Research and Application of Polypropylene Carbonate Composite Materials: A Review
Abstract
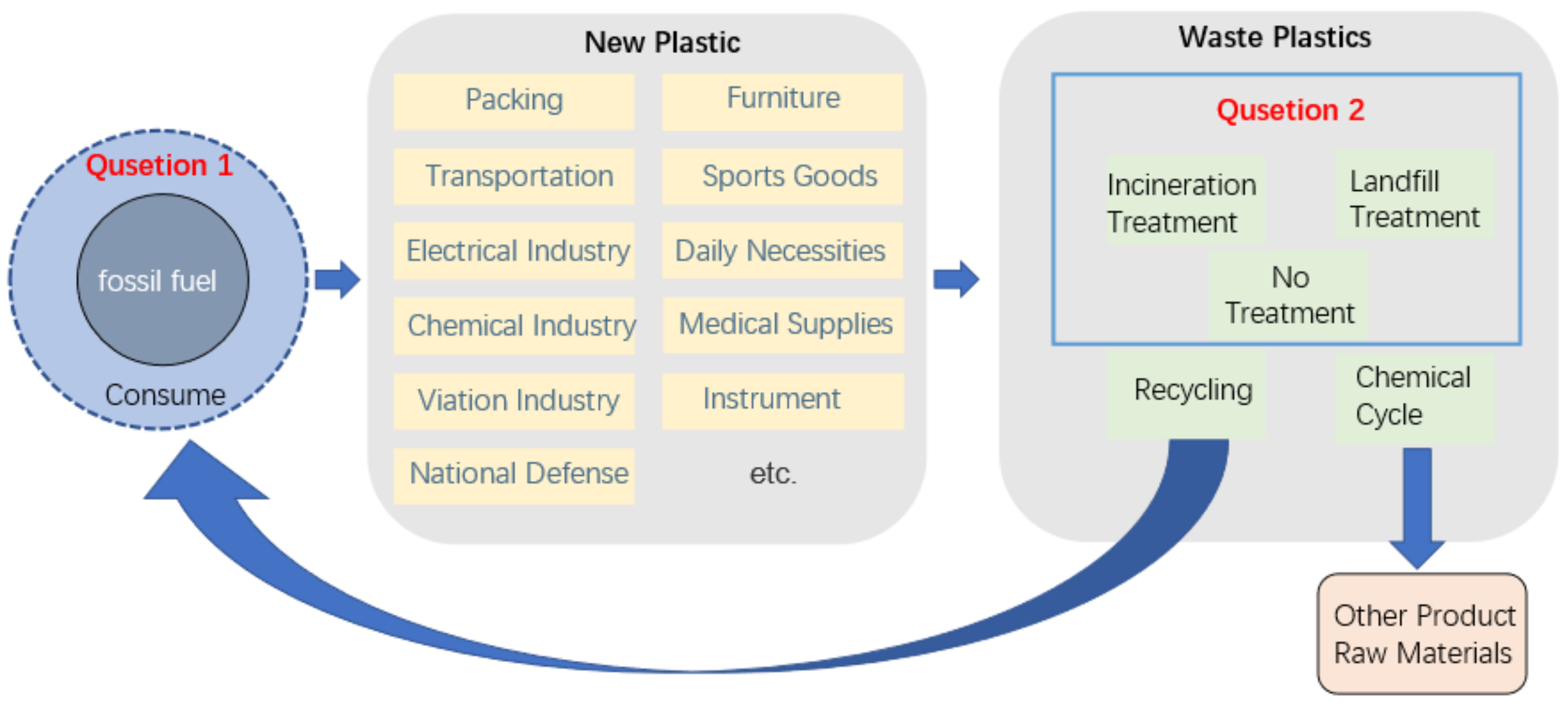
:1. Introduction
2. Polypropylene Carbonate
2.1. Synthesis of PPC
2.2. Structure of PPC
2.3. Performance of PPC
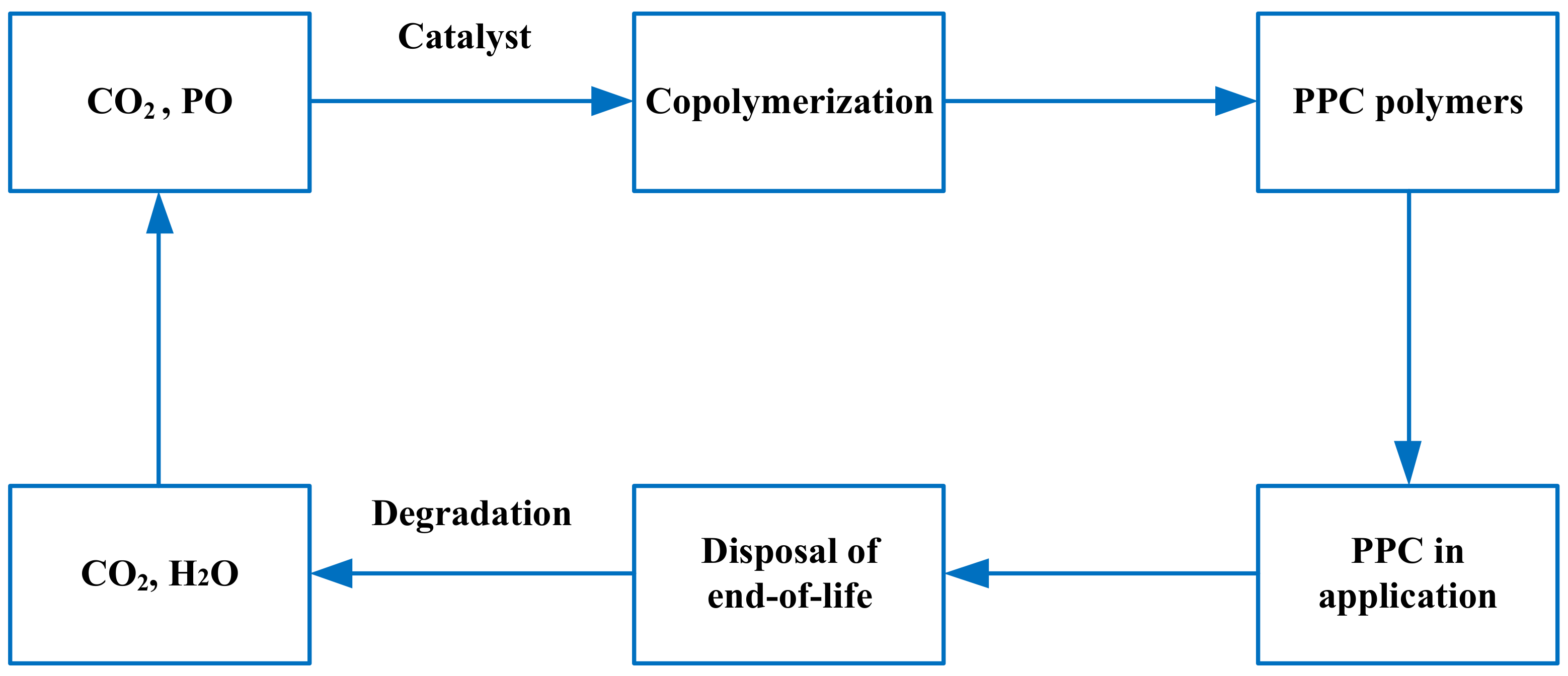
2.4. The Cyclicality and Sustainability of PPC
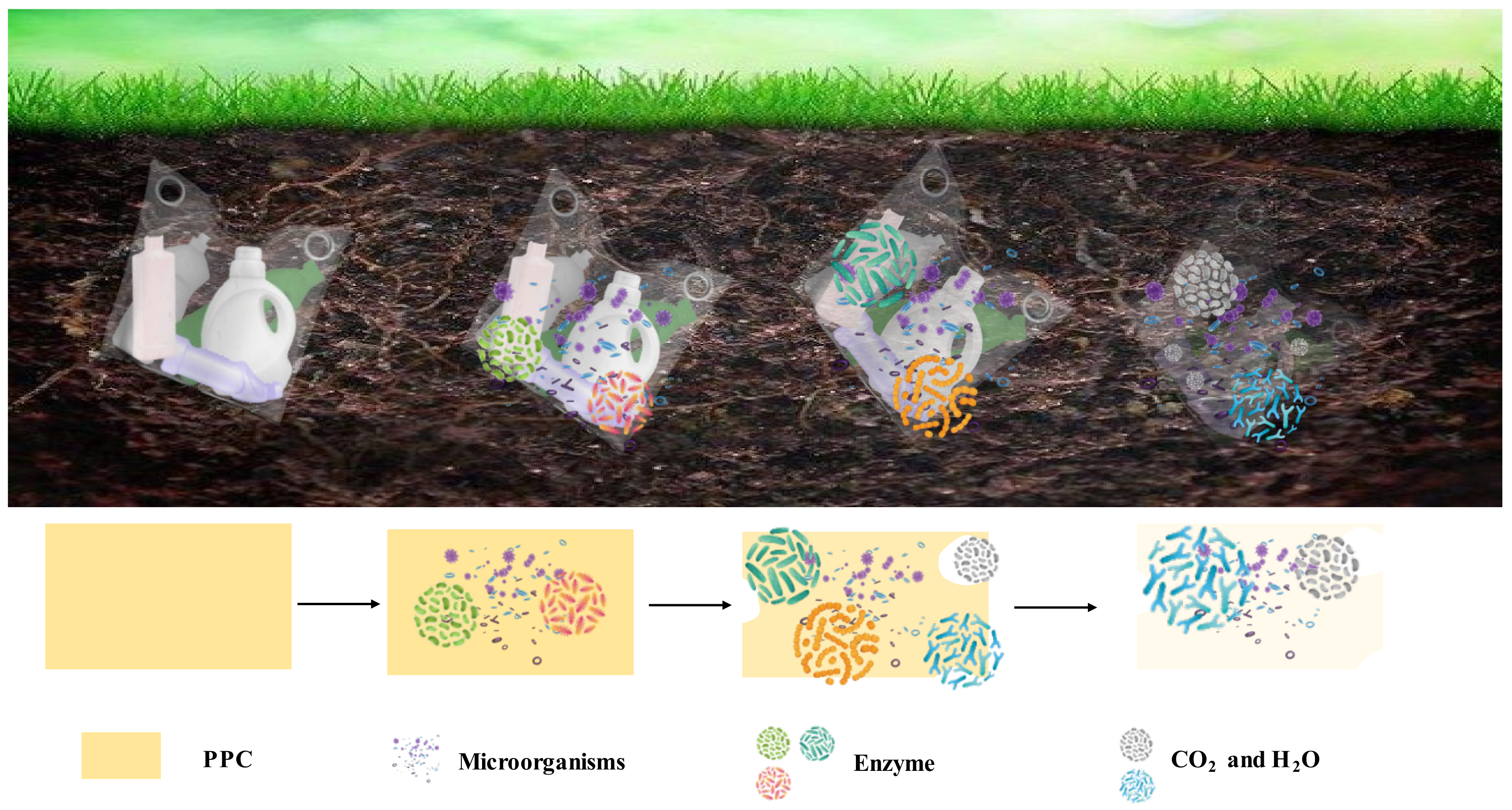
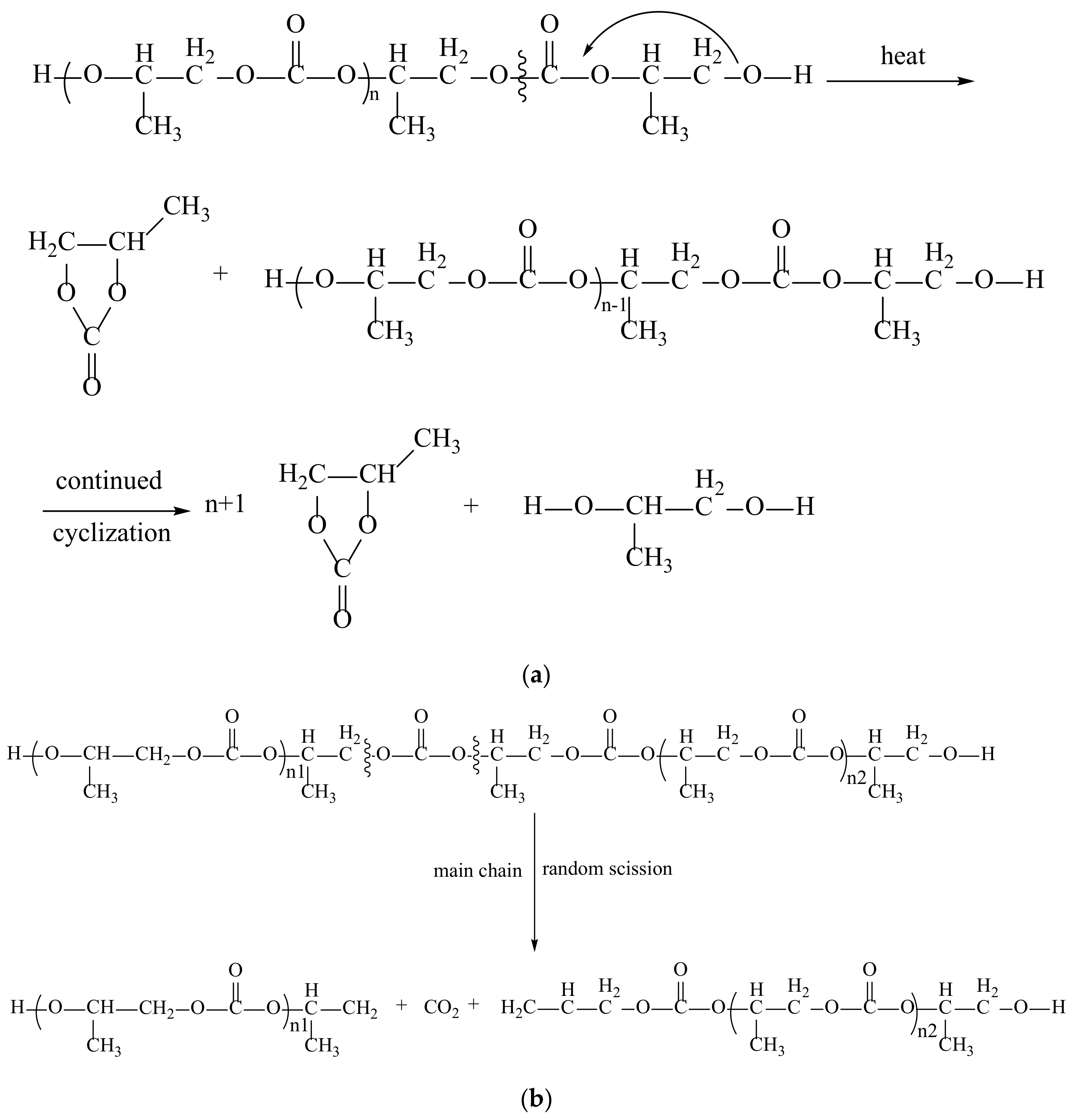
2.5. Degradation of PPC
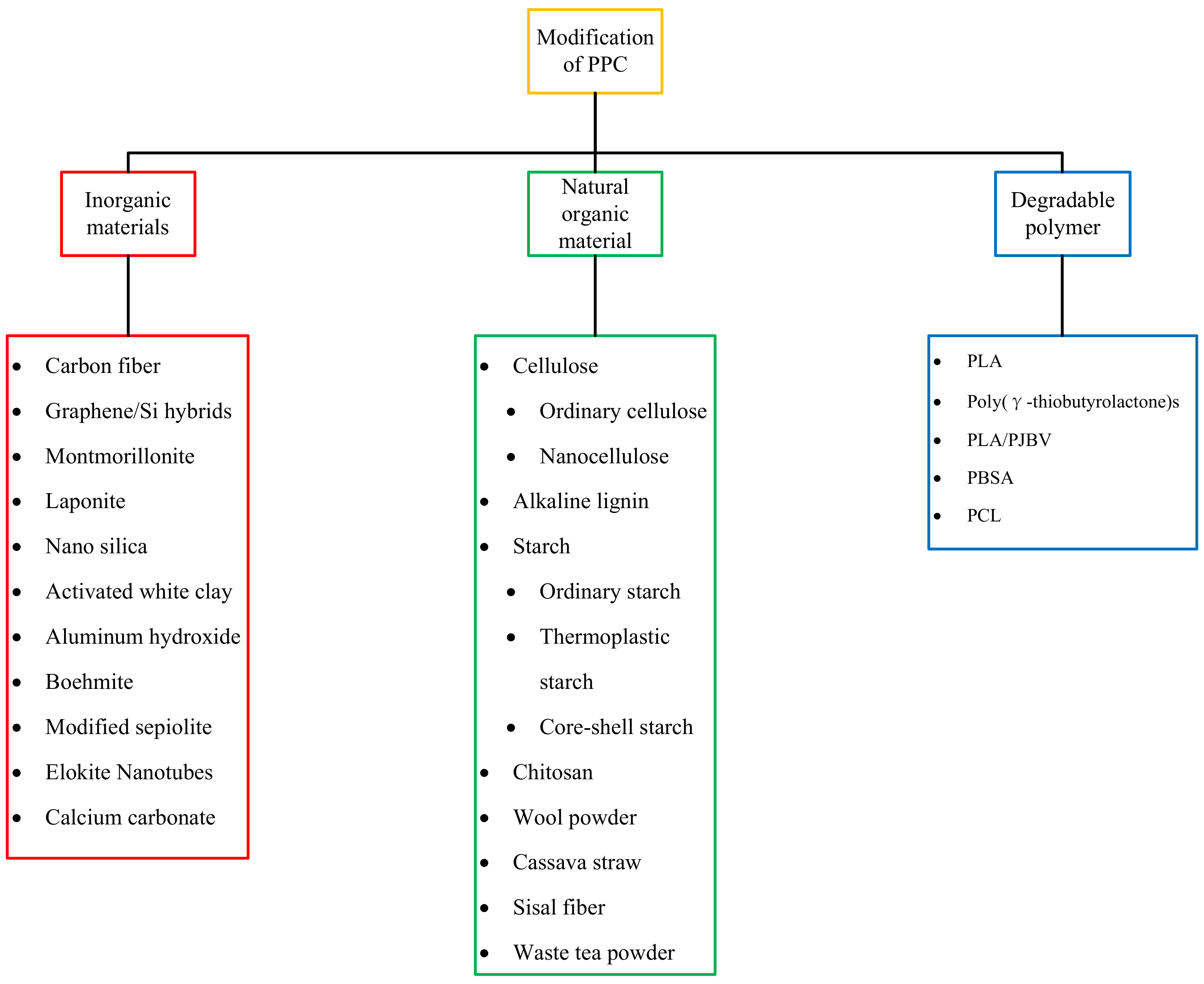
3. Modification of PPC
3.1. Inorganic Material Modification
3.2. Modification of Natural Organic Polymer Materials
3.3. Degradable Polymer Modification
3.4. Other Modification Methods
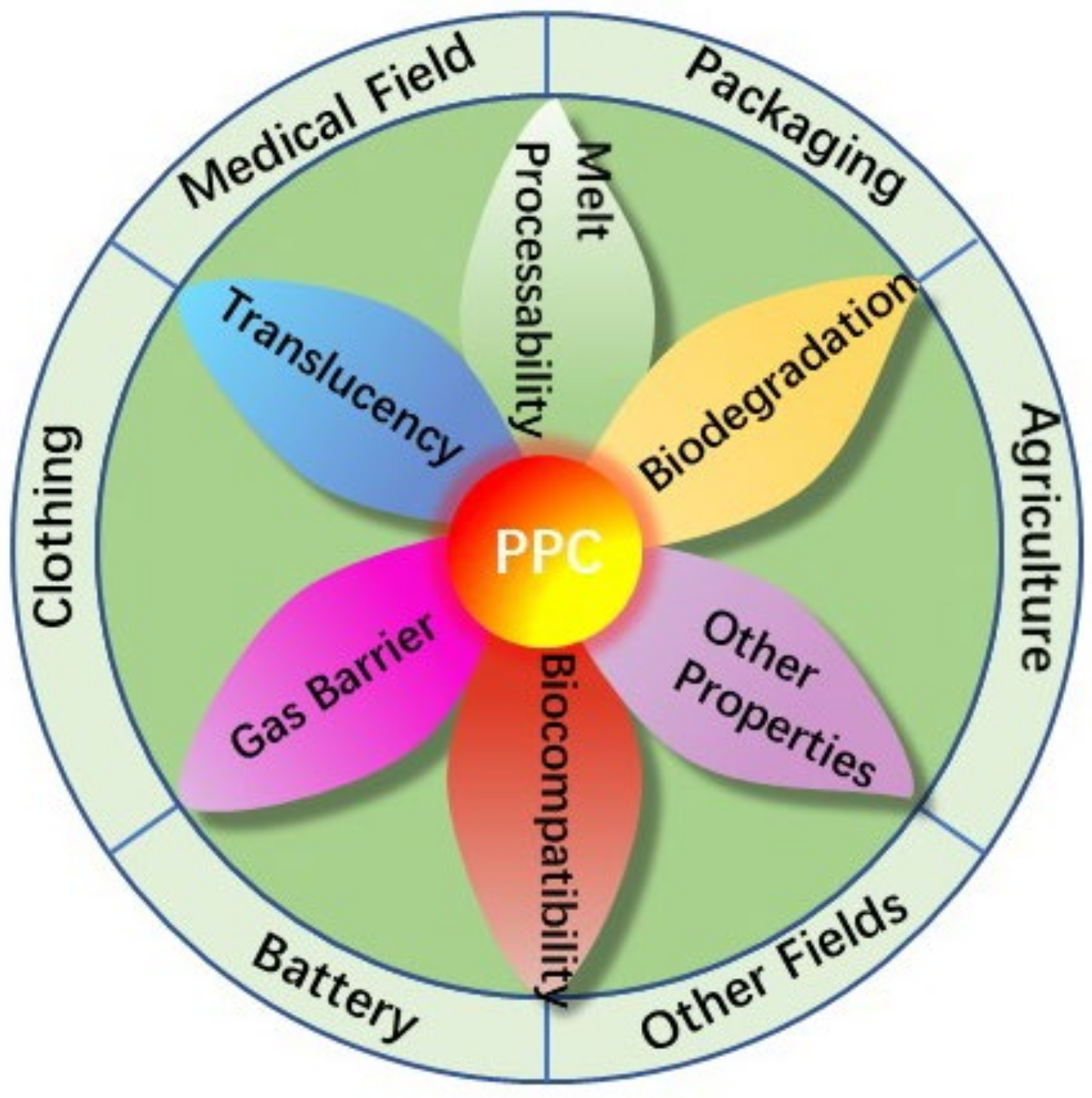
4. Applications of PPC
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| First Appearance | Shorthand | Full Name | First Appearance | Shorthand | Full Name |
|---|---|---|---|---|---|
| 1 | CO2 | Carbon dioxide | 11 | MMT | Montmorillonite |
| 2 | CH4 | Methane | 11 | AC | Activated white clay |
| 2 | pH | Hydrogen ion concentration | 11 | ATH | Aluminum hydroxide |
| 2 | TPS | Thermoplastic starch | 12 | CS | Chitosan |
| 2 | PHA | Polyhydroxyalkanoate | 12 | NCC | Nanocellulose |
| 2 | PGA | Polyglutamic acid | 13 | AL | Alkaline lignin |
| 2 | PBS | Polybutylene succinate | 13 | BLF | Black liquor lignin |
| 2 | PBAT | Polybutyleneadipate-co-terephthalate | 13 | HBL | Hydroxy black liquor lignin |
| 2 | PCL | Polycaprolactone | 13 | PPCMA | Maleic anhydride grafted PPC |
| 2 | PPC | Polypropylene carbonate | 13 | TPOS | Thermoplastic starch oxide |
| 2 | PLA | Polylactic acid | 13 | DL-TPOS | Aluminate pretreated starch oxide |
| 2 | PE | Polyethylene | 14 | CSS NPs | Core-shell starch nanoparticles |
| 2 | PP | Polypropylene | 14 | PMA | Poly (methyl acrylate) |
| 3 | PBSA | Polybutylene succinate-co-butylene adipate | 14 | PHBV | β-hydroxyvalerate copolymer |
| 4 | PVA | Polyvinyl alcohol | 14 | WP | Wool powder |
| 4 | PO | Propylene oxide | 14 | LCHBP | Long-chain hyperbranched polymer |
| 6 | Tg | Glass transition temperature | 16 | CA | Cellulose acetate |
| 6 | Tm | Melting temperature | 17 | HAP | Hydroxyapatite |
References
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution. Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef] [PubMed]
- Millican, J.M.; Agarwal, S. Plastic pollution: A material problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- Nie, H. Study on the Growth of Energy Consumption in China and Countermeasures. Ph.D. Thesis, Jilin University, Changchun, China, 2013. [Google Scholar]
- Zheng, Q. Plastic and “white pollution” rumination. Polym. Bull. 2022, 4, 1–10. [Google Scholar]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhou, Y.; Huang, Y. A review of population exposure routes of microplastics and their possible health hazards. Asian J. Ecotoxicol. 2021, 16, 221–227. [Google Scholar]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Sharma, K.; Park, Y.K.; Nadda, A.K.; Nadda, A.K.; Banerjee, P.; Singh, P.; Raizada, P.; Banat, F.; Bharath, G.; Jeong, S.M.; et al. Emerging chemo-biocatalytic routes for valorization of major greenhouse gases (GHG) into industrial products: A comprehensive review. J. Ind. Eng. Chem. 2022, 109, 1–20. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Peters, R.L.; Darling, J. The greenhouse effect and nature reserves: Global warming would diminish biological diversity by causing extinctions among reserve species. Bioscience 1985, 35, 707–717. [Google Scholar] [CrossRef]
- Demir, Z.; Yapcolu, P. Investigation of GHG emission sources and reducing GHG emissions in a municipal wastewater treatment plant. Greenh. Gases Sci. Technol. 2019, 9, 948–964. [Google Scholar] [CrossRef]
- Zeenat, E.A.; Bkhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Zhang, J.P. Study on Modification of Poly(Propylene Carbonate) and Its Application Properties in Packaging Field. Master’s Thesis, Dalian Polytechnic University, Dalian, China, 2016. [Google Scholar]
- Xiang, X.W. Microbial degradable packaging materials. J. Hunan Univ. Technol. 1995, 9, 31–38. [Google Scholar]
- Wang, Z.Y.; Zhang, J.; Su, T.T. Research and development of biodegradable plastics. Environ. Prot. Circ. Econ. 2003, 4, 9–11. [Google Scholar]
- Zhang, S.H.; Xiao, Y.; Jiang, Y.M. Research status and development trend of biodegradable plastics. J. Guangxi Norm. Univ. 1999, S1, 189–193. [Google Scholar]
- Chang, Q.; Zhu, D.; Hu, L.; Kim, H.; Liu, Y.; Cai, L. Rapid photo aging of commercial conventional and biodegradable plastic bags. Sci. Total Environ. 2022, 822, 153235. [Google Scholar] [CrossRef]
- Jin, Y.; Cai, F.F.; Wang, L.G.; Song, C.; Jin, W.X.; Sun, J.F.; Liu, G.Q.; Chen, C. Advance in degradation of biodegradable plastics in different environments. Chin. J. Biotechnol. 2021, 37, 1–25. [Google Scholar] [CrossRef]
