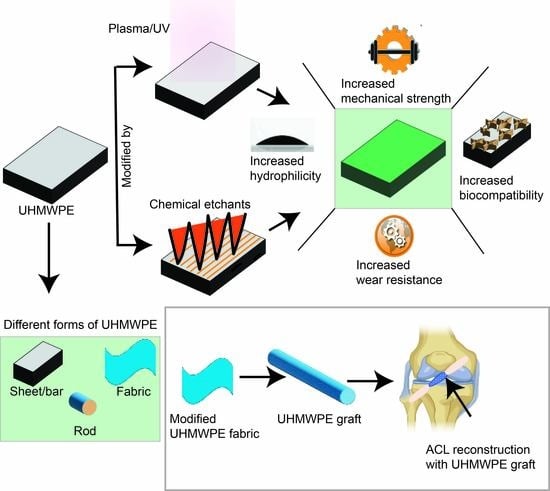
Functional Ultra-High Molecular Weight Polyethylene Composites for Ligament Reconstructions and Their Targeted Applications in the Restoration of the Anterior Cruciate Ligament
Abstract
:1. Introduction
2. Background of UHMWPE as Orthopedic Implants
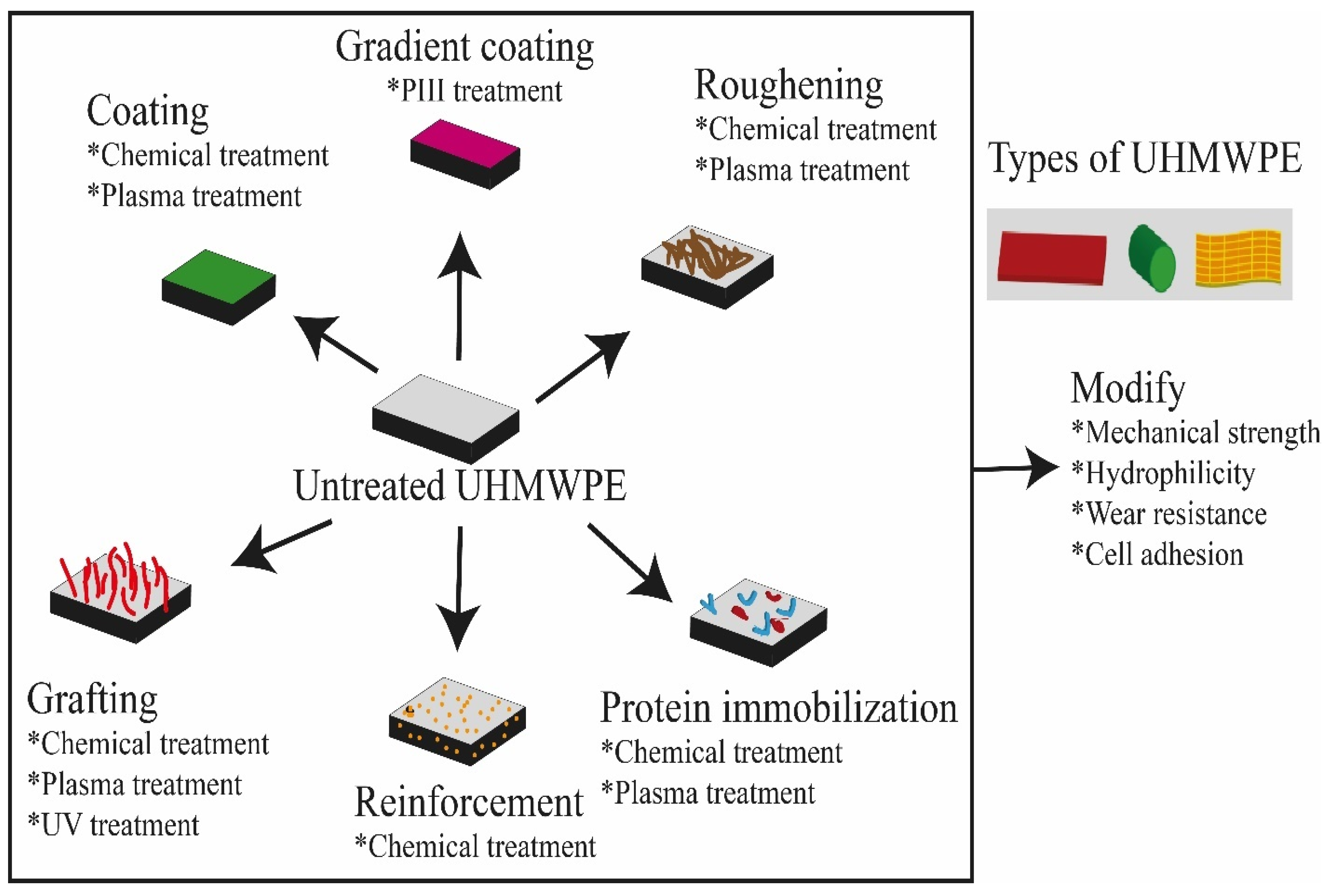
3. Methodologies for Surface Modification of UHMWPE
3.1. Chemical Treatment
3.2. Surface Modification of UHMWPE by Different Plasma (DBD, PACVD, ECR, CAP, PIII) and Gamma Irradiation Methods
3.2.1. Cold Atmospheric Plasma (CAP)
3.2.2. Plasma-Assisted Chemical Vapor Deposition (PACVD)
3.2.3. Electron Cyclotron Resonance (ECR) Plasma
3.2.4. Dielectric Barrier Discharge (DBD) Plasma
3.2.5. Grafting on UHMWPE by Gamma Irradiation
3.2.6. Plasma Immersion Ion Implantation (PIII)
3.2.7. Nano Reinforcement
4. Biofunctionalization of UHMWPE by Protein Immobilization
4.1. Protein Immobilization by Chemical Methods
4.2. Protein Immobilization by Plasma Methods
5. Relationship between Human Ligament and UHMWPE
5.1. Structure of Anterior Cruciate Ligament (ACL)
5.2. ACL Injury and Selection of UHMWPE for ACL Reconstruction
6. Fixation of UHMWPE Graft Animal Models for Ligament Reconstruction
7. Conclusions
8. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobbi, S.J.; Gobbi, J.V.; Rocha, Y. Requirements for Selection/Development of a Biomaterial. Biomed. J. Sci. Tech. Res. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Muratoglu, O.K.; Evans, M.; Edidin, A.A. Advances in the Processing, Sterilization, and Crosslinking of Ultra-High Molecular Weight Polyethylene for Total Joint Arthroplasty. Biomaterials 1999, 20, 1659–1688. [Google Scholar] [CrossRef]
- Oosterom, R.; Ahmed, T.J.; Poulis, J.A.; Bersee, H.E.N. Adhesion Performance of UHMWPE after Different Surface Modification Techniques. Med. Eng. Phys. 2006, 28, 323–330. [Google Scholar] [CrossRef]
- Diabb Zavala, J.M.; Leija Gutiérrez, H.M.; Segura-Cárdenas, E.; Mamidi, N.; Morales-Avalos, R.; Villela-Castrejón, J.; Elías-Zúñiga, A. Manufacture and Mechanical Properties of Knee Implants Using SWCNTs/UHMWPE Composites. J. Mech. Behav. Biomed. Mater. 2021, 120, 104554. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Cao, L.; Wang, J.; Qu, Y.; Wang, X.; Qiu, R.; Di, X.; Wang, Z.; Liang, B. Response of MG63 Osteoblast Cells to Surface Modification of Ti-6Al-4V Implant Alloy by Laser Interference Lithography. J. Bionic Eng. 2017, 14, 448–458. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of Topography and Composition of Titanium Surface Oxides on Osteoblast Responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef]
- Gilman, A.B.; Piskarev, M.S.; Kuznetsov, A.A.; Ozerin, A.N. Modification of Ultrahigh-Molecular-Weight Polyethylene by Low-Temperature Plasma (Review). High Energy Chem. 2017, 51, 136–144. [Google Scholar] [CrossRef]
- Ge, S.; Wang, Q.; Zhang, D.; Zhu, H.; Xiong, D.; Huang, C.; Huang, X. Friction and Wear Behavior of Nitrogen Ion Implanted UHMWPE against ZrO2 Ceramic. Wear 2003, 255, 1069–1075. [Google Scholar] [CrossRef]
- Rodrigues, M.M.; Fontoura, C.P.; Dotta Maddalozzo, A.E.; Leidens, L.M.; Quevedo, H.G.; Dos Santos Souzac, K.; Da Silva Crespo, J.; Michels, A.F.; Figueroa, C.A.; Aguzzoli, C. Ti, Zr and Ta Coated UHMWPE Aiming Surface Improvement for Biomedical Purposes. Compos. Part B Eng. 2020, 189, 107909. [Google Scholar] [CrossRef]
- Duraccio, D.; Strongone, V.; Malucelli, G.; Auriemma, F.; De Rosa, C.; Mussano, F.D.; Genova, T.; Faga, M.G. The Role of Alumina-Zirconia Loading on the Mechanical and Biological Properties of UHMWPE for Biomedical Applications. Compos. Part B Eng. 2019, 164, 800–808. [Google Scholar] [CrossRef]
- Reynolds, S.E.; Malkani, A.L.; Ramakrishnan, R.; Yakkanti, M.R. Wear Analysis of First-Generation Highly Cross-Linked Polyethylene in Primary Total Hip Arthroplasty. An Average 9-Year Follow-Up. J. Arthroplast. 2012, 27, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.L.; Forster, A.M.; Chin, J.W.; Peng, J.S.; Lin, C.C.; Petit, S.; Kang, K.L.; Paulter, N.; Riley, M.A.; Rice, K.D.; et al. Long-Term Stability of UHMWPE Fibers. Polym. Degrad. Stab. 2015, 114, 45–51. [Google Scholar] [CrossRef]
- Silverstein, M.S.; Breuer, O. Relationship between Surface Properties and Adhesion for Etched Ultra-High-Molecular-Weight Polyethylene Fibers. Compos. Sci. Technol. 1993, 48, 151–157. [Google Scholar] [CrossRef]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A Review of Biomimetic Surface Functionalization for Bone-Integrating Orthopedic Implants: Mechanisms, Current Approaches, and Future Directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Wang, J.-H. The relationship between zeta-potential and pull-out shear strength on modified UHMWPE fiber reinforced epoxy composites. Polym. Compos. 1998, 19, 347–351. [Google Scholar] [CrossRef]
- Sharma, V.; Bose, S.; Kundu, B.; Bodhak, S.; Mitun, D.; Balla, V.K.; Basu, B. Probing the Influence of γ-Sterilization on the Oxidation, Crystallization, Sliding Wear Resistance, and Cytocompatibility of Chemically Modified Graphene-Oxide-Reinforced HDPE/UHMWPE Nanocomposites and Wear Debris. ACS Biomater. Sci. Eng. 2020, 6, 1462–1475. [Google Scholar] [CrossRef]
- Wang, J.; Liang, G.; Zhao, W.; Lü, S.; Zhang, Z. Studies on Surface Modification of UHMWPE Fibers via UV Initiated Grafting. Appl. Surf. Sci. 2006, 253, 668–673. [Google Scholar] [CrossRef]
- Serro, A.P.; Degiampietro, K.; Colaço, R.; Saramago, B. Adsorption of Albumin and Sodium Hyaluronate on UHMWPE: A QCM-D and AFM Study. Colloids Surf. B Biointerfaces 2010, 78, 1–7. [Google Scholar] [CrossRef]
- Kostov, K.G.; Ueda, M.; Tan, I.H.; Leite, N.F.; Beloto, A.F.; Gomes, G.F. Structural Effect of Nitrogen Plasma-Based Ion Implantation on Ultra-High Molecular Weight Polyethylene. Surf. Coat. Technol. 2004, 186, 287–290. [Google Scholar] [CrossRef]
- Kar, R.; Barve, S.A.; Singh, S.B.; Barve, D.N.; Chand, N.; Patil, D.S. Diagnostics of Microwave ECR Generated Plasma: Effect of Contaminated Reference Electrode. Vacuum 2010, 85, 151–155. [Google Scholar] [CrossRef]
- Struszczyk, M.H.; Puszkarz, A.K.; Wilbik-Hałgas, B.; Cichecka, M.; Litwa, P.; Urbaniak-Domagała, W.; Krucinska, I. The Surface Modification of Ballistic Textiles Using Plasma-Assisted Chemical Vapor Deposition (PACVD). Text. Res. J. 2014, 84, 2085–2093. [Google Scholar] [CrossRef]
- Tomita, N.; Kitakura, T.; Onmori, N.; Ikada, Y.; Aoyama, E. Prevention of Fatigue Cracks in Ultrahigh Molecular Weight Polyethylene Joint Components by the Addition of Vitamin E. J. Biomed. Mater. Res. 1999, 48, 474–478. [Google Scholar] [CrossRef]
- Ahmadi, M.; Masoomi, M.; Safi, S. Mechanical Property Characterization of Carbon Nanofiber/Epoxy Nanocomposites Reinforced by GMA-Grafted UHMWPE Fibers. Compos. Part B Eng. 2015, 83, 43–49. [Google Scholar] [CrossRef]
- Da Silva Chagas, N.P.; De Oliveira Aguiar, V.; Da Costa Garcia Filho, F.; Da Silva Figueiredo, A.B.H.; Monteiro, S.N.; Huaman, N.R.C.; Marques, M.D.F.V. Ballistic Performance of Boron Carbide Nanoparticles Reinforced Ultra-High Molecular Weight Polyethylene (UHMWPE). J. Mater. Res. Technol. 2022, 17, 1799–1811. [Google Scholar] [CrossRef]
- Stürzel, M.; Kempe, F.; Thomann, Y.; Mark, S.; Enders, M.; Mülhaupt, R. Novel Graphene UHMWPE Nanocomposites Prepared by Polymerization Filling Using Single-Site Catalysts Supported on Functionalized Graphene Nanosheet Dispersions. Macromolecules 2012, 45, 6878–6887. [Google Scholar] [CrossRef]