- Sisti, L.; Totaro, G.; Celli, A.; Marek, A.A.; Verney, V.; Leroux, F. Chain extender effect of 3-(4-hydroxyphenyl) propionic acid/layered double hydroxide in biopolyesters containing the succinate moiety. New J. Chem. 2020, 44, 10127–10136. [Google Scholar] [CrossRef]
- Wang, G.X.; Huang, D.; Ji, J.H.; Völker, C.; Wurm, F.R. Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution. Adv. Sci. 2021, 8, 2001121. [Google Scholar] [CrossRef]
- Wang, W.; Mo, T.; Wang, Y. Better self and better us: Exploring the individual and collective motivations for China’s Generation Z consumers to reduce plastic pollution. Resour. Conserv. Recycl. 2022, 179, 106111. [Google Scholar] [CrossRef]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Bandyopadhyay, J.; Ray, S.S. Synthetic biopolymers and their composites: Advantages and limitations—An overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide with organometallic compounds. Macromol. Chem. Phys. 1969, 130, 210–220. [Google Scholar] [CrossRef]
- Sasanuma, Y.; Takahashi, Y. Structure–Property Relationships of Poly (ethylene carbonate) and Poly (propylene carbonate). ACS Omega 2017, 2, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.X. Production Technology and Market Application Status and Development Prospects of Aliphatic Polycarbonate. Pe-Troleum Petrochem. Today 2007, 15, 28–31 + 50. [Google Scholar]
- Fu, L.; Li, Q.S.; Bu, Z.W.; Chang, H.B. Copolymerization of carbon dioxide and epoxides catalyzed by zinc acetate. Chem. Res. 2017, 28, 752–756. [Google Scholar]
- Zhuo, C.; Qin, Y.; Wang, X.; Wang, F. Temperature-responsive Catalyst for the Coupling Reaction of Carbon Dioxide and Propylene Oxide. Chin. J. Chem. 2018, 36, 299–305. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X. Carbon dioxide-based copolymers: Environmental benefits of PPC, an industrially viable catalyst. Biotechnol. J. 2010, 5, 1164–1180. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerization—New strategies and cooperative mechanisms. Coord. Chem. Rev. 2010, 255, 1460–1479. [Google Scholar] [CrossRef]
- Zhao, L. Study on the Structure and Properties of Biodegradable Poly(Propylene Carbonate) Blends. Master’s Thesis, Changchun University of Technology, Changchun, China, 2021. [Google Scholar]
- Rokicki, A.; Kuran, W. Copolymerization of carbon dioxide with propylene oxide in the presence of catalysts based on alkylmetal compounds and pyrogallol. Macromol. Chem. Phys. 1979, 180, 2153–2161. [Google Scholar] [CrossRef]
- Marbach, J.; Nörnberg, B.; Rahlf, A.F.; Luinstra, G.A. Zinc glutarate-mediated copolymerization of CO2 and PO—Parameter studies using design of experiments. Catal. Sci. Technol. 2017, 7, 2897–2905. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.M.; Huang, K.; Long, S.Y.; Huang, R.B. Copolymerization of Carbon Dioxide and Propylene Oxide with Co-Zn Double Metal Cyanide Complex/Zinc Glutarate Composite Catalysts. Polym. Mater. Sci. Eng. 2014, 30, 24–28. [Google Scholar]
- Luo, L.; Wang, W.Z.; Wang, L.; Li, L.L.; Zhang, Y.L.; Zhao, S.D. Copolymerization of CO2, propylene oxide, and itaconic anhydride with double metal cyanide complex catalyst to form crosslinked polypropylene carbonate. E-Polymers 2021, 21, 854–868. [Google Scholar] [CrossRef]
- Quan, Z.; Min, J.; Zhou, Q.; Xie, D.; Liu, J.; Wang, X.; Zhao, X.; Wang, F. Synthesis and properties of carbon dioxide–epoxides copolymers from rare earth metal catalyst. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Anderson, C.E.; Vagin, S.I.; Xia, W.; Jin, H.; Rieger, B. Cobaltoporphyrin-Catalyzed CO2/Epoxide Copolymerization: Selectivity Control by Molecular Design. Macromolecules 2012, 45, 6840–6849. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Wildeson, J.R.; Yarbrough, J.C.; Reibenspies, J.H. Bis 2,6-difluorophenoxide Dimeric Complexes of Zinc and Cadmium and Their Phosphine Adducts: Lessons Learned Relative to Carbon Dioxide/Cyclohexene Oxide Alternating Copolymerization Processes Catalyzed by Zinc Phenoxides. J. Am. Chem. Soc. 2000, 122, 12487–12496. [Google Scholar] [CrossRef]
- Koning, C.; Wildeson, J.; Parton, R.; Plum, B.; Steeman, P.; Darensbourg, D.J. Synthesis and physical characterization of poly (cyclohexane carbonate), synthesized from CO2 and cyclohexene oxide. Polymer 2001, 42, 3995–4004. [Google Scholar] [CrossRef]
- Pilz, M.F.; Limberg, C.; Lazarov, B.B.; Hultzsch, K.C.; Ziemer, B. Dinuclear zinc complexes based on parallel β-diiminato binding sites: Syntheses, structures, and properties as CO2/epoxide copolymerization catalysts. Organometallics 2007, 26, 3668–3676. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yang, G.W.; Wu, G.P. A bifunctional β-diiminate zinc catalyst with CO2/epoxides copolymerization and RAFT polymerization capacities for versatile block copolymers construction. Macromolecules 2018, 51, 3640–3646. [Google Scholar] [CrossRef]