- Hamedi, G.H.; Ranjbar Pirbasti, Z. The Effects of UHMWPE/Nanoclay on Rheological Properties of Modified Asphalt Binder. Pet. Sci. Technol. 2020, 38, 309–315. [Google Scholar] [CrossRef]
- Hussain, O.; Ahmad, B.; Saleem, S. Tribological Performance of Biomedical Grade UHMWPE/Nano-Al2O3/Vitamin-C Hybrid Composite for Cartilage Replacements. Mater. Lett. 2021, 291, 129515. [Google Scholar] [CrossRef]
- Xiong, D.; Yang, Y.; Deng, Y. Bio-Tribological Properties of UHMWPE against Surface Modified Titanium Alloy. Surf. Coat. Technol. 2013, 228, S442–S445. [Google Scholar] [CrossRef]
- Kumar, N.N.; Yap, S.L.; Bt Samsudin, F.N.D.; Khan, M.Z.; Srinivasa, R.S.P. Effect of Argon Plasma Treatment on Tribological Properties of UHMWPE/MWCNT Nanocomposites. Polymers 2016, 8, 295. [Google Scholar] [CrossRef] [Green Version]
- Bracco, P.; Brunella, V.; Luda, M.P.; Zanetti, M.; Costa, L. Radiation-Induced Crosslinking of UHMWPE in the Presence of Co-Agents: Chemical and Mechanical Characterisation. Polymer 2005, 46, 10648–10657. [Google Scholar] [CrossRef]
- Shen, J.; Costa, L.; Xu, Y.; Cong, Y.; Cheng, Y.; Liu, X.; Fu, J. Stabilization of Highly Crosslinked Ultra High Molecular Weight Polyethylene with Natural Polyphenols. Polym. Degrad. Stab. 2014, 105, 197–205. [Google Scholar] [CrossRef]
- More, S.E.; Dave, J.R.; Makar, P.K.; Bhoraskar, S.V.; Premkumar, S.; Tomar, G.B.; Mathe, V.L. Surface Modification of UHMWPE Using ECR Plasma for Osteoblast and Osteoclast Differentiation. Appl. Surf. Sci. 2019, 506, 144665. [Google Scholar] [CrossRef]
- Liu, H.; Xie, D.; Qian, L.; Deng, X.; Leng, Y.X.; Huang, N. The Mechanical Properties of the Ultrahigh Molecular Weight Polyethylene (UHMWPE) Modified by Oxygen Plasma. Surf. Coat. Technol. 2011, 205, 2697–2701. [Google Scholar] [CrossRef]
- Bach, J.S.; Detrez, F.; Cherkaoui, M.; Cantournet, S.; Ku, D.N.; Corté, L. Hydrogel Fibers for ACL Prosthesis: Design and Mechanical Evaluation of PVA and PVA/UHMWPE Fiber Constructs. J. Biomech. 2013, 46, 1463–1470. [Google Scholar] [CrossRef]
- Ianuzzi, A.; Mkandawire, C. Applications of UHMWPE in Total Ankle Replacements, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Ai, C.; Sheng, D.; Chen, J.; Cai, J.; Wang, S.; Jiang, J.; Chen, S. Surface Modification of Vascular Endothelial Growth Factor-Loaded Silk Fibroin to Improve Biological Performance of Ultra-High-Molecular-Weight Polyethylene via Promoting Angiogenesis. Int. J. Nanomed. 2017, 12, 7737–7750. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Xiong, D.; Wang, K. Biotribological Properties of UHMWPE Grafted with AA under Lubrication as Artificial Joint. J. Mater. Sci. Mater. Med. 2013, 24, 2085–2091. [Google Scholar] [CrossRef]
- Sun, R.; Feng, Y.; Zhang, Z.; Yan, L.; He, Z.; Zhang, Z.; Yan, L. Research Progress on Surface Modification and Application Status of UHMWPE Fiber. J. Phys. Conf. Ser. 2022, 2263, 012016. [Google Scholar] [CrossRef]
- Silverstein, M.S.; Breuer, O.; Dodiuk, H. Surface Modification of UHMWPE Fibers. J. Appl. Polym. Sci. 1994, 52, 1785–1795. [Google Scholar] [CrossRef]
- Sa, R.; Wei, Z.; Yan, Y.; Wang, L.; Wang, W.; Zhang, L.; Ning, N.; Tian, M. Catechol and Epoxy Functionalized Ultrahigh Molecular Weight Polyethylene (UHMWPE) Fibers with Improved Surface Activity and Interfacial Adhesion. Compos. Sci. Technol. 2015, 113, 54–62. [Google Scholar] [CrossRef]
- Hu, J.; Feng, X.; Liu, Z.; Zhao, Y.; Chen, L. Surface Amine-Functionalization of UHMWPE Fiber by Bio-Inspired Polydopamine and Grafted Hexamethylene Diamine. Surf. Interface Anal. 2017, 49, 640–646. [Google Scholar] [CrossRef]
- Corradetti, B.; Taraballi, F.; Martinez, J.O.; Minardi, S.; Basu, N.; Bauza, G.; Evangelopoulos, M.; Powell, S.; Corbo, C.; Tasciotti, E. Hyaluronic Acid Coatings as a Simple and Efficient Approach to Improve MSC Homing toward the Site of Inflammation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.R.; Ashby, P.D.; Pruitt, L.A. Characterization and Tribology of PEG-like Coatings on UHMWPE for Total Hip Replacements. J. Biomed. Mater. Res. Part A 2010, 92, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, S.; Yu, J.; Zhang, W.; Dai, J.; Zhang, Y.; Zhang, G. The Construction of a Hydrophilic Inorganic Layer Enables Mechanochemically Robust Super Antifouling UHMWPE Composite Membrane Surfaces. Polymers 2020, 12, 569. [Google Scholar] [CrossRef] [Green Version]
- Denes, F.; Young, R.A.; Sarmadi, M. Surface Functionalization of Polymers under Cold Plasma Conditions-A Mechanistic Approach. J. Photopolym. Sci. Technol. 2008, 10, 91–112. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, Z.; Wang, C.; Zang, C.; Zhang, Y.; Xu, L. Influence of DBD Plasma Pretreatment on the Deposition of Chitosan onto UHMWPE Fiber Surfaces for Improvement of Adhesion and Dyeing Properties. Appl. Surf. Sci. 2017, 396, 1571–1579. [Google Scholar] [CrossRef]
- Cools, P.; Van Vrekhem, S.; De Geyter, N.; Morent, R. The Use of DBD Plasma Treatment and Polymerization for the Enhancement of Biomedical UHMWPE. Thin Solid Film. 2014, 572, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Sun, D.C.; Yau, S.; Edwards, B.; Sokol, M.; Essner, A.; Polineni, V.K.; Stark, C.; Dumbleton, J.H. Orientation softening in the deformation and wear of ultra-high molecular weight polyethylene. Wear 1997, 204, 230–241. [Google Scholar] [CrossRef]
- Huot, J.C.; Van Citters, D.W.; Currier, J.H.; Collier, J.P. The Effect of Radiation Dose on the Tensile and Impact Toughness of Highly Cross-Linked and Remelted Ultrahigh-Molecular Weight Polyethylenes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 327–333. [Google Scholar] [CrossRef]
- Xia, B.; Xie, M.; Yang, B. Surface Modification of Ultrahigh Molecular Weight Polyethylene by the Poly(Ethylene Glycol)-Grafted Method and Its Effect on the Adsorption of Proteins and the Adhesion of Blood Platelets. J. Biomed. Mater. Res. Part A 2013, 101, 54–63. [Google Scholar] [CrossRef]
- Saikko, V. Effect of Serum Dilution Fluids on the Wear of Unirradiated and High Dose Gamma-Irradiated, Vitamin E Stabilized UHMWPE. Wear 2019, 430–431, 76–80. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wu, J.Y.; Tsai, C.S.; Hsieh, K.H.; Yeh, J.T.; Chen, K.N. Effects of Argon Plasma Treatment on the Adhesion Property of Ultra High Molecular Weight Polyethylene (UHMWPE) Textile. Surf. Coat. Technol. 2013, 231, 507–511. [Google Scholar] [CrossRef]
- Rossi, J.O.; Ueda, M.; Silva, A.R.; Neto, L.P.S. Improvement on UHMWPE and PMMA Surface Flashover Under Atmospheric Pressure Using PIII Processing. IEEE Trans. Plasma Sci. 2020, 48, 3386–3391. [Google Scholar] [CrossRef]
- Gan, B.K.; Kondyurin, A.; Bilek, M.M.M. Comparison of Protein Surface Attachment on Untreated and Plasma Immersion Ion Implantation Treated Polystyrene: Protein Islands and Carpet. Langmuir 2007, 23, 2741–2746. [Google Scholar] [CrossRef]
- Kondyurin, A.; Nosworthy, N.J.; Bilek, M.M.M. Effect of Low Molecular Weight Additives on Immobilization Strength, Activity, and Conformation of Protein Immobilized on PVC AndUHMWPE. Langmuir 2011, 27, 6138–6148. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Domínguez-Díaz, M.; Paniagua, R.; Hernández-Pérez, J.A.; Martínez, H. Microhardness Modification of Ultrahigh-Molecular-Weight Polyethylene by Oxygen Plasma: Effect of the Polymer Crosslinking. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 445, 8–12. [Google Scholar] [CrossRef]