- Roznowska, A.; Dyduch, K.; Lee, B.Y.; Michalak, A. Theoretical study on preference of open polymer vs. cyclic products in CO2/epoxide copolymerization with cobalt (III)-salen bifunctional catalysts. J. Mol. Model. 2020, 26, 113. [Google Scholar] [CrossRef]
- Ree, M.; Hwang, Y.; Kim, J.S.; Kim, H.; Kim, G.; Kim, H. New findings in the catalytic activity of zinc glutarate and its application in the chemical fixation of CO2 into polycarbonates and their derivatives. Catal. Today 2006, 115, 134–145. [Google Scholar] [CrossRef]
- Ang, R.R.; Sin, L.T.; Bee, S.T.; Tee, T.T.; Kadhum, A.A.H.; Rahmat, A.R.; Wasmi, B.A. Determination of zinc glutarate complexes synthesis factors affecting production of propylene carbonate from carbon dioxide and propylene oxide. Chem. Eng. J. 2017, 327, 120–127. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Z.; Zhang, Y. New catalytic systems for the fixation of carbon dioxide. 1. Copolymerization of carbon dioxide and propylene oxide with new rare-earth catalysts-RE(P204)3-Al(i-Bu)3-R(OH)n. Macromolecules 1991, 24, 5305–5308. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Wang, X.; Wang, F. Copolymerization of carbon dioxide and propylene oxide with Ln (CCl3COO)3-based catalyst: The role of rare-earth compound in the catalytic system. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2751–2754. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, X.; Chen, X.; Zhao, X.; Wang, F. Regio-regular structure high molecular weight poly (propylene carbonate) by rare earth ternary catalyst and Lewis base cocatalyst. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4451–4458. [Google Scholar] [CrossRef]
- Zhou, T.; Zou, Z.; Gan, J.; Chen, L.; Zhang, M. Copolymerization of epoxides and carbon dioxide by using double metal cyanide complex (DMC) with high crystallinity. J. Polym. Res. 2011, 18, 2071–2076. [Google Scholar] [CrossRef]
- Dai, W.L.; Yin, S.F.; Guo, R.; Luo, S.L.; Du, X.; Au, C.T. Synthesis of propylene carbonate from carbon dioxide and propylene oxide using Zn-Mg-Al composite oxide as high-efficiency catalyst. Catal. Lett. 2010, 136, 35–44. [Google Scholar] [CrossRef]
- Cui, S.; Li, L.; Wang, Q. Fabrication of (PPC/NCC)/PVA composites with inner-outer double constrained structure and improved glass transition temperature. Carbohydr. Polym. 2018, 191, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, M.; Feng, J.; Zhang, S.; Wang, X. High oxygen barrier property of poly (propylene carbonate)/polyethylene glycol nanocomposites with low loading of cellulose nanocrytals. ACS Sustain. Chem. Eng. 2017, 5, 11246–11254. [Google Scholar] [CrossRef]
- Chen, L.J. Modification of Poly(Propylene Carbonate)Based on Hydrogen Bonding Interaction. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2011. [Google Scholar]
- Udipi, K.; Gillham, J.K. Poly(Ethylene Carbonate) and Poly(Propylene Carbonate): Transitions and Thermomechanical Spectra. J. Appl. Polym. Sci. 1974, 18, 1575–1580. [Google Scholar] [CrossRef]
- Lin, S.; Yu, W.; Wang, X.; Zhou, C. Study on the thermal degradation kinetics of biodegradable poly (propylene carbonate) during melt processing by population balance model and rheology. Ind. Eng. Chem. Res. 2014, 53, 18411–18419. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, X.; Zhao, X.; Li, J.; Wang, F. Double propagation based on diepoxide, a facile route to high molecular weight poly (propylene carbonate). Polymer 2006, 47, 7368–7373. [Google Scholar] [CrossRef]
- Wang, Q. Study on Properties of High-Barrier Composite Film of Carbon Dioxide-Propylene Oxide Copolymers. Master’s Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]
- Cheng, M.P.; Li, Z.; Liu, B.H.; Song, L.N. Degradation and stability of PPC. China Synth. Resin Plast. 2019, 36, 80–85. [Google Scholar]
- Li, J.M.; Huang, X.L.; Su, H.Q. Effect of processing conditions in melt blending on properties of poly propylene carbonate. Chem. Reag. 2014, 36, 210–214. [Google Scholar]
- Shi, P.; Zhong, M.M.; Li, F.Z.; Ou, Y.L.; Liu, Y.J. Effect of processing conditions in melt blending on properties of poly propylene carbonate. J. Funct. Mater. 2015, 46, 15090–15094. [Google Scholar]
- Varghese, J.K.; Na, S.J.; Park, J.H.; Woo, D.; Yang, I.; Lee, B.Y. Thermal and weathering degradation of poly (propylene carbonate). Polym. Degrad. Stab. 2010, 95, 1039–1044. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, Q.; Meng, Y. Kinetic analysis of thermal decomposition of poly(propylene carbonate). Polym. Degrad. Stab. 2005, 89, 282–288. [Google Scholar] [CrossRef]
- Tran, T.N.; Mai, B.T.; Setti, C.; Athanassiou, A. Transparent bioplastic derived from CO2-based polymer functionalized with oregano waste extract toward active food packaging. ACS Appl. Mater. Inter. 2020, 12, 46667–46677. [Google Scholar] [CrossRef]
- Peng, C.; Wang, J.; Liu, X.; Wang, L. Differences in the Plastispheres of Biodegradable and Non-biodegradable Plastics: A Mini Review. Front. Microbiol. 2022, 13, 849147. [Google Scholar] [CrossRef]
- Liu, B.H.; Zhang, M.; Yu, A.F.; Chen, L.B. Degradation mechanism of poly(propylene carbonate) polyols. Polym. Mater. Sci. Eng. 2004, 20, 76–79. [Google Scholar]
- Du, L.C.; Meng, Y.Z.; Wang, S.J.; Tjong, S.C. Synthesis and degradation behavior of poly (propylene carbonate) derived from carbon dioxide and propylene oxide. J. Appl. Polym. Sci. 2004, 92, 1840–1846. [Google Scholar] [CrossRef]
- Dhanraj, N.D.; Hatha, A.; Jisha, M.S. Biodegradation of petroleum based and bio-based plastics: Approaches to increase the rate of biodegradation. Arch. Microbiol. 2022, 204, 258. [Google Scholar] [CrossRef]
- Bano, K.; Kuddus, M.R.; Zaheer, M.; Zia, Q.F.; Khan, M.; Gupta, A.; Aliev, G. Microbial enzymatic degradation of biodegradable plastics. Curr. Pharm. Biotechnol. 2017, 18, 429–440. [Google Scholar]
- Sang, L.Y.; Yan, H.; Hu, Z.D.; Dai, J.; Yang, J.J. Degradation properties of poly (propylene carbonate)/poly (lactic acid) blends under natural light irradiation. China Plast. 2018, 32, 116–124. [Google Scholar]
- Sang, L.Y.; Yan, H.; Hu, Z.D.; Dai, J.; Yang, J.J. Degradation behavior and kinetics analysis of poly (propylene carbonate)/poly (lactic acid) blends in alkaline environment. China Plast. Ind. 2018, 41, 37–41. [Google Scholar]
- Sang, L.Y.; Yan, H.; Hu, Z.D.; Xue, M. Degradation properties of poly (propylene carbonate)/poly (lactic acid) blends in marine en-vironment. China Plast. Ind. 2018, 32, 122–130. [Google Scholar]
- Yang, X.; Steck, J.; Yang, J.; Wang, Y.; Suo, Z. Degradable Plastics Are Vulnerable to Cracks. Engineering 2021, 7, 624–629. [Google Scholar] [CrossRef]
- Wang, W.; Han, C.; Wang, X.; Zhou, G.; Xu, M. Enhanced rheological properties and heat resistance of poly (propylene carbonate) composites with carbon fiber. Compos. Commun. 2020, 21, 100422. [Google Scholar] [CrossRef]
- Qu, H.; Wang, Y.; Ye, Y.S.; Zhou, W.; Bai, S.P.; Zhou, X.P.; Peng, H.Y.; Xie, X.L.; Mai, Y.W. A promising nanohybrid of silicon carbide nanowires scrolled by graphene oxide sheets with a synergistic effect for poly (propylene carbonate) nanocomposites. J. Mater. Chem. A 2017, 5, 22361–22371. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Zhang, X.; Zhang, J.; Yu, J.; Zhang, J. Poly (propylene carbonate)/clay nanocomposites with enhanced mechanical property, thermal stability and oxygen barrier property. Compos. Commun. 2020, 22, 100520. [Google Scholar] [CrossRef]
- Xie, D.; Wang, X.; Wu, L.L.; Zhang, C.C. Preparation of poly (propylene carbonate)/nano-silica composite. China Plast. Ind. 2018, 46, 24–29. [Google Scholar]
- Li, M.Q.; Zhao, X.R.; Zhang, J.; Li, R.M.; Zhang, Y.D.; Chang, H.B.; Li, X.H.; Zhang, Z.J. Preparation and properties of activated clay/PPC composites. Chem. Res. 2021, 32, 514–518. [Google Scholar]
- Zhang, Y.X.; Yan, L.; Lu, X.L. Study on mechanical properties and thermal stability of aluminum hydroxide reinforced PPC composites. Plast. Sci. Technol. 2019, 47, 20–24. [Google Scholar]
- Liu, Y.M.; Zhang, C.L.; Hai, S.S.; Zhang, W.N.; Yu, X. Preparation and characterization of flame retardant and Reinforced PPC composites with boehmite. Plast. Sci. Technol. 2018, 46, 73–77. [Google Scholar]
- Zheng, F.; Mi, Q.H.; Zhang, K.; Xu, J. Synthesis and characterization of poly(propylene carbonate)/modified sepiolite nanocom-posites. Polym. Compos. 2016, 37, 21–27. [Google Scholar] [CrossRef]
- Chen, T.T.; Yu, W. Modification of poly (propylene carbonate) by halloysite nanotubes. China Plast. 2017, 31, 21–27. [Google Scholar]
- Zhu, J.Y.; Dong, X.M.; Wang, N.; Yuan, N.X.; Sun, H.Y. Effects of calcium carbonate on mechanical properties and thermal stability of cassava straw flour/PPC composites. Chin. J. Wood Sci. Technol. 2020, 34, 27–31. [Google Scholar]
- Guo, Y.Y. Research progress of degradable plastic materials. China Pet. Chem. Stand. Qual. 2017, 37, 74–75. [Google Scholar]
- Tan, L.C. Synthesis of Biodegradable Copolyesters and Antibacterial Polyesters via Melting Transesterification and Their Nanocomposites. Ph.D. Thesis, Nanchang University, Nanchang, China, 2012. [Google Scholar]
- Guo, C.Q.; Yang, Q.F. Studies on modifying poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Chem. Adhes. 2002, 5, 217–219 + 240. [Google Scholar]
- An, J.J.; Liu, W.R.; Ke, Y.C.; Huang, G.B.; Ma, Y.M.; Wang, F.S. Preparation and characterization of poly (propylene carbonate)/cellulose composite. Plastics 2014, 43, 65–68. [Google Scholar]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion properties of nanocellulose: A review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef]
- Pan, L.S.; Xiong, Y.L.; Pang, S.J.; Dai, H.X.; Xu, N.; Lin, Q. Modification of poly (propylene carbonate) by blending lignin. China Pulp Pap. 2011, 30, 26–30. [Google Scholar]