- Puértolas, J.A.; Kurtz, S.M. Evaluation of Carbon Nanotubes and Graphene as Reinforcements for UHMWPE-Based Composites in Arthroplastic Applications: A Review. J. Mech. Behav. Biomed. Mater. 2014, 39, 129–145. [Google Scholar] [CrossRef]
- Kurtz, S.M. The Origins of UHMWPE in Total Hip Arthroplasty, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 1982. [Google Scholar] [CrossRef]
- Patel, K.; Chikkali, S.H.; Sivaram, S. Ultrahigh Molecular Weight Polyethylene: Catalysis, Structure, Properties, Processing and Applications. Prog. Polym. Sci. 2020, 109, 101290. [Google Scholar] [CrossRef]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Sobieraj, M.C.; Rimnac, C.M. Ultra High Molecular Weight Polyethylene: Mechanics, Morphology, and Clinical Behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Kopova, I.; Kronek, J.; Bacakova, L.; Fencl, J. A Cytotoxicity and Wear Analysis of Trapeziometacarpal Total Joint Replacement Implant Consisting of DLC-Coated Co-Cr-Mo Alloy with the Use of Titanium Gradient Interlayer. Diam. Relat. Mater. 2019, 97, 107456. [Google Scholar] [CrossRef]
- Avinash Patil, N.; Njuguna, J.; Kandasubramanian, B. UHMWPE for Biomedical Applications: Performance and Functionalization. Eur. Polym. J. 2020, 125, 109529. [Google Scholar] [CrossRef]
- Jiang, J.H.; Zhu, L.P.; Li, X.L.; Xu, Y.Y.; Zhu, B.K. Surface Modification of PE Porous Membranes Based on the Strong Adhesion of Polydopamine and Covalent Immobilization of Heparin. J. Membr. Sci. 2010, 364, 194–202. [Google Scholar] [CrossRef]
- Nomura, K.; Terwilliger, P. Self-Dual Leonard Pairs Modifications on the Properties and Their Composites. Spec. Matrices 2019, 7, 40–49. [Google Scholar]
- Chhetri, S.; Bougherara, H. A Comprehensive Review on Surface Modification of UHMWPE Fiber and Interfacial Properties. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106146. [Google Scholar] [CrossRef]
- Firouzi, D.; Youssef, A.; Amer, M.; Srouji, R.; Amleh, A.; Foucher, D.A.; Bougherara, H. A New Technique to Improve the Mechanical and Biological Performance of Ultra High Molecular Weight Polyethylene Using a Nylon Coating. J. Mech. Behav. Biomed. Mater. 2014, 32, 198–209. [Google Scholar] [CrossRef]
- Kang, K.; Choi, I.S.; Nam, Y. A Biofunctionalization Scheme for Neural Interfaces Using Polydopamine Polymer. Biomaterials 2011, 32, 6374–6380. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Surface Modification of UHMWPE/Fabric Composite Membrane via Self-Polymerized Polydopamine Followed by MPEG-NH2 Immobilization. J. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Amirgoulova, E.V.; Groll, J.; Heyes, C.D.; Ameringer, T.; Röcker, C.; Möller, M.; Nienhaus, G.U. Biofunctionalized Polymer Surfaces Exhibiting Minimal Interaction towards Immobilized Proteins. ChemPhysChem 2004, 5, 552–555. [Google Scholar] [CrossRef]
- Smolko, E.; Romero, G. Studies on Crosslinked Hydroxyapatite-Polyethylene Composite as a Bone-Analogue Material. Radiat. Phys. Chem. 2007, 76, 1414–1418. [Google Scholar] [CrossRef]
- James, S.P.; Oldinski, R.K.; Zhang, M.; Schwartz, H. UHMWPE/Hyaluronan Microcomposite Biomaterials, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Wu, H.; Pei, M. Meniscus, Articular Cartilage and Nucleus Pulposus: A Comparative Review of Cartilage-like Tissues in Anatomy, Development and Function. Cell Tissue Res. 2017, 370, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Kondyurin, A.; Bao, S.; Bilek, M.M.M.; Ye, L. Plasma Immersion Ion Implantation of Polyurethane Shape Memory Polymer: Surface Properties and Protein Immobilization. Appl. Surf. Sci. 2017, 416, 686–695. [Google Scholar] [CrossRef]
- Nichols, S.P.; Koh, A.; Storm, W.L.; Shin, J.H.; Schoenfisch, M.H. Biocompatible Materials for Continuous Glucose Monitoring Devices. Chem. Rev. 2013, 113, 2528–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In Vitro Corrosion and Biocompatibility of Binary Magnesium Alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface Modification of Polymers by Plasma Treatments for the Enhancement of Biocompatibility and Controlled Drug Release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Schoonen, L.; Van Hest, J.C.M. Functionalization of Protein-Based Nanocages for Drug Delivery Applications. Nanoscale 2014, 6, 7124–7141. [Google Scholar] [CrossRef] [PubMed]
- Poncin-Epaillard, F.; Legeay, G. Surface Engineering of Biomaterials with Plasma Techniques. J. Biomater. Sci. Polym. Ed. 2003, 14, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.C.; Akhavan, B.; Hung, J.; Bao, S.; Jang, J.H.; Wise, S.G.; Bilek, M.M.M. Multifunctional Protein-Immobilized Plasma Polymer Films for Orthopedic Applications. ACS Biomater. Sci. Eng. 2018, 4, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Y.; Li, J. The Graphene/Nucleic Acid Nanobiointerface. Chem. Soc. Rev. 2015, 44, 6954–6980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedela, O.; Slepicka, P.; Švorcík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Ratner, B.D. The Engineering of Biomaterials Exhibiting Recognition and Specificity. J. Mol. Recognit. 1996, 9, 617–625. [Google Scholar] [CrossRef]
- Terpilowski, K.; Rymuszka, D. Surface Properties of Glass Plates Activated by Air, Oxygen, Nitrogen and Argon Plasma. Glas. Phys. Chem. 2016, 42, 535–541. [Google Scholar] [CrossRef]
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma Treatment of Polymers for Surface and Adhesion Improvement. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 281–286. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Del Val, J.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Laser Surface Modification of Ultra-High-Molecular-Weight Polyethylene (UHMWPE) for Biomedical Applications. Appl. Surf. Sci. 2014, 302, 236–242. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Abang, S.; Poncelet, D.; Chan, E.S.; Ravindra, P. Production of Biodiesel Using Immobilized Lipase-A Critical Review. Crit. Rev. Biotechnol. 2008, 28, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Kong, M.G.; Prokopovich, P. Cold Atmospheric Pressure Gas Plasma Enhances the Wear Performance of Ultra-High Molecular Weight Polyethylene. Acta Biomater. 2012, 8, 1357–1365. [Google Scholar] [CrossRef]
- Van Vrekhem, S.; Vloebergh, K.; Asadian, M.; Vercruysse, C.; Declercq, H.; Van Tongel, A.; De Wilde, L.; De Geyter, N.; Morent, R. Improving the Surface Properties of an UHMWPE Shoulder Implant with an Atmospheric Pressure Plasma Jet. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Dufay, M.; Jimenez, M.; Degoutin, S. Effect of Cold Plasma Treatment on Electrospun Nanofibers Properties: A Review. ACS Appl. Bio Mater. 2020, 3, 4696–4716. [Google Scholar] [CrossRef]
- Rodrigues, M.M.; Fontoura, C.P.; Garcia, C.S.C.; Martins, S.T.; Henriques, J.A.P.; Figueroa, C.A.; Roesch-Ely, M.; Aguzzoli, C. Investigation of Plasma Treatment on UHMWPE Surfaces: Impact on Physicochemical Properties, Sterilization and Fibroblastic Adhesion. Mater. Sci. Eng. C 2019, 102, 264–275. [Google Scholar] [CrossRef]
- Liang, H.; Shi, B.; Fairchild, A.; Cale, T. Applications of Plasma Coatings in Artificial Joints: An Overview. Vacuum 2004, 73, 317–326. [Google Scholar] [CrossRef]
- Cvrček, L.; Horáková, M. Plasma Modified Polymeric Materials for Implant Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Das, S.; Jana, S.; De, D.; Gangopadhyay, U.; Mondal, A. Low Temperature Growth of Diamond-like Nanocomposite Films Prepared by PACVD from Ar Diluted Siloxane Plasma. Mater. Res. Express 2019, 6, 115614. [Google Scholar] [CrossRef]
- Buchkremer-Hermanns, H.; Long, C.; Weiss, H. ECR Plasma Polishing of CVD Diamond Films. Diam. Relat. Mater. 1996, 5, 845–849. [Google Scholar] [CrossRef]
- Mwale, F.; Wang, H.T.; Nelea, V.; Luo, L.; Antoniou, J.; Wertheimer, M.R. The Effect of Glow Discharge Plasma Surface Modification of Polymers on the Osteogenic Differentiation of Committed Human Mesenchymal Stem Cells. Biomaterials 2006, 27, 2258–2264. [Google Scholar] [CrossRef]
- Cho, E.H.; Lee, S.G.; Kim, J.K. Surface Modification of UHMWPE with γ-Ray Radiation for Improving Interfacial Bonding Strength with Bone Cement (II). Curr. Appl. Phys. 2005, 5, 475–479. [Google Scholar] [CrossRef]
- Rhim, J.W. Characterization of Biopolymer and Chitosan-Based Nanocomposites with Antimicrobial Activity. In Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 355–382. [Google Scholar]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, L.; Yang, W. Photo-Induced Living/Controlled Surface Radical Grafting Polymerization and Its Application in Fabricating 3-D Micro-Architectures on the Surface of Flat/Particulate Organic Substrates. Polymer 2011, 52, 4159–4173. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Gao, Q.; Xu, L.; Zhang, M.; Xing, Z.; Guo, X.; Zhang, K.; Wu, G. Significant Improvement in Thermal and UV Resistances of UHMWPE Fabric through in Situ Formation of Polysiloxane-TiO2 Hybrid Layers. ACS Appl. Mater. Interfaces 2016, 8, 23311–23320. [Google Scholar] [CrossRef]
- Koseki, H.; Yonekura, A.; Shida, T.; Yoda, I.; Horiuchi, H.; Morinaga, Y.; Yanagihara, K.; Sakoda, H.; Osaki, M.; Tomita, M. Early Staphylococcal Biofilm Formation on Solid Orthopaedic Implant Materials: In Vitro Study. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Puppulin, L.; Leto, A.; Hasegawa, M.; Pezzotti, G. A Comparative Microstructural Study of Vitamin-E Blended and Infused Highly Crosslinked UHMWPE for Total Knee Arthroplasty. J. Mech. Behav. Biomed. Mater. 2014, 39, 247–256. [Google Scholar] [CrossRef]
- Fu, J.; Shen, J.; Gao, G.; Xu, Y.; Hou, R.; Cong, Y.; Cheng, Y. Natural polyphenol-stabilised highly crosslinked UHMWPE with high mechanical properties and low wear for joint implants. J. Mater. Chem. B 2013, 1, 4727–4735. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Chen, Y.; Xie, M.; Yu, Z.; Yang, B. Surface Modification of Ultrahigh Molecular Weight Polyethylene by Plasma-Induced in-Situ Grafting with Vinyl Triethoxysilane. J. Biomed. Mater. Res. Part A 2018, 106, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Esbah Tabaei, P.S.; Ghobeira, R.; Cools, P.; Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Comparative Study between In-Plasma and Post-Plasma Chemical Processes Occurring at the Surface of UHMWPE Subjected to Medium Pressure Ar and N2 Plasma Activation. Polymer 2020, 193, 122383. [Google Scholar] [CrossRef]
- Aziz, G.; De Geyter, N.; Declercq, H.; Cornelissen, R.; Morent, R. Incorporation of Amine Moieties onto Ultra-High Molecular Weight Polyethylene (UHMWPE) Surface via Plasma and UV Polymerization of Allylamine. Surf. Coat. Technol. 2015, 271, 39–47. [Google Scholar] [CrossRef]
- Conrad, J.R. Method and Apparatus for Plasma Source Ion Implantation. U.S. Patent 4,764,394, 16 August 1988. [Google Scholar]
- Conrad, J.R.; Radtke, J.L.; Dodd, R.A.; Worzala, F.J.; Tran, N.C. Plasma Source Ion-Implantation Technique for Surface Modification of Materials. J. Appl. Phys. 1987, 62, 4591–4596. [Google Scholar] [CrossRef]
- Biederman, H. Polymer Films Prepared by Plasma Polymerization and Their Potential Application. Vacuum 1987, 37, 367–373. [Google Scholar] [CrossRef]
- Gutensohn, K.; Beythien, C.; Bau, J.; Fenner, T.; Grewe, P.; Koester, R.; Padmanaban, K.; Kuehnl, P. In Vitro Analyses of Diamond-like Carbon Coated Stents: Reduction of Metal Ion Release, Platelet Activation, and Thrombogenicity. Thromb. Res. 2000, 99, 577–585. [Google Scholar] [CrossRef]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A Review of Bioactive Glasses: Their Structure, Properties, Fabrication, and Apatite Formation. J. Biomed. Mater. Res. A 2013, 102, 254–274. [Google Scholar] [CrossRef]
- Mahapatro, A. Bio-Functional Nano-Coatings on Metallic Biomaterials. Mater. Sci. Eng. C 2015, 55, 227–251. [Google Scholar] [CrossRef]
- Kosobrodova, E.; Kondyurin, A.; McKenzie, D.R.; Bilek, M.M.M. Kinetics of Post-Treatment Structural Transformations of Nitrogen Plasma Ion Immersion Implanted Polystyrene. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 304, 57–66. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.Y.; Dong, H. Improved Wear Resistance of Ultra-High Molecular Weight Polyethylene by Plasma Immersion Ion Implantation. Wear 2001, 250, 544–552. [Google Scholar] [CrossRef]
- Chen, J.S.; Sun, Z.; Guo, P.S.; Zhang, Z.B.; Zhu, D.Z.; Xu, H.J. Effect of Ion Implantation on Surface Energy of Ultrahigh Molecular Weight Polyethylene. J. Appl. Phys. 2003, 93, 5103–5108. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Y.; Zhu, L.; Al-rubeai, M. Critical Reviews in Biotechnology Cold Atmospheric Plasma as an Interface Biotechnology for Enhancing Surgical Implants. Crit. Rev. Biotechnol. 2021, 41, 425–440. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Alvi, F.I.; Kang, Q.; Laberge, M.; Drews, M.J.; Zhang, J.; Matthews, M.A.; Arciola, C.R. Effects of Sterilization on Implant Mechanical Property and Biocompatibility. Int. J. Artif. Organs 2005, 28, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, J. Plasma Nitriding and Plasma Immersion Ion Implantation; Szczecin University of Technology: Szczecin, Poland, 2008. [Google Scholar]
- Day, J.S.; MacDonald, D.W.; Ramsey, M.L.; Abboud, J.A.; Kurtz, S.M. Quantitative Ultrahigh-Molecular-Weight Polyethylene Wear in Total Elbow Retrievals. J. Shoulder Elb. Surg. 2020, 29, 2364–2374. [Google Scholar] [CrossRef]
- Liu, R.; Dai, J.; Ma, L.; Chen, J.; Shi, X.; Du, Y.; Li, Z.; Deng, H. Low-Temperature Plasma Treatment-Assisted Layer-by-Layer Self-Assembly for the Modification of Nanofibrous Mats. J. Colloid Interface Sci. 2019, 540, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, M.; Masoomi, M.; Ahmadi, M.; Safi, S. Interfacial Shear Strength Characterization of GMA-Grafted UHMWPE Fiber/Epoxy/Nano Clay Hybrid Nanocomposite Materials. RSC Adv. 2016, 6, 41793–41799. [Google Scholar] [CrossRef]
- Mamidi, N.; Leija, H.M.; Diabb, J.M.; Lopez Romo, I.; Hernandez, D.; Castrejón, J.V.; Martinez Romero, O.; Barrera, E.V.; Elias Zúñiga, A. Cytotoxicity Evaluation of Unfunctionalized Multiwall Carbon Nanotubes-Ultrahigh Molecular Weight Polyethylene Nanocomposites. J. Biomed. Mater. Res. Part A 2017, 105, 3042–3049. [Google Scholar] [CrossRef]
- Manoj Kumar, R.; Rajesh, K.; Haldar, S.; Gupta, P.; Murali, K.; Roy, P.; Lahiri, D. Surface Modification of CNT Reinforced UHMWPE Composite for Sustained Drug Delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 748–759. [Google Scholar] [CrossRef]
- Dyachkova, T.; Gutnik, I.; Nagdaev, V.; Maksimkin, A.; Burakova, E.; Galunin, E.; Memetov, N.; Khan, Y.; Dayyoub, T. Studying the Surface of UHMWPE Films Modified with Graphene Nanoplatelets Using a Raman Mapping Method. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 561–564. [Google Scholar] [CrossRef]