- Xiao, X.; Jiang, C.; Zhang, Y.; Cai, Z.; Yu, P. Preparation and characterization of formaldehyde-modified black liquor lignin/poly (propylene carbonate) composites. Int. J. Polym. Anal. Charact. 2018, 23, 346–353. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, Y.; Xiao, X.; Cai, Z.; Yu, P. High-performance hydroxypropyl black liquor lignin/poly (propylene carbonate) bio-composites with enhanced natural degradability. Polym. Test. 2018, 72, 348–356. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Chang, P.R.; Chen, Y.; Anderson, D.P.; Stumborg, M. Properties and structural characterization of oxidized starch/PVA/α-zirconium phosphate composites. J. Appl. Polym. Sci. 2010, 115, 1089–1097. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Chen, J.; Chen, Y.; Anderson, D.P.; Chang, P.R. Oxidized pea starch/chitosan composite films: Structural characterization and properties. J. Appl. Polym. Sci. 2010, 118, 3082–3088. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, J.H.; Feng, J.; Huang, H.X.; Zhang, Y.D. Rheological behavior, morphology and mechanical property of poly (propylene car-bonate)/thermoplastic oxidized starch composites. CIESC J. 2015, 66, 2718–2724. [Google Scholar]
- Jiang, G.; Xu, J.; Zhao, N.; Zhang, S.; Huang, H. Influence of starch oxidization and modification on interfacial interaction, rheological behavior, and properties of poly (propylene carbonate)/starch blends. Polym. Plast. Technol. Eng. 2017, 56, 1084–1095. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Hu, Q.; Li, T.; Ma, P.; Zhang, H.; Du, M.; Chen, M.; Dong, W. Core–shell starch nanoparticles improve the mechanical and thermal properties of poly (propylene carbonate). ACS Sustain. Chem. Eng. 2019, 7, 13081–13088. [Google Scholar] [CrossRef]
- Pang, S.J.; Xu, N.; Pan, L.S.; Xu, G.; Wang, X. Study on melt blending modification of PPC/CS. Plast. Sci. Technol. 2013, 41, 73–77. [Google Scholar]
- Qin, Y.; Chen, L.; Wang, X.; Zhao, X.; Wang, F. Enhanced mechanical performance of poly (propylene carbonate) via hydrogen bonding interaction with o-lauroyl chitosan. Carbohydr. Polym. 2011, 84, 329–334. [Google Scholar] [CrossRef]
- Chang, H.; Li, Q.; Xu, C.; Li, R.; Wang, H.; Bu, Z.; Lin, T. Wool powder: An efficient additive to improve mechanical and thermal properties of poly (propylene carbonate). Compos. Sci. Technol. 2017, 153, 119–127. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Guo, J.X.; Wang, N.; Sun, H.Y.; Yuan, N.X. Effects of particle size and filling amount on mechanical properties of cassava straw pow-der/PPC composites. Chin. J. Wood Sci. Technol. 2019, 33, 5–8. [Google Scholar]
- Xia, G.; Reddy, K.O.; Maheswari, C.U.; Jayaramudu, J.; Zhang, J.; Zhang, J.; Rajulu, A.V. Preparation and properties of biodegradable spent tea leaf powder/poly (propylene carbonate) composite films. Int. J. Polym. Anal. Charact. 2015, 20, 377–387. [Google Scholar] [CrossRef]
- Liu, X.J.; Pang, S.J.; Xu, N.; Pan, L.S.; Lin, Q.; Ma, N.S.; Xu, R.Z.; Wang, Y.Y.; Yang, S.Y. Study of polyurethane prepolymers on the interface modification and performance of sisal fi-ber/poly(propylcarbonate)composites. China Plast. Ind. 2013, 41, 55–59. [Google Scholar]
- Feng, Y.; Ashok, B.; Madhukar, K.; Zhang, J.; Zhang, J.; Reddy, K.O.; Rajulu, A.V. Preparation and characterization of polypropylene carbonate bio-filler (eggshell powder) composite films. Int. J. Polym. Anal. Charact. 2014, 19, 637–647. [Google Scholar] [CrossRef]
- Gao, J.; Bai, H.; Zhang, Q.; Gao, Y.; Chen, L.; Fu, Q. Effect of homopolymer poly (vinyl acetate) on compatibility and mechanical properties of poly (propylene carbonate)/poly (lactic acid) blends. Express Polym. Lett. 2012, 6, 860–870. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.C.; Han, C.Y.; Wang, X.H.; Huang, D.X. Sustainable blends of poly (propylene carbonate) and stereocomplex polylactide with enhanced rheological properties and heat resistance. Chin. J. Polym. Sci. 2020, 38, 1267–1275. [Google Scholar] [CrossRef]
- Haneef, I.; Buys, Y.; Shaffiar, N.M.; Shaharuddin, S.I.; Khairusshima, M.N. Miscibility, mechanical, and thermal properties of polylactic acid/polypropylene carbonate (PLA/PPC) blends prepared by melt-mixing method. Mater. Today Proc. 2019, 17, 534–542. [Google Scholar] [CrossRef]
- Wang, S.F.; Tao, J.; Guo, T.Y.; Fu, T.; Yuan, C.Y.; Zheng, J.P.; Song, C.J. Thermal characteristics, mechanical properties and biodegradability of polycarbonates/poly(lactic acid)(PPC/PLA)blends. Ion Exch. Adsorpt. 2007, 23, 1–9. [Google Scholar]
- Kang, K.E.; Jin, Y.J.; Wang, B.; Weng, Y.X. Study on compatibilizing modification of PLA/PPC blends with long-chain hyperbranched polymer. China Plast. 2019, 33, 1–7. [Google Scholar]
- Wang, X.Y.; Sai, H.J.; Zhang, Y.X.; Wang, F.Y. Phase morphology and properties of ternary PLA/PPC/OMMT nanocomposties. China Plast. 2018, 32, 49–56. [Google Scholar]
- Tao, J.; Hu, D.; Liu, L.; Kong, M.M.; Song, C.J.; Wang, S.F. Thermal characteristics, mechanical properties and degradability of PLA/PPC/PHBV blends. Ion Ex Change Adsorpt. 2010, 26, 59–67. [Google Scholar]