- Liu, C.Y.; Ishigami, A.; Kurose, T.; Ito, H. Wear Resistance of Graphene Reinforced Ultra-High Molecular Weight Polyethylene Nanocomposites Prepared by Octa-Screw Extrusion Process. Compos. Part B Eng. 2021, 215, 108810. [Google Scholar] [CrossRef]
- Ahmadi, M.; Zabihi, O.; Masoomi, M.; Naebe, M. Synergistic Effect of MWCNTs Functionalization on Interfacial and Mechanical Properties of Multi-Scale UHMWPE Fibre Reinforced Epoxy Composites. Compos. Sci. Technol. 2016, 134, 1–11. [Google Scholar] [CrossRef]
- Aghvami-Panah, M.; Panahi-Sarmad, M.; Seraji, A.A.; Jamalpour, S.; Ghaffarian, S.R.; Park, C.B. LDPE/MWCNT and LDPE/MWCNT/UHMWPE Self-Reinforced Fiber-Composite Foams Prepared via Supercritical CO2: A Microstructure-Engineering Property Perspective. J. Supercrit. Fluids 2021, 174, 105248. [Google Scholar] [CrossRef]
- Azam, M.U.; Samad, M.A. Tribological Evaluation of a UHMWPE Hybrid Nanocomposite Coating Reinforced with Nanoclay and Carbon Nanotubes under Dry Conditions. J. Tribol. 2018, 140, 26–28. [Google Scholar] [CrossRef]
- Xin, X.; Wang, Y.; Meng, Z.; Yan, F. Improving Mechanical Properties and Fretting Wear Resistance of Ultra-High-Molecular-Weight-Polyethylene via Incorporation of Zeolitic Imidazolate Frameworks-Carbon Nano-Fiber. Polym. Adv. Technol. 2021, 32, 2622–2632. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Zhang, L.; Zhu, P.; Zhang, G. Design and Characterization of the Surface Porous Uhmwpe Composite Reinforced by Graphene Oxide. Polymers 2021, 13, 482. [Google Scholar] [CrossRef]
- Fang, Z.; Tu, Q.; Shen, X.; Yang, X.; Liang, K.; Pan, M.; Chen, Z. Biomimetic Surface Modification of UHMWPE Fibers to Enhance Interfacial Adhesion with Rubber Matrix via Constructing Polydopamine Functionalization Platform and Then Depositing Zinc Oxide Nanoparticles. Surf. Interfaces 2022, 29, 101728. [Google Scholar] [CrossRef]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein Immobilization Strategies for Protein Biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef]
- Puertas, S.; De Gracia Villa, M.; Mendoza, E.; Jiménez-Jorquera, C.; De la Fuente, J.M.; Fernández-Sánchez, C.; Grazú, V. Improving Immunosensor Performance through Oriented Immobilization of Antibodies on Carbon Nanotube Composite Surfaces. Biosens. Bioelectron. 2013, 43, 274–280. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Luo, R.; Maitz, M.F.; Jing, F.; Sun, H.; Huang, N. The Covalent Immobilization of Heparin to Pulsed-Plasma Polymeric Allylamine Films on 316L Stainless Steel and the Resulting Effects on Hemocompatibility. Biomaterials 2010, 31, 2072–2083. [Google Scholar] [CrossRef]
- Bilek, M.M.M. Biofunctionalization of Surfaces by Energetic Ion Implantation: Review of Progress on Applications in Implantable Biomedical Devices and Antibody Microarrays. Appl. Surf. Sci. 2014, 310, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Cruz, G.J.; Gómez, L.M.; Gonzalez-Torres, M.; Gonzalez-Salgado, F.; Basurto, R.; Colín, E.; Palacios, J.C.; Olayo, M.G. Polymerization Mechanisms in Plasma Polyallylamine. J. Mater. Sci. 2017, 52, 1005–1013. [Google Scholar] [CrossRef]
- Pfeiffer, Ã. The Hydrolysis/Condensation Behaviour of Methacryloyloxyalkylfunctional Alkoxysilanes: Structure-Reactivity Relations. Mon. Für Chem./Chem. Mon. 2003, 1092, 1081–1092. [Google Scholar] [CrossRef]
- Voggenreiter, G.; Hartl, K.; Assenmacher, S. Assesment of the Biological Activity of Chemically Immobilized RhBMP-2 on Titanium Surfaces in Vivo. Mater. Und Werkst. Mater. Sci. Eng. Technol. 2001, 32, 942–948. [Google Scholar] [CrossRef]
- Widmer, M.R.; Heuberger, M.; Vörös, J.; Spencer, N.D. Influence of Polymer Surface Chemistry on Frictional Properties under Protein-Lubrication Conditions: Implications for Hip-Implant Design. Tribol. Lett. 2001, 10, 111–116. [Google Scholar] [CrossRef]
- Lu, T.; Qiao, Y.; Liu, X. Surface Modification of Biomaterials Using Plasma Immersion Ion Implantation and Deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Kwok, D.T.K.; Tong, L.; Yeung, C.Y.; Remedios, C.G.do.; Chu, P.K. Hybrid Plasma Surface Modification and Ion Implantation of Biopolymers. Surf. Coat. Technol. 2010, 204, 2892–2897. [Google Scholar] [CrossRef]
- Kan, Y.; Cvjetinovic, J.; Statnik, E.S.; Ryazantsev, S.V.; Anisimova, N.Y.; Kiselevskiy, M.V.; Salimon, A.I.; Maksimkin, A.V.; Korsunsky, A.M. The Fabrication and Characterization of Bioengineered Ultra-High Molecular Weight Polyethylene-Collagen-Hap Hybrid Bone-Cartilage Patch. Mater. Today Commun. 2020, 24, 101052. [Google Scholar] [CrossRef]
- Teodoru, S.; Kusano, Y.; Rozlosnik, N.; Michelsen, P.K. Continuous Plasma Treatment of Ultra-High-Molecular-Weight Polyethylene (UHMWPE) Fibres for Adhesion Improvement. Plasma Process. Polym. 2009, 6, 375–381. [Google Scholar] [CrossRef]
- Ho, J.P.Y.; Nosworthy, N.J.; Bilek, M.M.M.; Gan, B.K.; McKenzie, D.R.; Chu, P.K.; dos Remedios, C.G. Plasma-Treated Polyethylene Surfaces for Improved Binding of Active Protein. Plasma Process. Polym. 2007, 4, 583–590. [Google Scholar] [CrossRef]
- Gan, B.K.; Bilek, M.M.M.; Kondyurin, A.; Mizuno, K.; McKenzie, D.R. Etching and Structural Changes in Nitrogen Plasma Immersion Ion Implanted Polystyrene Films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 247, 254–260. [Google Scholar] [CrossRef]
- Gan, B.K.; Nosworthy, N.J.; McKenzie, D.R.; Dos Remedios, C.G.; Bilek, M.M.M. Plasma Immersion Ion Implantation Treatment of Polyethylene for Enhanced Binding of Active Horseradish Peroxidase. J. Biomed. Mater. Res. Part A 2008, 85, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, N.; Bougherara, H.; Amleh, A. In-Vitro Evaluation of the Bioactivity and the Biocompatibility of a Novel Coated UHMWPE Biomaterial for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 101, 103409. [Google Scholar] [CrossRef] [PubMed]
- Zolotarevová, E.; Hudeček, J.; Špundová, M.; Entlicher, G. Binding of Proteins to Ultra High Molecular Weight Polyethylene Wear Particles as a Possible Mechanism of Macrophage and Lymphocyte Activation. J. Biomed. Mater. Res. Part A 2010, 95, 950–955. [Google Scholar] [CrossRef]
- Nečas, D.; Vrbka, M.; Křupka, I.; Hartl, M. The Effect of Kinematic Conditions and Synovial Fluid Composition on the Frictional Behaviour of Materials for Artificial Joints. Materials 2018, 11, 767. [Google Scholar] [CrossRef] [Green Version]
- Nečas, D.; Sawae, Y.; Fujisawa, T.; Nakashima, K.; Morita, T.; Yamaguchi, T.; Vrbka, M.; Křupka, I.; Hartl, M. The Influence of Proteins and Speed on Friction and Adsorption of Metal/UHMWPE Contact Pair. Biotribology 2017, 11, 51–59. [Google Scholar] [CrossRef]
- Anindyajati, A.; Boughton, P.; Ruys, A.J. Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant. Materials 2019, 16, 916. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Buda, R.; Cavallo, C.; Desando, G.; Facchini, A.; Grigolo, B.; Barbiano, V. Ligament Repair: A Molecular and Immunohistological Characterization. J. Biomed. Mater. Res. Part A 2008, 84, 117–127. [Google Scholar] [CrossRef]
- Vermeijden, H.D.; Jonkergouw, A.; Van der List, J.P.; DiFelice, G.S. The Multiple Ligament-Injured Knee: When Is Primary Repair an Option? Knee 2020, 27, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.G.; Bellincampi, L.D.; Tria, A.J.; Zawadsky, J.P. Preliminary Development of a Collagen–PLA Composite for ACL Reconstruction. J. Appl. Polym. Sci. 1997, 63, 1423–1428. [Google Scholar] [CrossRef]
- Sharp, J.W.; Kani, K.K.; Gee, A.; Mulcahy, H.; Chew, F.S.; Porrino, J. Anterior Cruciate Ligament Fixation Devices: Expected Imaging Appearance and Common Complications. Eur. J. Radiol. 2018, 99, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hertel, P.; Behrend, H.; Cierpinski, T.; Musahl, V.; Widjaja, G. ACL Reconstruction Using Bone-Patellar Tendon-Bone Press-Fit Fixation: 10-Year Clinical Results. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.K.Y.; Ou, Y.; Rattner, J.; Hart, D.A.; Marchuk, L.L.; Frank, C.B.; Rattner, J.B. The Cellular Networks of Normal Ovine Medial Collateral and Anterior Cruciate Ligaments Are Not Accurately Recapitulated in Scar Tissue. J. Anat. 2002, 200, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.B. Ligament Structure, Physiology and Function. J. Musculoskelet. Neuronal Interact. 2004, 4, 199–201. [Google Scholar] [PubMed]
- Rand, J.A. Knee Ligaments: Structure, Function, Injury, and Repair. Mayo Clin. Proc. 1990, 65, 1389–1390. [Google Scholar] [CrossRef]
- Vieira, A.C.; Guedes, R.M.; Marques, A.T. Development of Ligament Tissue Biodegradable Devices: A Review. J. Biomech. 2009, 42, 2421–2430. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.S.; Wang, C.J. Femoral Interference Screw Placement Through the Tibial Tunnel: A Novel Method Without Graft Damage. Arthrosc.-J. Arthrosc. Relat. Surg. 2006, 22, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Koh, I.J.; McGarry, M.H.; Patel, N.A.; Lin, C.C.; Lee, T.Q. Double-Bundle Anterior Cruciate Ligament Reconstruction with Lateral Extra-Articular Tenodesis Is Effective in Restoring Knee Stability in a Chronic, Complex Anterior Cruciate Ligament-Injured Knee Model: A Cadaveric Biomechanical Study. Arthrosc.-J. Arthrosc. Relat. Surg. 2021, 37, 2220–2234. [Google Scholar] [CrossRef]
- Foster, T.E.; Wolfe, B.L.; Ryan, S.; Silvestri, L.; Kaye, E.K. Does the Graft Source Really Matter in the Outcome of Patients Undergoing Anterior Cruciate Ligament Reconstruction?: An Evaluation of Autograft Versus Allograft Reconstruction Results: A Systematic Review. Am. J. Sports Med. 2010, 38, 189–199. [Google Scholar] [CrossRef]
- Chehab, E.L.; Flik, K.R.; Vidal, A.F.; Levinson, M.; Gallo, R.A.; Altchek, D.W.; Warren, R.F. Anterior Cruciate Ligament Reconstruction Using Achilles Tendon Allograft: An Assessment of Outcome for Patients Age 30 Years and Older. HSS J. 2011, 7, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Yang, F.; Goh, J.C.H.; Ramakrishna, S.; Lee, E.H. Biomaterials and Scaffolds for Ligament Tissue Engineering. J. Biomed. Mater. Res. Part A 2006, 77, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Van Oeveren, W.; Tielliu, I.F.; De Hart, J. Comparison of Modified Chandler, Roller Pump, and Ball Valve Circulation Models for in Vitro Testing in High Blood Flow Conditions: Application in Thrombogenicity Testing of Different Materials for Vascular Applications. Int. J. Biomater. 2012, 2012, 673163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.L.; Lew, W.D.; Engebretsen, L.; Hunter, R.E.; Kowalczyk, C. Factors Affecting Graft Force in Surgical Reconstruction of the Anterior Cruciate Ligament. J. Orthop. Res. 1990, 8, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Poulsson, A.H.C.; Mitchell, S.A.; Davidson, M.R.; Johnstone, A.J.; Emmison, N.; Bradley, R.H. Attachment of Human Primary Osteoblast Cells to Modified Polyethylene Surfaces. Langmuir 2009, 25, 3718–3727. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Han, C.M.; Lee, E.J.; Kim, H.W. Preparation of Highly Monodispersed Porous-Channeled Poly(Caprolactone) Microspheres by a Microfluidic System. Mater. Lett. 2016, 181, 92–98. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, V.G.; Velten, M.F.; Odatsu, T.; Ilyas, A.; Iqbal, S.M.; Aswath, P.B. Surface Modifications and Surface Characterization of Biomaterials Used in Bone Healing; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Vaquette, C.; Kahn, C.; Frochot, C.; Nouvel, C.; Six, J.-L.; De Isla, N.; Luo, L.-H.; Cooper-White, J.; Rahouadj, R.; Wang, X. Aligned Poly(L-Lactic-Co-e-Caprolactone) Electrospun Microfibers and Knitted Structure: A Novel Composite Scaffold for Ligament Tissue Engineering. J. Biomed. Mater. Res. A 2010, 94, 1270–1282. [Google Scholar] [CrossRef]
- Vaquette, C.; Viateau, V.; Guérard, S.; Anagnostou, F.; Manassero, M.; Castner, D.G.; Migonney, V. The Effect of Polystyrene Sodium Sulfonate Grafting on Polyethylene Terephthalate Artificial Ligaments on Invitro Mineralisation and Invivo Bone Tissue Integration. Biomaterials 2013, 34, 7048–7063. [Google Scholar] [CrossRef] [Green Version]
- Day, R.M. Bioactive Glass Stimulates the Secretion of Angiogenic Growth Factors and Angiogenesis in Vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Oréfice, R.; Hench, L.; Brennan, A. Evaluation of the Interactions between Collagen and the Surface of a Bioactive Glass during in Vitro Test. J. Biomed. Mater. Res. A 2009, 90, 114–120. [Google Scholar] [CrossRef]
- Hench, L.L.; Greenspan, D. Interactions between Bioactive Glass and Collagen: A Review and New Perspectives. J. Aust. Ceram. Soc. 2013, 49, 1–40. [Google Scholar]
- Rawlings, R.D. Bioactive Glasses and Glass-Ceramics. Clin. Mater. 1993, 14, 155–179. [Google Scholar] [CrossRef]
- Yunos, D.M.; Ahmad, Z.; Boccaccini, A.R. Fabrication and Characterization of Electrospun Poly-DL-Lactide (PDLLA) Fibrous Coatings on 45S5 Bioglass® Substrates for Bone Tissue Engineering Applications. J. Chem. Technol. Biotechnol. 2009, 85, 768–774. [Google Scholar] [CrossRef]
- Bi, L.; Jung, S.; Day, D.; Neidig, K.; Dusevich, V.; Eick, D.; Bonewald, L. Evaluation of Bone Regeneration, Angiogenesis, and Hydroxyapatite Conversion in Critical-Sized Rat Calvarial Defects Implanted with Bioactive Glass Scaffolds. J. Biomed. Mater. Res. A 2012, 100, 3267–3275. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Bioactive Sol-Gel Foams for Tissue Repair. J. Biomed. Mater. Res. 2002, 59, 340–348. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In Vitro Dissolution of Melt-Derived 45S5 and Sol-Gel Derived 58S Bioactive Glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive Glass in Tissue Engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Rahaman, M.N.; Fu, H.; Liu, X. Silicate, Borosilicate, and Borate Bioactive Glass Scaffolds with Controllable Degradation Rate for Bone Tissue Engineering Applications. I. Preparation and in Vitro Degradation. J. Biomed. Mater. Res. Part A 2010, 95A, 164–171. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Wang, Y.; Toh, S.L.; Goh, J.C.H. The Interaction between a Combined Knitted Silk Scaffold and Microporous Silk Sponge with Human Mesenchymal Stem Cells for Ligament Tissue Engineering. Biomaterials 2008, 29, 662–674. [Google Scholar] [CrossRef]
- Laflamme, M.; Lamontagne, J.; Guidoin, R. Anterior Cruciate Ligament Prostheses Using Biotextiles. In Biomedical Textiles for Orthopaedic and Surgical Applications; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar] [CrossRef]
- Li, M.; Mondrinos, M.J.; Chen, X.; Gandhi, M.R.; Ko, F.K.; Lelkes, P.I. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin Blends for Tissue Engineering Scaffolds. J. Biomed. Mater. Res. Part A 2006, 79, 963–973. [Google Scholar] [CrossRef]