- Jiang, G.; Yu, L. High Strength and Barrier Properties of Biodegradable PPC/PBSA Blends Prepared by Reaction Compatibilization for Promising Application in Packaging. Macromol. Mater. Eng. 2021, 306, 2000723. [Google Scholar] [CrossRef]
- Guillaume, S.M. Sustainable and degradable plastics. Nat. Chem. 2022, 14, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.L.; Wang, Z.J.; Tang, L.X. Structure and properties of PPC/Si-g-PCL nanocomposites. Polym. Mater. Sci. Eng. 2020, 36, 74–80. [Google Scholar]
- Zhang, Y.L.; Wang, W.Z.; Li, L.L.; Zhang, K.Y. Research progress of ternary polymerization of carbon dioxide and epoxides. Polym. Bull. 2021, 8, 19–25. [Google Scholar]
- Yang, J.; Zhang, X.; Li, T.; Wang, Y.; Xia, B.; Jiang, J.; Chen, M.; Dong, W. A novel biodegradable poly (propylene carbonate) with enhanced thermal and mechanical properties by incorporating tannic acid. Polym. Adv. Technol. 2022, 33, 1341–1347. [Google Scholar] [CrossRef]
- Tong, Y.; Cheng, R.; Yu, L.; Liu, B. New strategies for synthesis of amino-functionalized poly (propylene carbonate) over SalenCo (III) Cl catalyst. J. Polym. Sci. 2020, 58, 1325–1337. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, S.L.; Wang, Z.P.; Chen, Y.J.; Bian, J.J.; Han, L.J.; Zhang, H.L.; Dong, L.S. Thermal, Rheological and Mechanical Properties of Biodegradable Poly (propylene carbonate)/Epoxidized Soybean Oil Blends. Chin. J. Polym. Sci. 2021, 39, 1572–1580. [Google Scholar] [CrossRef]
- Huang, M.; Gao, L.; Feng, J.; Huang, X.; Li, Z.; Huang, Z.; Wang, L. Cross-linked networks in poly (propylene carbonate) by incorporating (maleic anhydride/cis-1, 2, 3, 6-tetrahydrophthalic anhydride) oligomer in CO2/propylene oxide copolymerization: Improving and tailoring thermal, mechanical, and dimensional properties. ACS Omega 2020, 5, 17808–17817. [Google Scholar] [CrossRef]
- Li, K. Strengthening and Toughening of Poly(Propylene Carbonate) Using Non-Isocyanate Urethane. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2018. [Google Scholar]
- Wang, P.; Zhang, W.; Pi, Q.; Chen, W.F. Progress in the synthesis and application of polymethylene-ethylene-carbonate. Shandong Chem. Ind. 2016, 45, 33–35. [Google Scholar]
- Liu, Z.; Hu, J.; Gao, F.; Cao, H.; Zhou, Q.; Wang, X. Biodegradable and resilient poly (propylene carbonate) based foam from high pressure CO2 foaming. Polym. Degrad. Stab. 2019, 165, 12–19. [Google Scholar] [CrossRef]
- Zeng, B.; Li, Y.; Wang, L.; Zheng, Y.; Shen, J.; Guo, S. Body temperature-triggered shape-memory effect via toughening sustainable poly (propylene carbonate) with thermoplastic polyurethane: Toward potential application of biomedical stents. ACS Sustain. Chem. Eng. 2020, 8, 1538–1547. [Google Scholar] [CrossRef]
- Didwal, P.N.; Singhbabu, Y.N.; Verma, R.; Sung, B.J.; Lee, G.H.; Lee, J.S.; Chang, D.R.; Park, C.J. An advanced solid polymer electrolyte composed of poly (propylene carbonate) and mesoporous silica nanoparticles for use in all-solid-state lithium-ion batteries. Energy Storage Mater. 2021, 37, 476–490. [Google Scholar] [CrossRef]
- Hu, J.L.; Gao, C.; Mi, W.T.; Bao, W. Application progress of polypropylene carbonate. China Plast. Ind. 2021, 49, 1–4 + 80. [Google Scholar]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, L.S.; Xu, N.; Pang, S.J.; Lin, C.; Chen, L.M. Preparation and performance of biodegradable poly(propylene carbonate) spun-bonded non-woven. Plastics 2018, 47, 64–68 + 71. [Google Scholar]
- Zhang, Z.Q.; Xu, N.; Pan, L.S.; Pang, S.J.; Xu, R.Z.; Ma, N.S.; Wei, T.Y.; Chen, Z.; Chen, L.M. Preparation and property of poly(lactic acid)/poly(propylene carbonate) spunlaid nonwoven slice. New Chem. Mater. 2015, 43, 40–43. [Google Scholar]
- Wang, X.; Pan, H.; Jia, S.; Lu, Z.; Han, L.; Zhang, H. Mechanical properties, thermal behavior, miscibility and light stability of the poly (butylene adipate-co-terephthalate)/poly (propylene carbonate)/polylactide mulch films. Polym. Bull. 2022, 79, 1–17. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, J.; Yao, Z.; Luo, S.; Tian, L.; Tian, C.; Sun, Y. Preliminary Findings of Polypropylene Carbonate (PPC) Plastic Film Mulching Effects on the Soil Microbial Community. Agriculture 2022, 12, 406. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.S.; Xiao, X.F.; Luo, Z.J.; Xia, L.L.; Jiang, C. Preparation and characterization of cellulose acetate/polypropylene carbonate complex films. Plastics 2018, 47, 64–66 + 71. [Google Scholar]
- Cheng, P.F.; Liang, M.; Yun, X.Y.; Dong, T. Biodegradable blend films of poly (ε-caprolactone)/poly (propylene carbonate) for shelf life extension of whole white button mushrooms. J. Food Sci. Technol. 2022, 59, 144–156. [Google Scholar] [CrossRef]