- Zhang, P.; Han, F.; Chen, T.; Wu, Z.; Chen, S. “Swiss Roll”-Like Bioactive Hybrid Scaffolds for Promoting Bone Tissue Ingrowth and Tendon-Bone Healing After Anterior Cruciate Ligament Reconstruction. Biomater. Sci. 2020, 8, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C.; Luan, J.; Guan, G.; Lin, J.; Wang, F.; Wang, L. Polycaprolactone/Ultra-High Molecular Weight Polyethylene Partially Absorbable Suture with Improved Mechanical Performances for Tendon and Ligament Repair. Text. Res. J. 2020, 90, 2123–2135. [Google Scholar] [CrossRef]
- Tong, S.Y.; Wang, Z.; Lim, P.N.; Wang, W.; Thian, E.S. Uniformly-Dispersed Nanohydroxapatite-Reinforced Poly (ε-Caprolactone) Composite Films for Tendon Tissue Engineering Application. Mater. Sci. Eng. C 2017, 70, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.C.; Georgescu, H.I.; Kwoh, C.K.; Blomstrom, G.L.; Engle, C.P.; Larkin, L.A.; Evans, C.H.; Woo, S.L. Effect of Growth Factors on the Proliferation of Fibroblasts from the Medial Collateral and Anterior Cruciate Ligaments. J. Orthop. Res. 1995, 13, 184–190. [Google Scholar] [CrossRef]
- Molloy, T.; Wang, Y.; Murrell, G.A. The Roles of Growth Factors in Tendon and Ligament Healing. Sport Med. 2003, 33, 381–394. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Lingelbach, J.; Masakowski, V.R.; Griffin, G.L.; Senior, R.M.; Deuel, T.F. Platelet-Derived Growth Factor and Transforming Growth Factor-β Enhance Tissue Repair Activities by Unique Mechanisms. J. Cell Biol. 1989, 109, 429–440. [Google Scholar] [CrossRef]
- Jones, J.I. Insulin-like Growth Factors and Their Binding Proteins: Biological Actions. Endocr. Rev. 2004, 16, 3–34. [Google Scholar] [CrossRef]
- Marui, T.; Niyibizi, C.; Georgescu, H.I.; Cao, M.; Kavalkovich, K.W.; Levine, R.E.; Woo, S.L.Y. Effect of Growth Factors on Matrix Synthesis by Ligament Fibroblasts. J. Orthop. Res. 1997, 15, 18–23. [Google Scholar] [CrossRef]
- Nau, T.; Teuschl, A. Regeneration of the Anterior Cruciate Ligament: Current Strategies in Tissue Engineering. World J. Orthop. 2015, 6, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Hirukawa, M.; Katayama, S.; Sato, T.; Yamada, M.; Kageyama, S.; Unno, H.; Suzuki, Y.; Miura, Y.; Shiratsuchi, E.; Hasegawa, M.; et al. Development of Tissue-Engineered Ligaments: Elastin Promotes Regeneration of the Rabbit Medial Collateral Ligament. Artif. Organs 2018, 42, E102–E113. [Google Scholar] [CrossRef]
- Leong, N.L.; Arshi, A.; Kabir, N.; Nazemi, A.; Petrigliano, F.A.; Wu, B.M.; McAllister, D.R. In Vitro and in Vivo Evaluation of Heparin Mediated Growth Factor Release from Tissue-Engineered Constructs for Anterior Cruciate Ligament Reconstruction. J. Orthop. Res. 2015, 33, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Johnson, V.M.; Murray, M.M. Effects of Age and Platelet-Rich Plasma on ACL Cell Viability and Collagen Gene Expression. J. Orthop. Res. 2012, 30, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegen, S.; Carmeliet, G. Vascular Endothelial Growth Factor and Bone–Vascular Interactions; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Mifune, Y.; Matsumoto, T.; Ota, S.; Nishimori, M.; Usas, A.; Kopf, S.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Therapeutic Potential of Anterior Cruciate Ligament-Derived Stem Cells for Anterior Cruciate Ligament Reconstruction. Cell Transplant. 2012, 21, 1651–1665. [Google Scholar] [CrossRef] [Green Version]
- Mifune, Y.; Matsumoto, T.; Takayama, K.; Terada, S.; Sekiya, N.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Tendon Graft Revitalization Using Adult Anterior Cruciate Ligament (ACL)-Derived CD34+ Cell Sheets for ACL Reconstruction. Biomaterials 2013, 34, 5476–5487. [Google Scholar] [CrossRef] [PubMed]
- Robbe, R.; Paletta, G.A. Soft-Tissue Graft Fixation in Anterior Cruciate Ligament Reconstruction. Oper. Tech. Sports Med. 2004, 12, 188–194. [Google Scholar] [CrossRef]
- Anderson, A.F.; Snyder, R.B.; Lipscomb, A.B. Anterior Cruciate Ligament Reconstruction A Prospective Randomized Study of Three Surgical Methods. Am. J. Sports Med. 2001, 29, 272–279. [Google Scholar] [CrossRef]
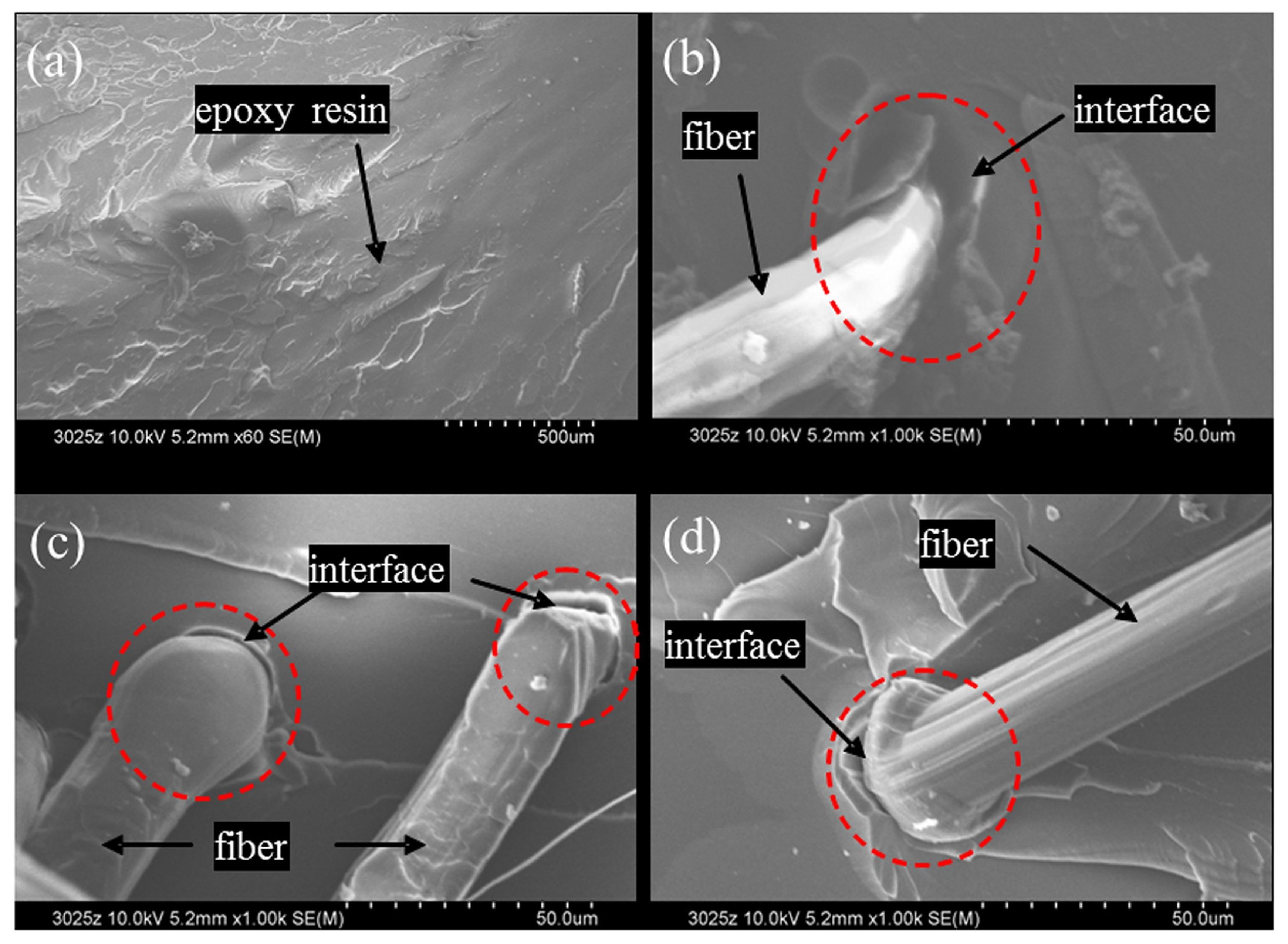
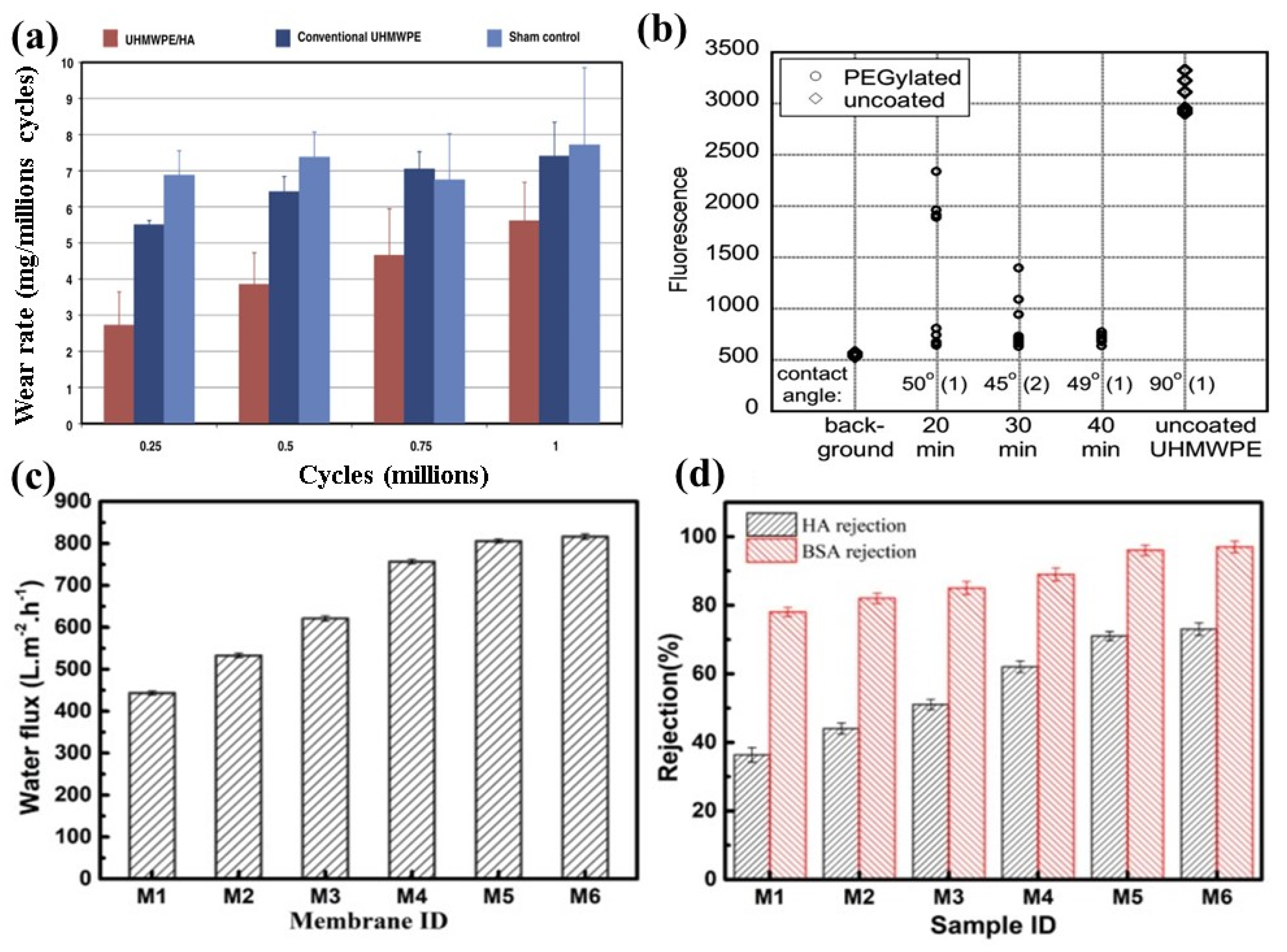
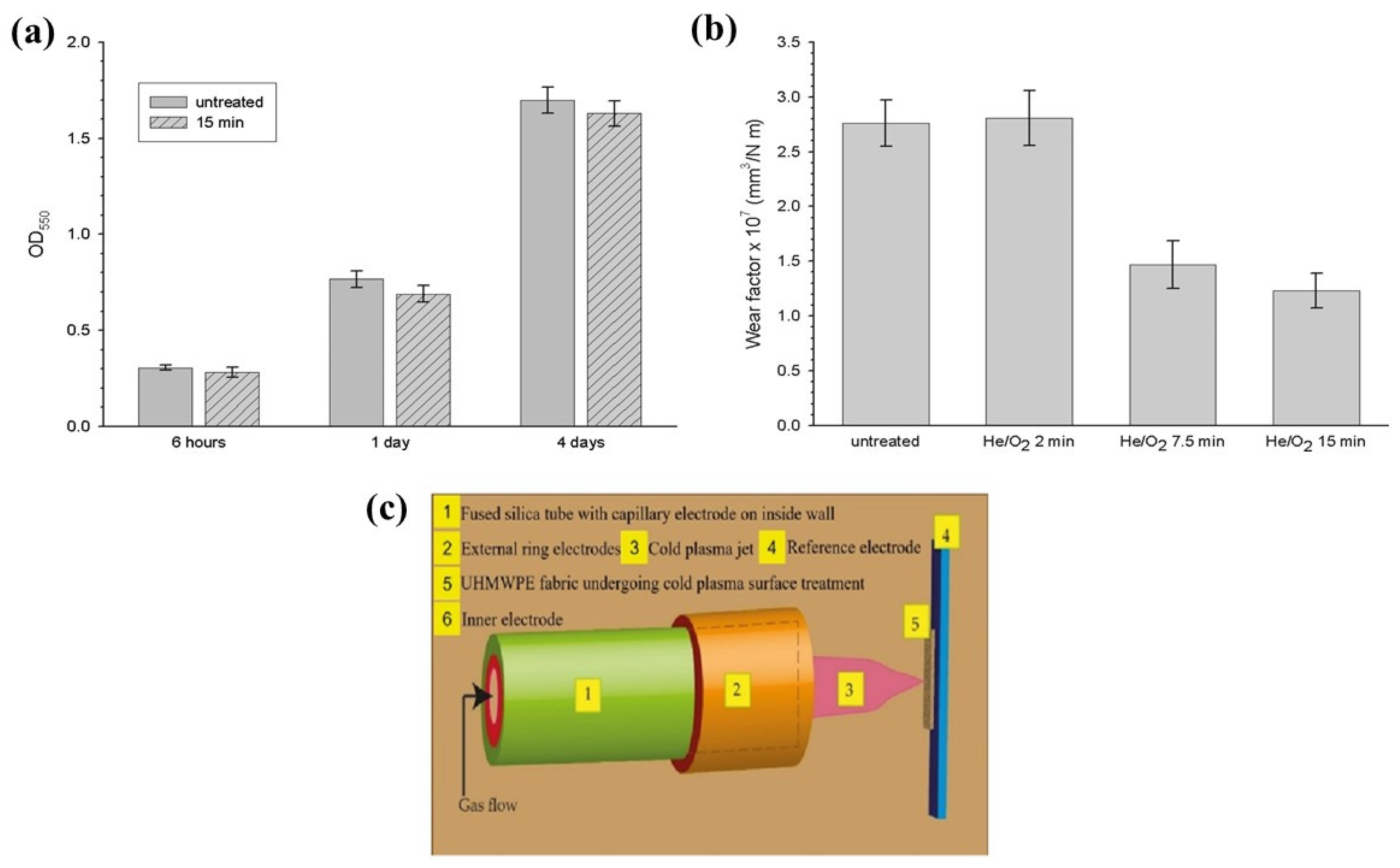

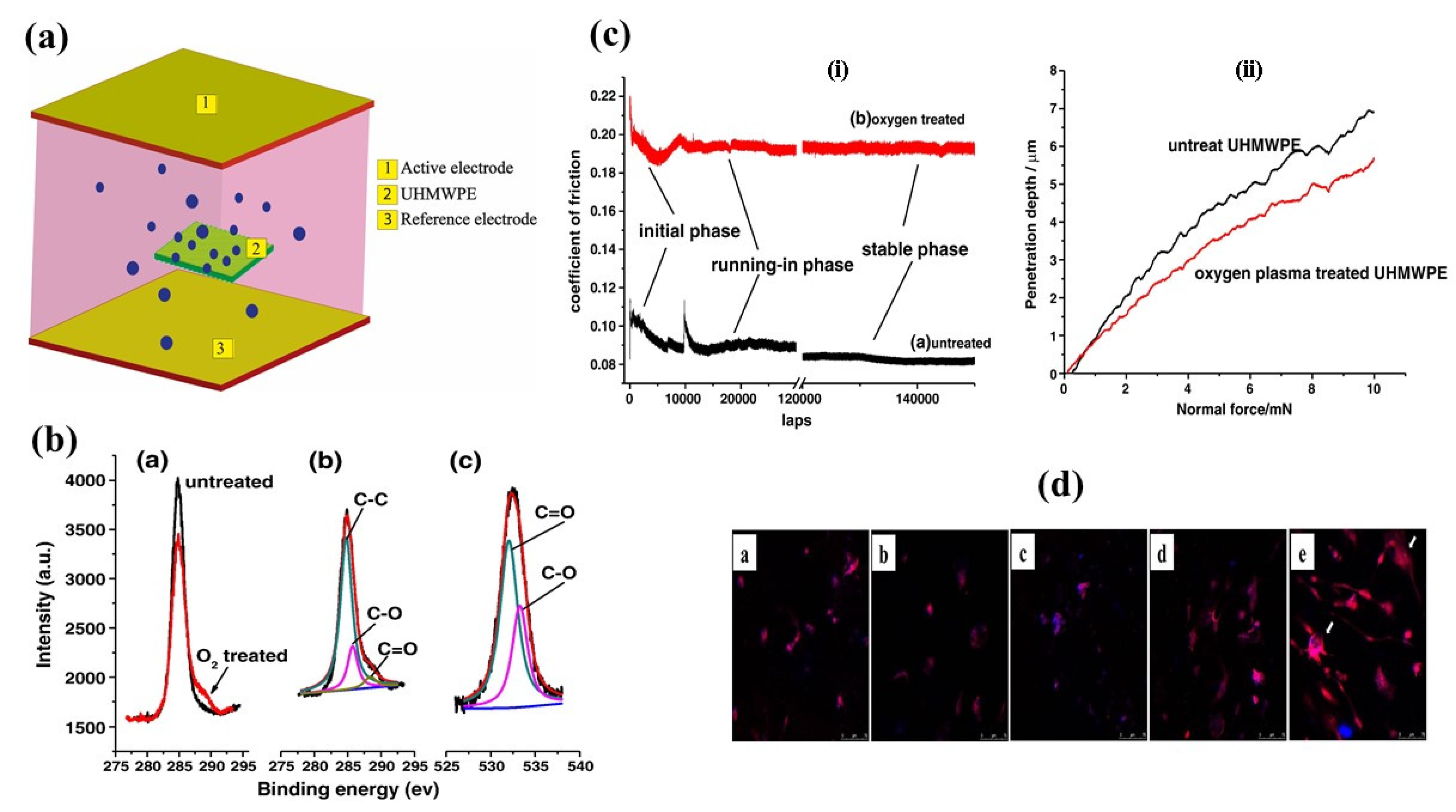
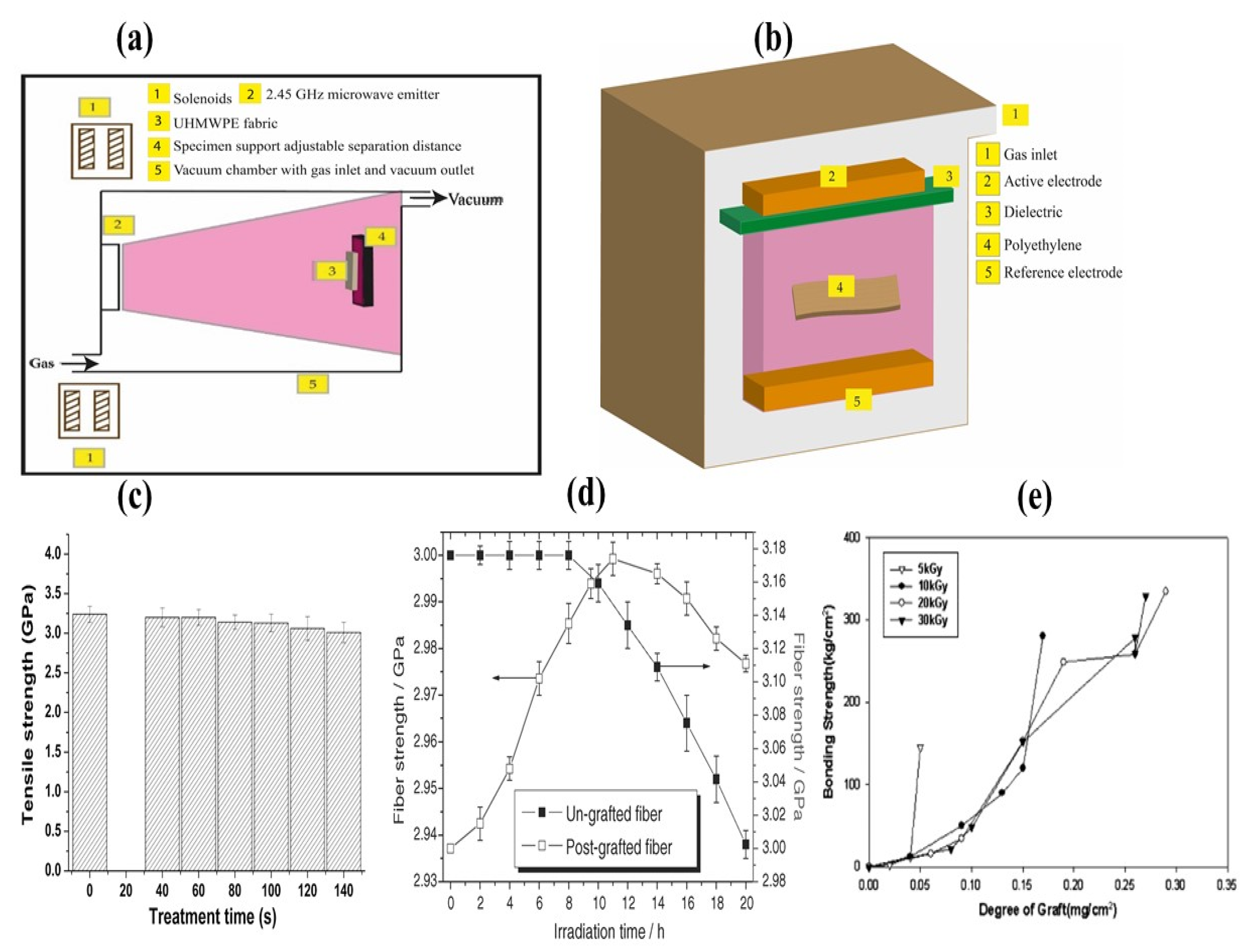
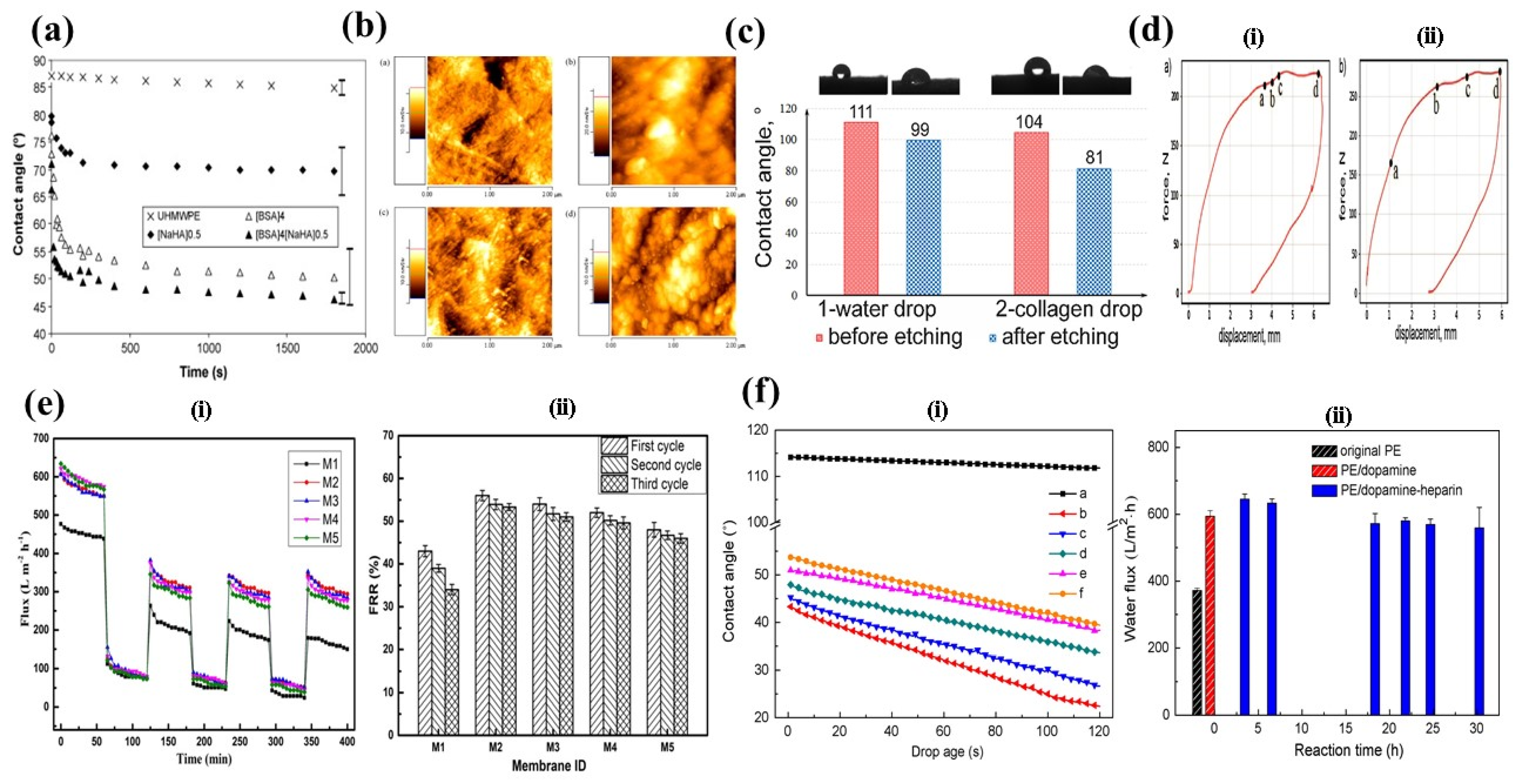
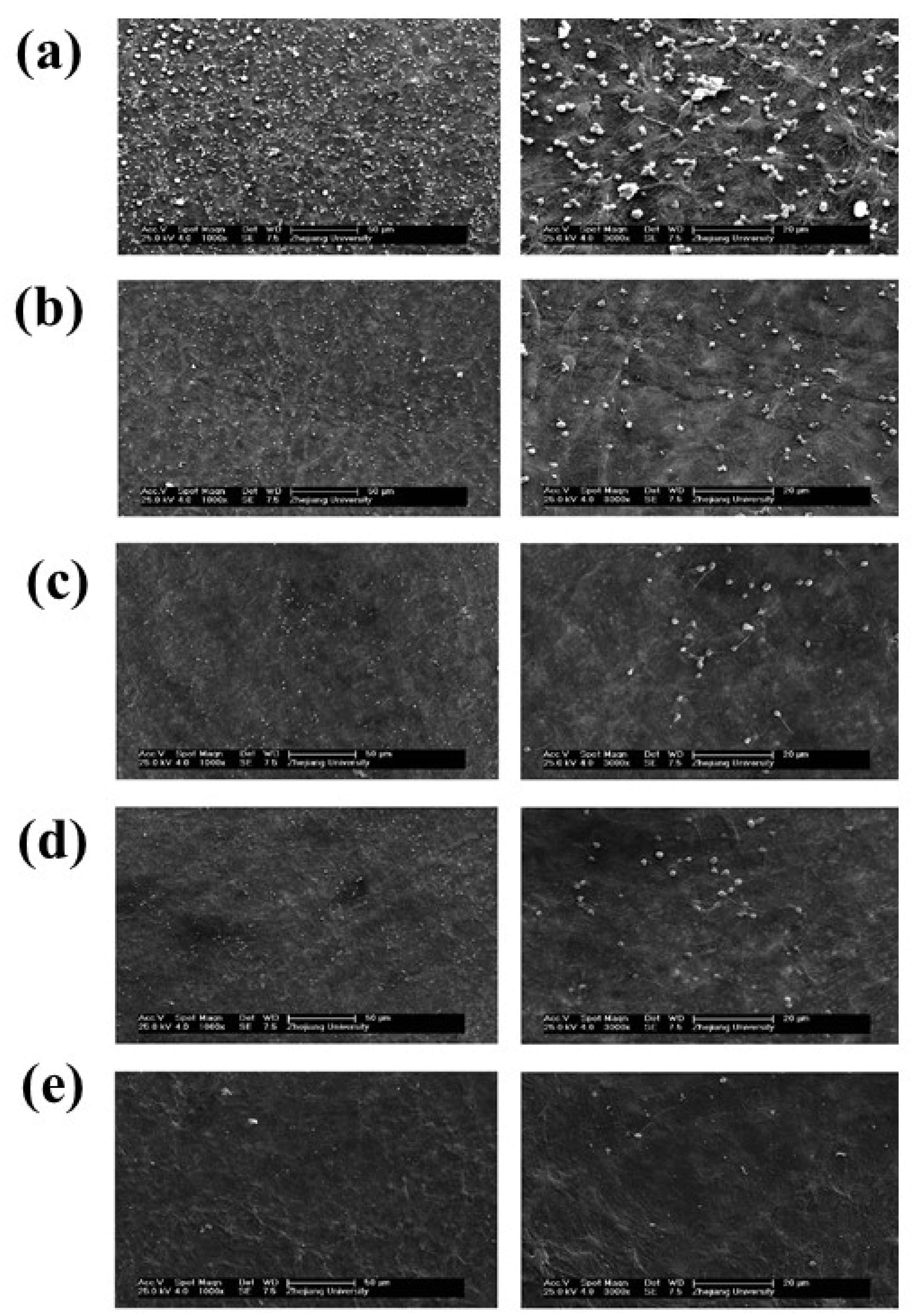
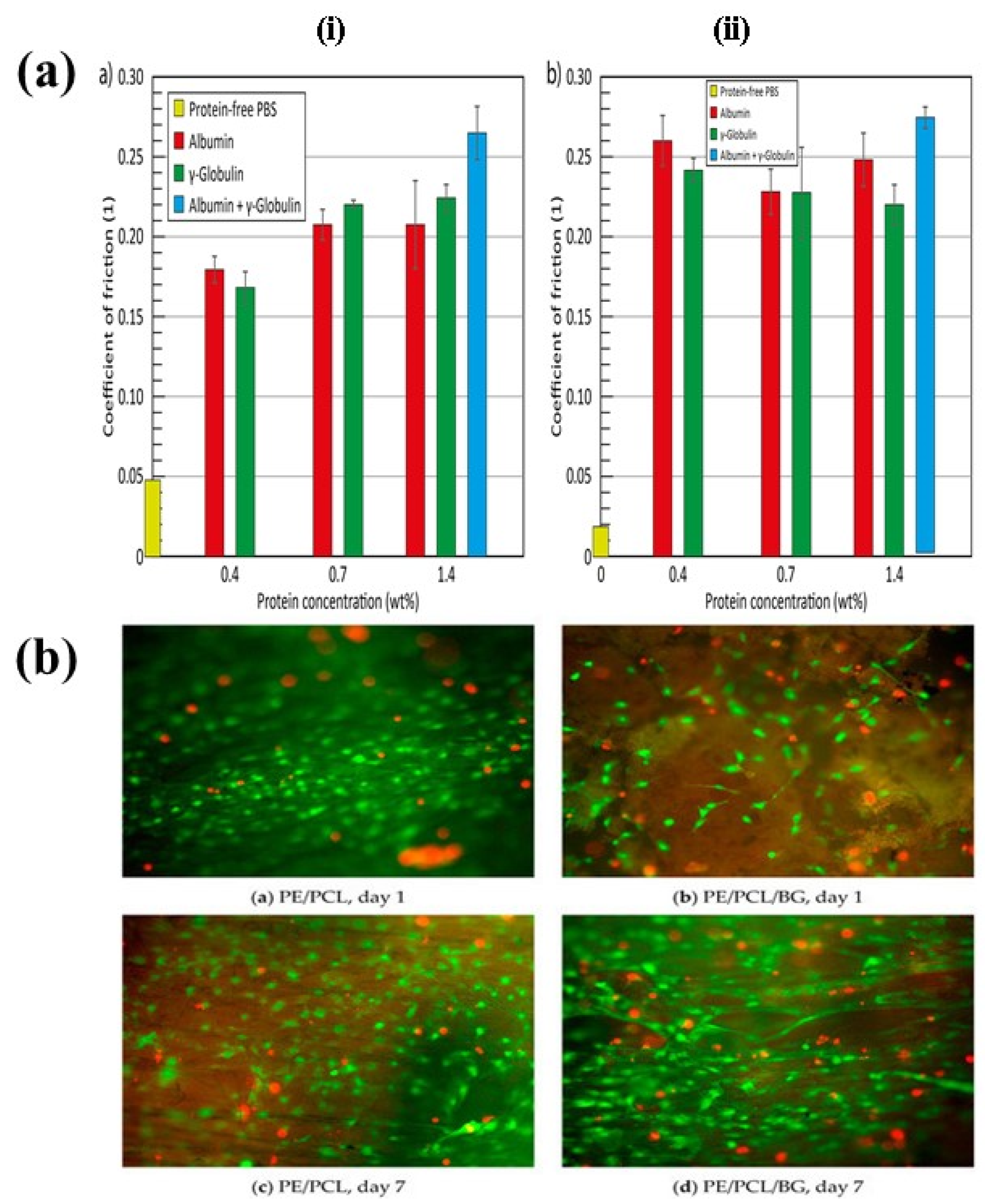
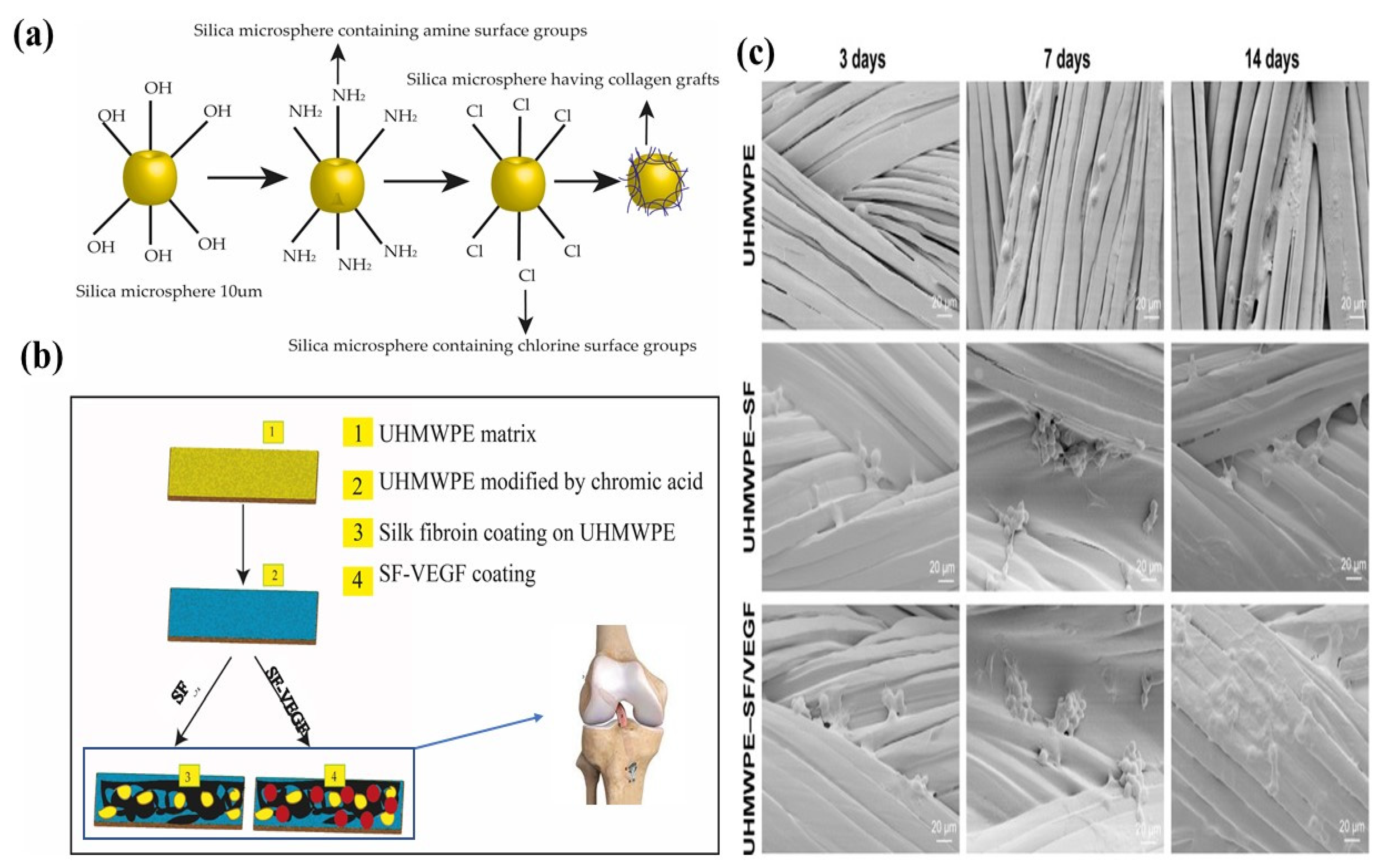
| Treatment | Parameters | Additional Peaks Found after Treatment | Elemental Analysis after Oxidation on the Surface (%) | Surface and Tribological Properties | Mechanical Properties | References | |||
|---|---|---|---|---|---|---|---|---|---|
| C | O | N | Others | ||||||
| Chemical | Chromic acid, hydrogen per oxide, potassium permanganate | 89.6, 73.6, 91.6 | 9.6, 16.5, 6.6 | - | Improvement in surface adhesion properties | Improvement in interfacial tensile strength | [40] | ||
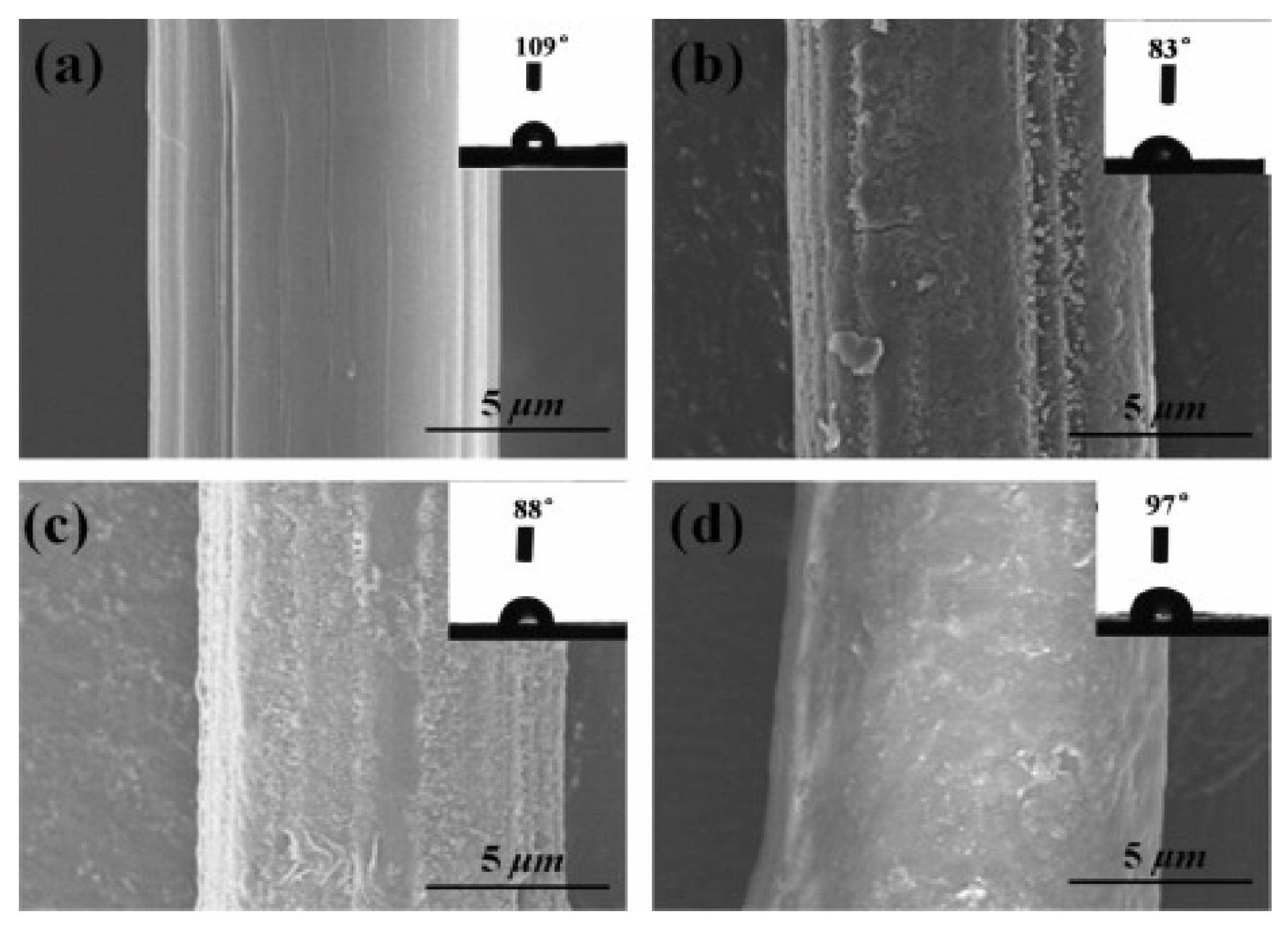
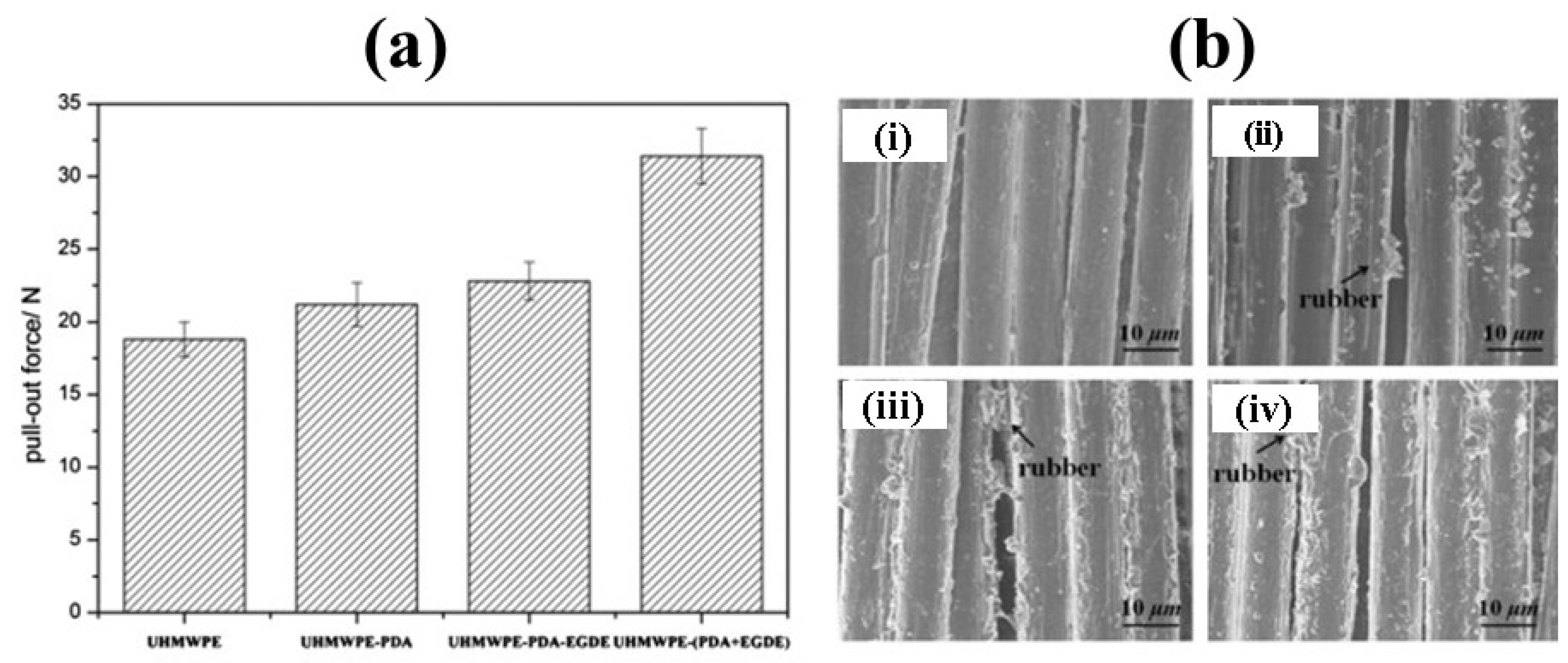
| Chemical | Polydopamine, Ethylene glycol diglycidyl ether (EGDGE) | Water contact angle (WCA) decreased from 109° to 97° | 67.5% improvement in mechanical strength | [41] | |||||
| Chemical | Polydopamine (PDA), hexamethylene diamine (HMDA) | C-OH, -NH, C=C stretching | 73.14, 74.39, 74.39 | 21.3, 18.65 | 5.13, 6.96 | Shear strength 0.920 MPa | [42] | ||
| Chemical | Hyaluronic acid | WCA decreased from 80° to 50°, increased crystallinity | High wear resistance | [43] | |||||
| Chemical | PEG-like coating | -OH stretch, C-O-C, C-OH | 68.8 | 31.2 | - | WCA decreased from 90.3° to 44.8° | [44] | ||
| Chemical | VTMS and SiO2 | Si-C, Si-O | 40.39 | 35.38 | 24.23 | WCA decreased from 123° to 43°, increased antifouling properties | [45] | ||
| Cold plasma | He and O2 gas | C-C stretching, C-N, Hydrogenated amorphous C | Increased surface hydrophilicity and cell adhesion | High wear resistance | [46] | ||||
| Cold plasma | H2 and O2 gas | CH vibrations, C-O stretching, C=O | WCA decreased from 102° to 43°, Improved cell adhesion property, Roughness increased from 588 nm to 687 nm | [46] | |||||
| PACVD plasma | Air and Ar gas | Tear resistance, 40% increase in tensile strength | [22] | ||||||
| ECR plasma | H2 and N2 gas | C-O/C-OH, C-C, C=C, C=O | 60 | 37 | 8 | Increased surface roughness | Increased surface hardness and elastic modulus | [33] | |
| ECR plasma | (N2 + H2) and O2 gas | C-N, C-O Stretching, N-H bending, C=O Stretching | WCA decreased from 96° to 22°, improvement in surface cell adhesion | [21] | |||||
| ECR plasma | Peroxides, acrylic acid, itaconic acid, collagen | C-O, -COOH, -NH, C-N | [34] | ||||||
| DBD + chitosan treatment | (Ar + O2) gas and chitosan | C=O, -COO, C-O/C-N | 93.15, 82.1, 74.0 | 5.8, 15.2, 2.7 | 1.1, 2.7, 3.0 | Surface adhesion increased by 72.2%, WCA decreased from 101° to 82° | Decreased tensile strength by 5.6% | [47] | |
| DBD plasma | (He + Ar + air + N2 + H2) gas | C-C, C-C=O, O=C-O | 88.3 | 6.3 | 6.9 | WCA decreased from 95.2° to 70° | [48] | ||
| DBD plasma | Ar gas | C-H, -OH, C-C, C=O | WCA decreased from 91° to 67.4° | ||||||
| DBD plasm | Ar gas, Multi walled carbon nanotube | C-H stretching, C-C, O-H, C=O | Wear volume was reduced by 73.3%, surface roughness was reduced by 17%, hardness of the composite increased by 45% | [30] | |||||