- Bahramian, B.; Chrzanowski, W.; Kondyurin, A.; Thomas, N.; Dehghani, F. Fabrication of antimicrobial poly (propylene carbonate) film by plasma surface modification. Ind. Eng. Chem. Res. 2017, 56, 12578–12587. [Google Scholar] [CrossRef]
- Kim, G.; Ree, M.; Kim, H.; Kim, I.J.; Kim, J.R.; Lee, J.I. Biological affinity and biodegradability of poly (propylene carbonate) prepared from copolymerization of carbon dioxide with propylene oxide. Macromol. Res. 2008, 16, 473–480. [Google Scholar] [CrossRef]
- Zhu, Y.H. An Injectable Hydroxyapatite/Poly(Lactide-Co-Glycolide) Composite Reinforced by Micro/Nano-Hybrid Poly(Glycolide) Fibers for Bone Repair. Ph.D. Thesis, Jilin University, Changchun, China, 2018. [Google Scholar]
- Li, G.; Liu, X.N.; Zhang, D.H.; He, M.; Qin, S.H.; Yu, J. Preparation and properties of nano-hydroxyapatite/polylactic acid porous bioengineering materials. Polym. Mater. Sci. Eng. 2018, 34, 158–164. [Google Scholar]
- Zhang, Y.X.; Yan, L.; Lu, X.L. Preparation and Properties of PPC/Nano-hydroxyapatite Composites. Plast. Sci. Technol. 2019, 47, 7–11. [Google Scholar]
- Jiang, Y.; Yang, S.P.; Gang, H.L.; Xi, M.; He, J.W. Preparation and properties of PPC/diatomite composite fiber membrane. China Elastomerics 2020, 30, 1–5. [Google Scholar]
- Chen, X.; Zhao, S.; Chu, S.; Liu, S.; Yu, M.; Li, J.; Gao, F.; Liu, Y. A novel sustained release fluoride strip based Poly (propylene carbonate) for preventing caries. Eur. J. Pharm. Sci. 2022, 171, 106128. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, F.; Li, H.; Zhang, S.; Liu, X.; Xu, Q. Ionic liquid-modified poly (propylene carbonate)-based electrolyte for all-solid-state lithium battery. Ionics 2020, 26, 5503–5511. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, L.; Ju, B.; Lei, G.; Zhou, Q.; Liao, H.; Yin, A.; Deng, Z.; Tang, Y.; Qin, S.; et al. Ameliorating Interfacial Issues of LiNi0. 5Co0. 2Mn0. 3O2/Poly (propylene carbonate) by Introducing Graphene Interlayer for All-Solid-State Lithium Batteries. Chem. Sel. 2020, 5, 2291–2299. [Google Scholar]
- Zhu, Z.; Xia, K.; Fu, J.; Du, C.; Zhang, H.; Lou, H.; Xu, Z. Bonding of aluminum coated silicon wafers based on polypropylene carbonate and as a multi-functional sensor. Org. Electron. 2018, 63, 296–299. [Google Scholar] [CrossRef]
- Qi, X.; Jing, M.; Liu, Z.; Dong, P.; Liu, T.; Fu, Q. Microfibrillated cellulose reinforced bio-based poly (propylene carbonate) with dual-responsive shape memory properties. RSC Adv. 2016, 6, 7560–7567. [Google Scholar] [CrossRef]
- Zeng, B.; Cao, M.; Shen, J.; Yang, K.; Zheng, Y.; Guo, S. Tuning the dual-and triple-shape-memory effect of thermoplastic polyurethane/polylactic acid/poly (propylene carbonate) ternary blends via morphology control. Polymer 2022, 242, 124546. [Google Scholar] [CrossRef]
- Ge, J.; Li, F.; Gao, Y.; Jin, J.; Jiang, W. A high-performance structural material based on maize straws and its biodegradable composites of poly (propylene carbonate). Cellulose 2021, 28, 11381–11395. [Google Scholar] [CrossRef]


| Type | Abbreviation | Structural Formula | Application |
|---|---|---|---|
| Thermoplastic starch | TPS | 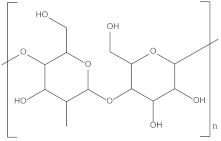 | Packaging, shopping bags, garbage bags, mulch films, disposable tableware and disposable medical products. |
| Polyhydroxyalkanoate | PHA | 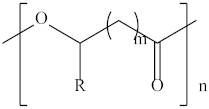 | Tissue engineering, medical implants, controlled drug delivery systems, packaging, mulch films and disposable medical products. |
| Polyglutamic acid | PGA |  | Food thickeners, stabilisers, surgery, food, cosmetics, pharmaceutical industry, agriculture. |
| Poly (lactic acid) | PLA | 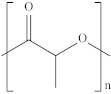 | Packaging, shopping bags, garbage bags, mulch films, disposable tableware, disposable medical products, building materials and textiles. |
| Poly (butylene succinate) | PBS |  | Packaging, shopping bags, garbage bags, pesticide and fertilizer sustained-release materials, mulch films and disposable tableware. |
| Poly (butylene succinate-co-butylene adipate) | PBSA | 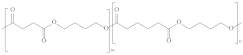 | Packaging, shopping bags, garbage bags and mulch films. |
| Poly (butylene adipate-co-terephthalate) | PBAT |  | Packaging, shopping bags, garbage bags, mulch films and disposable tableware. |
| Polycaprolactone | PCL | 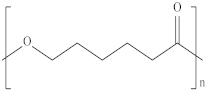 | Medical implants, controlled drug delivery systems, absorbable surgical sutures and cryogenic packaging. |
| Poly (propylene carbonate) | PPC | 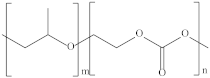 | Cryogenic packaging, mulch films, foam materials, controlled drug delivery systems and high-barrier materials. |
| Poly (vinyl alcohol) | PVA |  | Soluble packaging, high-barrier materials and medical implant. |
| Category | Typical Catalyst | Features | |
|---|---|---|---|
| Heterogeneous catalysts | ZnEt2-active hydrogen [25,30,31] | (1) | Low catalytic activity. |
| (2) | High price. | ||
| Zinc carboxylic acid [32] | (1) | Easy preparation and low cost. | |