| Gamma | Thermal treatment + gamma dose | Toughness increased by 67%, | [49] | ||||||
| Gamma | Increased cross-linking, wear rate 37% | [50] | |||||||
| Gamma | UV + PEG grafting | C=C, C-Si, C=O | Crystallinity increased, resistance to protein adsorption increased | [51] | |||||
| Gamma | Irradiation + Vitamin E | Reduced crystallinity, wear resistance | [52] | ||||||
| PIII treatment | Ar gas | C=C, -OH, COOH and COO- | WCA decreased from 80° to 28° | [53] | |||||
| PIII treatment | N2 + gas | A small decrease in WCA, surface roughness increased from 39 nm to 71 nm | [54] | ||||||
| PIII treatment | N2 + gas + HRP protein | 71.1 | 15.5 | 13.4 | Roughness increased from 528° to 1130°, surface area increased from 101.9 nm to 15.6 nm | [55] | |||
| PIII treatment | N2 + HRP + PVC | C=O, C-O, -OH | WCA decreased from 90° to 58° | [56] | |||||
| Property | HDPE | UHMWPE |
|---|---|---|
| Molecular weight (×106 g/mol) | 0.05–0.25 | 3.5–7.5 |
| Tensile ultimate strength (MPa) | 22–31 | 39–48 |
| Tensile ultimate elongation (%) | 10–1200 | 350–525 |
| Scheme | C (%) | O (%) |
|---|---|---|
| Untreated | 74.2 | 21.9 |
| Chromic acid | 89.6 | 9.6 |
| Potassium permanganate | 73.6 | 16.5 |
| Hydrogen peroxide | 91.6 | 6.6 |
| Ar Gas Flow (mL/min) | LP (w) | Adhesion Strength (MPa) | Load Applied (N) |
|---|---|---|---|
| 25 | 180 | 12 | 65.8 |
| 50 | 225 | 33 | 187.3 |
| 75 | 290 | 37 | 209.2 |
| 100 | 260 | 20 | 112.6 |
| 175 | 255 | 12 | 70.6 |
| Samples | Amount of Fibre (vol%) | Tensile Strength MPa |
|---|---|---|
| Raw UHMWPE | 34.6 | 526.7 |
| DBD-UHMWPE | 34.2 | 543.4 |
| BHHBP-DGEBA-UHMWPE | 34.5 | 537.3 |
| Sample | Coefficient of Wear (mm3/Nm) | Coefficient of Friction |
|---|---|---|
| UN | 19.6 × 10−9 | 0.060–0.063 |
| PE1 | 14.1 × 10−9 | 0.066–0.068 |
| PE2 | 8.45 × 10−9 | 0.065–0.066 |
| PE3 | 6.51 × 10−9 | 0.060–0.070 |
| Sample | Polar Components (dyne/cm) | Dispersive Components (dyne/cm) | Total Surface Energy (dyne/cm) |
|---|---|---|---|
| Untreated | 18.7 | 39.5 | 58.2 |
| PIII treated | 50.7 | 22.1 | 72.8 |
| Parameter | Effects |
|---|---|
| High temperature | Deteriorates the material [119] |
| High-intensity energy | Oxidizes the material, increases crystallinity, destroys the lifetime [120] |
| UV radiation | Oxidative degradation of the material can damage the material [17,60] |
| Chemical reagent | Changes surface chemistry, the formation of toxic residues and by-products including carcinogens [36] |
| Methods | Advantages | Disadvantages |
|---|---|---|
| DBD plasma | Atmospheric pressure Less time required | Sample could be contaminated |
| PACVD plasma | High deposition rate Low temperature Unique chemical properties of deposited film High solvent and corrosion resistance | Time consuming Unstable against ageing Very thin layer of deposition |
| ECR plasma | Low-pressure range High degree of ionization Provide sterile environment | Non-uniform etch surface Sample could be contaminated |
| Cold plasma | Can provide sterile environment Low temperature Suitable for porous structures Uniform surface treatment | Covers limited surface area Limited temperature range |
| PIII | Large treatment area High efficiency Can treat 3D objects Batch processing capability Small instrument footprint Provide uniformity in treated surface | Difficult to achieve accurate in situ dose monitoring |
| Sample | Roughness A° | Surface Area (µm2) | C (%) | N (%) | O (%) | Optical Density @450nm from HRP Activity |
|---|---|---|---|---|---|---|
| Untreated | 528 | 101.9 | 94.9 | Nil | 5.1 | 0.45 |
| PIII treated | 1130 | 105.6 | 71.1 | 13.4 | 15.5 | 0.52 |
| Samples | HRP Protein Remains after Wash (%) |
|---|---|
| Untreated | 19 |
| PIII treated | 84 |
| PIII treated + Plasticizer | 62–72 |
| Grafts | Ultimate Tensile Load (N) | Stiffness (N/mm) |
|---|---|---|
| Human ACL | 1730 | 242 |
| Human hamstring graft | 3790 | 776 |
| The human patellar tendon graft | 3790–4140 | 685 |
| Carbon fibers | 660 | 230 |
| Gore-Tex prosthesis | 5300 | 322 |
| Dacron | 3631 | 420 |
| Twisted silk matrix | 2337 | 354 |
| Parallel silk matrix | 1740 | 2214 |
| KLAD | 280 | 1500 |
| Trevira | 68.3 | 1866 |
| Leeds-Keio | 270 | 2000 |
| UHMWPE fabric | 52570 | 115 |
| Growth Factor | The Active Site of Growth Factors | Role | Reference |
|---|---|---|---|
| PDGF AA | Proliferation, remodeling | Controls DNA and protein synthesis at the injured site, controls the expression of other growth factors. | [193] |
| PDGF BB | Proliferation, remodeling | Controls DNA and protein synthesis at the injured site, controls the expression of other growth factors. | [195] |
| IGF-1 | Inflammation, proliferation | Supports the proliferation and migration of cells, triggers matrix production. | [196] |
| TGFβ | Inflammation | Controls cell migration, proteinase expression, fibronectin-binding interaction, and stimulation of collagen production. | [194] |
| VEGF | Proliferation, remodeling | Supports angiogenesis. | [194] |
| bFGF | Proliferation, remodeling | Supports cellular migration, angiogenesis. | [193] |
| IL1A | Proliferation induces pro-collagen type I and III synthesis | Supports proliferation, and induction. | [197] |
| BMP | Remodeling of impaired tissues | Supports angiogenesis. | [198] |
| GDF | Proliferation | Supports in ligament/tendon formation. | [198] |
| Elastin | Proliferation | Controls DNA and protein synthesis at the injured site. | [199] |
| Heparin | Proliferation | Supports the release of growth factors. | [200] |
| PRP (Platelet-rich plasma) | Increased cellular metabolic activity, reduced apoptotic rate, and stimulation of collagen production in the cells. | [201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahed, S.B.; Dunstan, C.R.; Boughton, P.C.; Ruys, A.J.; Faisal, S.N.; Wahed, T.B.; Salahuddin, B.; Cheng, X.; Zhou, Y.; Wang, C.H.; et al. Functional Ultra-High Molecular Weight Polyethylene Composites for Ligament Reconstructions and Their Targeted Applications in the Restoration of the Anterior Cruciate Ligament. Polymers 2022, 14, 2189. https://doi.org/10.3390/polym14112189
Wahed SB, Dunstan CR, Boughton PC, Ruys AJ, Faisal SN, Wahed TB, Salahuddin B, Cheng X, Zhou Y, Wang CH, et al. Functional Ultra-High Molecular Weight Polyethylene Composites for Ligament Reconstructions and Their Targeted Applications in the Restoration of the Anterior Cruciate Ligament. Polymers. 2022; 14(11):2189. https://doi.org/10.3390/polym14112189
Chicago/Turabian StyleWahed, Sonia B., Colin R. Dunstan, Philip C. Boughton, Andrew J. Ruys, Shaikh N. Faisal, Tania B. Wahed, Bidita Salahuddin, Xinying Cheng, Yang Zhou, Chun H. Wang, and et al. 2022. "Functional Ultra-High Molecular Weight Polyethylene Composites for Ligament Reconstructions and Their Targeted Applications in the Restoration of the Anterior Cruciate Ligament" Polymers 14, no. 11: 2189. https://doi.org/10.3390/polym14112189
APA StyleWahed, S. B., Dunstan, C. R., Boughton, P. C., Ruys, A. J., Faisal, S. N., Wahed, T. B., Salahuddin, B., Cheng, X., Zhou, Y., Wang, C. H., Islam, M. S., & Aziz, S. (2022). Functional Ultra-High Molecular Weight Polyethylene Composites for Ligament Reconstructions and Their Targeted Applications in the Restoration of the Anterior Cruciate Ligament. Polymers, 14(11), 2189. https://doi.org/10.3390/polym14112189