| (2) | Long reaction time. | ||
| Double metal cyanide complex [33,34] | (1) | High catalytic activity. | |
| (2) | Polymers with low Mn (a) and low CO2 fixation. | ||
| Ternary rare-earth catalyst [35] | (1) | Mn over 100 kg/mol in a relatively short time. | |
| (2) | Catalytic activity needs improvement. | ||
| Homogeneous catalyst | Metal-porphyrin [36] | (1) | High catalytic activity but very slow polymerization rate. |
| (2) | Catalyst structure is clear. | ||
| (3) | Simple to synthesize and easy to handle. | ||
| (4) | Product may have an undesirable color. | ||
| Zinc and cadmium phenoxides [37,38] | (1) | Catalyst structure is clear. | |
| (2) | Rapidly induced copolymerization. | ||
| (3) | Most of the polymers have a molecular weight of less than 100 kg/mol. | ||
| β-Diiminate zinc [39,40] | (1) | Catalyst structure is clear. | |
| (2) | Controlled ring opening. | ||
| Metal-salen or -salan complexes [41] | (1) | Catalyst structure is clear. | |
| (2) | High selectivity. | ||
| (3) | High catalytic activity. | ||
| (4) | Product may have an undesirable color. |
| No. | Catalyst | PPC Yield (a) | PPC Product | Ref. | |||
|---|---|---|---|---|---|---|---|
| Mn (b) | Mw (c) | PDI (d) | [η] (e),dL/g | ||||
| 1 | ZnGA | 83 (g polymer/g of catalyst) | 160 k | 60 k | 2.7 | - | [42] |
| 2 | ZnGA + GA | 68.25 (g polymer/g of catalyst) | - | - | 1.2815 | - | [43] |
| 3 | -RE(P204)3-Al(i-Bu)3-R(OH)n | 1672 (g/mol of Y (f)) | 46.9 × 10−4 (g/mol) | - | - | 3.82 | [44] |
| 4 | Nd(CCl3COO)3-ZnEt2-glycerol ternary catalyst | Improving | 62,282 | 73,412 | - | 0.76 | [45] |
| 5 | Lewis Base | 416.1 (g/(mol Zn)) | 11.0 × 10−4 (g/mol) | - | 2.9 | - | [46] |
| 6 | Zn3 [Co(CN)6]2-based Co-Zn DMC catalyst | 7488 (g polymer/g of catalyst) | 35,900 | - | 3.99 | - | [47] |
| 7 | Zn-Mg-Al composite oxide high-efficiency catalyst | 88.8% | - | - | - | - | [48] |
| Characteristic | Numerical Values |
|---|---|
| Glass transition temperature (°C) | 30, 33, 41 |
| Elastic modulus (MPa) | 993 |
| Tensile strength (MPa) | 33.2 |
| Density (103 kJ/kg) | 1.275, 1.3 |
| Permittivity (kHz) | 3.0 |
| Combustion heat (103 kJ/kg) | 18.5 |
| Refractive index, n | 1.463 |
| Hydroscopicity (23 °C, %) | 0.397 |
| Thermally decomposed temperature (°C) | 218 |
| Venting quality N2 (ml-cm10−12) | 5.3 |
| Material | H2O (g/m2/24h) | O2 (cm3/m2/d/atm) |
|---|---|---|
| PPC | 40–60 | 10–20 |
| Biaxially oriented polyethylene terephthalate | 100 | 60–100 |
| Bidirectional oriented polypropylene | - | 2000 |
| High-density Polyethylene | 20 | 1400 |
| Nylon-6 | 150 | 25–40 |
| Polyvinylidene chloride | 0.4–1 | <1 |
| Ethylene-vinyl alcohol copolymer | 20–70 | 0.1–1 |
| PBS | - | 1200 |
| PLA | 325 | 550 |
| Ecoflex (BASF) | 170 | 1400 |
| Ecoflex/PPC/PBS triple-coextruded film | 5 | 9.3 |
| Ecoflex/PPC/LDPE triple-coextruded film | 5.3 | 9.5 |
| No. | Materials | Preparation Method (a),(b) | Amount Added (wt%) | Performance Enhancement | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical Behavior | Thermal Properties | |||||||||
| Tensile Strength/MPa | Elongation at Break/% | Tg/°C | Td−5%/°C (c) | Td−10%/°C (d) | Tmax/°C(e) | |||||
| 1 | Carbon fiber | M | 0–20 | - | - | 42 | - | - | - | [71] |
| 2 | Graphene/Si hybrids | M | 0–5 | 35.5 ± 1.3 | 36.7 ± 1.5 | 34.2 | 289.5 | - | - | [72] |
| 3 | Elokite nanotubes | M | 0–10 | 22.6 | - | - | 285.1 | 311.3 | 311.3 | [73] |
| 4 | Montmorillonite | S | 0~10 | - | - | - | - | 280 °C | - | |
| 5 | Laponite | S | 0–10 | - | - | - | - | 250 | - | [74] |
| 6 | Activated white clay | S | 0–2 | 36.8 ± 1.7 | 92 ± 16 | 32.7 | 260 | - | 276 | [75] |
| 7 | Boehmite | M | 0–20 | 37.83 | lower | - | 399.9 | 410 | 439.4 | [76] |
| 8 | -modified sepiolite | S | 0–10 | 25.6 | 216 | 35.8 | 288.7 | - | 322.9 | [77] |
| 9 | Nanosilica | S | - | 15 | 498 | 37 | 238 | - | - | [78] |
| 10 | Calcium carbonate | M | 0–20 | 36.6 | - | - | 256 | - | 292 | [79] |
| 11 | Aluminum hydroxide | M | 0–20 | 31.54 | - | - | - | - | - | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Meng, L.; Zhang, Y.; Qin, Z.; Meng, L.; Li, C.; Liu, M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers 2022, 14, 2159. https://doi.org/10.3390/polym14112159
Li X, Meng L, Zhang Y, Qin Z, Meng L, Li C, Liu M. Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers. 2022; 14(11):2159. https://doi.org/10.3390/polym14112159
Chicago/Turabian StyleLi, Xiangrui, Lingyu Meng, Yinliang Zhang, Zexiu Qin, Lipeng Meng, Chunfeng Li, and Mingli Liu. 2022. "Research and Application of Polypropylene Carbonate Composite Materials: A Review" Polymers 14, no. 11: 2159. https://doi.org/10.3390/polym14112159
APA StyleLi, X., Meng, L., Zhang, Y., Qin, Z., Meng, L., Li, C., & Liu, M. (2022). Research and Application of Polypropylene Carbonate Composite Materials: A Review. Polymers, 14(11), 2159. https://doi.org/10.3390/polym14112159